Abstract
Aims: This study was designed to assess knowledge, attitude and practices of adverse drug reaction reporting among healthcare professionals in Hiwot Fana Specialized University Hospital (HFSUH). Method: Hospital based descriptive cross sectional study was conducted on healthcare professionals of HFSUH. Based on purposive sampling technique, all eligible healthcare professionals (nurses, physicians and pharmacists) were involved in the study. A pretested self-administered questionnaire was used to collect data. Data were coded, entered and analyzed using SPSS version 16. The test of association of selected categorical variables were done using cross tabulation and Pearson Chi-square test. Result: Our study indicated that about 297 participants provided their response to the distributed questionnaires which makes the response rate 91.4%. Of the total healthcare professionals involved in the study, 99 (33.6%) of them were able to understand the difference between adverse drug reaction (ADR) and side effects, of which pharmacists were significantly reported (95.24%, P<0.05). About 175(59.3%) of the respondents engaged in the study were reportedly knew the national ADR reporting system in Ethiopia. On the other hand, 181(61.36%) of the participants were recognized the presence of ADR reporting form while 114 (38.64%) of the respondents had no any information about its presence in the country. Conclusion: The study revealed that a gap in knowledge, awareness and practice of healthcare professionals on ADR reporting. Therefore, specific strategies should be designed in order to improve awareness, knowledge and practice of healthcare professionals to tackle issue related to under-reporting of ADR.
Keywords: adverse drug reactions, spontaneous ADR reporting, knowledge, attitude, practice
Introduction
Adverse drug reactions (ADRs) are one of the major problems associated with medicines. The World Health Organization (WHO) defined ADR as any response to a drug that is noxious and unintended, and that occurs at doses used in humans for prophylaxis, diagnosis, or therapy.1 It is an important cause of morbidity and mortality worldwide. In Sweden, ADRs are categorized into the top 10 principal factors that cause mortality, whereas in the United States, it was grouped with the 6 leading causes of mortality.2 However, in developing countries such as Africa, there is a limited study about the incidence ADR.3-5 ADRs also lead to huge financial problems to patients as well as to the country because of hospitalization and other health service required along with negative impact on the victim’s economy.6 The incidence of ADRs on health care and patients in Ethiopia is not available; nevertheless, it is likely that the problem is considerably widespread due to irrational drug use, including preference for injections, misuse of antibiotics and other traditional/herbal medicines, and extensive self-medication.7 Spontaneous and voluntary ADR reporting system is an integral component of drug safety surveillance program and also the most effective methods of collecting ADR-related information, especially the new and serious reactions.8,9 Spontaneous reporting of ADRs is one of the basic principle of pharmacovigilance, and it is crucial in maintaining patient safety.10 Health care professionals play an important role in the detection, assessment, and spontaneous reporting of ADRs.10-12 The establishment of pharmacovigilance system of Ethiopia under Food, Medicine and Health Care Administration and Control Authority (FMHACA) in 2002 paved the way for the country to become a member of the WHO program for international drug monitoring. This organization is empowered with following and monitoring ADR, facilitating and regulating the overall activities related to ADR documentation and report; despite that, only a few of ADR reports had received since its establishment.13 Therefore, the present study was concerned with the assessment of baseline knowledge, attitudes, and practices of health care professionals in Hiwot Fana Specialized University Hospital (HFSUH) on ADR reporting systems.
Methodology
Study Design and Study Period
A hospital-based descriptive cross-sectional study was conducted to assess knowledge, attitudes, and practices of ADR reporting among health care professionals working in HFSUH, Harar, which is located 526 km from the capital of Ethiopia, Addis Ababa. The study was conducted from February to March 2015.
Study Population
The target populations for this study were all nurses, physicians, and pharmacists working in HFSUH during the study periods. Health care professionals who were refused or not willing to participate in the study were excluded.
Sample Size Determination and Sampling Technique
The sampling technique was a purposive sampling including all health care professionals. As the target populations were very small in number, sampling is not necessary. Therefore, all those who are willing and qualified to participate were included in the study.
Data Collection Instruments
Data collection tools were a questionnaire adapted from reviewing different literatures, guidelines, and previous similar studies on the knowledge, attitudes, and practices of health care professionals on ADR reporting, with a little modification to suit the HFSUH setup. The prepared self-administered questionnaire contains 6 different parts which include sociodemographic characteristics, existing knowledge about ADRs, awareness about ADRs, attitudes, practices, and reasons for not reporting ADRs.
Validity of Data Collection Instruments
Pretesting of the questionnaire was carried out in a similar setting to ensure its consistency and clarity at one of the hospitals in Harari regional state other than HFSUH. Accordingly, 5% of the data collection instruments were distributed among similar target populations, and the results obtained from the pretest were used to make a necessary adjustment on the questionnaire that posed ambiguity or confusion among the respondents to collect appropriate data.
Data Quality Control and Analysis
The data were checked for completeness, accuracy, and consistency, and those found incomplete or missing in addressing important variables were discarded. Then, the data were coded with a sequential number and entered into SPSS version 16 for analysis. A descriptive analysis of collected data was conducted as well as some of the tests of association of selected categorical variables were conducted using cross tabulation and Pearson chi-square test.
Ethical Consideration
The ethical approval and clearance were obtained from School of Pharmacy, College of Health and Medical Sciences, Haramaya University, and a written letter was brought to the administrative body of HFSUH to get permission for the study. In addition, a brief explanation of the objective of study was given for health care professionals to avoid ambiguity and misunderstanding. The process of data collection was started after the willingness of the health care professionals was asked, and the formal written or verbal consent was taken.
