Abstract
Radiotherapy is the most important component of the comprehensive treatment of breast cancer, and immunocompromised patients respond with lower response rate. However, the role of programmed cell death protein-1, a critical immune molecule, in recurrence of breast cancer subjected to radiotherapy is unknown. A retrospective analysis was designed to explore the relevance. A number of 42 patients with early-stage breast cancer undergoing breast-conserving surgery and postoperative radiotherapy (18 recurrence and 24 nonrecurrence) were recruited, and clinical data were obtained. Immunohistochemistry was employed to detect programmed cell death protein-1, and Kaplan-Meier curves were used to analyze recurrence-free survival. The expression of programmed cell death protein-1 was higher in the recurrence group than recurrence-free group (P < .05). Meanwhile, the recurrence-free mean survival was significantly longer in programmed cell death protein-1 low-expression group (68 months) than that in programmed cell death protein-1 high-expression group (56 months). In addition, the levels of T lymphocytes were obviously lower in patients with breast cancer than healthy group, and natural killer showed an opposite tendency. CD4+ decreased significantly after 1 week radiotherapy and recovered rapidly 3 weeks after radiotherapy. Compared to recurrence-free group, the increment of T lymphocytes were inadequate in recurrence group. These experimental results indicated that the expression of programmed cell death protein-1 in tumor-infiltrating lymphocytes is related to immune disorder and recurrence of patients undergoing breast-preserving surgery and radiotherapy.
Keywords: breast cancer, radiotherapy, PD-1, immune, recurrence
Introduction
Breast cancer is the most common cancer and the leading cause of cancer-related deaths in females all around the world. Researchers calculated that there was an estimated 1.7 million breast cancer and 521 900 deaths every year.1 In the United States, ductal carcinoma in situ (DCIS) accounts for more than 20% of all breast cancer types. The recurrence rate of DCIS is 26% to 36% (without radiotherapy) or 9% to 23% (radiotherapy), and half of the recurrence of cancers are invasive.2 Surgery remains the mainstream method for early-stage breast cancer, but prognosis depends on its invasiveness and metastasis. In recent years, the radiotherapy, chemotherapy, and biotherapy have been used more and more in tumorous comprehensive therapy.3,4 For those cases undergoing breast-preserving surgery or with high risk of recurrence, radiotherapy can significantly reduce the recurrence rate. However, a certain percentage of these patients still have breast cancer recurrence after radiotherapy.
Radiotherapy is the common means for the treatment of malignant tumors. A growing number of research revealed that radiotherapy was closely related to tumor immunity.5-7 In the course of radiation therapy, the body’s immune system was inevitably accepted to a certain extent radiation, resulting in damage to immune cells. In addition, bone marrow tissue was highly sensitive to radiation and easily been suppressed. The origin of bone marrow cells generation reduced, including the original immune cell. However, recent studies found that the hypolymphemia caused by radiotherapy followed by rapid proliferation of immune cells for restoring immunity homeostasis. The phenomenon of killing and regeneration was benefit to eliminate invalid T cells. Proliferated cells had the characteristics of memory cells and effector cells, which was beneficial to the enhancement of antitumor effect. Another experiment showed that low-dose radiation had immune-promoting effect by reducing the proportion and number of Treg cells.
The immune response of tumor microenvironment determines the biological behavior of tumor cells. Immune system plays a protective role through eliminating or inhibiting the tumor cells. Recently, researches indicated that the damagement of immune microenvironment had a negative effect on radiotherapy in various tumors.8-11 Programmed cell death protein-1 (PD-1) plays an important role in tumor immunity. Cancer cells escape immune attack by upregulating the expression of PD-1, which combined with PD-L1 and restricted host immune response. Study revealed that PD-1 shortened the survival of melanoma-bearing mice treated with radiotherapy. However, PD-1 blocking antibody led to an opposite tendency in further experiment. And the combination of stereotactic ablative radiotherapy plus PD-1 blockade induced almost complete retrogression of the irradiated primary tumor, which was famous as synergistic effect.12 However, the role of PD-1 in recurrence of breast cancer subjected to radiotherapy is unknown. In this study, a retrospective analysis was designed to explore the relevance.
