Abstract
Background
Colonization with multidrug-resistant (MDR) bacteria is a major risk factor for developing subsequent MDR infections.
Methods
We performed a prospective surveillance study in hospitalized patients at Siriraj Hospital. Nasal cavity, throat, inguinal area and rectal swabs were obtained within the first 48-h after admission, on day-5 after hospitalization and then every 7 days until discharge. Target bacteria included extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL), carbapenem-resistant-P.aeruginosa (CR-PA), carbapenem-resistant-A.baumannii (CR-AB) and methicillin-resistant S.aureus (MRSA).
Results
From January 2013–December 2014, 487 patients were enrolled. The baseline prevalence of colonization by ESBL, CR-PA, CR-AB and MRSA at any site was 52.2%, 6.8%, 4.7% and 7.2%, respectively. After 3-week of hospitalization, the prevalence of colonization by ESBL, CR-PA, CR-AB and MRSA increased to 71.7%, 47.2%, 18.9% and 18.9%, respectively. Multivariable analysis revealed that diabetes mellitus and recent cephalosporin exposure were the independent risk factors for baseline colonization by ESBL. The independent risk factors for CR-AB and/or CR-PA colonization were cerebrovascular diseases, previous hospitalization, transfer from another hospital/a LTCF and previous nasogastric tube use, whereas those for MRSA colonization were previous fluoroquinolone exposure and previous nasogastric tube use.
Conclusions
The baseline prevalence of colonization by ESBL was relatively high, whereas the baseline prevalence of colonization by CR-PA, CR-AB and MRSA was comparable to previous studies. There was an increasing trend in MDR bacteria colonization after hospitalization.
Background
Antimicrobial resistance (AMR) is considered a major health threat. The consequences of multidrug-resistant (MDR) bacterial infections including high morbidity and mortality and economic loss have been well documented in many studies [1–3]. Colonization by MDR bacteria is considered a potential source of cross-transmission to other patients [4–6]. Moreover, colonization by MDR bacteria was found to be an independent risk factor for developing subsequent MDR bacterial infections in previous studies [5, 7].
The World Health Organization recognized AMR as a global health problem and recommended that Member States should strengthen the knowledge and evidence base through AMR surveillance and research in the global action plan on AMR [8]. Lack of AMR surveillance data contributes to underestimating the magnitude of AMR problem and halting the implementation of AMR control measures.
A surveillance study reported that the prevalence of rectal colonization by ESBL-producing Enterobacteriaceae among newly-hospitalized general medical patients in an Israel teaching hospital was only 8% [9]. After two weeks of hospitalization, the prevalence of colonization increased to 21% [9]. Surprisingly, the prevalence of rectal colonization by extended-spectrum beta-lactamase-producing Enterobacteriaceae in Thai community volunteers was remarkably high (32.0–66.5%) [10, 11].
Similar to ESBL-producing Enterobacteriaceae, the prevalence of Pseudomonas aeruginosa (PA) colonization varied across geographic locations. In a United States study, the prevalence of PA rectal colonization among intensive care unit (ICU) patients was 11.6% [12]. However, a recent Spanish study found that the prevalence of rectal colonization by non-drug resistant PA and extensive drug resistant PA in ICU patients was 27.0% and 4.0%, respectively [13].
Based on the data from a recent surveillance study performed in a medical ICU in Korea, active surveillance detected carbapenem-resistant-Acinetobacter baumannii (CR-AB) in 15.0% of patients, and approximately one-third of them later developed CR-AB infections [14]. Similar to the Korean study, the prevalence of CR-AB colonization in ICU patients at a US tertiary hospital was 13.5% [15].
Nasal colonization by methicillin-resistant Staphylococcus aureus (MRSA) has been widely investigated. The prevalence of MRSA nasal colonization varied from 4.1% in the US national surveillance in-patient data [16] to 9.0% among newly-hospitalized patients in an Israel teaching hospital [9]. Data on MRSA colonization at other sites in the body in addition to the nasal cavity is very limited.
Based on previous scientific evidence, the prevalence of MDR bacteria colonization varied across specific types of MDR bacteria, geographic regions and clinical settings [community, hospital or long-term care facility (LTCF)]. Although many studies have already investigated the prevalence of MDR bacteria colonization, most studies focused only on rectal or stool colonization by MDR gram-negative bacteria and nasal colonization by MRSA. Furthermore, these studies were not longitudinal studies that monitored changes in the prevalence of AMR bacteria colonization after hospitalization.
Given these considerations, we performed a prospective surveillance study for MDR bacteria colonization in hospitalized patients on admission and during hospitalization. The primary objective was to determine the prevalence of colonization by MDR bacteria in newly-hospitalized patients and the prevalence of new acquisition of MDR bacteria during hospitalization. The secondary objective was to identify risk factors for colonization by MDR bacteria and for new acquisition of MDR bacteria. Results from this study helped us determine the magnitude of AMR problem and the natural history of AMR colonization in hospitalized patients. Furthermore, the study could identify the patients at risk for MDR bacteria colonization who may subsequently develop infections due to these bacteria.
Methods
Study design and setting
During a 2-year study period (1 January 2013–31 December 2014), we performed a prospective surveillance study in eight general medical wards at Siriraj Hospital, which is a 2200-bed university hospital located in Bangkok, Thailand. The study protocol was approved by the Siriraj Institutional Review Board.
