Abstract
Aims
To evaluate the association of cumulative blood pressure (BP) from young adulthood to middle age with left atrial (LA) structure/function as assessed by three-dimensional echocardiography (3DE) in a large longitudinal bi-racial population study.
Methods and results
We conducted a prospective post hoc analysis of individuals enrolled at the Coronary Artery Risk Development in Young Adults, which is a multi-centre bi-racial cohort with 30 years of follow-up. Cumulative systolic and diastolic BP levels were defined by summing the product of average millimetres of mercury and the years between each two consecutive clinic visits over 30 years of follow-up. Multivariable linear regression analyses were used to assess the relationship between cumulative systolic and diastolic BP with 3DE LA structure and function, adjusting for demographics and traditional cardiovascular risk factors. A total of 1033 participants were included, mean age was 55.4 ± 3.5 years, 55.2% women, 43.9% blacks. Cumulative systolic BP had stronger correlations than cumulative diastolic BP. Higher cumulative systolic BP was independently associated with higher 3D LA volumes: maximum (β = 1.74, P = 0.004), pre-atrial contraction (β = 1.87, P < 0.001), minimum (β = 0.76, P = 0.04), total emptying (β = 0.98, P = 0.006), active emptying (β = 1.12, P < 0.001), and lower magnitude 3D LA early diastolic strain rate (β = 0.05, P = 0.02). Higher cumulative diastolic BP was independently associated with higher 3D LA active emptying volume (β = 0.66, P = 0.002), lower magnitude 3D LA early diastolic strain rate (β = 0.05, P = 0.004), and higher magnitude 3D LA late diastolic strain rate (β = −0.04, P = 0.05).
Conclusion
Higher cumulative BP from early adulthood throughout middle age was associated with adverse LA remodelling evaluated by 3D echocardiography.
Keywords: blood pressure , left atrium function , left atrium remodelling , 3D echocardiography
Introduction
Hypertension is a common disease affecting about one-third of the adult US population,1 and it is a major risk factor for cardiovascular (CV) disease, especially later in life when the prevalence of the disease is higher, and the cumulative blood pressure (BP) burden is more pronounced.2 In spite of advances in diagnosis and treatment, hypertension remains poorly controlled in 20–40% of patients.3
In recent years, cohort studies have investigated the association between BP and CV disorders by taking into consideration duration of exposure to high BP, as assessed by BP trajectories or cumulative exposure over a lifetime.4 This approach has provided insights into the long-term impact of BP exposure when compared with a single point in time evaluation.5 Also, higher cumulative BP has been independently associated with all-cause mortality, CV and cerebrovascular events, stroke, and worse cognitive performance.6
Recently, three-dimensional echocardiography (3DE) using speckle tracking echocardiography (STE) has allowed an accurate, reproducible and detailed assessment of cardiac structure and function, reducing the error caused by foreshortening, avoids geometric assumptions, and the out of plane motion phenomenon, which are inherent limitations in two-dimensional echocardiography (2DE) techniques.7
Prior investigations have shown that LA remodelling was independently associated with heart failure, the degree of left ventricular (LV) diastolic dysfunction, atrial fibrillation (AF), stroke, and long-term survival.8,9 Furthermore, other investigators have demonstrated that LA dysfunction was independently associated with mid to low levels of cumulative BP finding in high-normal BP, white coat hypertension, and masked hypertension.10
However, there is a paucity of data about the relationship of cumulative BP with LA structure and function assessed by 3DE. Therefore, this study aimed to evaluate the independent association of cumulative BP from young adulthood to middle age with LA structure and function evaluated by 3DE in a large longitudinal bi-racial population study.
Methods
Supplementary data online (S1, S2, and S3) describes in detail the study enrollment flowchart, the echocardiographic assessment, and the clinical covariates evaluation in the CARDIA study.
Study population
CARDIA is a multi-centre community-based cohort study sponsored by the National Heart, Lung, and Blood Institute, initiated between 1985 and 1986 that enrolled 5115 men and women, African-American, and white participants, aged 18–30 years at baseline from four USA field centres (Birmingham, Alabama; Oakland, California; Chicago, Illinois; and Minneapolis, Minnesota).11 After the baseline examination (year-0), eight subsequent examinations (Y2, Y5, Y7, Y10, Y15, Y20, Y25, and Y30) were performed. All participants provided written informed consent in each visit, and institutional review boards from each field centre and the co-ordinating centre approved the study annually. At the year-30 examination, 3358 participants (2015–16), 3184 underwent a standard 2DE evaluation, and 2834 had a 3DE assessment. Echocardiograms with a poor quality of 3D images as well as lacking BP or other covariates measurements were considered exclusion criteria, leaving 1033 subjects for a post hoc analysis of this proposed study. History of AF or episodes of AF during image acquisition were not classified as an exclusion criterion for this study.
