Abstract
Background
The mechanisms of activation and regulation of T lymphocytes and their cytokines in malaria caused by Plasmodium vivax are complex and poorly understood. Previous data suggest that T cells balance protective immune responses with immune mediated pathology in malaria. This study investigates the lymphocytic profile of patients infected with P. vivax by identifying and quantifying the specific sub-populations of Th1, Th2, Th17 and Treg cells and observing the correlation between parasitaemia and the number of platelets.
Methods
A cross-sectional study was carried out in an endemic area of the state of Acre, Brazil. In order to obtain identification and quantification of lymphocyte sub-populations through flow cytometry, blood samples were collected from 50 individuals infected with P. vivax and 20 non-infected controls. To differentiate Th1 from Th2, the presence of cytokines IL-4 and TNF was examined by enzyme-linked immunosorbent assay. Utilizing the Mann–Whitney and Spearman coefficient tests, comparison and correlation analysis were rendered to test the parasitaemia and the number of platelets relationship.
Results
The data indicate that individuals infected with P. vivax present a significant reduction in Th1, Th2 and Th17 cell sub-populations when compared to the non-infected control group. A negative correlation exists between parasitaemia and platelet counts in individuals infected with P. vivax. There is no correlation of parasitaemia or thrombocytopaenia with any sub-population of T lymphocytes analysed. Interestingly, patients with serum Th1 cytokine profile present inversely proportional parasitaemia to the increase in the number of Th1, Th2, Th17 and Treg cells while patients with serum Th2 cytokine profile present directly proportional parasitaemia to the increase in number of Th1 and Th2 cells. Regarding the number of platelets, patients with serum Th1 cytokine profile show a correlation directly proportional to the Th17 sub-population. In contrast, platelet counts are directly proportional only to Treg and activated Treg cells in patients with serum Th2 cytokine profile.
Conclusions
During the P. vivax infection patients with serum Th1 versus Th2 cytokine profile present different biological mechanisms for activating the immune system against parasite load.
Electronic supplementary material
The online version of this article (10.1186/s12936-018-2443-x) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium vivax, Parasitaemia, Platelets, Th1 cells, Th2 cells
Background
Plasmodium vivax is one of five parasites causing malaria in humans. There has been an increasing amount of documentation referring to the disease as a major health threat affecting the world’s most populous regions [1]. During the infection, patients present leukogram variations with values ranging from normal to leukopaenia [2]. Moreover, P. vivax has a greater capacity to elicit an inflammatory response, resulting in a lower pyrogenic threshold, and activation and dysfunction of T cells [3]. Plasmodium vivax blood-stage infection activates a substantially different type of immune response compared to Plasmodium falciparum and might have distinct contributions to the immune response to blood-stage infection [4].
Anaemia is a constant finding in malaria and progresses with disease proliferation [5]. Another finding frequently observed in P. vivax infection is thrombocytopaenia. Several hypotheses have already been postulated as causes of malaria-associated thrombocytopaenia, including but not limited to: disseminated intravascular coagulation, immune mechanisms, splenic sequestration, and the possible presence of the parasite in red blood cells in the bone marrow, with may lead to a decrease in the platelet population in circulation [6].
The pro‐inflammatory response against P. vivax gains more importance during periods of increased parasite burden [7]. Malaria parasites regulate the expression of selective Toll-like receptors (TLRs) on immune cells that induce a specific biological response against invasion of malaria parasites [8].
In malaria there is activation of both Th1 and Th2 cells. A balance between the cytokines produced by both cell profiles is required for the protection of the individual [9]. Elevated levels in Th1 and Th2 cytokines such as IFN-γ and IL-4 are associated with increased severity in some diseases [10]. Many individuals with asymptomatic malaria display multiple significant interactions involving IL-4 [11]. Studies have shown the protective role of the IL-4 cytokine as a negative regulator of the pro-inflammatory effects in malaria infection [11, 12]. IFN-γ is a cytokine of Th1 cells and plays an essential role in immunity against blood-stage Plasmodium infection [13]. Combined with TNF and CCL5 chemokine, they are proven to be crucial biomarkers in the profile of individuals with mild infection of P. vivax, and in contrast, there are positive correlations involving TNF and IFN-γ associated with pathogenicity in severe malaria [11].
Bueno and collaborators [14] have suggested the existence of different sub-populations of Treg cells during malaria infection, evidenced by the intracellular production of IFN-γ, IL-4 and IL-17 and the intermediate expression of the FOXP3 molecule. It should be emphasized, however, that not all FOXP3+ T cells are necessarily Treg cells and their activity may depend on the level of FOXP3 expression and isoforms of the protein expressed [15]. Some studies discuss the cellular and molecular factors that affect the development, the homeostasis and the function of the Treg cells and consequent immunity to self and non-self antigens [16]. The identification of CD127 receptor down-regulated in all human T cells after activation as a useful marker was heralded by genetic observations and a combination of CD4, CD25, and CD127 resulted in a highly purified population of Treg cells accounting for significantly more cells that previously identified based on other cell surface markers. These cells were highly suppressive in functional suppressor assays [15]. It is believed that Treg cells act to suppress the inflammatory response by obtaining direct contact with effector cells and via the production of cytokines such as IL-10 and TGF-β [17]. Also, Treg cells have been known to significantly modify cellular immune responses to various protozoan infections, including malaria [18]. However, these CD4+CD25+CD127−, once isolated, may be treated in vitro with TGF-β or other factors to enhance Treg cell function in these cells [15].
Another cell population recently associated with parasitic infections is Th17 cells. These cells are mediated by cytokines IL-17A and IL-17F, inducing the expression of pro-inflammatory cytokines as a consequence of the recruitment and activation of several leukocyte lines [19]. There are a few studies that described the importance of Th17 in the infection caused by malaria [20, 21]. Ishida and collaborators [21] demonstrated that the absence of IL-17 in transgenic animals favoured the development of cerebral malaria during Plasmodium berghei (ANKA strain) infection and point out the protective function of this cell in P. berghei ANKA malaria. Elevated IL-17 levels combined with high IL-4, IL-12α and IFN-γ levels may be a marker of protection. The mechanism may be controlled by host factor(s) [20].
Based on the literature, the aim of this study is to investigate the immunological profile of patients with acute malaria caused by P. vivax. The study analysed the association of platelets and the level of parasitaemia with the amount of sub-populations of lymphocytes: Th1, Th2, Th17 and Treg cells. The study also investigated haematological and biochemical parameters in patients with acute malaria caused by P. vivax, using haematological and biochemical markers. The final step compared the patients with P. vivax malaria to the non-infected control subjects.
Methods
Area of study
This is a cross-sectional descriptive study of patients in the acute phase of malaria caused by P. vivax, from September to December of 2016. Patients are located in the city of Cruzeiro do Sul, considered the second largest city in the state of Acre, Brazil.
Patients
The patients (n = 50) diagnosed with vivax malaria were analysed in the acute phase of the disease before receiving immunosuppressive or anti-malarial drugs and were between 18 and 83 years of age, both sexes, with no other acute or chronic infections or pregnancy.
The diagnosis of P. vivax infection was confirmed microscopically by a thick gauge stained with 5% Giemsa. Individuals with a positive diagnosis who agreed to participate in the study after reading and signing the informed consent form were included. The age, number of previous episodes of malaria, and the history of other infectious diseases of each participant were recorded in a standard questionnaire during their care. In addition, a peripheral blood sample (15 mL) was collected for laboratory tests and analyses. Patients were informed that whether or not they participated in the project, it would not affect their attendance at the health centres in the city of Cruzeiro do Sul-Acre.
Non-infected controls
In this study 20 healthy clinicians were selected for blood donation at the Haematology and Haemotherapy Centre of Mato Grosso-Brazil. Some of the requirements were no previous history of malaria and the clinicians had to be residents of regions considered non-endemic to the disease. Consent was obtained from all of the individuals evaluated for participation in the study. Haematological data were obtained for the comparison analyses. The exclusion criteria were the same as those used in the group of patients infected with P. vivax.
Parasitological diagnosis
Thick smears were performed on 1-cm2 of slide with 1 drop of peripheral blood. This slide was stained with 5% Giemsa solution and examined to determine the Plasmodium species by two trained microscopists from light microscopic images (Axio imager Z2, Carl Zeiss).
The diagnosis of the species and the quantification of parasitaemia were carried out by counting the number of parasites in fields of high magnification (1,000×), per 200 leukocytes. If 9 or fewer parasites were found, 300 leukocytes were counted in addition. The estimation of parasitaemia per mm3 of blood was expressed as the relation between parasites and leukocytes in the blood smears. The patients were classified according to the level of parasitaemia (low up to 750 parasites/mm3 and high above 751 parasites/mm3) [22, 23].