Study population
The eligible subjects were all adults aged ≥18 years who had been hospitalized in general medical wards for less than 24 h. Subjects who were expected to be discharged or dead within 48 h or those with any contraindications for obtaining clinical specimens (i.e. a neutropenic patient (digital rectal examination or rectal swab culture was contraindicated), or having local infection at the site of surveillance culture) were excluded. Only subjects who agreed to participate and signed informed consent forms were enrolled.
Microbiological surveillance of AMR bacteria
Clinical specimens from four sites including the nasal cavity, throat, skin at the inguinal area and rectum or stool were obtained from each patient within 48 h after hospitalization (time-1). Clinical specimens were subsequently obtained on day 5 ± 1 of hospitalization (time-2) and then every 7 days until the patient left the hospital (time-3, time-4 and so on). All clinical specimens were transferred in Stuart transport medium to the Laboratory of Division of Infectious Diseases, Department of Medicine.
The targeted MDR bacteria were ESBL-producing Enterobacteriaceae, CR-PA, CR-AB and MRSA. MacConkey agar supplemented with ceftriaxone for the isolation of MDR gram-negative bacteria and Mannitol Salt agar for the isolation of staphylococci were used for inoculating the clinical specimens collected from all sites.
Species identification and antimicrobial susceptibility tests were performed according to the performance standards for antimicrobial susceptibility testing recommended by the Clinical and Laboratory Standards Institute 2013 [17]. Species identification was performed using conventional biochemical tests. Identification of ESBL-producing bacteria was confirmed using the combination disc method. MRSA strains were determined using cefoxitin disc (30 mg) screening. Antimicrobial susceptibility testing was performed using the disc diffusion method.
Results of microbiology surveillance were directly reported to the study team and available (per request) for the service team (i.e. a responsible physician, an infectious disease consultant, etc.). However, there was no special infection control intervention for patients with colonization by target MDR-bacteria.
Data collection
Medical records for the enrolled patients were reviewed for demographics, co-morbidities and clinical course. Data on any hospitalization, medication used, intervention and catheter use in the preceding 90 days prior to hospitalization were also obtained. Previous hospitalization included any stay at observation or emergency rooms for periodic monitoring and/or short-term treatment for longer than 24 h within 3 months prior to the index hospitalization.
Statistical analysis
Categorical variables were summarized by frequency and proportion, whereas continuous variables were summarised by mean, median, standard deviation and range as appropriate. The prevalence of colonization by MDR bacteria was reported as percentage with a 95% confidence interval (95% CI). Wilcoxon-type test for trend analysis was performed to identify an increasing trend of colonization over time after hospitalization.
Multivariate logistic analysis was performed to identify the risk factors for colonization by MDR bacteria (at any site) and for new acquisition of MDR bacteria (at any site). A separate model was built for each MDR pathogen including 1) ESBL-producing Enterobacteriaceae; 2) CR-PA and/or CR-AB and 3) MRSA. Primary analysis was performed to compare cases with the specific MDR pathogens to controls without the given pathogen. Additionally, we performed a secondary analysis by comparing cases with the specific MDR pathogen to controls without any colonization.
Any associated variable with a p-value ≤0.20 was entered in a forward stepwise manner into the model. Any associated variables with a p-value < 0.20 was entered into the model. The likelihood ratio test was performed to confirm the model fit. For all calculations, a two-tailed p-value of < 0.05 was considered statistically significant. All calculations were performed using STATA version 14.0 (Stata Corp, College Station, TX).
Results
Baseline characteristics of patients
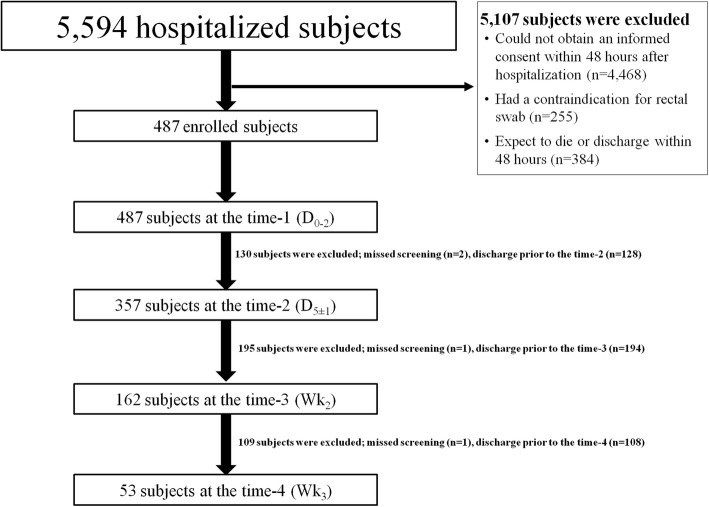
During the study period, 487 patients were enrolled in the study as shown in Fig. 1. The baseline characteristics of patients prior to hospitalization are shown in Table 1. Nearly half (45.4%) of the patients were male, with an average age of 61.7 ± 17.8 years. Previous hospitalization was documented in 43.3% of patients. Additionally, 11.0% and 1.2% of patients had been transferred from another hospital or a LTCF, respectively. Majority of patients (94.4%) had at least one underlying disease.
Fig. 1.