3DE acquisition and analysis
All 3D images were acquired with a matrix array PST-25SX 2.0–4.0 MHz transducer in a single breath hold over four consecutive cardiac cycles following the ASE 3DE guidelines.12 The images were analysed offline by trained and certified readers using a wall motion tracking software package (UltraExtend™, version 3.0, Toshiba Medical System) following a standardized protocol. A volumetric dataset was acquired, with mean volume rate of 25.3 ± 1.70 volume/s for the left atrium.
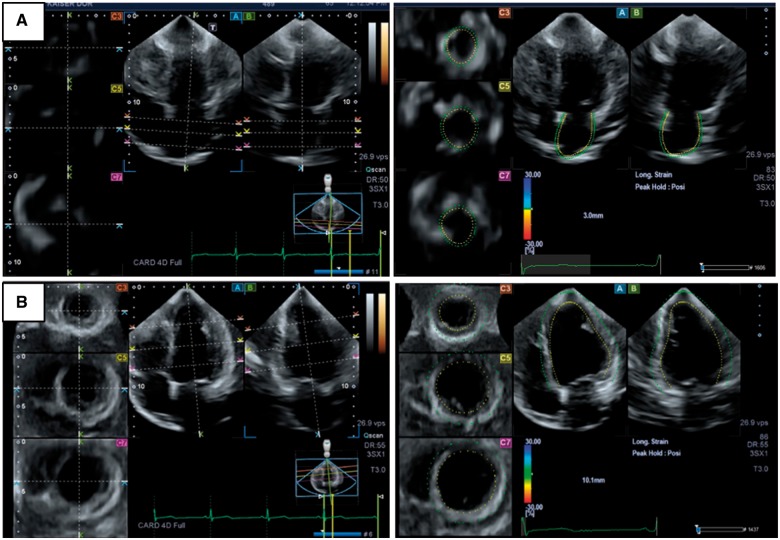
Analysis of LA parameters was performed at the end diastole using a multi-plane display, including three cross-sectional slices (basal, mid, and roof; planes C1, C2, and C3, respectively) and two longitudinal orthogonal planes (plane A = four-chamber view; plane B = a plane orthogonal to the plane A). The endocardial border was identified in a counterclockwise direction, from the medial to the lateral mitral annulus, excluding the LA appendage and the ostium of the pulmonary veins. A semi-automatic border contours detection was performed, with manual adjustments made when necessary. The LA wall thickness was fixed to 3 mm. The 3D endocardial shell of the entire LA was automatically rendered with quantification of LA phasic volumes, emptying volumes, emptying fraction, and strain parameters (Figure 1A, Supplementary data online, S7-3D STE Movie 1).
Figure 1.
Multi-plane display of the left atrial (A) and left ventricle (B) 3D full volume showing five plane views composed of three cross-sectional slices [(C7), (C5), and (C3)], and two longitudinal orthogonal planes (four-chamber (A) and two-chamber (B) views).
The LV volumetric image was also displayed at end diastole in multiple simultaneous views, including three cross-sectional slices (base, mid, and apex; planes C1, C2, and C3 respectively) and two longitudinal orthogonal planes (plane A = four-chamber view; plane B = a plane orthogonal to the plane A). Anatomical landmarks were identified in the longitudinal planes (mitral annulus and apex), and a semi-automatic border contour detection was performed, with manual adjustments made when was necessary. The 3D endocardial shell of the entire left ventricle was automatically rendered with measurement of LV volumes, mass, ejection fraction (EF), and strain parameters. (Figure 1B, Supplementary data online, S7-3D STE Movie 2).
The 3D image quality control score was composed by subjective image quality (poor, fair, good, and excellent), numbers of the segments excluded (16 segments model), and the presence of artefacts (blurring, shadowing, stitching, and rib artefacts). Exams with poor image quality, or with more than seven segments with poor tracking, or with a significant artefact were excluded from the analysis.