Laboratory analysis
The haemogram was performed on the same day of blood collection, from 3 mL of venous blood, collected in a Vacutainer® tube with anticoagulant EDTA. Erythrocytes (millions/mm3), hemoglobin (g/dL), haematocrit (%), platelets (mm3) and total/differential leukocytes (%), were evaluated by automatic counting of blood cells (ABX Pentra 90, Horiba Diagnostics, Kyoto, Japan).
To make a biochemical analysis, a sample of 5 mL of venous blood was collected in a tube Vacutainer® with separator gel to obtain the serum. After the clot formation, the serum was removed and the biochemical tests were immediately dosed, using specific Labtest kits of the following parameters: bilirubin, UV urea liquiform, creatinine, alanine amino transaminase (ALT/GPT) liquiform and aspartate amino transaminase (AST/GOT) liquiform in automatic analyzer Labmax 240 Premium (Labtest Diagnostica S/A, Brazil).
All of these procedures were performed at the Clinical Analysis Laboratory of the Juruá Women’s and Children’s Hospital in the city of Cruzeiro do Sul-AC by local professionals. The classification of severity of the patients analysed, followed the criteria for classification of severe falciparum malaria established by the World Health Organization (WHO). This model was chosen because there is not yet in the literature a specific gamut of severity for malaria caused by P. vivax.
Cell immunophenotyping
For immunophenotyping analysis, venous blood (7 mL) was collected in tubes containing EDTA. Then, peripheral blood leukocytes isolation was obtained by centrifugation of whole blood. Then, the ring area of leukocytes was collected. Erythrocytes were lysed with a lyse kit (Sigma, USA). While cells were resuspended (1 × 107 per 900 µL) in RPMI media with 20% of autologous serum and 10% DMSO (Sigma). Lastly, cell suspension was placed in cryotubes and frozen at − 80 °C [24] and transported to UFMT, Cuiabá-MT, by air transportation in appropriate temperature conditions and packaging.
For the immunophenotyping of T cell subpopulations: Th1 (CD3+CD4+IFN-γ+TNF-α+), Th2 (CD3+CD4+IL-4+), Th17 (CD3+CD4+IL-17+), Treg (CD4+CD25+CD127+FOXP3+) and activated Treg (CD4+CD25+CD127−FOXP3+), the blood samples (1 mL) were naturally thawed at room temperature and incubated for 5 min with 14 mL of lysis solution (NH4Cl/KHCO3/tetrasodium EDTA), centrifuged for 5 min at 300×g. The supernatant was aspirated and the pellet was resuspended in 5 mL of ice cold PBS and centrifuged for 5 min at 300×g. Again, the supernatant was aspirated and the pellet was resuspended in 1 mL ice-cold PBS solution [25].
Once cell lysis has taken place, the cells were resuspended in 100 μL of FACS solution (PBS + 0.5% BSA) containing specific combinations and concentrations of distinct fluorochromes labelled with monoclonal antibodies for simultaneous analysis of cell surface markers (Additional file 1: Table S1), and incubated for 30 min on ice and sheltered from light. After this period, 100 μL of Reagent A (Medium Fix & Perm, BD-Becton–Dickinson) solution was added and incubated for 15 min in the dark. Two 5-min washings at 350×g in FACS solution were performed and the cell contents resuspended in 100 μL of Reagent B (Permeabilization Medium-Fix & Perm, BD-Becton–Dickinson) solution and the specific volume in μL (Additional file 1: Table S1) for each intracellular labelled conjugated antibody was incubated for 20 min on ice and sheltered from light. Subsequently, the cells were centrifuged for 5 min at 350×g and resuspended in 200 μL FACS solution for immediate reading on the Accuri™C6 BD flow cytometer (Becton–Dickinson, USA), at the UFMT-Cuiabá Research Laboratory and the markers were performed according to the protocols proposed by the manufacturers (Additional file 1: Table S1).
Eighty thousand events were acquired and analysed by BD Accuri™ C6 software (BD Biosciences, USA). Isotype controls labeled with FITC, PE, PerCP, PerCP-CyTM 5.5 and APC were used in all experiments. The data obtained (percentage of fluorescent cells) were interpolated with the initial lymphocyte count obtained from the leukogram. The results are expressed as cells/mm3.
Determination of patient immunological type
Patients were differentiated as Th1 or Th2 immunological type by the level of the TNF-α or IL-4 cytokines present in the patients’ serum, determined by indirect Enzyme-Linked Immunosorbent Assay (ELISA) kit (BD, Biosciences-Pharmingen, San Diego, CA, USA) and measured by an automatic microplate reader, V-Max (Molecular Devices, Sunnyvale, USA). When patients have levels twofold or higher of one cytokine compared to the other, they were considered serum Th1 or Th2 cytokine profile.
Statistical analysis
Statistical analysis was performed using the GraphPad PRISM 5.04 program (La Jolla, CA, USA). The data relating to haematological parameters and the proportion of T cells and immunophenotyping of infected patients compared to non-infected controls were analysed by Mann–Whitney t test. Results were expressed as mean ± SEM (mean standard error). Values of p < 0.05 were considered statistically significant. For the analysis of correlation between parasitaemia and platelet count with different sub-populations of lymphocytes, the Spearman correlation coefficient test 95% CI, was performed.
Results
Haematological, biochemical and parasitological profile of patients infected with Plasmodium vivax
The data demonstrated that all patients with P. vivax infection evaluated in this study had some degree of thrombocytopaenia; some patients presented varying degrees of anaemia and some biochemical alterations (Table 1). All patients in this study reported more than one episode of malaria in their lifetime (n = 50). The data demonstrated that no patient had a severe malaria profile, according to the protocol established by the WHO.
Table 1.
Haematological, biochemical and parasitological parameters of patients infected by Plasmodium vivax