Study flow chart
Table 1.
Baseline characteristics of 487 patients
| Baseline characteristics prior to hospitalization | n (%) |
|---|---|
| Mean age ± SD (years) | 61.7 ± 17.8 |
| Male gender | 221 (45.4%) |
| Mean length of hospital stay, days (±SD) | 14.5 ± 18.5 |
| Median length of hospital stay, days (range) | 10.0 (2.0–303.0) |
| Previous hospitalization | 211 (43.3%) |
| Transfer status | |
| From another hospital | 56 (11.5%) |
| From a long-term care facility | 6 (1.2%) |
| Underlying diseases | |
| Any underlying disease | 420 (86.2%) |
| Hypertension | 290 (59.6%) |
| Diabetes mellitus | 184 (37.8%) |
| Cardiovascular diseases | 133 (27.3%) |
| Cerebrovascular diseases | 97 (19.9%) |
| Chronic liver diseases | 88 (18.0%) |
| Chronic renal diseases | 64 (13.1%) |
| Chronic lung diseases | 63 (12.9%) |
| Malignancy | 87 (17.9%) |
| Solid malignancy | 75 (15.4%) |
| Hematologic malignancy | 12 (2.5%) |
| Hematologic diseases | 47 (9.7%) |
| Prior organ transplantation | 7 (1.4%) |
| Receipt of any immunosuppressive agent within 90 days | 75 (15.4%) |
| HIV infection | 16 (3.3%) |
| Previous antibiotic exposure within 90 days after hospitalization | |
| Any antibiotic | 148 (30.4%) |
| Penicillins | 19 (3.9%) |
| Cephalosporins | 60 (12.3%) |
| Carbapenems | 33 (6.8%) |
| Beta-lactam/beta-lactamase inhibitors | 27 (5.5%) |
| Fluoroquinolones | 46 (9.4%) |
| Macrolides | 19 (3.9%) |
| Others | 42 (8.6%) |
| Previous use of indwelling catheters within 90 days | |
| Urinary catheter | 73 (15.0%) |
| Nasogastric tube | 45 (9.2%) |
The most common underlying disease was hypertension (59.6%), followed by diabetes mellitus (DM) (37.8%) and cardiovascular disease (27.3%). One-third (30.4%) of patients had previously been exposed to at least one type of antibiotics within the past 3 months. Approximately 15% of patients had a long-term urinary catheter inserted prior to hospitalization. The mean length of stay (LOS) was 14.5 ± 18.5 days, whereas the median LOS was 10 (2–303) days.
Colonization by MDR bacteria in newly-hospitalized patients
The prevalence of colonization by MDR bacteria in newly-hospitalized patients stratified by MDR bacteria species and by colonization site is shown in Table 2. Of the 487 patients evaluated, only 197 were free of colonization (40.5%). The rest (59.5%) were colonized by at least one specific MDR pathogen.
Table 2.
Prevalence of colonization of MDR bacteria in newly-hospitalized patients (N = 487) stratified by the specific MDR bacteria and by the specimen collection site
| MDR bacteria | All sites, n (%) | Nasal cavity, n (%) | Throat, n (%) | Inguinal area, n (%) | Rectum, n (%) |
|---|---|---|---|---|---|
| ESBL-producing Enterobacteriaceae | 254 (52.2%) | 13 (2.7%) | 42 (8.6%) | 80 (16.4%) | 232 (47.6%) |
| E. coli | 206 (42.3%) | 5 (1.0%) | 14 (2.9%) | 58 (11.9%) | 189 (38.8%) |
| K. pneumoniae | 81 (16.6%) | 9 (1.8%) | 31 (6.4%) | 29 (6.0%) | 60 (12.3%) |
| Other Enterobacteriaceae | 8 (1.6%) | 0 | 1 (0.2%) | 3 (0.6%) | 7 (1.4%) |
| A. baumannii | |||||
| Carbapenem-susceptible | 88 (18.0%) | 13 (2.7%) | 43 (8.8%) | 34 (7.0%) | 32 (6.6%) |
| Carbapenem-resistant | 63 (12.9%) | 13 (2.7%) | 16 (3.3%) | 32 (6.6%) | 28 (5.8%) |
| P. aeruginosa | |||||
| Carbapenem-susceptible | 33 (6.8%) | 10 (2.1%) | 11 (2.3%) | 18 (3.7%) | 12 (2.5%) |
| Carbapenem-resistant | 23 (4.7%) | 9 (1.9%) | 18 (3.7%) | 2 (0.4%) | 6 (1.2%) |
| Staphylococcus aureus | |||||
| Methicillin-susceptible | 49 (10.0%) | 29 (6.0%) | 21 (4.3%) | 8 (1.6%) | 11 (2.3%) |
| Methicillin-resistant | 35 (7.2%) | 21 (4.3%) | 17 (3.5%) | 6 (1.2%) | 12 (2.5%) |
More than half of the patients had ESBL-producing Enterobacteriaceae colonization in at least one body site, primarily in the rectum (47.6%) followed by the inguinal area (16.4%), throat (8.6%) and nasal cavity (2.7%). ESBL-producing E. coli (42.3%) were more prevalent than ESBL-producing K. pneumoniae (16.6%).