The inter- and intra-reader reproducibility was assessed by four readers in a random subset of 20 participants. Re-readings were performed 30 days after the initial reading, blinded to the original analysis.
3DE LA parameters
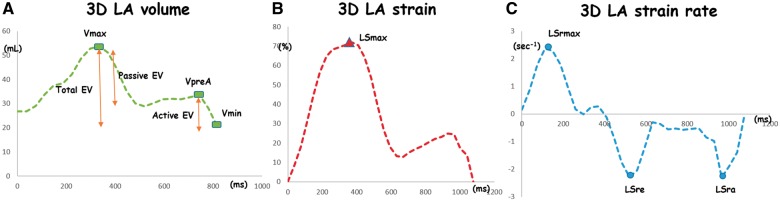
The LA volumes evaluated were: LAVmax: LA maximum volume at end systole (just before the mitral valve opens), LAVpreA: LA pre-atrial contraction volume at the onset of the P wave on electrocardiogram (ECG), and LAVmin: LA minimum volume at the end diastole (just after the mitral valve closes). The LA phasic volumes were index by body surface area. Three emptying volumes were calculated based on those volumes to evaluate LA phasic function: total emptying volume (LAtEV) = (LAVmax − LAVmin), passive emptying volume (LApEV) = (LAVmax − LAVpreA), and active emptying volume (LAaEV) = (LAVpreA − LAVmin). The LA global longitudinal strain (LSmax) was measured at the end of systole. Early LA strain rate (LASre) was defined as the negative peak of the LA strain rate in early diastole, whereas late LA strain rate (LASra) was determined as the negative peak of LA strain in late diastole, after the onset of the P wave on ECG (Figure 2).
Figure 2.
Multimodality tissue tracking volume analysis (A, green line), strain analysis (B, red line), and strain rate analysis (C, blue line). Vmax, maximum volume; Vmin, minimum volume; VpreA, pre-atrial contraction volume; EV, emptying volume; Smax, global longitudinal strain; Srmax, global maximum longitudinal strain rate; Sre, early negative diastolic peak of the LA strain rate; Sra, late negative diastolic peak of the LA strain rate.
3DE LV parameters
The 3D LV parameters assessed were: end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV) = (EDV − ESV), mass, EF = ((SV/EDV)*100), global longitudinal strain (GLS), global circumferential strain (GCS), and global endocardial surface area change strain (GAS = ((diastolic area − systolic area)/diastolic area)*100). All global strain measurements are averaged of peak systolic strain of regional 16 segments model (Figure 2).
Blood pressure and cumulative BP
Three BP measurements were obtained at one-minute intervals from the right arm of seated participant after 5 min of rest using Hawksley random-zero sphygmomanometer (0–15 year; W A Baum Company, Copiague, NY, USA) and a BP monitor (20–30 year; Omron Healthcare Inc. Lake Forest, IL, USA). The average of the final two measures was calculated. A calibration study was performed, and values calibrated to the sphygmomanometric measures were used for 20–30 year BP measurements.13
Cumulative BP levels were defined by summing the product of average millimetres of mercury and the years between each two consecutive clinic visits over 30 years of follow-up. To obtain the cumulative BP estimate, each participant was required to have at least the year O measure as well as at least three other BP measures from the follow-up exams.
Covariates
Assessments of CV risk factors in the CARDIA study were obtained through standard protocols across all field centres and in each examination as previously described.14,15
Statistical analysis
Continuous variables were described as a mean ± standard deviation and compared using Student’s t-test. Categorical variables were presented as an absolute value (percentage) and compared using χ2 statistics. The 3DE inter- and intra-reader reproducibility were assessed by the intraclass correlation coefficient (ICC) on a two-way mixed effect model. Multivariable linear regression analyses were used to assess the relationship between cumulative systolic and diastolic BP with 3DE LA structure and function parameters, evaluated by three analytic models: Model 1: unadjusted; Model 2: adjusted for age, race, and gender at year 30 examination; Model 3: included Model 2 and adjustment for body mass index (BMI), heart rate, physical activity, educational level, alcohol intake, diabetes mellitus, smoking status, total cholesterol, and LDL-cholesterol at year 30 examination. Exploratory analysis using product terms in the regression models were done to test for effect modification by race and sex.
All statistical analyses were conducted using SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA). A P-value of <0.05 was considered statistically significant.