Operational Definition
ADRs are any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy.3,14
Side effect refers to unintended effect occurring at a normal dose related to the pharmacological properties of the drugs.3
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding, and prevention of ADR or any other medicine-related problems to improve the safety of medicines.15
Results
Sociodemographic Data of Health Care Professionals
Out of 325 participants, 297 respondents filled and returned the questionnaire with a response rate of 91.4%. However, 2 questionnaires were found to be incomplete and discarded to make the actual study participants only 295. From 295 health care professionals, 230 (78%) were nurses, 44 (14.9%) were physicians, and 21 (7.1%) were pharmacists. In terms of age, about 99 (33.6%) respondents were in the age range of 26 to 30 years. Of the total respondents, the number of males was 158 (53.6%), whereas the number of females was 137 (46.4%). About 140 (47.5%) of the total respondents had 0 to 4 years of experience (Table 1).
Table 1.
Sociodemographic Characteristics of Health Care Professionals of HFSUH, Harar, Eastern Ethiopia, From February to March 2015.
| Variables | Category | N = 295 | % |
|---|---|---|---|
| Age | 20-25 | 86 | 29.2 |
| 26-30 | 99 | 33.6 | |
| 31-35 | 54 | 18.3 | |
| 36-40 | 31 | 10.5 | |
| 41-45 | 13 | 4.4 | |
| 46-50 | 7 | 2.4 | |
| ≥51 | 5 | 1.7 | |
| Sex | Female | 137 | 46.4 |
| Male | 158 | 53.6 | |
| Professional | Nurse | 230 | 78 |
| Physician | 44 | 14.9 | |
| Pharmacist | 21 | 7.1 | |
| Years of experience | 0-4 | 140 | 47.5 |
| 5-9 | 109 | 36.9 | |
| 10-14 | 26 | 8.8 | |
| 15-20 | 13 | 4.4 | |
| 21-29 | 6 | 2 | |
| 30-40 | 1 | 0.3 |
Note. N = number of participants of the study; HFSUH = Hiwot Fana Specialized University Hospital.
Knowledge of ADR Reporting Among Health Care Professionals
Among 295 respondents, only 99 (33.6%) respondents were able to differentiate ADR from side effect, of which pharmacists were better able to distinguish ADR from side effect (95.24%, P < .05). Of 295 respondents, 175 (59.3%) and 181 (61.36%) knew the national ADR reporting system and availability of ADR reporting form in Ethiopia, respectively. Regarding professionals, pharmacists (80.95%, P < .05) and physicians (84.1%, P < .05) significantly reported that they knew the national ADR reporting system in Ethiopia. However, pharmacists (80.95%, P < .05) significantly identified the availability of ADR reporting form in relation to other professionals. One hundred fourteen (38.64%) of the respondents had no awareness about the availability of the national ADR reporting form. On the other hand, majority of the respondents, 208 (70.5%), were not familiar with the term of pharmacovigilance. Despite that, pharmacists (71.43%, P < .05) had significant knowledge about pharmacovigilance compared with the remaining health care professionals. With respect to the means of ADR reporting, 182 (61.69%) of the respondents knew at least one of the means of ADR reporting (telephone, post, and e-mail), whereas 113 (38.3%) participants did not know any methods of ADR reporting. On the other hand, some of the participants also selected multiple answer for methods used for ADR reporting (Table 2).
Table 2.
Knowledge of ADR Reporting Among Health Care Professionals in HFSUH, Harar, Eastern Ethiopia, From February to March 2015.
| Variables | Profession |
Total 295 (%) |
Pearson chi-square | P value | ||
|---|---|---|---|---|---|---|
| Nurses 230 (%) |
Physicians 44 (%) |
Pharmacists 21 (%) |
||||
| Do you think that ADR is the same with side effect? | ||||||
| Yes | 83 (36.1) | 15 (34.1) | 1 | 99 (33.6) | 8.475 | .014 |
| No | 147 (63.9) | 29 (65.9) | 20 (95.24) | 196 (66.4) | ||
| Do you know pharmacovigilance? | ||||||
| Yes | 50 (21.74) | 22 (50) | 15 (71.43) | 87 (29.5) | 33.309 | .000 |
| No | 180 (78.3) | 22 (50) | 6 | 208 (70.5) | ||
| Do you know national ADR reporting system? | ||||||
| Yes | 121 (52.6) | 37 (84.1) | 17 (80.95) | 175 (59.3) | 19.55 | .000 |
| No | 109 (47.4) | 7 (15.9) | 4 | 120 (40.7) | ||
| How are ADRs reported? | ||||||
| Those who know any method(s) of ADR reporting (by telephone, post, and e-mail)a | 128 (55.7) | 36 (81.82) | 18 (85.71) | 182 (61.69) | 16.220 | .000 |
| Those who do not know any methods of ADR reporting | 102 (44.4) | 8 (18.2) | 3 | 113 (38.3) | ||
| Do you know availability of ADR reporting form? | ||||||
| Yes | 130 (56.5) | 34 (77.27) | 17 (80.95) | 181 (61.36) | 10.370 | .006 |
| No | 100 (34.5) | 10 (27.72) | 4 | 114 (38.64) | ||
| Do you think that ADRs are well documented at the time a drug is marketed? | ||||||
| Yes | 116 (50.4) | 26 (59.1) | 10 (47.62) | 152 (51.53) | 1.246 | .536 |
| No | 114 (49.6) | 18 (40.9) | 11 (52.38) | 143 (48.47) | ||
Note. Association is done using Pearson chi-square test, P < .05 considered to be statistically significant. HFSUH = Hiwot Fana Specialized University Hospital; ADR = adverse drug reaction.
Indicates some participants selected more than 1 answer.