| Gender | Age (years) | RBCs (106/mm3) | Hb g/dL | HCT % | Plat. mm3 | Leuc. % | Lynf. % | Paras. mm3 | GPT UI/L | GOT UI/L | Urea mg/dL | Cr mg/dL | BT mg/dL | TNF-α pg/mL | IL-4 pg/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 29 | 4.35 | 13 | 38.5 | 66 | 6 | 34 | 305 | 47 | 26 | 22 | 0.78 | 0.4 | 9.4 | 110.4 |
| M | 18 | 4.46 | 14 | 40.1 | 96 | 7.5 | 14.7 | 235 | 82 | 50 | 22 | 0.65 | 0.9 | 9.2 | 127.3 |
| F | 19 | 4.09 | 11.1 | 33.4 | 109 | 4.7 | 18.4 | 1007 | 13 | 32 | 17 | 0.39 | 1.1 | 7.4 | 65.2 |
| F | 19 | 3.91 | 11.3 | 33.6 | 174 | 6.7 | 20.8 | 1012 | 13 | 27 | 20 | 0.4 | 0.62 | 10.5 | 62.9 |
| F | 22 | 3.63 | 11.4 | 34.2 | 82 | 8.1 | 24.9 | 505 | 25 | 24 | 24 | 0.62 | 0.43 | 60.8 | 24.3 |
| M | 38 | 4.81 | 14.7 | 43 | 122 | 6.1 | 32.2 | 1092 | 58 | 33 | 31 | 0.59 | 0.89 | 70.6 | 24.0 |
| M | 21 | 5.16 | 14.9 | 43.5 | 85 | 4.7 | 20.5 | 508 | 36 | 30 | 36 | 0.68 | 1.29 | 16.2 | 127.3 |
| F | 49 | 5.37 | 15.4 | 45.9 | 180 | 5.4 | 20.4 | 220 | 51 | 44 | 22 | 0.49 | 0.62 | 9.0 | 157.8 |
| F | 25 | 5.01 | 13.8 | 39.9 | 118 | 9.7 | 32.2 | 328 | 15 | 31 | 24 | 0.56 | 1.28 | 7.4 | 34.7 |
| F | 25 | 4.03 | 13.9 | 43 | 117 | 6.8 | 33 | 1020 | 12 | 23 | 23 | 0.34 | 0.7 | 7.1 | 88.9 |
| M | 19 | 4.25 | 11.3 | 34.7 | 129 | 7.3 | 23 | 513 | 11 | 17 | 17 | 0.43 | 0.57 | 10.6 | 96.8 |
| M | 36 | 5.36 | 15.4 | 44.4 | 84 | 9.5 | 16.3 | 355 | 68 | 44 | 24 | 0.53 | 0.97 | 11.4 | 235.7 |
| F | 50 | 4.68 | 14.2 | 41.8 | 73 | 6.9 | 10.5 | 1663 | 55 | 50 | 31 | 0.55 | 0.58 | 12.6 | 164.6 |
| F | 47 | 4.6 | 13.4 | 40.2 | 119 | 4.6 | 14.7 | 186 | 57 | 38 | 33 | 0.9 | 1.23 | 90.4 | 18.4 |
| M | 47 | 4.82 | 13.5 | 42.4 | 62 | 6.2 | 12.8 | 195 | 16 | 32 | 25 | 0.62 | 0.88 | 12.1 | 82.1 |
| M | 44 | 3.8 | 11.4 | 34.5 | 128 | 11.2 | 12.6 | 518 | 72 | 75 | 21 | 0.68 | 0.75 | 8.1 | 95.7 |
| M | 34 | 4.59 | 12.9 | 38.8 | 124 | 6 | 15.1 | 300 | 47 | 34 | 33 | 0.98 | 1.2 | 8.5 | 72.0 |
| M | 37 | 4.82 | 14.7 | 42.2 | 177 | 8.4 | 11.3 | 520 | 29 | 31 | 36 | 0.7 | 3.94 | 8.3 | 20.0 |
| F | 35 | 4.69 | 13.4 | 40 | 151 | 9.3 | 10.5 | 200 | 17 | 22 | 26 | 0.89 | 1.86 | 9.7 | 97.9 |
| F | 23 | 4.88 | 15.3 | 44.6 | 78 | 7.1 | 10.3 | 451 | 21 | 32 | 30 | 0.86 | 0.3 | 99.0 | 21.2 |
| M | 24 | 4.63 | 13.8 | 40.6 | 83 | 6 | 16.8 | 530 | 21 | 22 | 29 | 0.94 | 0.9 | 5.1 | 18.9 |
| F | 24 | 4.26 | 11.2 | 34.9 | 85 | 4.6 | 27.8 | 8380 | 35 | 33 | 27 | 0.67 | 1.16 | 14.4 | 62.9 |
| F | 34 | 3.7 | 10.3 | 32.3 | 128 | 5.6 | 19.7 | 270 | 12 | 19 | 15 | 0.45 | 0.4 | 9.0 | 65.2 |
| M | 20 | 5.29 | 16.3 | 48.5 | 90 | 8.5 | 14.7 | 1119 | 20 | 30 | 51 | 0.98 | 0.68 | 81.1 | 20.0 |
| M | 27 | 4.56 | 13.7 | 38.9 | 124 | 6.7 | 24.9 | 400 | 21 | 31 | 39 | 1.12 | 1.46 | 97.9 | 10.5 |
| F | 19 | 4.9 | 14 | 40.5 | 146 | 11.6 | 7.2 | 508 | 14 | 21 | 38 | 0.98 | 0.8 | 7.8 | 21.2 |
| M | 83 | 3.99 | 11.5 | 35.2 | 88 | 5 | 23 | 560 | 22 | 18 | 33 | 0.8 | 0.5 | 5.8 | 18.9 |
| F | 29 | 4.09 | 10.9 | 32.8 | 128 | 7.1 | 35.1 | 270 | 9 | 21 | 23 | 1.1 | 0.4 | 13.5 | 62.9 |
| F | 45 | 4.4 | 12 | 36.5 | 219 | 13.2 | 28.3 | 285 | 20 | 26 | 25 | 0.55 | 0.61 | 12.1 | 65.2 |
| F | 18 | 4.14 | 10 | 31.2 | 139 | 4.8 | 35.3 | 300 | 35 | 40 | 17 | 0.4 | 1.26 | 94.0 | 17.8 |
| F | 67 | 4.73 | 13.7 | 41 | 144 | 8.1 | 19 | 235 | 43 | 35 | 30 | 0.9 | 0.65 | 54.1 | 16.6 |
| F | 32 | 4.33 | 13.6 | 39.4 | 101 | 4.6 | 28.7 | 420 | 27 | 31 | 19 | 0.72 | 0.46 | 8.7 | 17.8 |
| M | 41 | 3.77 | 11.4 | 33.6 | 106 | 7.9 | 17.4 | 582 | 33 | 55 | 24 | 0.55 | 0.69 | 11.4 | 16.6 |
| M | 18 | 4.41 | 12.7 | 38.4 | 109 | 11.1 | 13 | 2304 | 38 | 29 | 46 | 0.8 | 0.7 | 7.4 | 66.3 |
| F | 23 | 5.11 | 13.3 | 41.6 | 77 | 5.9 | 12.1 | 1157 | 15 | 24 | 30 | 0.9 | 1.2 | 6.5 | 127.3 |
| M | 23 | 4.6 | 11 | 33 | 102 | 10.8 | 42 | 415 | 22 | 25 | 28 | 0.85 | 1.1 | 7.6 | 23.4 |
| F | 28 | 4.87 | 10.8 | 34.9 | 89 | 4.7 | 44 | 301 | 16 | 23 | 17 | 0.8 | 1.09 | 7.2 | 12.1 |
| M | 25 | 5.8 | 12.2 | 42 | 79 | 10 | 39.5 | 335 | 53 | 47 | 20 | 0.72 | 1.13 | 10.5 | 39.2 |
| M | 31 | 5.2 | 11.5 | 39.9 | 146 | 5.8 | 27 | 300 | 14 | 32 | 27 | 0.87 | 1.92 | 53.0 | 14.4 |
| M | 21 | 3.45 | 11.7 | 41.2 | 98 | 7.2 | 31.8 | 899 | 12 | 28 | 22 | 0.79 | 1.12 | 64.1 | 24.2 |
| F | 32 | 4.4 | 10.4 | 40 | 114 | 11.5 | 33.4 | 340 | 6 | 33 | 19 | 0.7 | 1.8 | 8.6 | 55.0 |
| M | 43 | 4.9 | 13.1 | 37.8 | 65 | 13 | 29 | 590 | 24 | 26 | 32 | 0.85 | 1.34 | 6.4 | 32.5 |
| F | 23 | 5.9 | 12.9 | 34.3 | 176 | 9.5 | 29.9 | 1010 | 30 | 28 | 24 | 0.6 | 0.7 | 6.8 | 11.0 |
| F | 19 | 5.7 | 13.9 | 44 | 53 | 4 | 15.2 | 300 | 15 | 40 | 27 | 0.5 | 0.5 | 8.4 | 92.3 |
| M | 25 | 5.12 | 14.6 | 43.6 | 78 | 11.4 | 12 | 460 | 12 | 24 | 33 | 0.9 | 1.4 | 12.9 | 58.4 |
| M | 29 | 4.25 | 12.6 | 37.9 | 47 | 2.7 | 21.5 | 654 | 28 | 24 | 31 | 1.0 | 1.48 | 16.3 | 43.7 |
| F | 37 | 4.09 | 13 | 38 | 70 | 3.9 | 18.3 | 623 | 13 | 30 | 18 | 0.87 | 0.9 | 26.6 | 43.7 |
| M | 44 | 4.46 | 12.2 | 38.3 | 98 | 4.3 | 22 | 465 | 18 | 32 | 19 | 0.8 | 0.85 | 6.1 | 35.8 |
| F | 43 | 5.14 | 14.3 | 44.1 | 90 | 4.5 | 30.3 | 355 | 20 | 26 | 21 | 0.74 | 1.23 | 10.4 | 64.3 |
| F | 59 | 5.03 | 14.8 | 45.4 | 110 | 4.4 | 32 | 420 | 18 | 27 | 27 | 0.5 | 0.6 | 8.2 | 80.1 |
Regarding the parasitaemia, 39 individuals presented mild parasitaemia (< 750 parasites/mm3), and 11 patients had high parasitaemia (> 751 parasites/mm3) (Table 1).
The age group of patients infected with P. vivax included 27 patients between ages 18 and 30 (54%) and 23 patients between ages 31 and 83 (46%). The number of infected men and women were virtually identical (n = 24 and 26, respectively).
Comparative analysis of the number of platelets between groups and correlation of number of platelets and parasitaemia in patients infected with Plasmodium vivax
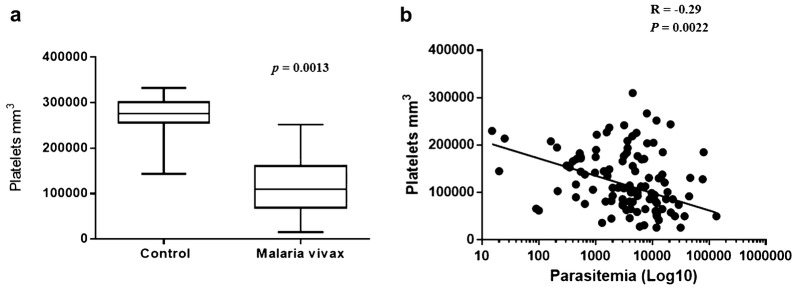
Patients with P. vivax infection presented thrombocytopaenia when compared to non-infected controls (p = 0.0013; Fig. 1a). Based on this result, to verify the correlation between the parasitaemia of patients with malaria caused by P. vivax and the number of platelets found in the peripheral blood at the time of diagnosis, the Spearman correlation coefficient test was performed. The data obtained indicated a negative correlation between the parasitaemia and the number of platelets of patients infected by P. vivax (Fig. 1b).
