Abstract
The definition of fascia includes tissues of mesodermal derivation, considered as specialized connective tissue: blood and lymph. As water shapes rocks, bodily fluids modify shapes and functions of bodily structures. Bodily fluids are silent witnesses of the mechanotransductive information, allowing adaptation and life, transporting biochemical and hormonal signals. While the solid fascial tissue divides, supports, and connects the different parts of the body system, the liquid fascial tissue feeds and transports messages for the solid fascia. The focus of this article is to reconsider the model of biotensegrity because it does not take into account the liquid fascia, and to try to integrate the fascial continuum with the lymph and the blood in a new model. The name given to this new model is RAIN—Rapid Adaptability of Internal Network.
Keywords: fascia, myofascial, biotensegrity, manual therapy, osteopathic, chiropractic
The fascial tissue is equally distributed throughout the entire body, enveloping, interacting with, and permeating blood vessels, nerves, viscera, meninges, bones, and muscles, creating various layers at different depths and forming a 3-dimensional metabolic and mechanical matrix. The fascia becomes an organ that can affect an individual’s health.1,2
We can identify 3 large groups of academics who try to define what a fascia is. The Federative Committee on Anatomical Terminology (FCAT), created in 1989 by the General Assembly of the International Federation of Associations of Anatomists.3 FCAT introduced the terms “fascia superficialis” (superficial fascia) and “fascia profunda” (deep fascia): the superficial fascia is considered to be the “whole loose layer of subcutaneous tissue lying superficial to the denser layer of fascia profunda.”4 The deep fascia, according to this definition, is located under the superficial fascia, emphasizing 2 fascial layers. In 2011, the Federative International Programme on Anatomical Terminologies (FIPAT), in agreement with FCAT, gave this definition of fascia: “A fascia is a sheath, a sheeth, or any other dissectible aggregation of connective tissue that forms beneath the skin to attach, enclose, and separate muscles and other internal organs.”5 FIPAT provided the anatomic terminology text: “Anatomic Terminology.”5 In the second definition, you can more specifically find the term “connective tissue,” which has the function of dividing, separating, and supporting different structures. FIPAT states that the connective tissue or fascia originates underneath the skin, excluding the epidermis from the fascial system.
The third group of scientists who are involved in giving a definition for fascia is the Fascia Nomenclature Committee (2014), created by the Fascia Research Society, founded in 2007.5
The committee gave the following definition of fascia:
The fascial system consists of the three-dimensional continuum of soft, collagen containing, loose and dense fibrous connective tissues that permeate the body. It incorporates elements such as adipose tissue, adventitiae and neurovascular sheaths, aponeuroses, deep and superficial fasciae, epineurium, joint capsules, ligaments, membranes, meninges, myofascial expansions, periostea, retinacula, septa, tendons, visceral fasciae, and all the intramuscular and intermuscular connective tissues including endo-/peri-/epimysium. The fascial system interpenetrates and surrounds all organs, muscles, bones and nerve fibers, endowing the body with a functional structure, and providing an environment that enables all body systems to operate in an integrated manner.5
It is emphasized the concept of the continuum of the structure containing collagen/connective, the diversity of cells that creates the fascia, and that the continuum itself guarantees the health of the body system.
From an embryological perspective, the fascia originates in the mesoderm, although, according to some authors, this connective network can be partially found in the neural crests (ectoderm), with particular reference to the cranial and cervical areas.6,7
All the tissues considered as “specialized connective tissues” of mesodermal derivation, such as blood, bone, cartilage, adipose tissue, hematopoietic tissue, and lymphatic tissue, are regarded as part of the fascial system.5
The ordinary movements of the body are possible thanks to the presence of the fascial tissues and to their inseparable interconnection that allows the sliding of muscular framework, the sliding of nerves and vessels between contractile districts and joints, as well as all the organs that can slide and move, influenced by the position of the body.8 The fascial continuum allows the correct distribution of the tensional information produced by different tissues enveloped and supported by the fascia, so that the whole body system can interact in real time.9
One of the fundamental characteristics of the fascia is the ability to adapt to mechanic stress, remodeling the cellular/tissue structure, and mirroring the functional necessity of the environment where the tissue lays.2,6
Of the scientific data deriving from cellular microscopy, we can avoid citing the anatomic subdivision of the different fascial depths (superficial and deep), because we know that a clear separation of the structures does not exist in vivo; there is a functional continuum.10
We can subdivide the fascial tissue into solid fascia and liquid fascia. The first one is all that is considered connective tissue, while the second is what is considered as specialized connective tissue: blood and lymph.5
Method
We have tried to redefine the concept of biotensegrity of the current fascial model, in a narrative review.
We checked articles on PubMed that had the fascia as their theme, using keywords such as fascia, myofascial, fascial, biotensegrity, fibroblast, connective tissue, and collagen.
We checked articles on PubMed that contained manual work on fascia in their theme, using keywords such as manual therapy, osteopathic, chiropractic, and physiotherapy.
We included the more recent articles and the most significant texts in the scientific literature. We did not use texts that repeated the same concepts or were too dated. We took a year to do this screening.
Result
At the end of the screening, we included 103 articles with the keywords used.
Of these 103 articles, we used only 37 for the topic of fascia and biotensegrity. The rest of the articles mentioned in the text cover topics such as blood and lymph (physiology, structure, and function).
In the 37 articles there was no mention of a biotensegrity model involving the fascial fluid tissue, such as blood and lymph, despite these fluids being of strategic importance for the transmission of mechanometabolic information. From these results emerges the need to try to formulate a new hypothesis on a fascial biotensegretive model that also takes into account blood and lymph.
Discussion
The discussion will examine the knowledge on the solid and liquid fascia and the concept of the biotensegrity of the solid and liquid fascia. Finally, we will formulate a hypothesis that integrates the solid and liquid fascia in the biotensegrity.