Results
Participant’s characteristics
A total of 1033 participants were included, mean age was 55.4 ± 3.5 years, 55.2% women, 43.9% blacks. Compared with men, women had lower weight, systolic BP, cumulative systolic and diastolic BP, and physical activity; a lower proportion of them were also smokers and drinkers. Compared with men, women had on average higher education levels, total cholesterol, and HDL-cholesterol. Participant’s characteristics are displayed in Table 1. Our study populations seem to be healthier in comparison with the all cohort underwent a CARDIA year 30 examination (see Supplementary data online, Table S4).
Table 1.
Participant’s characteristics by gender at CARDIA year 30 exam
| n = 1033 | Men (n = 461) | Woman (n = 572) | P-value |
|---|---|---|---|
| Age (years) | 55.1 ± 3.5 | 55.2 ± 3.7 | 0.50 |
| Whites | 258 (56) | 321 (56) | 0.96 |
| Weight (kg) | 85.9 ± 14.1 | 75.2 ± 17.4 | <0.001 |
| Body mass index (kg/m2) | 27.2 ± 4.1 | 27.6 ± 6.2 | 0.22 |
| Resting heart rate (bpm) | 64.0 ± 9.1 | 65.0 ± 10.1 | 0.064 |
| SBP (mmHg) | 119.3 ± 13.0 | 117.1 ± 16.0 | 0.02 |
| DBP (mmHg) | 72.0 ± 9.5 | 71.2 ± 11 | 0.25 |
| Cumulative SBP (mmHg × year) | 3435 ± 239 | 3289 ± 300 | <0.001 |
| Cumulative DBP (mmHg × year) | 2153 ± 198 | 2088 ± 231 | <0.001 |
| Diabetes mellitus | 49 (10.6) | 59 (10.3) | 0.87 |
| Current smoker | 81 (17.6) | 70 (12.2) | 0.02 |
| Drinker | 306 (66.4) | 336 (58.7) | 0.01 |
| Total cholesterol (mg/dL) | 187.4 ± 36.0 | 198.1 ± 34.4 | <0.001 |
| HDL-cholesterol (mg/dL) | 54.4 ± 14.1 | 70.1 ± 18.4 | <0.001 |
| LDL-cholesterol (mg/dL) | 112.3 ± 33.6 | 111.0 ± 30.8 | 0.52 |
| Physical activity (EU) | 438 ± 315 | 325 ± 251 | <0.001 |
| Education (years) | 15.3 ± 2.8 | 15.7 ± 2.5 | 0.02 |
Values are mean ± standard deviation or n (%). Comparison across gender was using Student’s t-test and χ2 test.
DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
3D LA and LV echocardiography characteristics
Compared with men, women had similar LA index volumes, lower LA emptying volumes, higher magnitude LA global longitudinal systolic strain, and higher magnitude early diastolic LA strain rate. Also, compared with men, women had lower LV EDV, ESV, and mass; higher LV EF; and lower magnitude global longitudinal strain (Table 2).
Table 2.
Participant’s 3D echocardiogram characteristics by gender at CARDIA year 30 exam
| n = 1033 | Men (n = 461) | Woman (n = 572) | P-value |
|---|---|---|---|
| 3D LA Vmax index (mL/m2) | 25.59 ± 7.27 | 25.94 ± 7.15 | 0.47 |
| 3D LA Vmin index (mL/m2) | 13.88 ± 4.39 | 13.99 ± 4.50 | 0.71 |
| 3D LA VpreA index (mL/m2) | 20.22 ± 5.73 | 20.49 ± 6.07 | 0.49 |
| 3D LA total EV (mL) | 24.17 ± 9.42 | 22.29 ± 9.10 | 0.002 |
| 3D LA passive EV (mL) | 11.39 ± 7.14 | 10.44 ± 6.84 | 0.04 |
| 3D LA active EV (mL) | 13.00 ± 5.85 | 12.01 ± 7.17 | 0.02 |
| 3D LA LSmax (%) | 26.68 ± 7.24 | 29.47 ± 7.47 | <0.001 |
| 3D LA LSRe (s−1) | −1.29 ± 0.50 | −1.37 ± 0.55 | 0.02 |
| 3D LA LSRa (s−1) | −0.87 ± 0.52 | −0.93 ± 0.51 | 0.06 |
| 3D LV EDV index (mL/m2) | 53.51 ± 13.19 | 50.38 ± 11.44 | <0.001 |
| 3D LV ESV index (mL/m2) | 22.37 ± 7.82 | 20.08 ± 6.92 | <0.001 |
| 3D LV SV index (mL/m2) | 31.14 ± 7.40 | 30.30 ± 6.42 | 0.05 |
| 3D LV mass index (g/m2) | 84.39 ± 23.15 | 76.55 ± 21.54 | <0.001 |
| 3D LV EF (%) | 58.76 ± 7.62 | 60.81 ± 7.70 | <0.001 |
| 3D LV GLS (%) | −14.95 ± 2.73 | −15.76 ± 2.68 | <0.001 |
| 3D LV GCS (%) | −28.29 ± 5.92 | −29.61 ± 6.62 | <0.001 |
| 3D LV GRS (%) | 27.94 ± 12.38 | 30.22 ± 12.16 | 0.003 |
| 3D LV GAS (%) | −39.66 ± 6.40 | −41.18 ± 6.87 | <0.0001 |
Values are mean ± standard deviation. Comparison across gender was using Student’s t-test.