CR-PA was identified in only 4.7% of patients, primarily in the throat (3.7%). Baseline colonization by CR-AB was documented in 12.9% of patients, primarily in the inguinal area (6.6%) followed by the rectum (5.8%), throat (3.3%) and nasal cavity (2.7%).
MRSA was documented in 7.2% of patients, unlike MDR gram-negative bacteria, which primarily colonized in the nasal cavity (4.3%). The risk factors for baseline colonization by each MDR bacteria are reported in the next section.
Colonization by MDR bacteria during hospitalization
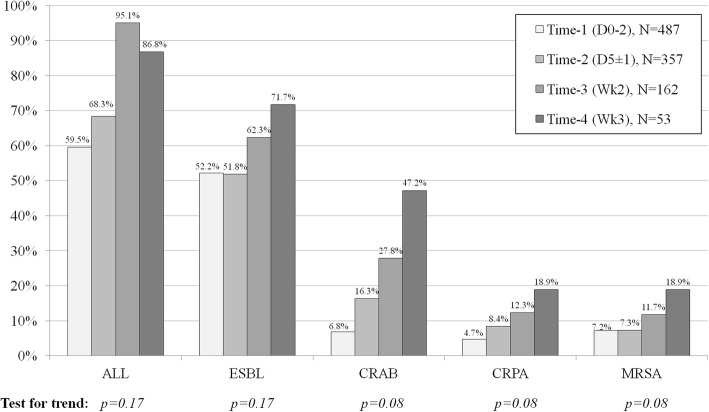
Given that some patients were discharged or dead before subsequent clinical specimens were obtained, the number of follow-up specimens decreased over time. Collection of the subsequent specimens was successfully completed in 357 patients (73.3%) at time-2, 162 patients (33.3%) at time-3 and 53 patients (10.9%) at time-4. Overall colonization and colonization by all species of MDR bacteria showed an increasing trend over time as shown in Fig. 2. However, this increasing trend did not reach statistical significance in the test for trend analysis (all p-values > 0.05). The details of colonization by MDR bacteria stratified by MDR bacteria species, colonization site and specimen collection time are shown in Table 3. Due to the small number of new MDR bacteria acquisitions, we did not further investigate the risk factors for new acquisitions of these bacteria.
Fig. 2.
Prevalence of colonization by MDR bacteria stratified by the specific MDR bacteria and by the specimen collection time
Table 3.
Prevalence of colonization on admission and during hospitalization stratified by the specific MDR bacteria, the surveillance culture site and the time of specimen collection
| Time | Any site, (%) | Nasal cavity, (%) | Throat, (%) | Inguinal area, (%) | Rectum, (%) |
|---|---|---|---|---|---|
| ESBL-producing Enterobacteriaceae | |||||
| Time-1 (N = 487) | 52.2 | 2.7 | 9.8 | 17.5 | 50.4 |
| Time-2 (N = 357) | 51.8 | 3.4 | 15.1 | 17.5 | 46.2 |
| Time-3 (N = 162) | 62.3 | 8.3 | 25.0 | 21.9 | 46.2 |
| Time-4 (N = 53) | 71.7 | 12.0 | 28.6 | 27.1 | 53.8 |
| ESBL-producing E. coli | |||||
| Time-1 (N = 487) | 42.3 | 1.0 | 2.9 | 11.9 | 38.8 |
| Time-2 (N = 357) | 41.2 | 1.3 | 3.7 | 10.9 | 34.3 |
| Time-3 (N = 162) | 39.5 | 0.6 | 3.3 | 10.3 | 33.1 |
| Time-4 (N = 53) | 41.5 | 2.0 | 4.1 | 16.7 | 32.7 |
| ESBL-producing Klebsiella spp. | |||||
| Time-1 (N = 487) | 16.6 | 1.9 | 6.4 | 5.9 | 12.3 |
| Time-2 (N = 357) | 19.1 | 2.2 | 10.7 | 5.9 | 10.4 |
| Time-3 (N = 162) | 34.6 | 7.1 | 19.7 | 9.7 | 19.4 |
| Time-4 (N = 53) | 45.3 | 1.0 | 22.5 | 19.4 | 19.2 |
| CR-AB | |||||
| Time-1 (N = 487) | 6.8 | 2.1 | 2.3 | 3.7 | 2.5 |
| Time-2 (N = 357) | 16.3 | 4.7 | 6.7 | 10.4 | 8.6 |
| Time-3 (N = 162) | 27.8 | 8.3 | 13.2 | 15.5 | 10.0 |
| Time-4 (N = 53) | 47.2 | 26.0 | 24.5 | 22.9 | 19.2 |
| CR-PA | |||||
| Time-1 (N = 487) | 4.7 | 1.6 | 3.7 | 0.4 | 1.2 |
| Time-2 (N = 357) | 8.4 | 2.2 | 5.4 | 1.8 | 2.1 |
| Time-3 (N = 162) | 12.3 | 5.8 | 7.2 | 3.2 | 3.8 |
| Time-4 (N = 53) | 18.9 | 10.0 | 12.2 | 2.1 | 1.9 |
| MRSA | |||||
| Time-1 (N = 487) | 7.2 | 4.3 | 3.5 | 1.2 | 2.5 |
| Time-2 (N = 357) | 7.3 | 3.1 | 2.2 | 1.1 | 2.2 |
| Time-3 (N = 162) | 11.7 | 4.9 | 2.6 | 3.7 | 1.2 |
| Time-4 (N = 53) | 18.9 | 7.5 | 5.7 | 3.7 | 1.8 |
Abbreviations: ESBL Extended-Spectrum Beta-Lactamase, CR-AB Carbapenem-Resistant Acinetobacter baumannii, CR-PA Carbapenem-Resistant Pseudomonas aeruginosa, MSSA Methicillin-Susceptible Staphylococcus aureus, MRSA Methicillin-Resistant Staphylococcus aureus
Risk factors for colonization by ESBL-producing Enterobacteriaceae in hospitalized patients
The risk factors for baseline colonization by ESBL-producing Enterobacteriaceae in 254 patients compared with 233 patients without ESBL-producing Enterobacteriaceae colonization are shown in Table 4. The independent risk factors from the primary multivariate analysis [Odds Ratio (OR); 95% CI; p-value] included underlying DM [1.45;1.00–2.10; p = 0.05] and previous exposure to cephalosporin [2.00;1.13–3.54; p = 0.02] as shown in Table 7. Secondary analysis identified similar risk factors with a similar OR as shown in the Table 7.