Fig. 1.
Comparison analysis of platelet counts among groups and correlation between platelet counts and parasitaemia in patients with malaria caused by Plasmodium Vivax. a Number of platelets (mm3) in patients infected with P. vivax (n = 50) and non-infected controls (n = 20). Mann–Whitney t test. b Presence of negative correlation between parasitaemia (mm3) and platelet counts in patients infected with P. vivax. Spearman correlation coefficient test
Comparison of the cell profile of patients infected with Plasmodium vivax and non-infected controls T cells subpopulations CD3+CD4+IFN-γ+TNF-α+, CD3+CD4+IL-4+, CD3+CD4+IL-17+, CD4+CD25+CD127+FOXP3+ and CD4+CD25+CD127−FOXP3+
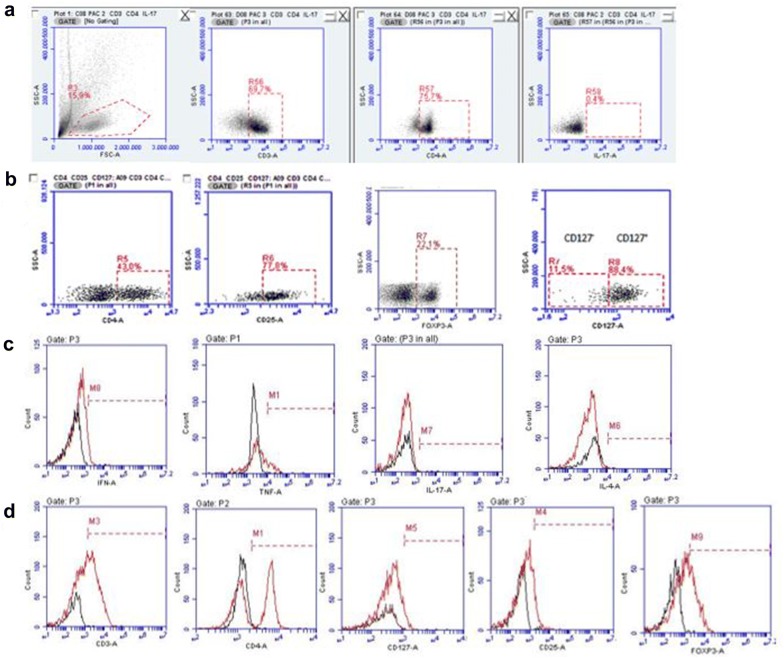
The leukogram of patients with malaria were evaluated and compared to the ones of healthy individuals and no significant statistical differences were noticed (Table 1). However, to evaluate the possible alterations in the different lymphocyte populations, the Th1, Th2, Th17, Treg, and activated Treg cells were investigated in the peripheral blood of the two groups, by flow cytometry as in the scheme below (Fig. 2).
Fig. 2.
CD3+CD4+IL-17+, CD4+CD25+CD127+FOXP3+ and CD4+CD25+CD127−FOXP3+ cells analysis model used in flow cytometry. a Representative scheme of the analysis used to identify CD3+CD4+IL-17+ T cell sub-population through the identification of its cytokine and dot plots illustrate isotype control used to make the quadrants. b Representative scheme of the analysis used to identify Treg cell (CD4+CD25+CD127+FOXP3+) and activated Treg cells (CD4+CD25+CD127−FOXP3+). c, d Flow cytometry histograms show the isotype controls and cells with positive reactivity with human monoclonal antibodies. Black line shows the isotype control and red line shows cells
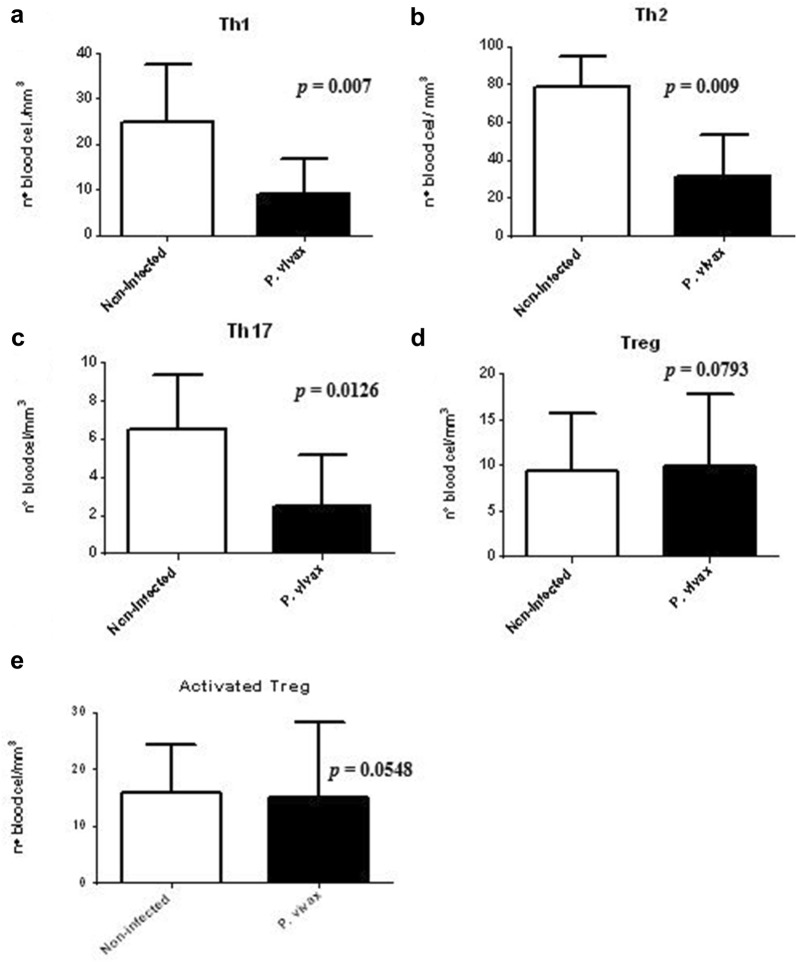
The data obtained indicate that malaria patients presented a significant reduction in the cellular sub-populations of Th1 (p = 0.007), Th2 (p = 0.009) and Th17 (p = 0.0126, Fig. 3a–c). No statistical significance was demonstrated when comparing the number of Treg cell and activated Treg cells sub-population between groups of infected patients and noninfected controls (p > 0.05; Fig. 3d, e).
Fig. 3.
Analysis of the Th1, Th2, Th17, Treg and activated Treg cells profile. a Number of cells IFN-γ+/TNF-α+, b IL-4+, c IL-17+, d, e Tregs respectively, in the T cell sub-populations: Th1, Th2, Th17, Treg and activated Treg cells of patients infected with P. vivax (n = 50) and non-infected controls (n = 20). Mann–Whitney t test
Correlation between parasitaemia and T cell sub-populations: CD3+CD4+IFN-γ+TNF-α+, CD3+CD4+IL-4+, CD3+CD4+IL-17+, CD4+CD25+CD127+FOXP3+ and CD4+CD25+CD127−FOXP3+ in patients infected with Plasmodium vivax
The Spearman correlation coefficient test was performed to verify the presence of correlation between parasitaemia and T lymphocyte sub-populations identified in patients infected with P. vivax. However, the results did not present a statistically significant correlation in any subpopulation of T lymphocytes analysed (p > 0.05).
Next, to investigate the profile of the infectious process triggered by P. vivax in patients diagnosed with malaria, patients were divided into two groups using serum TNF-α and IL-4 levels to focus on the serum cytokine profile. It is known that high levels of serum TNF-α and IL-4 have correlated with Th1 and Th2 cytokine profile, respectively. Therefore, these two groups were nominated serum Th1 and Th2 cytokine profile, respectively. The same T cell sub-populations described above were analysed in each serum cytokine profile.