3D, three-dimension; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; EV, emptying volume; GAS, global area-change strain; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LA, left atrium; LASra, second negative diastolic peak strain rate; LASre, first negative diastolic peak strain rate; LSmax, global longitudinal strain; LV, left ventricle; SV, stroke volume; Vmax, maximum volume; Vmin, minimum volume; VpreA, pre-atrial contraction volume.
Cumulative systolic BP and 3D LA parameters
In the fully adjusted model, higher cumulative systolic BP was independently associated with higher: 3D LA volumes: maximum volume (β = 1.74, P = 0.004), pre-atrial contraction volume (β = 1.87, P < 0.001), minimum volume (β = 0.76, P = 0.04), total emptying volume (β = 0.98, P = 0.006), and active emptying volume (β = 1.12, P < 0.001); and lower magnitude early diastolic LA strain rate (β = 0.05, P = 0.02) (Table 3).
Table 3.
Association of cumulative systolic BP with 3D LA structure and function
| Cumulative systolic blood pressure |
||||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||
| β | P-value | β | P-value | β | P-value | |
| 3D LA Vmax (mL) | 3.39 | <0.001 | 2.86 | <0.001 | 1.74 | 0.004 |
| 3D LA Vmin (mL) | 1.39 | <0.001 | 1.19 | 0.001 | 0.76 | 0.04 |
| 3D LA VpreA (mL)a | 3.26 | <0.001 | 2.91 | <0.001 | 1.87 | <0.001 |
| 3D LA total EV (mL) | 1.99 | <0.001 | 1.66 | <0.001 | 0.98 | 0.006 |
| 3D LA passive EV (mL) | 0.28 | 0.27 | 0.08 | 0.78 | −0.10 | 0.72 |
| 3D LA active EV (mL)a | 1.80 | <0.001 | 1.65 | <0.001 | 1.12 | <0.001 |
| 3D LA LSmax (%) | −0.58 | 0.03 | −0.28 | 0.35 | −0.24 | 0.45 |
| 3D LA LSRe (s−1) | 0.06 | <0.001 | 0.06 | 0.006 | 0.05 | 0.02 |
| 3D LA LSRa (s−1) | −0.04 | 0.05 | −0.04 | 0.06 | −0.04 | 0.06 |
The multivariable linear regression model is showing coefficients per 300 mmHg-years for cumulative SBP and P-values.
Model 1: unadjusted; Model 2: model 1 + adjusted for age, sex, and race at year 30 examination; Model 3: model 2 + adjusted for physical activity, heart rate, educational level, body mass index, alcohol intake, diabetes, smoke, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol at year 30 examination.
3D, three-dimension; DBP, diastolic blood pressure; EV, emptying volume; LA, left atrium; LSmax, global longitudinal strain; LASra, second negative diastolic peak strain rate; LASre, first negative diastolic peak strain rate; SBP, systolic blood pressure; Vmax, maximum volume; Vmin, minimum volume; VpreA, preatrial contraction volume.
Race-specific statistically significant interactions.
Cumulative diastolic BP and 3D LA parameters
In the fully adjusted model, higher cumulative diastolic BP was independently associated with higher 3D LA active emptying volume (β = 0.66, P = 0.002), lower magnitude early diastolic LA strain rate (β = 0.05, P = 0.004), and higher magnitude early diastolic LA strain rate (β = −0.04, P = 0.05) (Table 4).