Materials and Methods
Patient Data
The retrospective study included 42 patients with breast cancer from January 2012 to June 2017 in our department, whose diagnosis was confirmed by surgery and pathology. The total 42 cases contained 18 recurrent cases and 24 recurrence-free cases. The inclusion criteria in the study were as follows: (1) newly diagnosed, unilateral breast cancer, and underwent surgery. (2) Accepted postoperative radiotherapy, 5 times a week and 2 Gy every time. Carry out the radiation dose of 50 Gy and append 10 Gy to tumor bed. (3) Availability of at least 1 block of formalin-fixed and paraffin-embedded tumor tissue containing viable breast cancer tissue, used for immunohistochemistry (IHC). (4) Availability of full clinical follow-up data, no prior chemotherapy or radiotherapy before surgery. We followed up for 5 years and the median follow-up time was 62 months. The clinical data of age, gender, tumor location, tumor size, histological type, and TNM stage were collected. The study was approved by the ethics committee of The Affiliated Jiangning Hospital of Nanjing Medical University (#20170302), and informed consent was obtained from all patients and volunteers.
Immunohistochemistry Analysis
The tissue sections from available tumors were collected for IHC. Four-micrometer sections were cut and mounted on SuperFrost Plus electrostatically charged glass slides with the help of pathology department. Sections were dried overnight in a 37°C incubator followed by PD-1 staining. The sections were treated lightly according to the instructions of immunostain streptavidin–peroxidase and SP kit before incubation of antibody. For PD-1 staining, the slides were incubated with a monoclonal rabbit anti-PD-1 antibody (Cell Signaling Technology, Massachusetts, USA) applied at a 1/200 dilution and detected with the UltraView Universal DAB Detection Kit. All slides were counterstained for 4 minutes with hematoxylin followed by a 4-minute incubation with Bluing Reagent. The stained sections were dehydrated, and glass coverslips were mounted. The PD-1 expression of tumor-infiltrating lymphocytes (TILs) on IHC-stained tissues was independently performed by 2 trained pathologists who were blinded to the clinical and experimental data. The expression of PD-1 was assessed as a continuous variable based on the percentage of tissue area occupied by PD-1 positive T cells. Discordant results were jointly reevaluated by the pathologists.
T Lymphocyte Subsets Detection
T lymphocyte subsets (CD3+, CD4+, CD8+, and natural killer [NK]) of peripheral blood were detected in patients and 20 health people who took physical examination by laboratory. Those subsets determined before and after postoperative radiotherapy when at the time of the primary treatment. Before radiotherapy, 1 week after radiotherapy and 3 weeks after radiotherapy were detection time point, respectively.
Statistical Analysis
All statistical analyses were performed using SPSS software (IBM SPSS Statistics for Macintosh version 20.0). The Student t test and the χ2 test were used to evaluate statistical differences of PD-1 expression in different samples and examine the relationship between PD-1 expression and clinicopathological features. The recurrence-free survival of patient was estimated using the Kaplan-Meier method. In all cases, P < .05 was considered statistically significant.
Results
Correlation of PD-1 Expression With Clinicopathological Features
The clinical characteristics of patients and grouping were summarized in Table 1. A total of 24 recurrence-free and 18 recurrence female patients were enrolled. All these patients were at an early stage of breast cancer (8 stage I and 34 stage II according to TNM stage). The PD-1 was detected, and the median expression level was used to separate the patients into 2 subgroups (low-expression group [n = 21] and high-expression group [n = 21]). We found recrudesce was obviously associated with the expression of PD-1 in TILs (P = .013). There was no difference between PD-1 and other clinicopathological characteristics, such as age, TNM stage, and differentiation (Table 2).
Table 1.
Patient Characteristics.
| Feather | RFG | RG | P Value |
|---|---|---|---|
| Total cases | |||
| 42 | 24 | 18 | - |
| Gender | |||
| Male | 0 | 0 | - |
| Female | 24 | 18 | - |
| Age | |||
| ≤35 | 7 | 5 | .921 |
| >35 | 17 | 13 | |
| TNM stage | |||
| I | 4 | 4 | .955 |
| II | 20 | 14 | |
| Differentiation | |||
| Well | 4 | 3 | 1.000 |
| Moderate | 17 | 13 | .921 |
| Poorly | 3 | 2 | 1.000 |
| Herceptin | |||
| Yes | 8 | 5 | .700 |
| No | 16 | 13 | |
| Endocrine therapy | |||
| Yes | 15 | 10 | .650 |
| No | 9 | 8 | |
| PD-1 | |||
| Low | 16 | 5 | .013* |
| High | 8 | 13 | |
Abbreviations: PD-1, programmed cell death protein-1; RFG, recurrence-free group; RG, recurrence group.
Table 2.