Awareness Regarding ADR Reporting Among Health Care Professionals in HFSUH
Of 295 respondents, 160 (54.2%, P < .05) of them believed that ADR should be reported to drug therapeutic committee (DTC) of the respective health facilities, whereas 68 (23.05%) of the respondents believed that ADR should be reported to FMHACA. However, 227 (76.9%, P < .05) of the respondents fail to identify FMHACA as a responsible organization to which ADR is to be reported in the country. Being a pharmacist, 12 (57.14%), accounted for a higher percentage of awareness on ADR report to FMHACA relative to other health care professionals. From the total respondents, 187 (63.4%, P < .05) believed that physicians were responsible in reminding and following up the clients, whereas about 186 (63.1%, P < .05) of the participants believed that it is the responsibility of pharmacists to remind and follow up the patients. As a source of information, about 155 (52.5%) of the participants were used national drug formulary and standard treatment guidelines. According to the respondents’ opinion, 232 (78.6%, P > 0.05) of the respondents expected that prescription error is a major predisposing factor to ADR, whereas 199 (67.5%, P > .05) of the study participants believed dispensing error responsibly predisposes the patients to ADR, even though both cases were not statistically significant. In addition, some of the respondents were considering multiple answer for questions concerning the individual who is responsible to remind the patients about drug side effects, sources of information for ADR, factors predisposing patients to develop ADR, and area where ADR is to be reported (Table 3).
Table 3.
General Awareness Regarding ADR Reporting Among Health Care Professionals at HFSUH, Harar, Eastern Ethiopia, From February to March 2015.
| Variables | Professions |
Total 295 (%) |
Pearson chi-square | P value | ||
|---|---|---|---|---|---|---|
| Nurses 230 (%) |
Physicians 44 (%) |
Pharmacists 21 (%) |
||||
| To whom do you think that ADRs should be reported?a | ||||||
| Manufacturer | 12 (5.22) | 1 | — | 13 (4.41) | 1.803 | .406 |
| Minister of health of the country | 28 (12.2) | 7 | 5 | 40 (13.6) | 2.466 | .291 |
| Drug therapeutic committee of respective health facility | 126 (54.8) | 30 (68.2) | 4 | 160 (54.2) | 13.952 | .001 |
| FMHACA | 49 (21.3) | 7 | 12 (57.14) | 68 (23.05) | 15.421 | .000 |
| Pharmacy department | 17 (7.4) | — | 1 | 18 (6.1) | 3.593 | .166 |
| Who do you think is primarily responsible to remind and follow up patients about side effects of drugs they are given? | ||||||
| Pharmacistsa | 142 (61.74) | 25 (56.8) | 19 (90.5) | 186 (63.1) | 7.684 | .021 |
| Physiciansa | 150 (65.2) | 30 (68.2) | 7 | 187 (63.4) | 8.941 | .011 |
| Nursesa | 63 (27.4) | 7 | 3 | 73 (24.75) | 3.943 | .139 |
| What is your source of information about ADR? | ||||||
| National drug formulary and STGa | 115 (50) | 27 (61.4) | 13 (61.9) | 155 (52.5) | 2.707 | .258 |
| Standard textbooka | 76 (33) | 13 (29.5) | 9 (42.9) | 98 (33.2) | 1.150 | .563 |
| Note from traininga | 37 (16.1) | 4 | — | 41 (13.9) | 5.498 | .240 |
| Drug salesmana | 4 | 1 | — | 5 | 0.453 | .797 |
| What possible factor(s) do you think predispose(s) a patient to ADR? | ||||||
| Dispensing errora | 153 (66.5) | 31 (70.5) | 15 (71.4) | 199 (67.5) | 0.423 | .810 |
| Overdosea | 105 (45.7) | 19 (43.2) | 16 (76.2) | 140 (47.5) | 7.507 | .023 |
| Prescription errora | 182 (47.53) | 36 (81.8) | 14 (66.7) | 232 (78.6) | 2.090 | .352 |
| Lifestyle of the patienta | 86 (37.4) | 19 (43.2) | 9 (42.9) | 114 (38.6) | 0.692 | .708 |
| Nonadherence to drug regimena | 93 (40.4) | 19 (43.2) | 11 (52.4) | 123 (41.7) | 1.177 | .555 |
Note. Association is done using Pearson chi-square test, P < .05 considered to be statistically significant. HFSUH = Hiwot Fana Specialized University Hospital; ADR = adverse drug reaction; FMHACA = Food, Medicine and Health Care Administration and Control Authority; STG = standard treatment guideline.
Indicates some participants selected multiple answer.
Association of Years of Experience With Knowledge on ADR Reporting
According to the findings of this study, health care professionals with the years of experience between 10 and 14 significantly reported that they have awareness about the national ADR reporting system (84.6%, P < .05). On the other hand, with regard to the availability of ADR reporting form, health care professionals in the range of 5 to 9 (71.6%, P < .05) and 10 to 14 years of experience (84.6%, P < .05) suggested that they knew the availability of ADR reporting form (Table 4).
Table 4.
Association of Years of Experience With Knowledge of ADR Reporting Among Health Care Professionals at HFSUH, Harar, Eastern Ethiopia, From February to March 2015.