Solid Fascia
Collagen makes up more than the 30% of the protein mass of the human body. Its most common shape is the collagen fibril, made up of around 300 nm of tropocollagen (polypeptide triple helices). The fibril is highly organized and provides the framework for the extracellular matrix, tendons, bones, and other load-bearing structures.11 Collagen fibrils resemble self-assembling cables on a nanometric scale. The biosynthesis of the collagen takes place thanks to different types of cells, depending on the tissue. For example, osteoblasts form collagen in bones, while fibroblasts form collagen in the tendon.2
There are different types of connective tissue, classified according to morphologic and functional criteria. We can find dense connective tissue (fibrous or elastic), where collagen is arranged in regular and irregular structures, and loose connective tissue (fibrous, reticular, or elastic), which stands out because of the abundance of amorphous substance compared with the quantity of the fibrous component.12 In dense connective tissue, we can mainly find collagen types I, III, XII, and XIV and elastin, while in the loose connective tissue, we can mainly find collagen types I, III, IV, V, VII, XII, and XIV.12
Fibroblasts are the main cellular component of connective tissue and secrete components of extracellular matrix such as collagen and matrix, glycosaminoglycan, elastic and reticular fibers, and glycoproteins.13 Fibroblasts communicate among each other and are fundamental for managing perceived and produced tension.2
They play a fundamental role in conveying tension and can dynamically affect mechanical tension, rapidly remodeling their cytoskeletons; the fibroblast’s cytoskeleton is made of microtubules, namely, actin filaments and intermediate filaments; specifically, the flexibility of actin enables a more rapid adaptation of the fibroblasts in the presence of compressive forces, due to the lengthening of the fascia.2 If the mechanical information is present for only a short period of time, any morphological variation is reversible, and the cytoskeleton of the fibroblast can be restored to its original state.2
The fibroblasts play a significant active role in stimulating inflammatory processes, because they are responsible for a suitable cleaning, repair, and replacement of the elements of the fascial continuum that have been and are affected by traumas resulting from daily use.2
Fibroblasts largely constitute the microvacuoles. The microvacuoles have a diameter ranging from a few microns up to 200 nanometers, with their size probably depending on the cells that they incorporate and/or on the considered body area.10 The fibrils vary in size between 5 and 70 nanometers in diameter, reaching a length of 10 to 100 nanometers.10 About 70% of these fibrils are represented by collagen types I, III, and IV, and about 20% by elastin, with around 4% of lipids. The microvacuoles are rich in water, thanks to the hydrophilic properties of the lipids and, in particular, of proteoglycans (approximately 72%). The core of these molecules is a protein with one or more covalent bonds with polysaccharides (glycosaminoglycans); the negative charge of glycosaminoglycans attracts water molecules, facilitating their passage through the membrane of microvacuoles and ensuring hydration. Hydration allows to maintain constant pressure of the volumes.10
Connective tissue derives from mesenchyme.4,5 During embryonal development, connective tissue probably influences the shape (morphogenesis) of the structures that it will contain and connect.14
Embryonal mesenchyme or embryonal connective or undifferentiated mesenchyme is made up of branched star-shaped cells, with a high mitotic rate (high reproductive ability); they are considered as pluripotent stem cells, because they have the ability of differentiating into different tissues. The embryonal mesenchyme is the source not only of many connectival structures, but also of stromal stem cells; over the course of the development process, they occupy the spaces between germ layers, connecting various structures, and making up the stroma of the organs.15–17
Mesenchyme is present in and derives from all of the 3 embryological layers (ectoderm, mesoderm, endoderm), especially from mesoderm and ectoderm.18,19 The fascia that forms part of the head (muscles, bones, skin, etc) and part of the cervical spine derive from mesoderm and ectoderm.20,21
Fascial tissues are described as layered, but it is a habit widespread that comes from anatomic dissection.22 The layers are inseparable and they move and answer/react in unison to the presence of mechanical/metabolic information.10,23
Liquid Fascia
Blood and lymph derive from mesoderm and are considered as connectival tissues.5 Blood and lymphatic vessels are solid fascial structures; what they carry is liquid fascia.
In addition to the nutritive functions, blood also provides a way of linking to different organs that can communicate among each other with hormones and chemical mediators, guaranteeing the integration of the functions of the organism. It is the vehicle of immune cells and platelets that can reach places in which their presence is necessary (eg, areas of inflammation), of antibodies and proteins of the clotting system, and of the numerous transport proteins (eg, lipoproteins, transferrin, ceruloplasmin, and albumin) to which the water-insoluble compounds that circulate in blood are attached.24 Human blood is a liquid that can be ruby red (clean), or purplish red (dirty); its viscosity is around 4 times higher than the viscosity of water; its specific weight is 1.041 to 1.062 g/cm3. It makes up around 7.7% of the human body weight; its temperature is around 37/38°C (it varies depending on internal and external factors), and its pH (in arteries) is 7.38 to 7.42 (the pH of an optimal saline solution should be 7.383). In men, it is made up of a liquid part (55%) called plasma, and a corpuscular part (45%) that consists of cells or cell fragments (average values for a healthy adult male), while in women, the liquid part takes up 60% and the corpuscular part takes up 40%. This ratio is called hematocrit and evaluates the volume of corpuscular blood elements under normal conditions.24
Plasma is a pale-yellow liquid made up of water (90%), organic substances, and dissolved salt (10%).
Blood is a connective tissue.5 It consists of cells and cell fragments in suspension in an extracellular matrix of complex composition. The unusual characteristic of blood is the fact that the extracellular matrix is a liquid, which means that blood is a fluid connective tissue. In blood, there are 2 different components that can be separated by centrifugation: a fluid matrix called plasma and corpuscles, which are cells or cell fragments.24
Corpuscles are erythrocytes, platelets, and leukocytes. Only leukocytes are complete cells: erythrocytes are anucleate cells and platelets are cell fragments. Erythrocytes are present in larger quantities than the other elements, which is why they influence the value of the hematocrit much more than leukocytes or platelets, which make up around 1% of the total volume. Erythrocytes, just like the other elements, are generated by pluripotent stem cells located in the bone marrow, particularly in ribs, sternum, pelvis, and vertebrae. There are different kinds of leukocytes.
Granulocytes are characterized by the presence of big granules in the cytoplasm. They are visible in the optical microscope after coloring and are divided into neutrophils (with an affinity to neutral coloring), eosinophils (they color with acid coloring), and basophils (with an affinity to basic coloring).24
Lymphocytes, which include lymphocytes T, lymphocytes B, and natural killer cells, participate in specific defense: first they recognize a pathogen, target it, and then attack it.