Correlation Between PD-1 and Clinicopathological Characteristics.
| Feather | PD-1 Low | PD-1 High | P Value |
|---|---|---|---|
| Total cases | |||
| 42 | 21 | 21 | - |
| Gender | |||
| Male | 0 | 0 | - |
| Female | 21 | 21 | |
| Age | |||
| ≤35 | 5 | 7 | .495 |
| >35 | 16 | 14 | |
| TNM stage | |||
| I | 5 | 3 | .694 |
| II | 16 | 18 | |
| Differentiation | |||
| Well | 5 | 2 | .408 |
| Moderate | 15 | 15 | 1.000 |
| Poorly | 1 | 4 | 0.341 |
| Herceptin | |||
| Yes | 8 | 5 | .317 |
| No | 13 | 16 | |
| Endocrine therapy | |||
| Yes | 15 | 10 | .116 |
| No | 6 | 11 | |
Abbreviation: PD-1, programmed cell death protein-1.
Association of PD-1 Expression and Recurrence-Free Survival
Mean follow-up time was 62 months. The recurrence-free mean survival in PD-1 low-expression group was 68 months, and the recurrence-free mean survival in PD-1 high-expression group was 56 months. Recurrence and June 2017 acted as the end of the follow-up. As shown in Figure 1, Kaplan-Meier curves showed that the recurrence-free survival was significantly longer in PD-1 low-expression group than that in PD-1 high-expression group.
Figure 1.
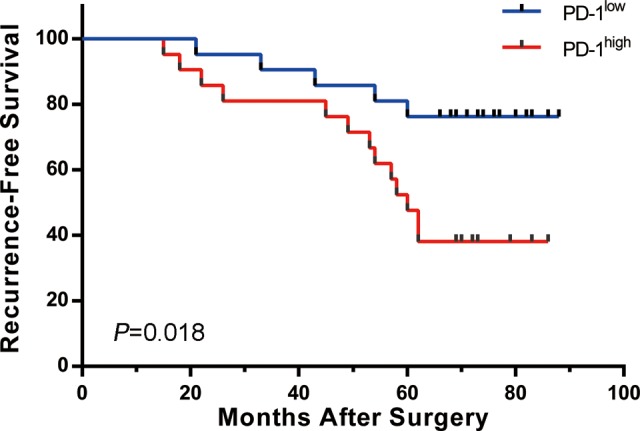
Recurrence-free survival. Kaplan-Meier curves showing recurrence-free mean survival time of programmed cell death protein-1 (PD-1) low-expression group and PD-1 high-expression group.
Association of T-Lymphocyte Subsets and PD-1 Expression
The levels of CD4+ and CD4+/CD8+ were obviously lower in patients with breast cancer than healthy group (P < .05; Table 3). On the contrary, the NK expression was higher (P < .05). CD4+ decreased significantly after 1 week’s radiotherapy and recovered rapidly 3 weeks after radiotherapy. Compared to that in recurrence-free group (RFG), the expression of CD4+ was lower in the recurrence group (RG) both at 1 week after radiotherapy point and 3 weeks after radiotherapy point (P < .05). The increments in most T lymphocytes were significantly lower (P < .05) in RG than that in RFG. Compared to that in PD-1 low group, the expression of CD4+ was lower PD-1 high group both at 1 week after radiotherapy point and 3 weeks after radiotherapy point (P < .05; Table 4). The increments of CD4+ were significantly lower (P < .05) in PD-1 high group than that in PD-1 low group.
Table 3.
Correlation Between T Lymphocyte Subsets and Radiotherapy.a
| Groups | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | NK |
|---|---|---|---|---|---|
| HG | 72.2 ± 4.4 | 53.7 ± 2.3 | 33.2 ± 3.5 | 1.62 ± 0.21 | 8.1 ± 2.6 |
| Before radiotherapy | |||||
| RFG | 68.9 ± 4.2 | 43.5 ± 1.9b | 32.5 ± 3.2 | 1.34 ± 0.12b | 11.9 ± 3.4b |
| RG | 67.8 ± 3.6 | 41.1 ± 2.2b | 35.9 ± 4.3 | 1.14 ± 0.19b | 12.1 ± 2.9b |
| 1 week after radiotherapy | |||||
| RFG | 66.6 ± 5.2 | 38.7 ± 1.8 b,c | 35.6 ± 5.6 | 1.08 ± 0.25b,c | 9.8 ± 2.1 |
| RG | 65.3 ± 4.7b | 35.9 ± 3.6 b,c,e | 37.1 ± 4.8b | 0.96 ± 0.24b | 9.3 ± 3.2 |
| 3 week after radiotherapy | |||||
| RFG | 66.9 ± 3.9 | 43.3 ± 2.8b,d | 33.7 ± 4.3 | 1.28 ± 0.22b | 10.8 ± 4.9 |
| RG | 66.1 ± 4.6b | 39.2 ± 4.1b,d,e | 36.3 ± 3.9b | 1.07 ± 0.23b,e | 11.7 ± 4.8b,d |
Abbreviations: HG, healthy group; NK, natural killer; RFG, recurrence-free group; RG, recurrence group.