Table 4.
Association of cumulative diastolic BP with 3D LA structure and function
| Cumulative diastolic blood pressure |
||||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||
| β | P-value | β | P-value | β | P-value | |
| 3D LA Vmax (mL) | 1.90 | <0.001 | 1.44 | 0.004 | 0.26 | 0.60 |
| 3D LA Vmin (mL) | 0.62 | 0.03 | 0.47 | 0.11 | 0.004 | 0.99 |
| 3D LA VpreA (mL) | 2.07 | <0.001 | 1.75 | <0.001 | 0.66 | 0.11 |
| 3D LA total EV (mL) | 1.27 | <0.001 | 0.97 | 0.001 | 0.26 | 0.38 |
| 3D LA passive EV (mL) | −0.06 | 0.77 | −0.22 | 0.34 | −0.39 | 0.10 |
| 3D LA active EV (mL) | 1.40 | <0.001 | 1.24 | <0.001 | 0.66 | 0.002 |
| 3D LA LSmax (%) | −0.41 | 0.07 | −0.29 | 0.22 | −0.23 | 0.37 |
| 3D LA LSRe (s−1) | 0.06 | <0.001 | 0.05 | 0.001 | 0.05 | 0.004 |
| 3D LA LSRa (s−1) | −0.04 | 0.008 | −0.04 | 0.02 | −0.03 | 0.05 |
The multivariable linear regression model is showing coefficients per 200 mmHg-years for cumulative DBP and P-values.
Model 1: unadjusted; Model 2: model 1 + adjusted for age, sex, and race at year 30 examination; Model 3: model 2 + adjusted for physical activity, heart rate, educational level, body mass index, alcohol intake, diabetes, smoke, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol at year 30 examination.
3D, three-dimension; DBP, diastolic blood pressure; EV, emptying volume; LA, left atrium; LASra, second negative diastolic peak strain rate; LASre, first negative diastolic peak strain rate; LSmax, global longitudinal strain; SBP, systolic blood pressure; Vmax, maximum volume; Vmin, minimum volume; VpreA, preatrial contraction volume.
Effect modification by race
In the fully adjusted models, there was a significant race interaction in the association of cumulative systolic BP with LAVpreA (P = 0.03) and LAaEV (P = 0.02), (see Supplementary data online, S6-Table 2). For those 3D LA echo parameters, which had significant race interaction, the association was only significant for African-Americans and not for whites (Table 3 and Supplementary data online, S5-Table 2).
3DE reproducibility
The 3DE LA inter-reader ICCs were 0.83–0.91 for phasic volumes and 0.64–0.69 for the strains (see Supplementary data online, S6-Table 3).
Discussion
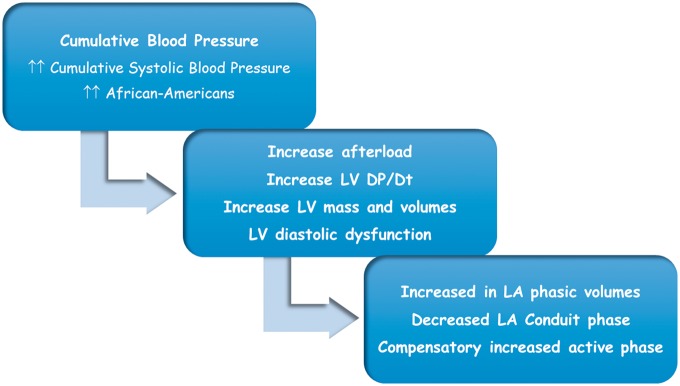
This study demonstrates that higher cumulative BP was independently associated with LA adverse remodelling showed by higher LA phasic volumes, lower LA passive function (conduit phase), and higher LA active function (burst phase) assessed by 3DE. Those associations were independent of age, race, sex, BMI, heart rate, physical activity, educational level, alcohol intake, diabetes, smoking, total cholesterol, and LDL-cholesterol (Figure3).
Figure 3.
Proposal pathophysiologic mechanism for subclinical LA dysfunction due to the higher cumulative blood pressure.