The targeted answer implies almost always the production of proteins circulating in blood, called antibodies. Monocytes are the biggest leukocytes, characterized by a big horseshoe-shaped nucleus.24
The lymphatic system effectively removes the excess of interstitial fluids, solutes, and various cells, guiding them toward the bloodstream, maintaining the volume of plasma and interstitial fluids in constant balance.25 The lymphatic system originates from the interstitial tissue called “initial lymphatics,” small capillaries delimited by discontinuous endothelium and basement membrane, offering low resistance to the flow of fluids and substances (hydrophiles, cells, viruses, and bacteria). They attach to the external surface of the cells through collagen fibrils (collagen type VII).25 This collagen allows the transmission of mechanical forces toward the lumen of the lymphatic vessel; there is autonomous contraction in some vessels, thanks to the presence of filaments similar to actin. These initial lymphatics become wider, creating collecting ducts that consist of collagen and smooth muscle cells and elastic fibers.25 Lymphatic vessels have got their own tone and, probably, their own intrinsic contraction autonomy, according to recent data, with a high ability of sensibility to flow variation (sensory functions). They are surrounded by nerves of the autonomous system (mainly sympathetic fibers), which could act to better coordinate the lymphatic transport. Lymphatic vessels adapt and change their elastic capacity, improving or worsening the function of lymphatic transport.26
We can identify primary valves, formed by the cytoplasmic extent of the adjacent endothelial cells linked by close connections. The valves of these cells protrude toward the inside; this way, what goes in cannot go out. Finally, interluminal valves (weaker), 2 sheets attached to the opposite sides of the lymphatic vessel, and connected to zonules (perimeter junction involving a band that surrounds the cell).25 Lymph flows thanks to external mechanical compressions, for example, the one caused by muscle contraction and to its own intrinsic contraction abilities.
The lymphatic system is subject to aging, losing its elasticity and creating “aneurysms” over time, or decreasing the number of blood vessels or of lymphangions (the lymphatic functional unit).27 Recent evidence reveals that lymphatic vessels are supported by a nervous system, of vagal cholinergic type and sympathetic type, able to modulate the contraction (peristalsis, also helped by the breathing and pulsation of arteries) of vessels endowed with contractile fibers (with an actin-like protein).26 These thin nerves reach the external layer of the lymphatic vessel and then reach the deepest endothelial layer; this nerve network deteriorates in elderly people. Probably, the presence of both the parasympathetic system and the sympathetic one acts not only as tension or vessel tone modulator but also as sensor of the contractile layer of the vessel itself.26
The dural system has a lymphatic system called glymphatic system. The cerebrospinal fluid is not only drained through the venous system but also through the lymphatic system.28,29
Dural lymphatic vessels, placing themselves side by side to the veins and arteries of the brain, more specifically, come out of the skull following the reverse path of the pterygopalatine artery and a branch of the internal carotid, and travel through external vein paths to the skull and through cranial nerves that come out of the skull.28
Lymphatic vessels follow vein paths of the cribriform plate toward the nasal mucosa, following ways exiting the cerebrospinal fluid.28 The glymphatic system absorbs the interstitial liquid and the cerebrospinal fluid from the subarachnoid space and transports it outside of the skull, more specifically from the base, up to the cervical spine.28 This mechanism is stronger during sleep.
Biotensegrity
Tensegrity, the tension that preserves integrity, comes from a principle of architecture thanks to Fuller in 1961.30 A structure is stabilized by the balance of a constant tension and by the presence of a discontinuous compression. This organization is self-stabilizing, allowing to manage tension variations with a certain degree of flexibility, transferring the forces applied to the whole structure30 (see Figure 1).
Figure 1.

Tensegrity. A structure is stabilized by the balance of a constant tension and by the presence of a discontinuous compression. This organization is self-stabilizing, allowing to manage tension variations with a certain degree of flexibility, transferring the forces applied to the whole structure. The photo shows the sculpture by artist Kenneth Snelson.
The architectonic principle can be found in the living, from the body system to the single cell. The first transposition of the concept on the body system dates back to 1977, when the tensegretive vision was applied to the column (see Figure 2).30
Figure 2.
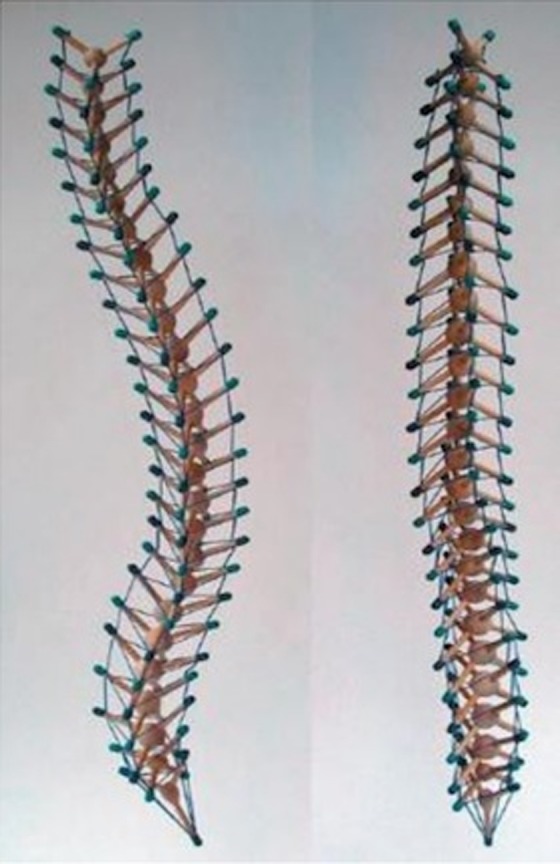
Spine tensegrity. The picture shows the concept of tensegrity, with a shape that resembles the spine, with the tendons and ligaments.