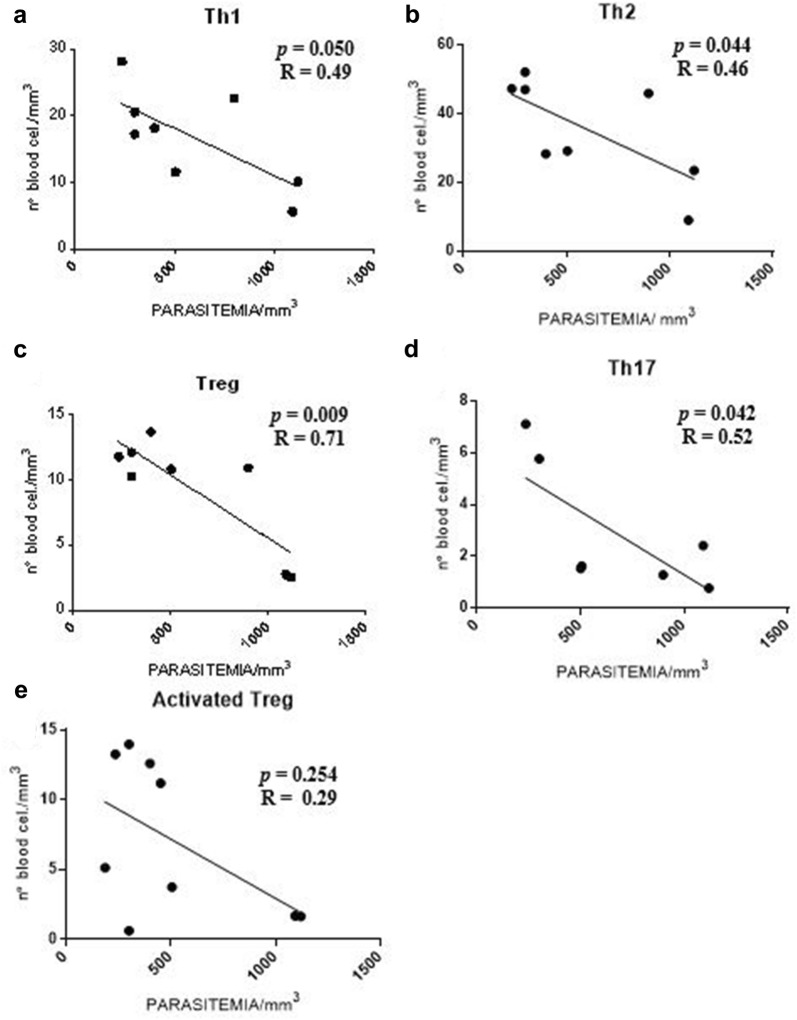
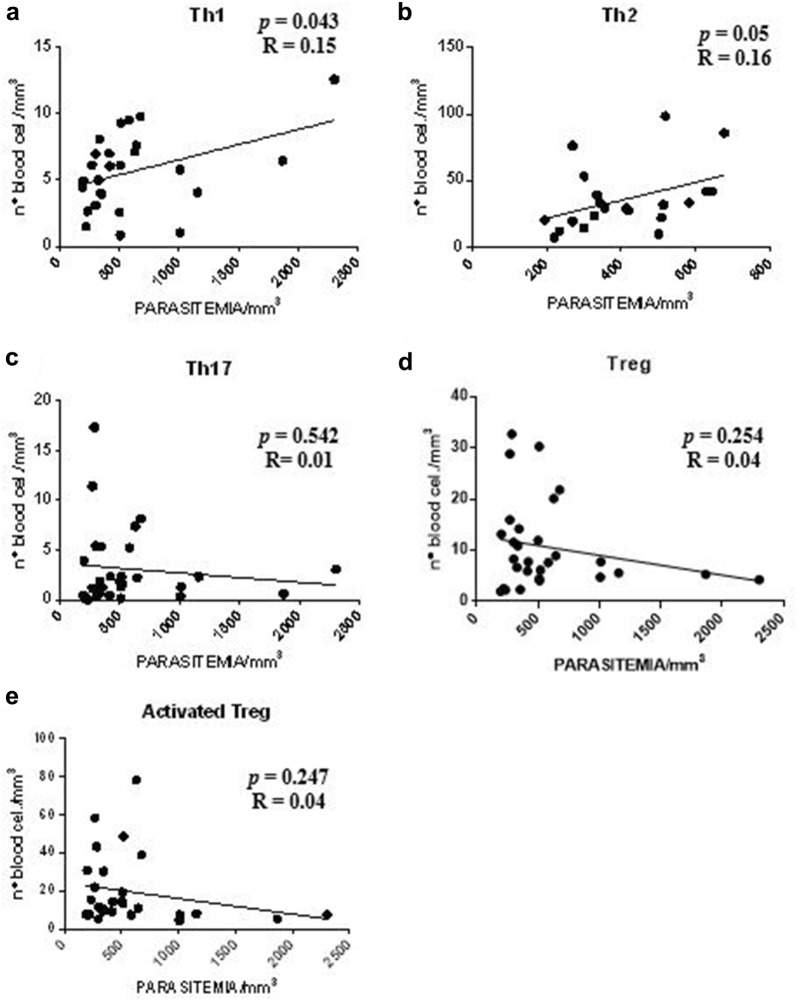
Initially, evaluating patients with serum Th1 cytokine profile, the data indicated a negative correlation between the level of parasitaemia with the following T cell sub-populations: Th1 (p = 0.05), Th2 (p = 0.044), Treg (p = 0.009), and Th17 (p = 0.042, Fig. 4a–d, respectively). Only the activated Treg cells sub-population did not present a statistical significance with the level of parasitaemia (p > 0.05; Fig. 4e).
Fig. 4.
Correlation analysis between parasitaemia and T cell sub-populations in patients with serum Th1 cytokine profile. a–d Presence of negative correlation between parasitaemia (mm3) and T cell sub-populations Th1, Th2, Treg and Th17. e Absence of correlation between parasitaemia (mm3) and number of cells corresponding to the activated Treg cells subpopulation (n = 10). Spearman correlation coefficient test
It was verified that the data obtained indicated the presence of a positive correlation between the level of parasitaemia and the sub-populations of Th1 and Th2 cells (p = 0.043 and p = 0.05; Fig. 5a, b, respectively) in patients with serum Th2 cytokine profile. No statistically significant difference was observed within the sub-populations of Th17, Treg and activated Treg cells (p > 0.05; Fig. 5c–e respectively).
Fig. 5.
Correlation analysis between parasitaemia and T lymphocyte sub-populations in patients with serum Th2 cytokine profile. a, b Presence of positive correlation between parasitaemia (mm3) and sub-populations of Th1 and Th2 cells. c–e Absence of correlation between the level of parasitaemia (mm3) and the number of cells corresponding to the sub-populations of Th17, Treg and activated Treg cells (n = 40). Spearman correlation coefficient test
Correlation between platelets and T cell sub-populations: CD3+CD4+IFN-γ+TNF-α+, CD3+CD4+IL-4+, CD3+CD4+IL-17+, CD4+CD25+CD127+FOXP3+ and CD4+CD25+CD127−FOXP3+ in patients infected with Plasmodium vivax
The Spearman correlation coefficient test was performed to verify the presence of a correlation between the number of platelets and the T lymphocyte sub-populations, identified in patients infected with P. vivax. Therefore, no statistically significant correlation was found in patients infected with P. vivax (p > 0.05) when evaluating all of the T cell sub-populations described above.
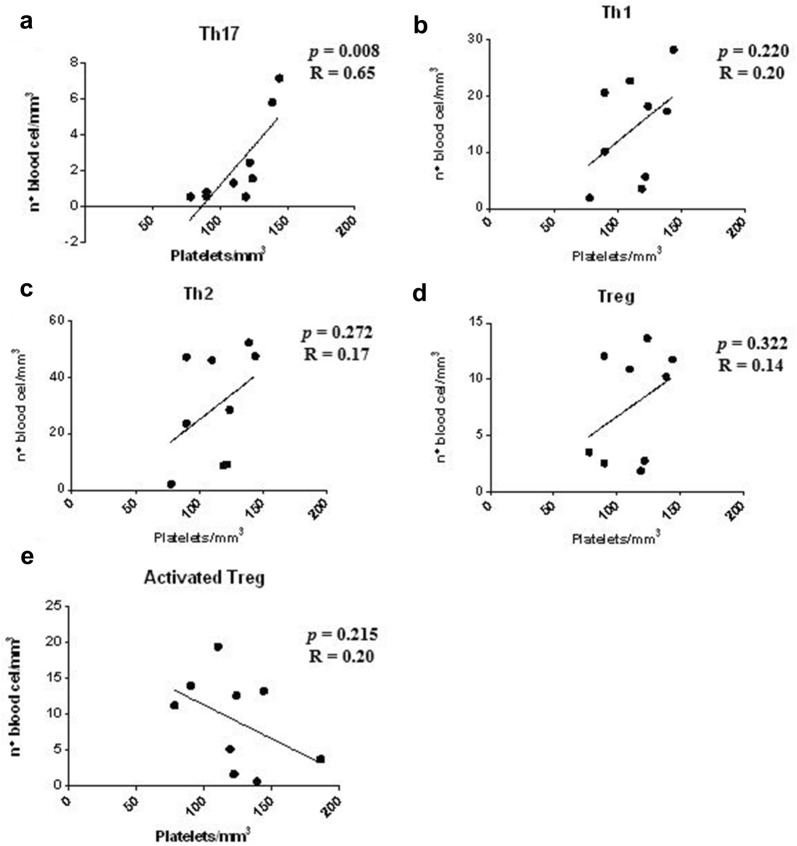
However, when patients with serum Th1 cytokine profile were evaluated separately, the data obtained indicated a positive correlation between the number of platelets and the Th17 sub-population (p = 0.008; Fig. 6a). Furthermore, the sub-populations of T cells: Th1, Th2, Treg, and activated Treg cells (Fig. 6b–e, respectively) showed no correlation with the platelet counts of patients infected with P. vivax. (p > 0.05).