Table 4.
Baseline characteristics prior to hospitalization for 254 patients (ESBL-producing Enterobacteriaceae) and 233 controls (no ESBL-producing Enterobacteriaceae)
| Baseline characteristics | ESBL+ (N = 254) |
ESBL- (N = 233) |
p-value |
|---|---|---|---|
| Mean age ± SD (years) | 61.4 ± 18.2 | 61.9 ± 17.4 | 0.76 |
| Male gender | 120 (47.2%) | 101 (43.4%) | 0.39 |
| Previous hospitalization | 115 (45.3%) | 96 (41.2%) | 0.37 |
| Transfer status | |||
| From another hospital | 30 (11.8%) | 26 (11.2%) | 0.82 |
| From a long-term care facility | 4 (1.6%) | 2 (0.9%) | 0.47 |
| Underlying diseases | |||
| Any underlying disease | 225 (88.6%) | 195 (83.7%) | 0.12 |
| Hypertension | 115 (61.0%) | 135 (57.9%) | 0.49 |
| Diabetes mellitus | 106 (41.7%) | 78 (33.5%) | 0.06 |
| Cardiovascular disease | 74 (29.1%) | 59 (25.3%) | 0.35 |
| Cerebrovascular disease | 56 (22.0%) | 41 (17.6%) | 0.22 |
| Chronic liver disease | 43 (16.9%) | 45 (19.3%) | 0.50 |
| Chronic renal disease | 37 (14.6%) | 27 (11.6%) | 0.33 |
| Chronic lung disease | 32 (12.6%) | 41 (17.6%) | 0.82 |
| Malignancy | 46 (18.1%) | 41 (17.6%) | 0.88 |
| Solid malignancy | 42 (16.5%) | 33 (14.1%) | 0.47 |
| Hematologic malignancy | 4 (1.6%) | 8 (3.4%) | 0.19 |
| Hematologic diseases | 26 (10.2%) | 21 (9.0%) | 0.65 |
| Prior organ transplantation | 2 (0.8%) | 5 (2.2%) | 0.21 |
| Receipt of any immunosuppressive agent | 33 (13.0%) | 26 (11.2%) | 0.54 |
| HIV infection | 10 (3.9%) | 6 (2.6%) | 0.40 |
| Previous antibiotic exposure within 90 days after hospitalization | |||
| Any antibiotic | 84 (33.1%) | 64 (27.5%) | 0.18 |
| Penicillins | 12 (4.7%) | 7 (3.0%) | 0.33 |
| Cephalosporins | 40 (15.8%) | 20 (8.6%) | 0.02 |
| Carbapenems | 16 (6.3%) | 17 (7.3%) | 0.67 |
| Beta-lactam/beta-lactamase inhibitors | 10 (3.9%) | 17 (7.3%) | 0.11 |
| Fluoroquinolones | 26 (10.2%) | 20 (8.6%) | 0.53 |
| Macrolides | 11 (4.3%) | 8 (3.4%) | 0.61 |
| Others | 24 (9.5%) | 18 (7.7%) | 0.50 |
| Previous use of indwelling catheters | |||
| Urinary catheter | 40 (15.8%) | 33 (14.2%) | 0.63 |
| Nasogastric tube | 26 (10.2%) | 19 (8.2%) | 0.43 |
Table 7.