In 2007 the vision of the column as a tensegretive structure is taken into consideration again, suggesting the biotensegretive model, meaning the principle of self-regulation on the living, where constant tension is represented by musculature, and bones/joints play the role of discontinuous compression.31
The concept of biotensegrity applied to the cell derives from the work of Ingber, starting from 1993.32 The biotensegretive model allows the cell or the system to sense external or internal forces, to transmit that mechanical information to the inside/outside of the cell or system, in order to allow local and systemic functions to remain stable. When a cell deforms at the passing of a force vector, the change of the shape activates a series of metabolic and hormonal events, with the purpose of making the cell less fragile, allowing it to continue to function. This mechanism is called mechanotransduction.30
We can summarize the principle of biotensegrity with a thought by Bernstein: “The ability to find an action solution for any environmental situation – to solve adequately any emerging action problem.”33
The propagation of the mechanical information toward the cytoplasm and the DNA, deforming the cell at the passing of one or more force vectors, is an event as rapid as the speed of sound.33
In the inside of the cell, we can find microfilaments, straight and tight as a rope that form a geodesic triangular structure and slightly bent microtubules. The microfilaments are the elements that provide constant tension, while microtubules provide compression.30 The extracellular matrix plays a key role in the transmission of the force to and from the cell and in keeping a discontinuous compression in cooperation with the microtubules.30
The extracellular matrix creates a 3-dimensional net that surrounds all the cells, just like all the organs and tissues of the body.34 It is made up of a ground substance or gel (glycosaminoglycans, hyaluronan, glycoproteins, proteoglycans), and with a fibrous framework (reticulum, elastin, collagen fibers) in a way that helps maintain the tension of the cells of the different tissues: extracellular matrix has its own biotensegrity. The fibrous framework acts as tensional element, while the base substance acts as discontinuous compression.35
Extracellular matrix communicates with the cell and its DNA via transmembrane proteins (integrin) gathered together to form focal adhesion complexes; this system allows the creation of an informational network of all tissues.35
Biotensegrity of the Solid Fascia
The development of the biotensegretive model concerning the fascia comes from the observation of its influence on muscular coordination.33 The fascial continuum envelopes and permeates muscles (epimysium, perimysium, endomysium), and it links them to one another, directly or indirectly through bones and joints, tendons and ligaments, allowing systemic communication and the transmission of force expressed that involves the whole contractile tissue (with the cooperation of the neural system). There is transmission of the intramuscular, intermuscular, and extramuscular force (see Figure 3).36–39
Figure 3.
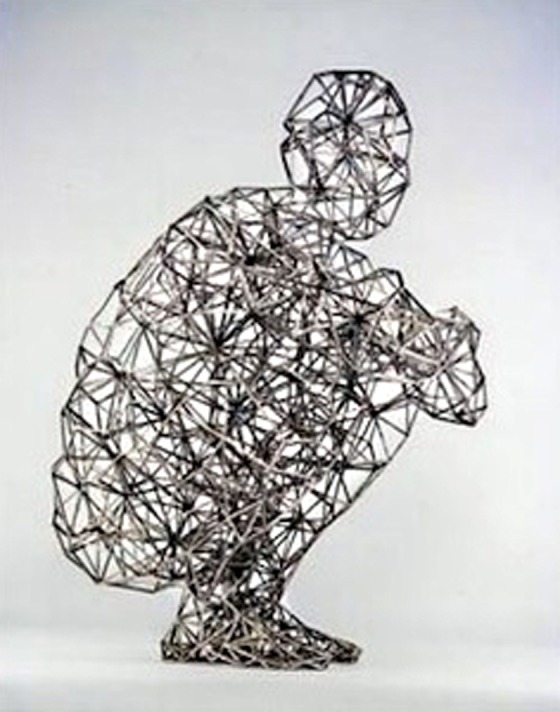
Biotensegrity model of fascial continuum. The picture shows a model of a man sitting in perfect balance, reflecting the concept of tensegretive continuity.
The myofascial tissue represent constant tension, while joints and bones play the role of discontinuous compression.30,33
This organization allows the real-time adaptation of the posture and the improvement of the expressed gesture: the fascial continuum acts in unison.33 The biotensegrity of the solid fascia allows to constantly tune the different body areas, reflecting current necessity, a property the tension elements have, called “prestress” or “pretension.”33
To summarize the concept of biotensegrity of the solid fascia, we quote Ingber:
This is a physically integrated framework that supports the weight of our bodies, allows us to rapidly adjust to resist external forces, and permits us to move freely in our environment. But without the aid of surrounding tension-generating muscles and tension-resisting tendons, ligaments and fascia, bones and cartilage would do little to support our upright forms.40
Biotensegrity of the Liquid Fascia
The erythrocyte is able to change its shape according to the mechanical information it receives, and to go back to its resting state at the cessation of the stress. Its membrane is made up of a double layer of lipids, reinforced by transmembrane proteins (spectrin family). These proteins connect the membrane with the cytoplasm, with the presence of a short actin filament, creating a complex net that reaches the DNA. The erythrocyte deforms according to the caliber of the vessel or to the speed and direction of the blood flow; the membrane changes its shape just like the cytoskeleton and, probably, thanks to the elastic accumulation of spectrins, the erythrocyte restores its original shape.41,42 Its biotensegretive organization allows to absorb the mechanical force it endures, to distribute it inside the cell, and to restore its shape, carrying out its function.30 Actin and spectrins provide constant tension, while the double layer of lipids provides compression.43
The ability to deform its shape is vital for its functions, like distributing oxygen to the body tissues and keeping its viscosity even in narrow vessels. The possibility to correctly sense the present tension and to allow the cell to change shape is probably due to the presence of some transmembrane proteins that act as ion channels (Piezo1, also known as Fam38a).44
The cell that represents the lymph the most is the lymphocyte, which changes its shape because it endures mechanical forces (flow speed, contractile tissues, etc) and it follows the principle of mechanotransduction.45 The cytoskeleton of the lymphocyte is very elastic, thanks to the presence of filaments like actin, alpha-actinin, calmodulins, spectrins, and myosin light chains.46 The mechanical vector that deforms the cell reaches the nucleus, deforming it as well. The nucleus would act as a brake to prevent further deformation.46 The shape of the lymphocytes is spherical, but with external stress it can even change into a semicircle; with structures in prestress (cytoskeleton), mechanical hysteresis restores its original shape.46 The membranes of the lymphocyte and of the nucleus represent compression elements, while the filaments of the cytoskeleton are the factors that determine constant tension. The transmembrane proteins of the lymphocyte, like integrins, syndecans, and different ion channels (Ca2+ activated potassium channels; voltage-gated Kv1.3; Ca+ release-activated Ca2+ or CRAC) make the process of mechanotransduction and the transmission of the mechanical information to the inside of the lymphocyte easier.47–49
The liquids of the body like blood and lymph cooperate for the well-being of body health.50,51 The lymph originates from the venous system, and it dies in the venous system, just like for an inevitable destiny the venous system transforms into the arterial system. This is the continuum of the liquid fascia.