Fig. 6.
Correlation analysis between platelet count and T lymphocyte subpopulations in patients with serum Th1 cytokine profile. a Presence of positive correlation between the number of platelets (mm3) and the number of cells corresponding to the Th17 cell sub-population. b–e Absence of correlation between the number of platelets (mm3) and the number of cells corresponding to the T cell sub-populations: Th1, Th2, and Treg and activated Treg cells (n = 10). Spearman correlation coefficient test
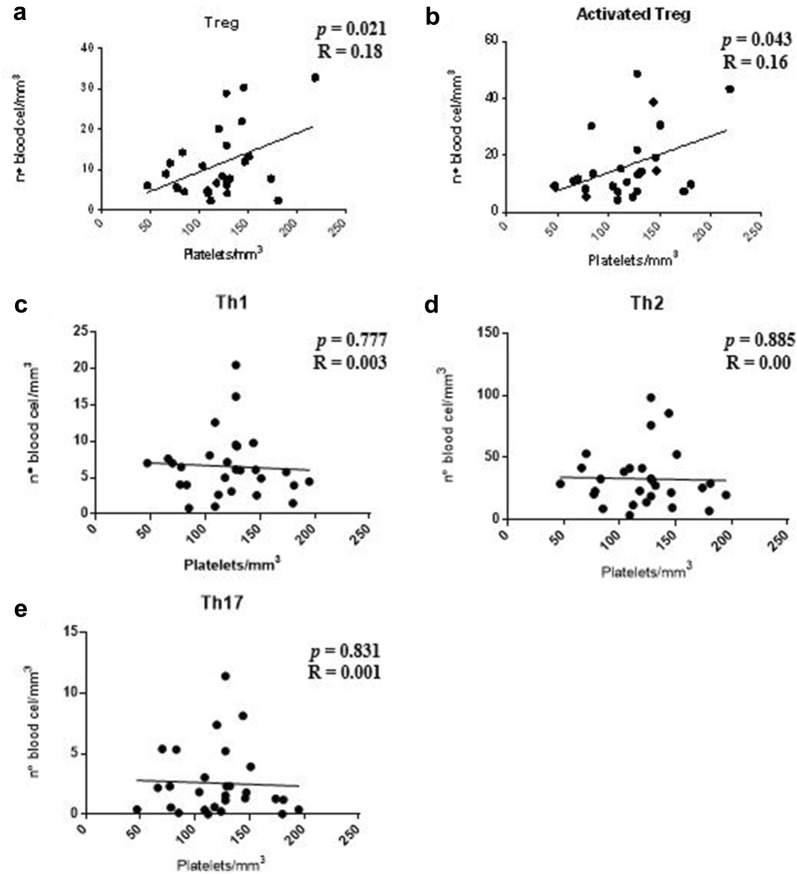
The data obtained when patients with serum Th2 cytokine profile were evaluated showed a positive correlation between the number of platelets and Treg sub-populations and activated Treg cells (p = 0.021 and p = 0.043; Fig. 7a, b, respectively). No statistically significant correlation was found between the number of platelets and the T cell sub-populations: Th1, Th2 and Th17 (p > 0.05; Fig. 7c–e, respectively).
Fig. 7.
Correlation analysis between platelet counts and T lymphocyte sub-populations in patients with serum Th2 cytokine profile. a, b Presence of positive correlation between the number of platelets (mm3) and sub-populations of Treg cell and activated Treg cells. c–e Absence of correlation between the number of platelets (mm3) and the number of cells corresponding to the T cell sub-populations: Th1, Th2 and Th17 (n = 40). Spearman correlation coefficient test
Discussion
The infectious process of malaria is characterized by a complex interaction between the host’s immune response and parasite survival strategy. This study contributes to the delimitation of haematological, parasitological and immunological aspects of individuals infected with P. vivax who live in a region of Brazil where malaria is actively transmitted. When analysed as a whole, patients showed a variation in T lymphocyte sub-populations. However, the stratification of serum Th1 and Th2 cytokine profile strengthens the correlation analysis of haematology, parasitology and immunology in patients.
In the pathogenic process of malaria, the cellular immune response is mediated by Th1 profile T cell lymphocytes with IFN-γ production and Th2 profile, with IL-4 and IL-5 cytokines, to eliminate the parasite [26]. In this study, the leukogram of patients with P. vivax infection was analysed and the total number of leukocytes was within the normal range. These data were in agreement with Grynberg and collaborators [27]. However, when the sub-populations of CD4+ T lymphocytes Th1, Th2, Th17 and Treg were evaluated, the data showed that the numbers of these cells in patients infected by P. vivax were significantly lower in relation to the control group. Similar results were observed by Gonçalves and collaborators [28], including Hojo-Souza and collaborators [29]. The literature describes that the decrease in peripheral blood lymphocytes is usually observed in patients with acute malaria caused by P. falciparum and P. vivax, and that may be induced by lymph nodes sequestration and also death by apoptosis [23, 30].
The data showed a predominance of low parasitaemia for most patients. A study conducted in Porto Velho, Brazil, was in agreement with these results [31]. The literature indicates that the parasitaemia of the infected patients in endemic regions tends to be low or moderate, which directly relates to the severity of the clinical manifestations. In regions where transmission is low or unstable, immunity is also low and individuals may present severe disease [32].
In this study, patients with serum Th1 cytokine profile were observed with parasitaemia inversely proportional to the increased number of Th1, Th2, Th17, and Treg cell. The information gathered suggests that these T cell sub-populations suffer a clonal deletion during this infectious process when the parasitaemia is elevated. In agreement with these results, a study with individuals infected with P. vivax and low parasitaemia, show evidence of high serum levels of IFN-γ [33]. In malaria, IFN-γ has direct antiparasitic action and may act in synergism with TNF-α. The action of IFN-γ, as well as other pro-inflammatory cytokines, should be controlled and able to eliminate infection without causing damage to the host [34].
In the initial phase of infection, CD4+ T lymphocytes participate in the reduction of parasite density. During the course of infection, the proportion of Th2 cells increases, favouring the development of antibody-mediated immunity and, consequently, the reduction of parasitaemia and the resolution of the patent infection [35, 36]. This study demonstrates that the number of Th1 and Th2 cells was directly proportional to the parasitaemia during the infection by P. vivax in patients with serum Th2 cytokine profile, extending the view that these immune cells may contribute to direct immunological anti-parasitic mechanisms.
The data established in this study, associated with the literature may indicate that in patients with a serum Th1 cytokine profile, activated Treg cells would be newly migrated cells that would not have a correlation with parasitaemia. The sub-population of Treg cells are possibly memory cells, which have an inverse correlation with parasitaemia. Studies indicate that by using CD127, the newly activated effector cells and the memory cells of the Treg population can be more clearly distinguished, since only newly activated Treg have a low expression of CD127. The memory cells have high expression of this marker and traditional effector cells rapidly re-express this marker after activation [15, 37]. These results are consistent with those recently published in a study with neonates of women infected with P. falciparum. In this situation, the frequency of both Treg cells (defined by the CD25+CD127+FOXP3− and CD25+CD127−FOXP3+ phenotypes, respectively) did not range from the placental parasitaemia, and decreased significantly as the level of placental inflammation increased [38].