| Variables | Years of experience |
Total 295 (%) |
Pearson chi-square | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0-4 140 (%) |
5-9 109 (%) |
10-14 26 (%) |
15-19 13 (%) |
20-29 6 (%) |
30-40 1 (%) |
||||
| Do you think that ADR is the same with side effect? | |||||||||
| Yes | 45 (32) | 36 (33) | 12 (46) | 5 | 1 | — | 99 (33.6) | 3.403 | .638 |
| No | 95 (67.9) | 73 (67) | 14 (54) | 8 (61.5) | 5 | 1 | 196 (66.4) | ||
| Do you know pharmacovigilance? | |||||||||
| Yes | 44 (31.4) | 33 (30.3) | 5 | 3 | 1 | 1 | 87 (29.5) | 4.724 | .450 |
| No | 96 (68.6 | 76 (69.7) | 21 (80.7) | 10 (76.9) | 5 | — | 208 (70.5) | ||
| Do you know national ADR reporting system? | |||||||||
| Yes | 67 (47.9) | 73 (67) | 22 (84.6) | 9 (69.2) | 3 | 1 | 175 (59.3) | 18.593 | .002 |
| No | 73 (52.1) | 36 (33) | 4 | 4 | 3 | — | 120 (40.7) | ||
| How are ADRs reported? | |||||||||
| Those who know any method(s) of reporting (by telephone, post, and e-mail) | 63 (45) | 39 (35.8) | 6 (23.1) | 1 | 4 | — | 113 (38.3) | 13.319 | .021 |
| Those who do not know any methods of ADR reporting | 77 (55) | 70 (64.2) | 20 (76.9) | 12 (92.3) | 2 | 1 | 182 (61.7) | ||
| Do you know availability of ADR reporting form? | |||||||||
| Yes | 72 (51.4) | 78 (71.6) | 22 (84.6) | 7 (53.8) | 1 | 1 | 181 (61.4) | 22.531 | .000 |
| No | 68 (48.6) | 31 (28.4) | 4 | 6 (46.2) | 5 | — | 114 (38.6) | ||
| Do you think that ADRs are well documented at the time a drug is marketed? | |||||||||
| Yes | 64 (45.7) | 64 (58.7) | 14 (53.8) | 7 (53.8) | 2 | 1 | 152 (51.5) | 5.969 | .309 |
| No | 76 (54.3) | 45 (41.3) | 12 (46.2) | 6 (46.2) | 4 | 0 | 143 (48.5) | ||
Note. Association is done using Pearson chi-square, P < .05 considered to be statistically significant. HFSUH = Hiwot Fana Specialized University Hospital; ADR = adverse drug reaction.
General Practices Regarding ADR Reporting
Out of 295 participants involved in the study, 145 (49.2%) encountered ADR in the past 12 months of their clinical practice. However, only 110 (37.3%) of the respondents recorded ADR in the patient follow-up chart. In terms of professions, a significant number of physicians (72.7%, P < .05) observed ADR during the last 12 months of their practice. Statistically significant differences were not identified among health care professionals in terms of number of patients with ADR that encountered and documentation of recognized ADR on the patient follow-up chart (P > .05) in the past 12 months. About 179 (60.68%) of the respondents reported ADR to the concerned body, in which a significant number of physicians (77.27%, P < .05) conducted the report compared with other health care professions. Of the respondents, 101 (34.24%) usually provide advice for their clients in the last 12 months, whereas 12 (4.07%) respondents do not offer any advice with regard to drugs for their clients. Moreover, among health care professionals, pharmacists (66.67%, P < .05) significantly reported that they usually provide advice on possible adverse effects of drugs prescribed, dispensed, or administered to their patients during the last 12 months (Table 5).
Table 5.
Practices of ADR Reporting Among Health Care Professionals at HFSUH, Harar, Eastern Ethiopia, From February to March 2015.
| Variables | Professions |
Total 295 (%) |
Pearson chi-square | P value | ||
|---|---|---|---|---|---|---|
| Nurses 230 (%) |
Physicians 44 (%) |
Pharmacists 21 (%) |
||||
| Have you ever encountered patients with ADR in your clinical practice in the last 12 months? | ||||||
| Yes | 102 (44.3) | 32 (72.7) | 11 (52.4) | 145 (49.2) | 11.816 | .002 |
| No | 127 (55.2) | 12 (27.3) | 10 (47.6) | 149 (50.5) | ||
| How many patients with ADR did you see? | ||||||
| One | 21 (9.13) | 6 | 3 | 30 (10.17) | 13.972 | .083 |
| Two | 30 (13.04) | 6 | 5 | 41 (13.9) | ||
| Three | 20 (8.7) | 13 (29.55) | 1 | 34 (11.53) | ||
| Four | 14 (6.1) | 6 | — | 20 (6.78) | ||
| Above four | 18 (7.83) | 1 | 2 | 21 (7.12) | ||
| Have you noted down the ADR you encountered on the patient clinical record? | ||||||
| Yes | 75 (32.61) | 28 (63.64) | 7 (33.3%) | 110 (37.3) | 4.150 | .386 |
| No | 28 (12.17) | 4 | 4 | 36 (12.2) | ||
| Have you ever reported the ADRs? | ||||||
| Yes | 134 (58.26) | 34 (77.27) | 11 (52.4) | 179 (60.68) | 6.139 | .046 |
| No | 95 (41.3) | 10 (22.73) | 10 (47.6) | 115 (38.98) | ||
| Where did you report that reaction? | ||||||
| Hospital | 67 (29.13) | 26 (59.1) | 9 (42.86) | 102 (34.58) | 15.125 | .019 |
| Pharmaceutical company | 33 (14.35) | 7 | 1 | 41 (13.9) | ||
| FMHACA | 2 (0.87) | — | — | 2 | ||
| Doctor | 37 (16.1) | 1 | 1 | 39 (13.22) | ||
| How often do you give advice to your patients on possible adverse effects of drugs you prescribed, dispensed, or administered? | ||||||
| Usually | 69 (30) | 18 (40.91) | 14 (66.67) | 101 (34.24) | 14.842 | .022 |
| Sometimes | 147 (63.91) | 26 (59.1) | 7 (33.33) | 180 (61.02) | ||
| Never | 12 (5.22) | — | — | 12 (4.07) | ||
Note. Association is done using Pearson chi-square test, P < .05 considered to be statistically significant. HFSUH = Hiwot Fana Specialized University Hospital; ADR = adverse drug reaction; FMHACA = Food, Medicine and Health Care Administration and Control Authority.