The LA’s primary physiological role is to regulate LV filling, serving as a reservoir for blood from the pulmonary veins during systole (reservoir phase), working as a passive conduct allowing the majority of the blood to pass into the left ventricle in early diastole (passive phase), and acting as a burst pump of the remaining blood in late diastole (active phase).16 Those complex and dynamic functions appear to be determined by pulmonary venous return, LA compliance and stiffness, LV compliance and stiffness, atrial contractility, and LV function.17
LA remodelling is an adaptive process in response to extensive cardiac stressors (pressure and volume overload, neurohormonal changes with the activation of renin-angiotensin-aldosterone), leading to anatomical, contractile, and electrophysiological changes, resulting in LA enlargement, reduced function, and myocardium fibrosis.17,18 The type and the degree of remodelling depend on the severity and the duration imposed by those stressors factors. The first step in this accommodative pathway appears to be a compensatory increase in the LA active function in late diastole, as a response to the decrease in the passive function in early diastole. A later adaption mechanism seems to be an increase in the LA volume, in an attempt to reduce the LA intracavitary pressure and to increase the total emptying fraction.
Traditionally, LA adverse remodelling has been evaluated in clinical practice by using measures of LA dimension, such as maximum diameter and area. However, the LA enlargement reflected by these structural parameters likely occurs at late stages of LA impairment. By using 3DE, LA phasic volumes and function can be directly measured by a non-invasive, portable and relatively inexpensive diagnostic tool, allowing a sensitive analysis of LA adverse remodelling.19 Nonetheless, the prognostic role of these early markers of LA dysfunction is not entirely established.
Race differences seem to exist in the correlation between cumulative BP and LA echocardiographic parameters, with blacks having a greater association than whites. Furthermore, those race disparities appear to be higher with systolic than diastolic cumulative BP. Higher arterial afterload combine with genetically determined myocardial hypersensibility among blacks may partly explain those racial divergences.20,21
The cumulative systolic BP seems to have a greater association with LA structure and function than the cumulative diastolic BP, affecting both volumes and function, and having higher β coefficients. The exact pathophysiological mechanism why this difference occurs is incompletely understood. Pulsatile and steady pressure overload related to higher cumulative systolic BP, and associated aging-related vascular stiffness, could be contributory pathophysiological mechanisms that partly explain this difference.22 Both the SPRINT trial and a systematic review and meta-analyses including 41 trials and 144 220 patients document lower rates of major CV events and death in those participants with a more intensive BP treatment, targeting systolic BP as low as 120 mmHg.23 Another recently published study, pooling data from three large recent US cohorts, demonstrated that >60% of incident CV events occurred among participants with BP <140/90 mmHg, suggesting the necessity of BP reduction beyond that threshold.24 However, more additional studies are needed to better understand differences between cumulative BP components, the effect of cumulative BP levels, as well as the racial differences in the development of subclinical cardiac dysfunction.
Strengths and limitations
A strength of this study is assessing a large bi-racial cohort with nine visits over 30 years of follow-up from early adulthood to middle age, using a careful and precise BP, and echocardiogram protocols. Also, the subclinical CV abnormalities were measured by the 3DE, which seems to be a more accurate, reproducible, and detailed estimation of cardiac structure and function compared with 2D traditional echocardiograph.25
There are several limitations to our study. Many participants were excluded from the analyses because of suboptimal 3D echocardiography images, leaving a place for selection bias towards to a health study population (see Supplementary data online, S1-Figure 2). ‘Therefore, we believe that our results may not apply to those very obese and with more CV risk factors.’ Moreover, a direct comparison between 2D and 3D STE echocardiography methods were not made, not allowing to demonstrate the diagnostic superiority of one technique over the other. Also, due to the left atrial thinner wall, there is inherent tracking limitation of the 3D STE LA analysis, which can be appreciated as a Videos in the Supplementary data online section. Finally, the influence of BP control, type of antihypertensive medication use, BP variability and BP trajectories over the BP cumulative effect on the subclinical CV disease were not investigated.
Conclusion
Higher cumulative systolic BP from early adulthood throughout middle age was associated with adverse LA remodelling reflected by higher LA volumes, lower LA passive function, and higher possible compensatory LA active function. Higher cumulative diastolic BP was associated with LA dysfunction identified by lower LA passive function and higher LA active function. The strength of association between cumulative BP and LA parameters was greater in blacks than whites. Cumulative systolic BP had a stronger correlation with LA variables when compared with diastolic cumulative BP. Our findings suggested that assessment of LA phasic functions by 3DE might be a valuable marker for subclinical CV disease due to chronic exposures to BP, potentially providing new insights into pathways linking cumulative BP with CV diseases.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the CARDIA study for their valuable contributions.