RAIN—Rapid Adaptability of Internal Network
The human body is made up of liquids of about 60%, approximately 40% of which (two thirds) is stored inside cells; the remaining 20% (one third) is located between the interstices of the cells.50 Just a small part of the fluids is transported by vessels, like blood and lymph.50
The arterial system transports biochemical and hormonal substances to the different tissues, with the ultimate aim of obtaining a correct body synergy, oxygen included. Arterial capillaries are permeable and the plasma exits the vessel toward the cellular interstice of the tissues; this is possible thanks to their hydrostatic pressure.50 The venous system gathers waste products and water from the tissues; the venous capillaries gather the biggest part of what is extravased from arterial capillaries, thanks to their oncotic pressure.50
A small amount of plasma stays in the cellular interstice, collected, eventually, by lymphatic vessels, thanks to their low hydrostatic pressure; this final drainage is also useful to buffer an excess accumulation of sodium, contrasting an imbalance of tension among liquids (hypertension).50
Blood and lymph determine the health of the tissues and, consequently, their shape.
This also happens during ontological development. According to the embryologist Blechschmidt, during embryologic development the pressures of the liquids (volume and speed), with different vectors and forces (direction), create the print and the direction for future tissues and organs.52 During embryogenesis and in the fully developed human body, the mechanical forces produced by the flows of blood and lymph or shear stress (laminar, turbulent, oscillatory flows) also assure the preservation of the shape and function of the vessels (and of the heart).50,53–57
The pressure generated by the liquid fascia can be decomposed in a triad: volume, direction, and speed. These are the components that determine the destiny of the shape and function of the solid fascia. If the solid fascia allows movement, the liquid fascia is the “intelligence” that allows its evolution and continuation.
The continuum of the liquid fascia is mirrored by the continuum of the solid fascia from transport system: vessels. To give some examples, if a lymph node is negatively affected by pathology and therapy, like in the case of cancers and radiotherapy, the whole lymphatic system is involved, implying the worsening of the transport of lymphatic material.58 With aging, the whole vascular tree suffers from thickening of the vessels with an increasing of stiffness (in particular with the thickening of the intimal and adventitious layer), even in absence of a manifest pathology like atherosclerosis.59 The venous system suffers a delay of its flow with aging, both in limbs and in the cerebral vascular system, with a high probability of developing different central and peripheral pathologies.60,61 Not only the tensegretive ability disappears, but when a local tensegretive discontinuity develops, the event will involve the whole blood and lymph net over time.
If the solid fascia (vessels) is functional, the liquid fascia will be as well; if the liquid fascia does not encounter any obstacle on its path, the solid fascia (vessels and body structures) will be able to carry out its functions.
This functional and biotensegretive synergy can be found in other examples, where the liquid fascia, seen in the new theoretic model, acts as “joints” for the solid fascia.
A joint allows the expression of the muscular strength, thanks to tendons and ligaments that improve the lever arm and create a fundamental focal point for the biotensegretive musculoskeletal model. At the same time, constant tension elements (muscles) determine joint stability.62–64 Compression and tension elements are vital to each other.
Blood and lymphatic flow (volume, speed, and direction) changes depending on present posture to compensate the functional necessities of the body in real time, both for the head and the trunk and for the limbs.65–71
These variations and liquid movements improve the biotensegretive and myofascial functions, protect the organs, and allow a better immune defense. There is a direct relation between the strength expressed by muscles and the quantity/speed of arterial blood used.72 An artificially induced reduction of the quantity of blood that reaches the musculature during sports training makes protein synthesis easier, with increase of muscle hypertrophy.73
The venous return is facilitated when the limbs are positioned higher than the heart, so that the function of the right atrium to receive venous blood is made easier.74 Venous fluctuations with respect to postural changes may put some outflows into trouble. In supine position, internal jugular veins are open and allow the outflow from the cerebral system (also facilitated by breathing). In orthostatism, internal jugular veins collapse and the venous outflow is readdressed toward vertebral veins and up until it reaches the superior vena cava.75
The lymphatic movement toward a specific district or tissue can not only mean a postural change but also an immunological necessity. Lymphatic structures react to the inflammatory stimulus by increasing the drainage and the quantity of lymph, probably to make up for a bigger liquid loss from the blood vessels. Unfortunately, if the cause that produces inflammation is not solved, the increase of lymph becomes counterproductive (secondary lymphatic edema).76
The new theoretic model conceived and suggested by our Department of Fascial Osteopathic Research (FORe), called RAIN (Rapid Adaptability of Internal Network), tries to complete the biotensegretive model of the solid fascia, implementing its maximal functionality with the liquid fascia. The liquid fascia has a high variability in changing the pressures at which it flows, both at rest and during postural changes, in order to improve the continuum of the solid fascia, concerning the movement, the function, and the shape.
RAIN reminds us of the liquid tissue, and as an acronym, it highlights the rapid adaptability of blood and lymph inside of the net of the solid fascia. The liquid fascia is another element of discontinuous compression inside of the biotensegretive myofascial model.
This is the first article in the world that considers the liquid fascia inside of the better-known model of the solid fascia.
The article presents points of strength and points of weaknesses. The first includes knowledge on the solid and liquid fascia; the role of connective tissue is recognized for both types of fascia and to be part of the body system or biotensegretive network. The weakness of the study lies in the same biotensegretive hypothesis. More articles will be needed to prove more realistically the effect that RAIN has on the biotensegretive myofascial model.
This study can be of inspiration for other researchers, with the aim of improving the manual and therapeutic approaches, and for the comprehension of the bodily dysfunctions, thanks to a different perspective in the general observation of the symptomatic framework.
Figure 4 illustrates the idea of the RAIN model. The circle represents the epidermis that closes the body; the epidermis is a constituent part of the fascial system.77 The red lines represent muscles and bones; the blue lines parallel to the red lines represent the vascular and lymphatic vessels; the yellow spider web represents the indissoluble connection of the different fascial layers. The blue color of the bottom and that fills the circle are liquids, including the interstitial ground substance.