Independent risk factors for baseline colonization by ESBL-producing Enterobacteriaceae, CR-AB and/or CR-PA and MRSA from the primary and secondary analyses
| Variables | Adjusted OR [95% CI; p-value] | |
|---|---|---|
| Primary Analysis (Case vs non-case) | Secondary Analysis (Case vs No MDR) | |
| 1. ESBL-producing Enterobacteriaceae | Case (n = 254) vs. non-case (n = 233) | Case (n = 254) vs. no MDR (n = 197) |
| Underlying diabetes mellitus | 1.45 [1.00–2.10; p = 0.05] | 1.49 [1.01–2.20; p = 0.05] |
| Previous cephalosporin exposure | 2.00 [1.13–3.54; p = 0.02] | 2.06 [1.11–3.81; p = 0.02] |
| 2. CR-AB and/or CR-PA | Case (n = 49) vs. non-case (n = 438) | Case (n = 49) vs. no MDR (n = 197) |
| Previous hospitalization | 2.21 [1.07–4.53; p = 0.03] | 2.96 [1.40–6.26; p = 0.004] |
| Transfer from another hospital | 2.67 [1.19–5.98; p = 0.02] | … |
| Transfer from a LTCF | 11.51 [1.84–71.83; p = 0.01] | … |
| Underlying cerebrovascular disease | 2.90 [1.37–6.16; p = 0.005] | 2.68 [1.08–6.64; p = 0.03] |
| Previous nasogastric tube use | 2.38 [1.002–5.67; p = 0.05] | 4.13 [1.27–13.47; p = 0.02] |
| 3. MRSA | Case (n = 35) vs. non-case (n = 452) | Case (n = 35) vs. no MDR (n = 197) |
| Previous fluoroquinolone exposure | 2.76 [1.13–6.74; p = 0.03] | 3.85 [1.26–11.80; p = 0.02] |
| Previous use of nasogastric tube | 6.60 [1.13–6.74; p < 0.001] | 12.86 [4.47–36.97; p < 0.001] |
Risk factors for colonization by CR-PA and/or CR-AB in hospitalized patients
Due to the small number of cases with baseline colonization by CR-AB and CR-PA, we combined data on colonization by these two MDR bacteria. A total of 49 patients (10.1%) had at least one clinical specimen that grew CR-PA and/or CR-AB at the baseline. The risk factors for baseline colonization by CR-PA and/or CR-AB in 49 patients compared with 438 patients without this colonization are shown in Table 5. The independent risk factors identified in the primary analysis [OR; 95% CI; p-value] included previous hospitalization [2.21;1.07–4.53; p = 0.03], transfer from another hospital [2.67;1.19–5.98; p = 0.02] or a LTCF [11.51;1.84–71.83; p = 0.01], underlying cerebrovascular diseases [2.90;1.37–6.16; p = 0.005] and previous nasogastric tube use [2.38;1.002–5.67; p = 0.05]. Secondary analysis identified only three independent risk factors, specifically previous hospitalization, underlying cerebrovascular disease and previous nasogastric tube use, with slightly higher ORs. The results for both primary and secondary analyses are shown in Table 7.
Table 5.
Baseline characteristics prior to hospitalization for 49 patients (with CR-AB and/or CR-PA) and 438 controls (without CR-AB and CR-PA)
| Baseline characteristics | CR-AB and/or CR-PA (n = 49) |
No CR-AB and CR-PA (n = 438) | p-value |
|---|---|---|---|
| Mean age ± SD (years) | 66.7 ± 18 | 61.1 ± 17.8 | 0.04 |
| Male gender | 18 (36.7%) | 203 (46.4%) | 0.20 |
| Previous hospitalization | 32 (65.3%) | 179 (40.9%) | 0.001 |
| Transfer status | |||
| From other hospital | 13 (26.5%) | 43 (9.8%) | 0.001 |
| From a long-term care facility | 4 (8.2%) | 2 (0.5%) | < 0.001 |
| Underlying diseases | |||
| Any underlying disease | 48 (98.0%) | 372 (84.9%) | 0.01 |
| Hypertension | 33 (67.4%) | 257 (58.7%) | 0.24 |
| Diabetes mellitus | 20 (40.8%) | 164 (37.4%) | 0.64 |
| Cardiovascular disease | 16 (32.7%) | 117 (26.7%) | 0.38 |
| Cerebrovascular disease | 23 (46.9%) | 74 (16.9%) | < 0.001 |
| Chronic liver disease | 9 (18.4%) | 79 (18.0%) | 0.95 |
| Chronic renal disease | 8 (16.3%) | 56 (12.8%) | 0.49 |
| Chronic lung disease | 7 (14.3%) | 56 (12.8%) | 0.77 |
| Malignancy | 7 (14.3%) | 80 (18.3%) | 0.49 |
| Solid malignancy | 7 (14.3%) | 68 (15.5%) | 0.82 |
| Hematologic malignancy | 0 | 12 (2.7%) | 0.24 |
| Hematologic diseases | 6 (12.2%) | 41 (9.4%) | 0.52 |
| Prior organ transplantation | 1 (2.0%) | 6 (1.4%) | 0.71 |
| Receipt of any immunosuppressive agent | 6 (12.2%) | - 53 (12.1%) | 0.98 |
| HIV infection | 1 (2.0%) | 15 (3.4%) | 0.61 |
| Previous antibiotic exposure within 90 days after hospitalization | |||
| Any antibiotic | 26 (53.1%) | 122 (27.9%) | < 0.001 |
| Penicillins | 1 (2.0%) | 18 (4.1%) | 0.48 |
| Cephalosporins | 10 (20.4%) | 50 (11.4%) | 0.07 |
| Carbapenems | 9 (18.4%) | 24 (5.5%) | 0.001 |
| Beta-lactam/beta-lactamase inhibitors | 7 (14.3%) | 20 (4.6%) | 0.005 |
| Fluoroquinolones | 8 (16.3%) | 38 (8.7%) | 0.08 |
| Macrolides | 4 (8.2%) | 15 (3.4%) | 0.10 |
| Others | 8 (16.3%) | 34 (7.8%) | 0.04 |
| Previous use of indwelling catheters | |||
| Urinary catheter | 19 (38.8%) | 54 (12.3%) | < 0.001 |
| Nasogastric tube | 16 (32.7%) | 29 (6.6%) | < 0.001 |
Risk factors for colonization by MRSA in hospitalized patients
Of the 487 enrolled patients, 35 (7.2%) had at least one clinical specimen that grew MRSA at the baseline. Baseline characteristics for the 35 patients with MRSA colonization and 452 patients without MRSA colonization are shown in Table 6. Independent risk factors for baseline colonization by MRSA [OR; 95% CI; p-value] were previous fluoroquinolone exposure [2.76; 1.13–6.74; p = 0.03] and previous nasogastric tube use [6.60; 1.13–6.74; p < 0.001]. Stronger association between these two factors and baseline colonization by MRSA was documented in secondary analysis as shown in Table 7.