Funding
This study was supported by the CARDIA contract is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005).
Conflict of interest: None declared.
Supplementary Material
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M. et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2. Harbaoui B, Courand PY, Defforges A, Khettab F, Milon H, Girerd N. et al. Cumulative effects of several target organ damages in risk assessment in hypertension. Am J Hypertens 2016;29:234–44. [DOI] [PubMed] [Google Scholar]
- 3. Fudim M, Vemulapalli S.. No time to waste: in support of aggressive and immediate management of hypertension. Curr Hypertens Rep 2016;18:26.. [DOI] [PubMed] [Google Scholar]
- 4. Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC. et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 2014;311:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petruski-Ivleva N, Viera AJ, Shimbo D, Muntner P, Avery CL, Schneider AL. et al. Longitudinal patterns of change in systolic blood pressure and incidence of cardiovascular disease: the atherosclerosis risk in communities study. Hypertension 2016;67:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang YX, Song L, Xing AJ, Gao M, Zhao HY, Li CH. et al. Predictive value of cumulative blood pressure for all-cause mortality and cardiovascular events. Sci Rep 2017;7:41969.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong AC, Liu K, Lewis CE, Sidney S, Colangelo LA, Kishi S. et al. Left atrial dimension and traditional cardiovascular risk factors predict 20-year clinical cardiovascular events in young healthy adults: the CARDIA study. Eur Heart J Cardiovasc Imaging 2014;15:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM.. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 2017;10:735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tadic M, Cuspidi C, Pencic B, Rihor B, Radojkovic J, Kocijanic V. et al. The influence of white-coat hypertension on left atrial phasic function. Blood Press 2017;26:102–8. [DOI] [PubMed] [Google Scholar]
- 11. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR. et al. Cardia: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T. et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3–46. [DOI] [PubMed] [Google Scholar]
- 13. Jacobs DR Jr, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S. et al. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension 2012;59:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nwabuo CC, Moreira HT, Vasconcellos HD, Ambale-Venkatesh B, Yoneyama K, Ohyama Y. et al. Association of aortic root dilation from early adulthood to middle age with cardiac structure and function: the CARDIA study. J Am Soc Echocardiogr 2017;30:1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reis JP, Allen N, Gunderson EP, Lee JM, Lewis CE, Loria CM. et al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity (Silver Spring) 2015;23:879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vieira MJ, Teixeira R, Goncalves L, Gersh BJ.. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr 2014;27:463–78. [DOI] [PubMed] [Google Scholar]
- 17. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA. et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm 2017;14:e3–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mottonen MJ, Ukkola O, Lumme J, Kesaniemi YA, Huikuri HV, Perkiomaki JS.. Cardiac remodeling from middle age to senescence. Front Physiol 2017;8:341.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoit BD. Left atrial remodeling: more than just left atrial enlargement. Circ Cardiovasc Imaging 2017;10:e006036.. [DOI] [PubMed] [Google Scholar]
- 20. Fernandes-Silva MM, Shah AM, Hegde S, Goncalves A, Claggett B, Cheng S. et al. Race-related differences in left ventricular structural and functional remodeling in response to increased afterload: the ARIC study. JACC Heart Fail 2017;5:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yano Y, Reis JP, Tedla YG, Goff DC Jr, Jacobs DR Jr, Sidney S. et al. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Cardiol 2017;2:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaroch J, Rzyczkowska B, Bociąga Z, Łoboz-Rudnicka M, Kruszyńska E, Rychard W. et al. Arterial-atrial coupling in untreated hypertension. Blood Press 2015;24:72–8. [DOI] [PubMed] [Google Scholar]
- 23. Soliman EZ, Ambrosius WT, Cushman WC, Zhang ZM, Bates JT, Neyra JA. et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation 2017;136:798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tajeu GS, Booth JN 3rd, Colantonio LD, Gottesman RF, Howard G, Lackland DT. et al. Incident cardiovascular disease among adults with blood pressure <140/90 mm Hg. Circulation 2017;136:798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badano LP, Miglioranza MH, Mihăilă S, Peluso D, Xhaxho J, Marra MP. et al. Left atrial volumes and function by three-dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two-dimensional echocardiographic measurements. Circ Cardiovasc Imaging 2016;9:e004229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.