Figure 4.
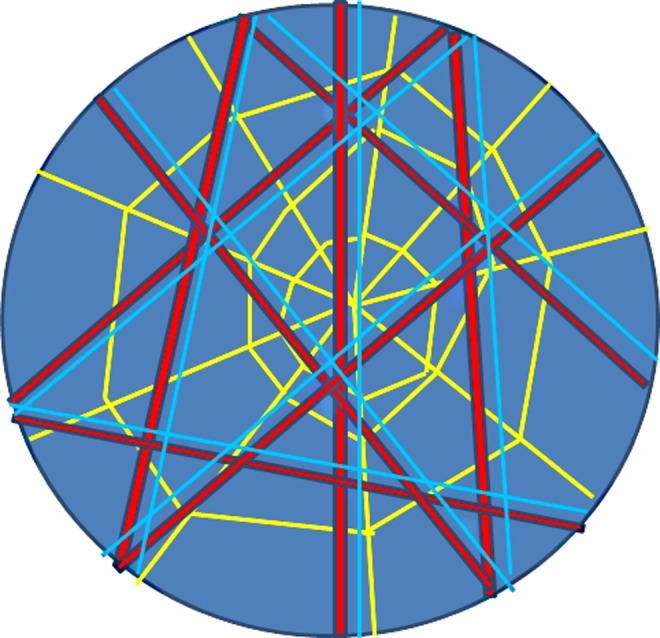
RAIN model. The circle represents the epidermis that closes the body. The red lines represent muscles and bones; the blue lines parallel to the red lines represent the vascular and lymphatic vessels; the yellow spider web represents the indissoluble connection of the different fascial layers. The blue color of the bottom and that fills the circle are liquids, including the interstitial ground substance.
Conclusion
Ordinary movements of the body are possible thanks to the presence of the fascial tissue and its inseparable interconnection that allows the sliding of the muscular framework, the sliding of nerves and vessels between contractile districts, influenced by the position of the body.
We can subdivide the fascial tissue into solid fascia and liquid fascia. The second one is what is considered as specialized, nonsolid connective tissue: blood and lymph.
The biotensegretive myofascial model describes the principle of self-regulation on the living, where constant tension is represented by musculature, and bones/joints play the role of discontinuous compression. The aim of this article is to reconsider the model of biotensegrity because it does not consider the liquid fascia, and to try to integrate the fascial continuum with lymph and blood in a new model. The name given to this new model is RAIN—Rapid Adaptability of Internal Network. We think that the liquid fascia is another element of discontinuous compression inside the biotensegretive myofascial model.
This is the first article in the world that considers the liquid fascia inside of the better-known model of the solid fascia. Since this is a hypothesis, other articles will be needed to prove more realistically the effect that RAIN has on the biotensegretive myofascial model.
Footnotes
Author Contributions: Bruno Bordoni wrote the article; Fabiola Marelli and Bruno Morabito corrected the article; and Roberto Castagna translated the text.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bruno Bordoni  http://orcid.org/0000-0002-4949-5126
http://orcid.org/0000-0002-4949-5126
Bruno Morabito  http://orcid.org/0000-0001-6156-8781
http://orcid.org/0000-0001-6156-8781
Ethical Approval: As this is a review article, there is no need for ethical approval.
References
- 1. Bordoni B, Marelli F. The fascial system and exercise intolerance in patients with chronic heart failure: hypothesis of osteopathic treatment. J Multidiscip Healthc. 2015;8:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bordoni B, Zanier E. Understanding fibroblasts in order to comprehend the osteopathic treatment of the fascia. Evid Based Complement Alternat Med. 2015;2015:860934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wendell-Smith CP. Fascia: an illustrative problem in international terminology. Surg Radiol Anat. 1997;19:273–277. [DOI] [PubMed] [Google Scholar]
- 4. Schleip R, Jäger H, Klingler W. What is “fascia?” A review of different nomenclatures. J Bodyw Mov Ther. 2012;16:496–502. [DOI] [PubMed] [Google Scholar]
- 5. Adstrum S, Hedley G, Schleip R, Stecco C, Yucesoy CA. Defining the fascial system. J Bodyw Mov Ther. 2017;21:173–177. [DOI] [PubMed] [Google Scholar]
- 6. Bordoni B, Zanier E. Clinical and symptomatological reflections: the fascial system. J Multidiscip Healthc. 2014;7:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bordoni B, Marelli F. Emotions in motion: myofascial interoception [in German]. Complement Med Res. 2017;24:110–113. [DOI] [PubMed] [Google Scholar]
- 8. Dawidowicz J, Matysiak N, Szotek S, Maksymowicz K. Telocytes of fascial structures. Adv Exp Med Biol. 2016;913:403–424. [DOI] [PubMed] [Google Scholar]
- 9. Chapman MA, Meza R, Lieber RL. Skeletal muscle fibroblasts in health and disease. Differentiation. 2016;92:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bordoni B, Marelli F, Morabito B, Sacconi B. The indeterminable resilience of the fascial system. J Integr Med. 2017;15:337–343. [DOI] [PubMed] [Google Scholar]
- 11. Dittmore A, Silver J, Sarkar SK, Marmer B, Goldberg GI, Neuman KC. Internal strain drives spontaneous periodic buckling in collagen and regulates remodeling. Proc Natl Acad Sci U S A. 2016;113:8436–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumka M. Kumka’s response to Stecco’s fascial nomenclature editorial. J Bodyw Mov Ther. 2014;18:591–598. [DOI] [PubMed] [Google Scholar]
- 13. Nithiananthan S, Crawford A, Knock JC, Lambert DW, Whawell SA. Physiological fluid flow moderates fibroblast responses to TGF-β1. J Cell Biochem. 2017;118:878–890. [DOI] [PubMed] [Google Scholar]
- 14. Dawidowicz J, Szotek S, Matysiak N, Mielańczyk Ł, Maksymowicz K. Electron microscopy of human fascia lata: focus on telocytes. J Cell Mol Med. 2015;19:2500–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breznik B, Motaln H, Vittori M, Rotter A, Turnšek TL. Mesenchymal stem cells differentially affect the invasion of distinct glioblastoma cell lines. Oncotarget. 2017;8:25482–25499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shirjang S, Mansoori B, Solali S, Hagh MF, Shamsasenjan K. Toll-like receptors as a key regulator of mesenchymal stem cell function: an up-to-date review. Cell Immunol. 2017;315:1–10. [DOI] [PubMed] [Google Scholar]
- 17. Young B, O’Dowd G, Woodford P. Wheater’s Functional Histology: A Text and Colour Atlas. 6th ed London, England: Churchill Livingstone; 2013:65. [Google Scholar]
- 18. Schmidt C, Stoeckelhuber M, McKinnell I, Putz R, Christ B, Patel K. Wnt 6 regulates the epithelisation process of the segmental plate mesoderm leading to somite formation. Dev Biol. 2004;271:198–209. [DOI] [PubMed] [Google Scholar]