Attitudes of Health Care Professionals Toward ADR Reporting
With respect to attitude, this study illustrated about 218 (73.9%) of the respondents agreed that ADR should be reported spontaneously on a regular basis and 179 (60.68%) of the respondents thought that ADR reporting is part of their duty. Among different health care professionals, pharmacists (80.95%, P < .05) significantly recognized that ADR reporting is part of their responsibility. Majority of the respondents, 246 (83.4%), believed that reporting drug safety is crucial for the public and 216 (73.2%) of the respondents agreed that reporting ADR is imperative for the health care system, even though in both cases statistically significant difference were not present among different health care professionals. On the other hand, about 200 (67.8%) of the participants of the study believed they need to be sure that ADR is related to the drug before reporting. In relation to other professions, pharmacists (85.7%, P < .05) significantly reported that they need to be clear with ADR observed before reporting. One hundred eighty-four (62.4%) of the respondents disagreed with the idea that ADR reporting is creating additional workloads as well as 116 (39.3%) of the participants were against of reporting only ADR causing persistent disability (Table 6).
Table 6.
Attitudes of Health Care Professionals Toward ADR Reporting in HFSUH, Harar, Eastern Ethiopia, From February to March 2015.
| Variables | Professions |
Total 295 (%) | Pearson chi-square | P value | ||
|---|---|---|---|---|---|---|
| Nurses 230 (%) |
Physicians 44 (%) |
Pharmacists 21 (%) |
||||
| ADRs should be reported spontaneously at regular basis | ||||||
| Agree | 166 (72.2) | 34 (72.3) | 18 (85.7) | 218 (73.9) | 5.718 | .221 |
| Neutral | 55 (23.9) | 9 (20.45) | 1 | 65 (22.03) | ||
| Disagree | 9 (3.91) | 1 | 2 | 12 (4.07) | ||
| Reporting ADR is part of duty of health professionals | ||||||
| Agree | 134 (58.26) | 28 (63.64) | 17 (80.95) | 179 (60.68) | 12.02 | .017 |
| Neutral | 91 (39.56) | 16 (36.36) | 2 | 109 (36.95) | ||
| Disagree | 5 | — | 2 | 7 (33.3) | ||
| Reporting drug safety is important for the public | ||||||
| Agree | 189 (82.17) | 36 (81.82) | 21 (100) | 246 (83.4) | 5.692 | .223 |
| Neutral | 36 (15.65) | 8 (18.2) | — | 44 (14.92) | ||
| Disagree | 5 | — | — | 5 | ||
| Reporting drug safety is important for the health care system | ||||||
| Agree | 165 (71.74) | 30 (68.2) | 21 (100) | 216 (73.2) | 8.762 | .067 |
| Neutral | 64 (27.83) | 14 (31.82) | — | 78 (26.4) | ||
| Disagree | 1 | — | — | — | ||
| There is a need to be sure that ADR is related to the drug before reporting | ||||||
| Agree | 155 (67.4) | 27 (61.36) | 18 (85.7) | 200 (67.8) | 10.398 | .034 |
| Neutral | 64 (27.83) | 17 (38.6) | 1 | 82 (27.8) | ||
| Disagree | 11 (4.78) | — | 2 | 13 (4.4) | ||
| Only ADR that causes persistent disability should be reported | ||||||
| Agree | 43 (18.7) | 4 | 6 (28.57) | 53 (17.97) | 6.700 | .153 |
| Neutral | 102 (44.35) | 19 (43.2) | 5 | 126 (42.7) | ||
| Disagree | 85 (36.96) | 21 (47.7) | 10 (47.62) | 116 (39.3) | ||
| Reporting creates additional workload | ||||||
| Agree | 29 (12.6) | 6 (13.64) | 7 (33.3) | 42 (14.2) | 6.812 | .146 |
| Neutral | 55 (23.9) | 10 (22.7) | 4 | 69 (23.4) | ||
| Disagree | 146 (63.5) | 28 (63.64) | 10 (47.62) | 184 (62.4) | ||
Note. P < .05 is considered statistically significant. ADR = adverse drug reaction; HFSUH = Hiwot Fana Specialized University Hospital.
Reasons for Not Reporting ADR
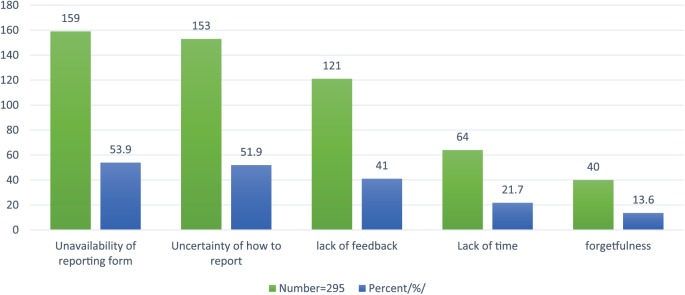
Among 295 participants of the study, 159 (53.9%) of the respondents did not report ADR mainly due to the unavailability of reporting form, and similarly, 153 (51.9%) of the respondents also unable to report it because of uncertainty of how to report. In addition, 121 (41%) of the health care professionals failed to report ADR due to the lack of feedback from the concerned body. Some of the individuals involved in the study were considering multiple reasons for why they failed to report ADR (Figure 1).
Figure 1.
Reasons for not reporting ADR among health care professionals at HFSUH from February to March 2015.
Note. Due to multiple responses, the sum of the respondents is greater than the actual number of the people involved in the study. ADR = adverse drug reaction; HFSUH = Hiwot Fana Specialized University Hospital.