a Results are expressed as the means ± SEM.
b P < .05 versus HG.
c P < .05 versus before radiotherapy.
d P < .5 versus 1 week after radiotherapy;
e P < .05 versus RFG.
Table 4.
Correlation Between PD-1 and T Lymphocyte Subsets When Receiving Radiotherapy.a
| Groups | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | NK |
|---|---|---|---|---|---|
| HG | 72.2 ± 4.4 | 53.7 ± 2.3 | 33.2 ± 3.5 | 1.62 ± 0.21 | 8.1 ± 2.6 |
| Before radiotherapy | |||||
| PD-1low | 66.2 ± 2.2 | 43.2 ± 1.7b | 33.0 ± 3.3 | 1.31 ± 0.14b | 11.5 ± 2.4b |
| PD-1high | 69.7 ± 4.6 | 41.6 ± 2.1b | 35.2 ± 4.4 | 1.18 ± 0.20b | 12.6 ± 2.9b |
| 1 week after radiotherapy | |||||
| PD-1low | 65.5 ± 4.4b | 38.4 ± 2.2b,c | 36.0 ± 5.8b | 1.01 ± 0.26b,c | 10.2 ± 3.1 |
| PD-1high | 66.6 ± 5.1 | 36.4 ± 3.2b,c,e | 36.5 ± 5.0b | 1.00 ± 0.22b | 8.7 ± 3.2 |
| 3 week after radiotherapy | |||||
| PD-1low | 65.8 ± 3.5b | 44.1 ± 2.7b,d | 34.0 ± 4.5 | 1.30 ± 0.19b,d | 10.4 ± 3.9 |
| PD-1high | 67.5 ± 4.2 | 39.5 ± 4.2b,d,e | 35.8 ± 3.7b | 1.10 ± 0.25b,e | 12.1 ± 2.8b,d |
Abbreviations: HG, healthy group; NK, natural killer; PD-1, programmed cell death protein-1; RFG, recurrence-free group.
a Results are expressed as the means ± SEM.
b P < .05 versus HG.
c P < .05 versus before radiotherapy.
d P < .5 versus 1 week after radiotherapy.
e P < .05 versus RFG.
Discussion
According to statistical information, the incidence of breast cancer in women in China was increasing year by year, and the mortality rate was the second. Surgical resection is the most direct and effective treatment of breast cancer. With people’s attention to their appearance, more and more patients choose breast-preserving surgery, especially in early stage. To further remove residual lesions, radiotherapy was an effective treatment and was applied in most patients.
A growing number of researches have revealed that radiotherapy could reduce the postoperative recurrence and improve prognosis in various cancers, including colorectal cancer, breast cancer, prostate cancer, and esophagus cancer.5,13 Postoperative breast irradiation was considered as standard after breast-preserving surgery for cancer.14 A 15-year follow-up study found that approximately 1 in 3 nonirradiated patients developed a local recurrence after breast-preserving excision for DCIS. However, adjuvant radiation therapy significantly reduced this risk.15 But, there were a part of women subjected to tumor recurrence after adjuvant radiation therapy and decreased survival quality.
It is generally known that immune system plays an important role in antitumor activity and immunocompromised subjects respond with lower response rate to treatment. In this study, a critical immune molecule PD-1 was detected to explore the role in recurrence of patients undergoing breast-preserving surgery and radiotherapy. This study demonstrated that the expression of PD-1 was significantly correlated with breast cancer recurrence. Kaplan-Meier curve analysis revealed that the recurrence-free mean survival was significantly longer in PD-1 low-expression group (68 months) than that in PD-1 high-expression group (56 months). Because overexpression of PD-1 could restrict host immune response and induce immune evasion of cancer cells, the rate of recurrence became higher. PD-1 may be a new therapeutic target for prevention of recurrence. In preclinical models of bladder cancer, study demonstrated anti-PD-1 or anti-PD-L1 antibodies could enhance radiotherapy-induced antitumor immunity.16 Another study revealed the synergistic antitumor immunity effect of radiotherapy and anti-PD-L1 antibody in non-small-cell lung cancer mouse model.17 The synergistic effect of anti-PD-1 antibodies with radiotherapy in breast cancer should been explored in the future.