Table 6.
Baseline characteristics prior to hospitalization for 35 patients (with MRSA) and 452 controls (without MRSA)
| Baseline characteristics | MRSA (n = 35) | No MRSA (n = 452) | p-value |
|---|---|---|---|
| Mean age ± SD (years) | 66.7 ± 20.1 | 61.3 ± 17.6 | 0.09 |
| Male gender | 22 (62.9%) | 244 (54.0%) | 0.31 |
| Previous hospitalization | 23 (65.7%) | 188 (41.6%) | 0.006 |
| Transfer status | |||
| From other hospital | 5 (14.3%) | 52 (11.3%) | 0.59 |
| From a long-term care facility | 0 | 6 (1.3%) | 1.00 |
| Underlying diseases | |||
| Any underlying disease | 31 (88.6%) | 389 (86.1%) | 0.68 |
| Hypertension | 22 (62.9%) | 268 (59.3%) | 0.68 |
| Diabetes mellitus | 13 (37.1%) | 172 (38.1%) | 0.92 |
| Cardiovascular disease | 12 (34.3%) | 121 (26.8%) | 0.34 |
| Cerebrovascular disease | 15 (42.9%) | 82 (18.1%) | < 0.001 |
| Chronic liver disease | 5 (14.3%) | 83 (18.4%) | 0.55 |
| Chronic renal disease | 3 (8.6%) | 61 (13.4%) | 0.60 |
| Chronic lung disease | 5 (14.3%) | 58 (12.8%) | 0.79 |
| Malignancy | 6 (17.1%) | 81 (17.9%) | 0.91 |
| Solid malignancy | 5 (14.3%) | 70 (15.5%) | 0.85 |
| Hematologic malignancy | 1 (2.9%) | 11 (2.4%) | 0.60 |
| Hematologic diseases | 3 (8.6%) | 44 (9.7%) | 1.00 |
| Prior organ transplantation | 0 | 7 (1.6%) | 1.00 |
| Receipt of any immunosuppressive agent | 4 (11.4%) | 55 (12.2%) | 1.00 |
| HIV infection | 1 (2.9%) | 15 (3.3%) | 1.00 |
| Previous antibiotic exposure within 90 days after hospitalization | |||
| Any antibiotic | 19 (54.3%) | 129 (28.5%) | 0.001 |
| Penicillins | 2 (5.7%) | 17 (3.8%) | 0.64 |
| Cephalosporins | 6 (17.1%) | 54 (12.0%) | 0.37 |
| Carbapenems | 6 (17.1%) | 27 (6.0%) | 0.01 |
| Beta-lactam/beta-lactamase inhibitor | 5 (14.3%) | 22 (4.9%) | 0.02 |
| Fluoroquinolones | 9 (25.7%) | 37 (8.2%) | 0.001 |
| Macrolides | 2 (5.7%) | 17 (3.8%) | 0.64 |
| Others | 5 (14.3%) | 37 (8.2%) | 0.22 |
| Previous use of indwelling catheters | |||
| Urinary catheter | 13 (37.1%) | 32 (7.1%) | < 0.001 |
| Nasogastric tube | 13 (37.1%) | 60 (13.3%) | < 0.001 |
Discussion
The present study revealed a remarkably high prevalence of baseline colonization by ESBL-producing Enterobacteriaceae compared with the prevalence from the Israel study (52.2% vs 8%) [9]. However, our baseline prevalence for faecal colonization by ESBL-producing Enterobacteriaceae (47.6%) was comparable with the prevalence of ESBL colonization among Thai community volunteers (32.0–66.5%) [10, 11].
Two important characteristics, namely DM and previous cephalosporin use, were identified as the independent risk factors for baseline colonization by ESBL-producing Enterobacteriaceae in this study. These findings were previously documented in many studies [9, 10]. Underlying DM may be a proxy for recurrent infections, previous antibiotic use and previous hospitalization [10, 18, 19]. Previous exposure to cephalosporin would result in selective pressure against non-ESBL-producing pathogens to become resistant to cephalosporin, leading to colonization in the patients [20].
This study revealed the comparable prevalence of CR-PA colonization (4.7%) compared with the results from the Spanish ICU study (4.0%) [13]. Additionally, the prevalence of CR-AB colonization (12.9%) was similar to the findings from previous studies performed in ICU patients (13.5–15.0%) [14, 15]. Although our study included only hospitalized patients in general medical wards, these patients were sicker than those hospitalized in a general medical ward in developed countries due to resource limitations. These statements could be confirmed due to a very high proportion of patients with co-morbidities (> 80%). Furthermore, approximately 40% of our enrolled patients had been previously hospitalized and more than 30% had a previous history of antibiotic exposure. These factors may explain the comparative prevalence of CR-PA and CR-AB colonization.