Treg cells failed to show statistically significant differences when analysed in patients with a serum Th2 cytokine profile. Perhaps these cells are not phenotypically dysregulated during P. vivax infection. Evidence indicates that Treg cells can suppress T cell responses by producing IL-10 and TGF-β. Also, Treg cells can suppress B cell maturation and differentiation directly or indirectly by regulating IL-2 or IL-4 production [39]. The absence of deregulation suggests that the potential of these cells is maintained and may contribute to infection control and clinical immunity to P. falciparum or P. vivax infection [40]. Another study conducted with DEREG mice analyses the effect of CD4+FOXP3+ Treg on the course of non-lethal Plasmodium yoelii infection in BALB/c mice. The study reveals that the depletion of Treg cells reduced the level of parasitaemia and increased the activation of CD4+ and CD8+ T cells, indicating that Treg cells play a crucial role in the control of immune responses to parasitic infection [41].
The findings in this study showed that patients infected by P. vivax presented a significant decrease of Th17 in relation to the control group. Another observation made, was the statistically significant decrease in the number of Th17 cells when correlated with parasitaemia in patients infected with P. vivax with serum Th1 cytokine profile. Those data do not provide any clear conclusion regarding the role of these cells in the protection or pathogenesis of this disease in relation to parasitaemia. Bueno and collaborators [42], in turn, showed that Th17 cells were detected in patients with infection by P. vivax in the state of Amazonas. These findings correlated with the high production of IFN-γ, IL-10 and TGF-β. The production of IL-17 by CD4+ T cells was previously described during malaria infection in Treg cells [17, 42]. A study demonstrated in vitro that there may be a relationship between Th17 and Treg, and the balance between these cells may lead to susceptibility or resistance against other models of inflammation [43].
When compared to the control group patients infected with P. vivax presented thrombocytopaenia and a negative correlation with the level of parasitaemia in the group of individuals infected with P. vivax. Similarly, studies present an increase in the number of platelets parallel to the reduction of parasitaemia in patients with malaria caused by P. falciparum [6] or by P. vivax [2].
A correlation analysis between the number of platelets and the specific lymphocyte subpopulations present in the infectious process of individuals with malaria caused by P. vivax, was performed to verify whether the activation of these sub-populations could contribute to thrombocytopaenia. Several studies have tried to clarify the origin of this vital haematological alteration. A concomitant increase in the number of Th17 and platelet cells in patients infected with P. vivax with serum Th1 cytokine profile was observed. The IL-17 induces the expression of various mediators of inflammation [19]. However, the inflammatory response to malaria results in erythrocyte modifications that may increase changes in blood flow, including loss of normal discoid shape, increased membrane stiffness and high permeability, facilitating the survival of the parasite within the host cell and increasing the virulence of the disease [44, 45]. One study demonstrated that symptomatic patients infected with P. vivax who were treated with IL-17 displayed a reduction in blood viscosity, indicating the possible use of this cytokine as a potential immunomodulatory agent [33].
Furthermore, the analysis performed in patients with serum Th2 cytokine profile, verified a significant positive correlation with the number of platelets and Treg cells and activated Treg cells in the patients infected with P. vivax. A study of children in Kenya, with P. falciparum infection, showed a significant and independent inverse correlation between platelet counts and plasma IL-10 [46]. It is speculated in this work that IL-10 could, by reducing the number of circulating platelets, prevent the adhesion of parasitized red blood cells to the vascular endothelium, as if thrombocytopaenia could represent a defense mechanism against severe malaria. In another study, thrombocytopaenia in patients with P. vivax infection was associated with increased IL-1, IL-6, IL-10, and TGF-β [47]. Thus, in addition to immunological profiles, other biological mechanisms may also be important for the regulation of thrombocytopaenia.
The epidemiological data and laboratory analysis of patients with P. vivax infection were verified. The literature reports more frequent cases of malaria caused by P. vivax in male individuals, however, it is correlated with economic activity, usually in garimpo and agriculture [48]. The area studied was an endemic urban area, therefore, there was no gender prevalence in the data obtained for this study. All patients in this study reported more than one malaria episode during their lifetime. However, no adjustments were made according to previous malaria experience, which may be a limitation for this study. With regard to the laboratory analysis, biochemical tests and blood count were performed to identify suggestive cases of severe malaria caused by P. vivax, using established standards for P. falciparum infection [49], as already done in other studies [50]. The most common complications in malaria are haematologic changes, which are involved in disease severity and fatality [51]. The biochemical and haematological data of the patients in this study did not reach severity parameters.
Conclusions
The data rendered in this study are related to the aspects of the immune response and its activation against parasite load and platelet activation during malaria caused by P. vivax. Considering the different defence mechanisms of the host against an infection, it is possible to hypothesize that the lymphocytic profile is dependent on parasitaemia and the number of platelets for its differentiation and activation. Further studies will be important to elucidate in more detail the mechanisms of action triggered by the immune system against P. vivax infection.
Additional file
Additional file 1: Table S1. Demonstration of monoclonal antibodies used in cellular immunophenotyping analyzes.
Authors’ contributions
SSO, QIB and DSAS collected the samples, performed the experiments, analyzed the data and wrote the manuscript. ASD designed the study, analyzed the data, performed the statistical analysis and wrote the manuscript. RMS and ECMM contributed with reagents/materials/analysis tools. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the participants of this study. We also thank the technicians’ team of the Hospital of the Woman and the Child of the Juruá for their invaluable assistance in helping to collect and process samples.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author per reasonable request.
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Ethics and Research Committee of Júlio Müller Hospital, CAAE: 30864514.0.0000.5541, No. 1.785.003, October 21, 2016, in compliance with Resolution 196/96 of the National Health Council. All participants signed a written informed consent, and after blood collection, all patients were treated according to the national malaria treatment policy in Brazil, as recommended in the Manual of Malaria Therapy.
Funding
This work was supported by the Conselho Nacional de Pesquisa (CNPq)—Brazil (Grant Number 555652/2009-2—PRONEX-Rede Malária, and 481626/2013-1) and Ministério da Saúde, Secretaria de Vigilância em Saúde (Grant Number 797613/2013). SSO and QIB are funded by MEC—Coordination of Superior Level Staff Improvement (CAPES)—Brazil (doctorate studentship). ASD is funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 311986/2014-5).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gething PW, Elyazar IRF, Moyes CL, Smith DL, Battle KE, Guerra CA, et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ourives SS, Dos Santos DSA, Alem LR, Rios-Santos F, Fontes CJF, Damazo AS. Analysis of parasitological and haematological parameters and of CD4+ and CD8+ cell number in patients with Plasmodium vivax malaria. Res J Parasitol. 2015;10:1–14. doi: 10.3923/jp.2015.1.14. [DOI] [Google Scholar]
- 3.Anstey NM, Douglas NM, Poespoprodjo JR, Price R. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201. doi: 10.1016/B978-0-12-397900-1.00003-7. [DOI] [PubMed] [Google Scholar]
- 4.Burel JG, Apte SH, McCarthy JS, Doolan DL. Plasmodium vivax but not Plasmodium falciparum blood-stage infection in humans is associated with the expansion of a CD8+ T cell population with cytotoxic potential. PLoS Negl Trop Dis. 2016;10:e0005031. doi: 10.1371/journal.pntd.0005031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes AAM, Carvalho LJM, Zanini GM, Ventura AMRS, Souza JM, Cotias PM, et al. Similar cytokine responses and degrees of anemia in patients with Plasmodium falciparum and Plasmodium vivax infections in the Brazilian Amazon Region. Clin Vacc Immunol. 2008;15:650–658. doi: 10.1128/CVI.00475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacerda MVG, Mourao MPG, Coelho HCC, Santos JB. Thrombocytopenia in malaria: who cares? Mem Inst Oswaldo Cruz. 2011;106:52–63. doi: 10.1590/S0074-02762011000900007. [DOI] [PubMed] [Google Scholar]
- 7.Zeyrek FY, Kurcer MA, Zeyrek D, Simsek Z. Parasite density and serum cytokine levels in Plasmodium vivax malaria in Turkey. Paras Immunol. 2006;28:201–207. doi: 10.1111/j.1365-3024.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 8.Jide C, Ying H, Wenyue X, Fusheng H. Toll-like receptors, a double-edged sword in immunity to malaria. J Med Coll PLA. 2009;24:118–124. doi: 10.1016/S1000-1948(09)60027-7. [DOI] [Google Scholar]
- 9.Mbugi EV, Meijerink M, Veenemans J, Jeurink PV, Mccall M, et al. Effect of nutrient deficiencies on in vitro Th1 and Th2 cytokine response of peripheral blood mononuclear cells to Plasmodium falciparum infection. Malar J. 2010;9:162. doi: 10.1186/1475-2875-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousefzadeh H, Jabbari Azad F, Rastin M, Banihashemi M, Mahmoudi M. Expression of Th1 and Th2 cytokine and associated transcription factors in peripheral blood mononuclear cells and correlation with disease severity. Rep Biochem Mol Biol. 2017;6:102–111. [PMC free article] [PubMed] [Google Scholar]
- 11.Mendonça VR, Queiroz AT, Lopes FM, Andrade BB, Barral-Netto M. Networking the host immune response in Plasmodium vivax malaria. Malar J. 2013;12:69. doi: 10.1186/1475-2875-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 13.Inoue S, Niikura M, Mineo S, Kobayashi F. Roles of IFN-γ and γδ T cells in protective immunity against blood-stage malaria. Front Immunol. 2013;4:1–9. doi: 10.3389/fimmu.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bueno LL, Morais CG, Araújo FF, et al. Plasmodium vivax: induction of CD4+CD25+FoxP3 regulatory T cells during infection are directly associated with the level of circulating parasite. PLoS ONE. 2010;5:e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Putnam AL, Xu-Yu Z, Szot LG, Lee MR, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43:395–401. doi: 10.1016/j.cyto.2008.07.469. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 18.Riley EM, Wahl S, Perkins DJ, et al. Regulating immunity to malaria. Parasit Immunol. 2006;28:35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helegbe GK, Huy NT, Yanagi T, et al. Elevated IL-17 levels in semi-immune anaemic mice infected with Plasmodium berghei ANKA. Malar J. 2018;17:169. doi: 10.1186/s12936-018-2257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida H, Matsuzaki-Moriya C, Imai T, et al. Development of experimental cerebral malaria is independent of IL-23 and IL-17. Biochem Biophys Res Commun. 2010;402:790–795. doi: 10.1016/j.bbrc.2010.10.114. [DOI] [PubMed] [Google Scholar]
- 22.Ministério da Saúde/SVS: Guia prático de tratamento da malária no Brasil. 3rd ed. Brasília/DF; 2010. p. 36.