Discussion
ADRs can result in a deleterious effect on the health of patients with increasing the risk of morbidity and mortality as well as hospitalization that lead to unnecessary health care expenditures. Therefore, monitoring of ADRs is considered as part of an integral component of patient care. To put these into effect, the contribution of health care professionals in early detection and reporting of ADR is indispensable.16 Taking this into consideration, our study prominently targeted on nurses, physicians, and pharmacists, those who are closely in contact with the patients. Accordingly, the proportion of health care professionals involved in this study indicated the nurses accounted for a major portion of the participants while it was followed by physicians and pharmacists. This finding is in support with study conducted at Adama Hospital Medical College.17
Regarding the knowledge to distinguish ADR from side effects, only 33.6% of respondents provided a positive response, whereas about 66.4% of them failed to do so. This finding is higher than other study conducted in Addis Ababa, the capital city of Ethiopia.13 However, it is not concordant with the finding reported from Adama Hospital Medical College in which majority of the respondents were able to differentiate ADR from side effects.17 Probably, this might indicate low knowledge of health care professionals on ADR which is related to poor attention and awareness creation about ADR and its consequences in the hospital. Nevertheless, pharmacists reported that they had better knowledge about the difference between ADR and side effects. This could be emanated from the fact that pharmacists have more access to information related to ADR as they are frequently dealing with drugs, than nurses and physicians, which enable them to better distinguish ADR from side effects. This finding is in line with the results reported from the study conducted in West Ethiopia at Nekemte Hospital.18
The study also indicated 59.3% of the respondents were clear with the national ADR reporting system, in which 80.95% (P < .05) of pharmacists and 84.1% (P < .05) of physicians were significantly reported. This could in fact indicate pharmacists and physicians have relatively adequate information about ADR and its reporting system. Besides to this, the study showed that 61.36% of the participants reportedly knew the availability of ADR reporting form in Ethiopia in which about 52.6% of nurses reported to have awareness about its presence. This finding is comparable with the results reported from the study conducted in West Ethiopia at Nekemte Hospital.18 However, it was higher than a study conducted in India.19 On the other hand, our findings demonstrated that about 38.64% of the study participants had no awareness about the availability of national ADR reporting form or yellow card and 40.7% of the respondents were also not clear with the ADR reporting system in the country. This finding is closely related to the study conducted in West Ethiopia at Nekemte Hospital.18 Furthermore, this study showed that only health care professionals between 10 and 14 years of experience adequately (P < .05) reported their awareness about the national ADR reporting system. However, the availability of ADR reporting system in the country was significantly reported by those with 5 to 9 (P < .05) and 10 to 14 (P < .05) years of experience. These gaps among health care professionals could probably be related to the absence of in-service training or orientation which enable them to acquire and consolidate their knowledge about the ADR reporting system throughout the period of their clinical service. This finding is not in line with the study conducted in Spain which stated that the tendency to report ADR increases with length of work experience20 as well as it is not consistent with the study conducted in West Ethiopia.18
On the other hand, our study showed that about 70.5% of the study participants had no information about pharmacovigilance. This could be attributed to the absence of ADR-related education, in-service training, and encouraging and establishing ADR reporting committee,21 and lack of motivation and feedback from FMHACA has its own impact. These could lead to underreporting of ADR which leads to high risk of serious ADR exposure among the individuals and negatively affect the quality of life of patients.22 Despite that, pharmacists were reported to be more knowledgeable than the remaining health care professionals (P < .05) with regard to the pharmacovigilance system. In fact, this may demonstrate that pharmacists have more access to information related to drugs and their negative consequence on the health of individuals compared with other health care professionals. This finding is concordant with the results reported from a study conducted at Nekemte Hospital, in which pharmacists were better able to recognize the term pharmacovigilance.18 However, our results are not in agreement with the study conducted in Jordan, in which Pharmacists had poor knowledge about the pharmacovigilance system.23
Our study also indicated that 61.69% of the respondents knew at least one of the means of ADR reporting, whereas 38.3% of the participants did not know any methods of ADR reporting. Although more than half of the respondents knew methods of ADR reporting, still knowledge gaps were observed among health care professionals that could undermine proper handling and reporting of ADR. In terms of professions, 81.82% of physicians and 85.71% of pharmacists were found to be more knowledgeable than nurses on how to report ADR. This might indicate poor team work and information sharing among these health care professionals that could lead to unequal distribution of knowledge on how to report ADR.
Concerning the knowledge on ADR documentation at the time of marketing, 52.53% of respondents believed that ADR is well documented at the time of marketing, whereas 48.47% of the participants did not agree it. This could be resulted from poor awareness about new ADRs likely to be associated with the drugs after marketing due to exposure of a large group of population with various characteristics and genetic makeup that contribute to unusual reactions not ever reported. Therefore, the presence of such perceptions among health care professionals attributed to undermining early detection and reporting of ADR to be encountered which may in turn end up with fatal consequences.
Regarding the concerned body to whom ADR is to be reported, most of the study participants suggested that ADR should be reported to DTC of the health facility, whereas only few of them believed that ADR should be reported to FMHACA. This result is in line with the report of the study conducted in West Ethiopia at Nekemte town.24 However, our finding is lower than the study conducted in Amhara region.25 Nevertheless, compared with other health care professionals, 57.14% of pharmacists participated in our study have better awareness with respect to ADR reporting to FMHACA. Probably, this could imply that pharmacists mainly deal with drugs and their related issues, and they are more likely to know where such problems are to be reported and addressed.
In terms of individual who is primarily responsible in reminding and following up of the clients about ADR of the drugs, around 63% of the respondents suggested that it is the responsibility of pharmacists and physicians. This may be due to the fact that physicians and pharmacists are mainly engaged in prescribing and dispensing the drugs, respectively, and they have an ample chance to discuss drug-related issues frequently with their clients.
Concerning the source of information about ADR, our study indicated 52.5% of the participants used national drug formulary and standard treatment guidelines, whereas 33.2% of the respondents prefer standard textbooks as a source of information for ADR. This finding is not in agreement with study conducted in Amhara region.25 On the other hand, according to the respondents’ opinion, 78.6% of respondents expected that prescription error is a major predisposing factor to ADR, whereas 67.5% of study participants believed dispensing error invariably predisposes the patients to ADR. Probably, this could be ascribed to the ignorance of physicians who are expected to select appropriate drugs for their patients based on potential and predictable ADRs related to the drugs with respect to their patient’s health status. Likewise, dispensing errors that arise from inadequate knowledge or inexperienced dispenser are not to be overlooked as it is likely to predispose patients to unnecessary effects of drugs.