In recent years, studies have shown that radiotherapy could not only suppress immunity but also activate antitumor immunity and cause bystander effect.12 The bystander effect can trigger systemic inflammatory reaction after local radiotherapy and promote tumor regression of distant nonradiation regional organization. In our study, compared to the healthy group, CD4+ and CD4+/CD8+ were obviously lower in the group with breast cancer. On the contrary, the NK expression was higher, which was consistent with the results of previous studies. Our results revealed that the radiotherapy could inhibit the immunity of the body. One week after radiotherapy, the expression of CD4+ was lower than that before radiotherapy, especially the RG. In addition, radiotherapy was proved to promote antitumor immunity, which mainly reflected in the following aspects, including T cell-enhancing tumor cell recognition, NK cell function activated, regulatory T cell function inhibited, apoptosis signal activated, and so on. Our research demonstrated an interesting phenomenon that CD3+, CD4+, and CD4+/CD8+ increased gradually 3 weeks after radiotherapy, which further validated the role of radiotherapy in promoting antitumor immunity.
Although this research was carefully prepared, there were some unavoidable limitations and shortcomings. First, the population of the experimental group was small, only 42 patients, and more works were necessary to confirm the results in larger samples in the future. Second, because of the single institute study, there was a potential selection bias to this study. It would be better if a multi-institute study was carried out.
Conclusions
The expression PD-1 in TILs is related to immune disorder and recurrence of patients undergoing breast-preserving surgery and radiotherapy.
Abbreviations
- DCIS
ductal carcinoma in situ
- IHC
immunohistochemistry
- NK
natural killer
- PD-1
programmed cell death protein-1
- RFG
recurrence-free group
- RG
recurrence group
- TILs
tumor-infiltrating lymphocytes
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Parikh U, Chhor CM, Mercado CL. Ductal carcinoma in situ: the whole truth. AJR Am J Roentgenol. 2018;210(2):246–255. [DOI] [PubMed] [Google Scholar]
- 3. Asselain B, Barlow W, Bartlett J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saiki H, Petersen IA, Scott CG, et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135(15):1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nardone V, Botta C, Caraglia M, et al. Tumor infiltrating T lymphocytes expressing FoxP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther. 2016;17(11):1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. [DOI] [PubMed] [Google Scholar]
- 7. Napolitano M, D’Alterio C, Cardone E, et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget. 2015;6(10):8261–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lhuillier C, Vanpouille-Box C, Galluzzi L, Formenti SC, Demaria S. Emerging biomarkers for the combination of radiotherapy and immune checkpoint blockers [published ahead of print December 16, 2017]. Semin Cancer Biol. doi: 10.1016/j.semcancer.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oweida A, Lennon S, Calame D, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(10):e1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahashi Y, Yasui T, Tamari K, et al. Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS One. 2017;12(12):e0189697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vanpouille-Box C, Lhuillier C, Bezu L, et al. Trial watch: immune checkpoint blockers for cancer therapy. Oncoimmunology. 2017;6(11):e1373237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park SS, Dong H, Liu X, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3(6):610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han J, Zhu W, Yu C, Zhou X, Li T, Zhang X. Clinical study of concurrent chemoradiotherapy or radiotherapy alone for esophageal cancer patients with positive lymph node metastasis. Tumori. 2012;98(1):60–65. [DOI] [PubMed] [Google Scholar]
- 14. Holli K, Hietanen P, Saaristo R, Huhtala H, Hakama M, Joensuu H. Radiotherapy after segmental resection of breast cancer with favorable prognostic features: 12-year follow-up results of a randomized trial. J Clin Oncol. 2009;27(6):927–932. [DOI] [PubMed] [Google Scholar]
- 15. Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31(32):4054–4059. [DOI] [PubMed] [Google Scholar]
- 16. Walshaw RC, Honeychurch J, Illidge TM, Choudhury A. The anti-PD-1 era - an opportunity to enhance radiotherapy for patients with bladder cancer. Nat Rev Urol. 2017;15(4):251–259. [DOI] [PubMed] [Google Scholar]
- 17. Gong X, Li X, Jiang T, et al. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12(7):1085–1097. [DOI] [PubMed] [Google Scholar]