- 23.Borges QI, Fontes CJF, Damazo AS. Analysis of lymphocytes in patients with Plamodium vivax malaria and its relation to the annexin-A1and IL-10. Malar J. 2013;12:455. doi: 10.1186/1475-2875-12-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauruschkat CD, Wurster S, Page L, et al. Susceptibility of A. fumigatus specific T-cell assays to pre-analytic blood storage and PBMC cryopreservation greatly depends on readout platform and analytes. Mycoses. 2018 doi: 10.1111/myc.12780. [DOI] [PubMed] [Google Scholar]
- 25.Dennis JY. Erythrocyte Lysis. In: UCSD Cancer center flow cytometry core facility. 2016. https://medschool.ucsd.edu/research/moores/sharedresources/flowcytometry/protocols/Pages/erythrocyte-lysis.aspx. Accessed 22 June 2016.
- 26.Torre D, Speranza F, Martegani R. Role of proinflammatory and anti-inflammatory cytokines in the immune response to Plasmodium falciparum malaria. Lancet Infect Dis. 2002;2:719–720. doi: 10.1016/S1473-3099(02)00449-8. [DOI] [PubMed] [Google Scholar]
- 27.Grynberg P, Fontes CJF, Braga EM. Association between particular polymorphic residues on apical membrane antigen 1 (AMA-1) and platelet levels in patients with vivax malaria. Clin Microbiol Infect. 2007;13:1089–1094. doi: 10.1111/j.1469-0691.2007.01815.x. [DOI] [PubMed] [Google Scholar]
- 28.Gonçalves RM, Salmazi KC, Santos BAN, Bastos MS, Rocha SC, et al. CD4+CD25+Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun. 2010;78:4763–4772. doi: 10.1128/IAI.00578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hojo-Souza NS, Pereira DB, Mendes TA, Passos L, Gazzinelli-Guimaraes AC, Gazzinelli-Guimaraes PH, et al. CD4+ T cells apoptosis in Plasmodium vivax infection is mediated by activation of both intrinsic and extrinsic pathways. Malar J. 2015;14:5. doi: 10.1186/1475-2875-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riccio EKP, Neves I, Riccio LRP, das Graças Alecrim M, Corte-Real S, Morgado M, et al. Malaria associated apoptosis is not significantly correlated with either parasitemia or the number of previous malaria attacks. Parasitol Res. 2003;90:9–18. doi: 10.1007/s00436-002-0816-z. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues-da-Silva RN, Lima-Junior JDAC, Fonseca BDEP, Antas PR, Baldez A, Storer FL, et al. Alterations in cytokines and haematological parameters during the acute and convalescent phases of Plasmodium falciparum and Plasmodium vivax infections. Mem Inst Oswaldo Cruz. 2014;109:154–162. doi: 10.1590/0074-0276140275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolfes MA, McCarra M, Magak NG, Ernst KC, Dent AE, Lindblade KA, et al. Development of clinical immunity to malaria in highland areas of low and unstable transmission. Am J Trop Med Hyg. 2012;87:806–812. doi: 10.4269/ajtmh.2012.11-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherer EF, Cantarini DG, Siqueira R, Ribeiro EB, Braga EM, Honório-França AC, et al. Cytokine modulation of human blood viscosity from vivax malaria patients. Acta Trop. 2016;158:139–147. doi: 10.1016/j.actatropica.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169:2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 35.Taylor-Robinson AW, Phillips RS, Severn A, Moncada S, Liew FY. The role of Th1 and Th2 cells in a rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 36.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 37.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soulard V, Amadoudji ZM, Fitting C, Ibitokou S, Oesterholt M, Luty AJ, et al. Placental malaria-associated suppression of parasite-specific immune response in neonates has no major impact on systemic CD4 T cell homeostasis. Infect Immun. 2011;79:2801–2809. doi: 10.1128/IAI.00203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura A, Wakabayashi Y, Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-β. J Biochem. 2010;147:781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kho S, Marfurt J, Handayuni I, Pava Z, Noviyanti R, Kusuma A, et al. Characterization of blood dendritic and regulatory T cells in asymptomatic adults with sub-microscopic Plasmodium falciparum or Plasmodium vivax infection. Malar J. 2016;15:328. doi: 10.1186/s12936-016-1382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abel S, Lückheide N, Westendorf AM, Geffers R, Roers A, Müller W, et al. Strong impact of CD4+Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. J Immunol. 2012;188:5467–5477. doi: 10.4049/jimmunol.1102223. [DOI] [PubMed] [Google Scholar]
- 42.Bueno LL, Morais CG, Lacerda MV, Fujiwara RT, Braga EM. Interleukin-17 producing T helper cells are increased during natural Plasmodium vivax infection. Acta Trop. 2012;123:53–57. doi: 10.1016/j.actatropica.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 43.Moore-Connors JM, Fraser R, Halperin SA, Wang J. CD4+CD25+Foxp3+ regulatory T cells promote Th17 responses and genital tract inflammation upon intracellular Chlamydia muridarum infection. J Immunol. 2013;191:3430–3439. doi: 10.4049/jimmunol.1301136. [DOI] [PubMed] [Google Scholar]
- 44.Cromer D, Best SE, Engwerda C, Haque A, Davenport M. Where have all the parasites gone? Modelling early malaria parasite sequestration dynamics. PLoS ONE. 2013;8:e55961. doi: 10.1371/journal.pone.0055961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohandas N, An X. Malaria and human red blood cells. Med Microbiol Immunol. 2012;201:593–598. doi: 10.1007/s00430-012-0272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casals-Pascual C, Kai O, Newton CR, Peshu N, Roberts DJ. Thrombocytopenia in falciparum malaria is associated with high concentrations of IL-10. Am J Trop Med Hyg. 2006;75:434–436. [PubMed] [Google Scholar]
- 47.Park JW, Park SH, Yeom JS, Huh AJ, Cho YK, Ahn JY, et al. Serum cytokine profiles in patients with Plasmodium vivax malaria: a comparison between those who presented with and without thrombocytopenia. Ann Trop Med Parasitol. 2003;97:339–344. doi: 10.1179/000349803235002416. [DOI] [PubMed] [Google Scholar]
- 48.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO. World malaria report 2016. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/world_malaria_report_2016/en/. Accessed 02 May 2016.
- 50.Gomes LT, Alves-Junior ER, Rodrigues-Jesus C, Nery AF, Gasquez-Martin TO, Fontes CJ. Angiopoietin-2 and angiopoietin-2/angiopoietin-1 ratio as indicators of potential severity of Plasmodium vivax malaria in patients with thrombocytopenia. PLoS ONE. 2014;9:e109246. doi: 10.1371/journal.pone.0109246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ministério da Saúde/SVS: Guia de vigilância em saúde. 2014. http://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_unicado.pdf. Accessed 14 Nov 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Demonstration of monoclonal antibodies used in cellular immunophenotyping analyzes.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author per reasonable request.