Regarding practice, 49.2% of participants of the study encountered ADR in the past 12 months of their clinical practice. This finding is higher than the result obtained from the study conducted in West Ethiopia at Nekemte Hospital18 and Nekemte town.24 However, only 37.3% of the respondents recorded ADR in the patient follow-up chart. This habit implies a poor practice of ADR documentation among health care professionals that could be contributed to masking critical problems posed by the drugs and undermining post–marketing assessment of drugs safety. Probably, this could be linked to the lack of the desired knowledge and awareness about significance of ADR reporting which in turn uphold and maintain safety of the patients. This result is comparable with the finding of the study conducted in Amhara region.25 In terms of professions, a significant number of physicians (P < .05) observed ADR during the last 12 months of their practice. This finding is in agreement with the result reported from a study carried out in Malaysia.26 Despite the poor knowledge and awareness, 60.68% of the respondents reported ADR to the concerned body, in which a significant number of physicians (P < .05) conducted the report compared with other health care professions. This finding is significantly higher than the result reported from the study conducted in a tertiary health care center in South India.27
With respect to attitude, most of our study participants demonstrate positive attitude toward spontaneous ADR reporting as well as considering reporting of ADR as part of their professional obligation. Accordingly, 73.9% of the respondents agreed that ADR should be reported spontaneously on a regular basis, and 60.68% of the respondents thought that ADR reporting is part of their duty. Providing training and education on ADR28,29 and feedback from the concerned organization together with imposing tight rules on them may encourage reporting among health care professionals, which in turn contributes a lot to the pharmacovigilance system. However, our findings are lower as compared with the study conducted in Amhara region,25 Malaysia,27 and in West Ethiopia at Nekemte Hospital.18 Moreover, our findings also showed about 83.4% of respondents believed reporting drug safety is crucial for the public, whereas 73.2% of them agreed that reporting ADR is imperative for the health care system of the community. Besides, 67.8% of respondents believed that they need to be sure whether ADR is related to the drug before reporting. This finding is closely in agreement with the study carried out in West Ethiopia at Nekemte Hospital.18 In relation to other professions, pharmacists (P < .05) significantly reported that they need to be sure about ADR associated with drugs before reporting. Probably, this may indicate that pharmacists have better knowledge about the properties of drugs and possible unintended reactions associated with the drugs that could enable them to look further whether the suspected problem(s) is/are likely to arise from the drug itself to avoid trivial reports.
Concerning the burden of ADR reporting on daily activities and types of ADR to be reported, 62.4% of respondents disagreed that ADR reporting is creating additional workloads on their daily activity. This might arise from strong positive attitudes of health care professionals to fulfill their commitment and societal obligation imposed on them to serve their community. In addition, 39.3% of respondents disagreed that only ADR causing persistent disability should be reported. This finding is lower than the result from other study, in which 52.2% of the participants strongly disagreed reporting of only ADR causing persistent disability.18 Moreover, the study conducted in Jordan also indicated that reaction characterized with serious consequences, unusual reaction, and reaction not yet reported get priority attention to avoid trivial ADR reporting.23
Regarding reasons for not reporting ADR, the present study reveals 53.9%, 51.9%, 41%, 21.7%, and 13.6% of respondents fail to report ADR due to the unavailability of reporting form, uncertainty of how to report, lack of feedback, lack of time, and forgetfulness, respectively. In a similar fashion, the study conducted in West Ethiopia at Nekemte town showed that uncertainty of how to report and unavailability of reporting form are suggested to be discouraging factors.24 In addition, our findings are closely related to the study conducted in tertiary hospitals of North India.30
Conclusion
The results of our study at HFSUH stated poor awareness, knowledge, and practice toward spontaneous ADR reporting system. In addition, most of the participants are not clear with the concerned body in the country who is responsible to address ADR-related issues. Therefore, we recommended in-service training to promote its related problems along with appropriate reporting system, which is at the heart of pharmacovigilance systems to enhance spontaneous and voluntary ADR reporting.
Acknowledgments
The authors are very grateful to Haramaya University for their unreserved support for the success of the study. We extend our sincere gratitude to our data collectors and the study participants for their kindness and willingness to involve in our study.