- 19. Grapin-Botton A, Melton DA. Endoderm development: from patterning to organogenesis. Trends Genet. 2000;16:124–130. [DOI] [PubMed] [Google Scholar]
- 20. Goodnough LH, Dinuoscio GJ, Ferguson JW, Williams T, Lang RA, Atit RP. Distinct requirements for cranial ectoderm and mesenchyme-derived Wnts in specification and differentiation of osteoblast and dermal progenitors. PLoS Genet. 2014;10:e1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mariani FV, Fernandez-Teran M2, Ros MA. Ectoderm-mesoderm crosstalk in the embryonic limb: the role of fibroblast growth factor signaling. Dev Dyn. 2017;246:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scarr G. Comment on “Defining the fascial system.” J Bodyw Mov Ther. 2017;21:178. [DOI] [PubMed] [Google Scholar]
- 23. Stecco C, Schleip R. A fascia and the fascial system. J Bodyw Mov Ther. 2016;20:139–140. [DOI] [PubMed] [Google Scholar]
- 24. Standring S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 41st ed Ontario, Canada: Elsevier; 2015. [Google Scholar]
- 25. Negrini D, Moriondo A. Lymphatic anatomy and biomechanics. J Physiol. 2011;589(pt 12):2927–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mignini F, Sabbatini M, Coppola L, Cavallotti C. Analysis of nerve supply pattern in human lymphatic vessels of young and old men. Lymphat Res Biol. 2012;10:189–197. [DOI] [PubMed] [Google Scholar]
- 27. Bridenbaugh EA, Nizamutdinova IT, Jupiter D, et al. Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol. 2013;11:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14:977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swanson RL., 2nd Biotensegrity: a unifying theory of biological architecture with applications to osteopathic practice, education, and research—a review and analysis. J Am Osteopath Assoc. 2013;113:34–52. [DOI] [PubMed] [Google Scholar]
- 31. Vora AJ, Doerr KD, Wolfer LR. Functional anatomy and pathophysiology of axial low back pain: disc, posterior elements, sacroiliac joint, and associated pain generators. Phys Med Rehabil Clin N Am. 2010;21:679–709. [DOI] [PubMed] [Google Scholar]
- 32. Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104(pt 3):613–627. [DOI] [PubMed] [Google Scholar]
- 33. Turvey MT. Action and perception at the level of synergies. Hum Mov Sci. 2007;26:657–697. [DOI] [PubMed] [Google Scholar]
- 34. Noguera R, Nieto OA, Tadeo I, Fariñas F, Alvaro T. Extracellular matrix, biotensegrity and tumor microenvironment. An update and overview. Histol Histopathol. 2012;27:693–705. [DOI] [PubMed] [Google Scholar]
- 35. Tadeo I, Berbegall AP, Escudero LM, Alvaro T, Noguera R. Biotensegrity of the extracellular matrix: physiology, dynamic mechanical balance, and implications in oncology and mechanotherapy. Front Oncol. 2014;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maas H, Huijing PA. Myofascial force transmission in dynamic muscle conditions: effects of dynamic shortening of a single head of multi-tendoned rat extensor digitorum longus muscle. Eur J Appl Physiol. 2005;94:584–592. [DOI] [PubMed] [Google Scholar]
- 37. Yucesoy CA, Koopman BH, Baan GC, Grootenboer HJ, Huijing PA. Extramuscular myofascial force transmission: experiments and finite element modeling. Arch Physiol Biochem. 2003;111:377–388. [DOI] [PubMed] [Google Scholar]
- 38. Huijing PA. Epimuscular myofascial force transmission: a historical review and implications for new research. International Society of Biomechanics Muybridge Award Lecture, Taipei, 2007. J Biomech. 2009;42:9–21. [DOI] [PubMed] [Google Scholar]
- 39. Huijing PA, van de Langenberg RW, Meesters JJ, Baan GC. Extramuscular myofascial force transmission also occurs between synergistic muscles and antagonistic muscles. J Electromyogr Kinesiol. 2007;17:680–689. [DOI] [PubMed] [Google Scholar]
- 40. Ingber DE. Tensegrity and mechanotransduction. J Bodyw Mov Ther. 2008;12:198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Svetina S, Kokot G, Kebe TŠ, Žekš B, Waugh RE. A novel strain energy relationship for red blood cell membrane skeleton based on spectrin stiffness and its application to micropipette deformation. Biomech Model Mechanobiol. 2016;15:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Armiger TJ, Spagnol ST, Dahl KN. Nuclear mechanical resilience but not stiffness is modulated by αII-spectrin. J Biomech. 2016;49:3983–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vera C, Skelton R, Bossens F, Sung LA. 3-D nanomechanics of an erythrocyte junctional complex in equibiaxial and anisotropic deformations. Ann Biomed Eng. 2005;33:1387–1404. [DOI] [PubMed] [Google Scholar]
- 44. Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflugers Arch. 2015;467:95–99. [DOI] [PubMed] [Google Scholar]
- 45. Makino A, Shin HY, Komai Y, et al. Mechanotransduction in leukocyte activation: a review. Biorheology. 2007;44:221–249. [PubMed] [Google Scholar]
- 46. Pasternak C, Elson EL. Lymphocyte mechanical response triggered by cross-linking surface receptors. J Cell Biol. 1985;100:860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strazza M, Azoulay-Alfaguter I, Pedoeem A, Mor A. Static adhesion assay for the study of integrin activation in T lymphocytes. J Vis Exp. 2014;(88). doi:10.3791/51646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teixé T, Nieto-Blanco P, Vilella R, Engel P, Reina M, Espel E. Syndecan-2 and -4 expressed on activated primary human CD4+ lymphocytes can regulate T cell activation. Mol Immunol. 2008;45:2905–2919. [DOI] [PubMed] [Google Scholar]