Footnotes
Author Contributions: HS involved in the development of the concept, preparation of proposal, and write-up. JA supervised throughout the study periods, critically evaluated the findings, involved in write up and prepared the final manuscript for publication. Both the authors read and approved the final version of the manuscript thoroughly.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Sivadasan S, Yuong NY, Chyi NW, et al. Knowledge and perception towards pharmacovigilance and adverse drug reaction reporting among medicine and pharmacy students. World J Pharm Pharmaceut Sci. 2014;3(3):1652-1676. [Google Scholar]
- 2. Srikanth BA, Hailu W, Admassie E, Patel I, Khan TM. Knowledge, attitude and practices of health professional towards adverse drug reactions reporting in Gondar University Hospital Northwest Ethiopia. Afr J Health Sci. 2014;27(1):46-56. [Google Scholar]
- 3. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255-1259. [DOI] [PubMed] [Google Scholar]
- 4. Oshikoya KA. Adverse drug reaction in children: types, incidence and risk factors. Niger J Paediatr. 2006;33:29-35. [Google Scholar]
- 5. Elkami RM, Hassali MA, Ibrahim MI, Liau SY, Awaisu A. A qualitative study exploring barriers and facilitators for reporting of adverse drug reactions among community pharmacists in Malaysia. J Pharm Health Serv Res. 2011;2:71-78. [Google Scholar]
- 6. Chetty S, Parida A, Adiga S, Bairy KL. Knowledge, attitude and practice of health care professionals towards adverse drug reaction reporting in a South Indian teaching hospital. World J Pharm Res. 2014;3(3):4263-4271. [Google Scholar]
- 7. Dilbato DD, Kuma ZG, Teklemariam S. A baseline survey on prescribing indicators and the underlying factors influencing prescribing in southern Ethiopia. Ethiop J Health Dev. 1998;12(2):87-93. [Google Scholar]
- 8. Waller PC. Making the most of spontaneous adverse drug reaction reporting. Basic Clin Pharmacol Toxicol. 2006;98:320-323. doi: 10.1111/j.1742-7843.2006.pto_286.x. [DOI] [PubMed] [Google Scholar]
- 9. Vallano A, Cereza G, Pedròs C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60(6):653-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrison-Griffiths S, Pirmohamed M. Specialist nurse reporting of adverse drug reactions. Prof Nurse. 2000;15(5):300-304. [PubMed] [Google Scholar]
- 11. Ulfvarson J, Mejyr S, Begman U. Nurses are increasingly involved in pharmacovigilance in Sweden. Pharmacoepidemiol Drug Saf. 2007;16(5):532-537. doi: 10.1002/pds.1336. [DOI] [PubMed] [Google Scholar]
- 12. Backstrom M, Mjorndal T, Dahlqvist R. Spontaneous reporting of adverse drug reactions by nurses. Pharmacoepidemiol Drug Saf. 2003;12(2):157-159. doi: 10.1002/pds.753. [DOI] [PubMed] [Google Scholar]
- 13. Drug Administration and Control Authority in collaboration with MSH/RPM plus/SPS. Report on the assessment of health care providers’ knowledge, attitude and practice on adverse drug reaction (ADR) reporting and its monitoring. Addis Ababa, Ethiopia: http://www.fmhaca.gov.et/documents/ADR_Study.pdf. Published 2008. Accessed October 4, 2017. [Google Scholar]
- 14. Wilke RA, Lin DW, Roden DM, et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. 2007;6(11):904-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Pharmacovigilance: Ensuring the Safe Use of Medicines. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 16. Iffat W, Shakeel S, Naseem S, Imam S, Khan M. Attitudinal survey to assess medical and dental students belief of ADR reporting in Pakistan. Int J Pharm Pharm Sci. 2014;6(5):279-283. [Google Scholar]
- 17. Bule MH, Hamido BA, Chala TS, Kefeni GT. Knowledge, attitudes and practices of healthcare professionals towards adverse drug reaction reporting in Adama Hospital Medical College, East Shoa zone, Oromia regional state, Ethiopia. Pharma Innovation. 2016;5(7):24-28. [Google Scholar]
- 18. Tariku B, Mulisa M, Tesema S. Health professionals’ knowledge, attitude and practices towards adverse drug reaction reporting in Nekemte Hospital, Ethiopia. MD-Medical Data. 2015;7(1): 041-046. [Google Scholar]
- 19. Ganesan S, Vikneswaran G, Reddy KC, Subrahmanyam DK, Adithan C. A survey on knowledge, attitude and practice of pharmacovigilance towards adverse drug reactions reporting among doctors and nurses in a tertiary care hospital in South India. J Young Pharm. 2016;8(4):471-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernandez-Diaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Safety. 2007;30(11):1073-1082. [DOI] [PubMed] [Google Scholar]
- 21. Kaur M, Kosey S, Kumar R. Knowledge, attitude, and practice of healthcare professionals about adverse drug reaction in major tertiary care teaching hospital in Punjab. Int J Basic Clin Pharmacol. 2015;4(5):993-998. [Google Scholar]
- 22. Aithal S, Hooli TV, Varun HV. Knowledge and attitude about adverse drug reaction reporting among doctors at a tertiary care hospital. Int J Pharm Bio Sci. 2014;5(1):108-113. [Google Scholar]
- 23. Suyagh M, Farah D, Farha RA. Pharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Saudi Pharm J. 2015;23(2):147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gurmesa LT, Dedefo MG. Factors affecting adverse drug reaction reporting of healthcare professionals and their knowledge, attitude, and practice towards ADR reporting in Nekemte Town, West Ethiopia. Biomed Res Int. 2016;2016:5728462. doi: 10.1155/2016/5728462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Necho W, Worku A. Assessment of knowledge, attitude and practice of health professionals towards adverse drug reaction reporting and factors associated with reporting. J Pharmacovigilance. 2014;2:135. doi: 10.4172/2329-6887.1000135. [DOI] [Google Scholar]
- 26. Tew MM, Teoh BC, Mohd Baidi AS, Saw HL. Assessment of knowledge, attitude and practices of adverse drug reaction reporting among doctors and pharmacists in primary healthcare. Adv Pharmacoepidemiol Drug Saf. 2016;5:206. doi: 10.4172/2167-1052.1000206. [DOI] [Google Scholar]
- 27. Thomas TM, Udaykumar P, Scandashree K. Knowledge, attitude and practice of adverse drug reaction reporting among doctors in a tertiary health care centre in South India. Int J Pharmacol Clin Sci. 2013;2(3):82-88. [Google Scholar]
- 28. Abubakar AR, Simbak NB, Haque M. A systematic review of knowledge, attitude and practice on adverse drug reactions and pharmacovigilance among doctors. J Appl Pharmaceut Sci. 2014;4(11):117-127. [Google Scholar]
- 29. Adhikary J, Bhandare B, Adarsh E, Satyanarayana V. A study to assess knowledge, attitude and practice of adverse drug reaction reporting among physicians in a tertiary care hospital. J Evol Med Dent Sci. 2013;2:1027-1034. [Google Scholar]
- 30. Bhati N, Gupta S, Kgosla PP. Assessment of knowledge, attitude and practices of health professionals towards adverse drug reactions and pharmacovigilance in tertiary hospital of North India. J Adv Manag Res. 2014;1(1). doi: 10.5176/2345-7201. [DOI] [Google Scholar]