- 49. Varga Z, Hajdu P, Panyi G. Ion channels in T lymphocytes: an update on facts, mechanisms and therapeutic targeting in autoimmune diseases. Immunol Lett. 2010;130:19–25. [DOI] [PubMed] [Google Scholar]
- 50. Planas-Paz L, Lammert E. Mechanical forces in lymphatic vascular development and disease. Cell Mol Life Sci. 2013;70:4341–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Minasyan HA. Erythrocyte and leukocyte: two partners in bacteria killing. Int Rev Immunol. 2014;33:490–497. [DOI] [PubMed] [Google Scholar]
- 52. Gasser RF, Blechschmidt E. Biokinetics and Biodynamics of Human Differentiation: Principles and Applications. Berkeley, CA: North Atlantic Books Berkeley; 2012. [Google Scholar]
- 53. Tarbell JM, Weinbaum S, Kamm RD. Cellular fluid mechanics and mechanotransduction. Ann Biomed Eng. 2005;33:1719–1723. [DOI] [PubMed] [Google Scholar]
- 54. Choi D, Park E, Jung E, et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J Clin Invest. 2017;127:1225–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kunert C, Baish JW, Liao S, Padera TP, Munn LL. Mechanobiological oscillators control lymph flow. Proc Natl Acad Sci U S A. 2015;112:10938–10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chatterjee S, Fisher AB. Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid Redox Signal. 2014;20:899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garita B, Jenkins MW, Han M, et al. Blood flow dynamics of one cardiac cycle and relationship to mechanotransduction and trabeculation during heart looping. Am J Physiol Heart Circ Physiol. 2011;300:H879–H891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baker A, Semple JL, Moore S, Johnston M. Lymphatic function is impaired following irradiation of a single lymph node. Lymphat Res Biol. 2014;12:76–88. [DOI] [PubMed] [Google Scholar]
- 59. Barodka VM, Joshi BL, Berkowitz DE, Hogue CW, Jr, Nyhan D. Review article: implications of vascular aging. Anesth Analg. 2011;112:1048–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Edgell H, Robertson AD, Hughson RL. Hemodynamics and brain blood flow during posture change in younger women and postmenopausal women compared with age-matched men. J Appl Physiol (1985). 2012;112:1482–1493. [DOI] [PubMed] [Google Scholar]
- 61. Viramo P, Luukinen H, Koski K, Laippala P, Sulkava R, Kivelä SL. Orthostatic hypotension and cognitive decline in older people. J Am Geriatr Soc. 1999;47:600–604. [DOI] [PubMed] [Google Scholar]
- 62. Masi AT, Nair K, Evans T, Ghandour Y. Clinical, biomechanical, and physiological translational interpretations of human resting myofascial tone or tension. Int J Ther Massage Bodywork. 2010;3:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pardehshenas H, Maroufi N, Sanjari MA, Parnianpour M, Levin SM. Lumbopelvic muscle activation patterns in three stances under graded loading conditions: proposing a tensegrity model for load transfer through the sacroiliac joints. J Bodyw Mov Ther. 2014;18:633–642. [DOI] [PubMed] [Google Scholar]
- 64. Ciszkiewicz A, Knapczyk J. Load analysis of a patellofemoral joint by a quadriceps muscle. Acta Bioeng Biomech. 2016;18:111–119. [PubMed] [Google Scholar]
- 65. Bulekbaeva LE, Makashev EK, Demchenko GA, Abdreshov SN. Transport function of lymph nodes in body antiorthostatic posture [in Russian]. Ross Fiziol Zh Im I M Sechenova. 2007;93:39–45. [PubMed] [Google Scholar]
- 66. Demchenko GA, Vovk EV. Effect of a passive orthostatic test on lymph circulation [in Russian]. Kosm Biol Aviakosm Med. 1991;25:18–20. [PubMed] [Google Scholar]
- 67. Garrett ZK, Pearson J, Subudhi AW. Postural effects on cerebral blood flow and autoregulation. Physiol Rep. 2017;5:e13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Leyk D, Essfeld D, Baum K, Stegemann J. Early leg blood flow adjustment during dynamic foot plantarflexions in upright and supine body position. Int J Sports Med. 1994;15:447–452. [DOI] [PubMed] [Google Scholar]
- 69. Łastowiecka-Moras E. How posture influences venous blood flow in the lower limbs: results of a study using photoplethysmography. Int J Occup Saf Ergon. 2017;23:147–151. [DOI] [PubMed] [Google Scholar]
- 70. Gisolf J, van Lieshout JJ, van Heusden K, Pott F, Stok WJ, Karemaker JM. Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol. 2004;560(pt 1):317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zaniewski M, Simka M. Biophysics of venous return from the brain from the perspective of the pathophysiology of chronic cerebrospinal venous insufficiency. Rev Recent Clin Trials. 2012;7:88–92. [DOI] [PubMed] [Google Scholar]
- 72. Wilson KE, Tat J, Keir PJ. Effects of wrist posture and fingertip force on median nerve blood flow velocity. Biomed Res Int. 2017;2017:7156489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187–200. [DOI] [PubMed] [Google Scholar]
- 74. Hickey M, Phillips JP, Kyriacou PA. The effect of vascular changes on the photoplethysmographic signal at different hand elevations. Physiol Meas. 2015;36:425–440. [DOI] [PubMed] [Google Scholar]
- 75. Laganà MM, Di Rienzo M, Rizzo F, et al. Cardiac, respiratory and postural influences on venous return of internal jugular and vertebral veins. Ultrasound Med Biol. 2017;431195–1204. [DOI] [PubMed] [Google Scholar]
- 76. Gousopoulos E, Proulx ST, Bachmann SB, et al. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight. 2016;1:e89081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bordoni B, Marelli F, Morabito B, Castagna R, Sacconi B, Mazzucco New proposal to define the fascial system [in German; published online March 19, 2018]. Complement Med Res. doi:10.1159/000486238. [DOI] [PubMed] [Google Scholar]