The independent risk factors for CR-AB and/or CR-PA colonization identified in this study were underlying cerebrovascular disease (CVA), previous hospitalization, transfer from another hospital or a LTCF and previous nasogastric tube use. Previous hospitalization and transfer from another hospital or a LTCF are well known risk factors for colonization by MDR bacteria. Neurologic disease was previously documented as an independent risk factor for PA colonization [12]. Furthermore, underlying CVA may be a proxy of aspiration pneumonia, previous nasogastric tube use, functional disability and previous hospitalization [21].
Our results for the baseline prevalence of MRSA colonization (7.2%) were comparable with results from previous studies [9, 16]. Significant risk factors for MRSA colonization identified in our study included previous fluoroquinolone exposure and previous nasogastric tube use. Previous fluoroquinolone exposure is well documented as an independent risk factor for MRSA colonization in many observational studies [22, 23]. Recent use of nasogastric tube was previously identified to be a significant risk factor for MRSA nasal colonization in end-stage renal disease patients [24].
The present study had several strengths. It was specifically designed to determine the prevalence of MDR bacteria colonization at various sites (nasal cavity, throat, skin at the inguinal area and rectum) and by a variety of important MDR bacteria (ESBL-producing Enterobacteriaceae, CR-PA, CR-AB and MRSA). Additionally, clinical specimens were collected at various time points to capture additional acquisition rates of colonization by MDR bacteria after hospitalization. Furthermore, we thoroughly collected all clinical characteristics that may be associated with baseline colonization by MDR bacteria.
The present study had some limitations. First, there was a small number of follow-up cultures, with only 53 specimens collected at time-4. Given that sicker patients are more likely to have a longer LOS with more collected clinical specimens, the prevalence of colonization after hospitalization may not represent the true prevalence. Second, the study results may be applicable to only tertiary care university hospitals. As we mentioned before, patients in our study were relatively sicker than those hospitalized at a general medical ward in developed countries.
Conclusion
The prevalence of baseline colonization by ESBL-producing Enterobacteriaceae was relatively high, whereas the prevalence of baseline colonization by CR-PA, CR-AB and MRSA was comparable with the results from previous studies in other geographical locations. There was a slightly increasing trend of MDR bacteria colonization by all important pathogens after hospitalization. However, these observations did not reach statistical significance. Previous antibiotic use and previous nasogastric tube use were the common risk factors for various species of MDR pathogens. The documented risk factors from our study may be used to identify patients who are at a risk for MDR bacterial infection. A study with a larger sample size would be needed to identify the risk factors for acquiring new MDR colonization after hospitalization. Measures to prevent or delay colonization by MDR bacteria in hospitalized patients should be employed.
Acknowledgements
The authors thank all nurses in eight general medical wards at Siriraj Hospital for their assistance.
Funding
This study was primarily supported by Faculty of Medicine Siriraj Hospital and Health Systems Research Institute (Thailand).
Availability of data and materials
Data is available upon request.
Abbreviations
- AMR
Antimicrobial resistance
- CR-AB
Carbapenem-resistant-A. baumannii
- CR-PA
Carbapenem-resistant-P. aeruginosa
- CVA
Cerebrovascular disease
- DM
Diabetes mellitus
- ESBL
Extended-spectrum beta-lactamase-producing
- HIV
Human immunodeficiency virus
- ICU
Intensive care unit
- LTCF
Long-term care facility
- MDR
Multidrug-resistant
- SD
Standard deviation
Authors’ contributions
PR was responsible for study design, data analysis, data interpretation and writing manuscript. CC and KT were responsible for data collection. TT and CS were responsible for performing all laboratory tests. VT was responsible for study design, data interpretation and writing manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study received ethical approval from the Institutional Review Board of Faculty of Medicine Siriraj Hospital, Mahidol University. Only subjects who agreed to participate and signed informed consent forms were enrolled.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pinyo Rattanaumpawan, Email: pinyo.rat@mahidol.ac.th.
Chatiros Choorat, Email: jaytiros@hotmail.com.
Kanchanaporn Takonkitsakul, Email: a_zoo@hotmail.com.
Teerawit Tangkoskul, Email: teerawit.tan@mahidol.ac.th.
Chakrapong Seenama, Email: chakrapong.see@mahidol.ac.th.
Visanu Thamlikitkul, Phone: 662 412 5994, Email: visanu.tha@mahidol.ac.th.
References
- 1.Sheng WH, Chie WC, Chen YC, Hung CC, Wang JT, Chang SC, et al. Impact of nosocomial infections on medical costs, hospital stay, and outcome in hospitalized patients. J Formos Med Assoc. 2005;104:318–326. [PubMed] [Google Scholar]
- 2.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:913–920. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 3.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 4.Hallgren A, Burman LG, Isaksson B, Olsson-Liljeqvist B, Nilsson LE, Saeedi B, et al. Rectal colonization and frequency of enterococcal cross-transmission among prolonged-stay patients in two Swedish intensive care units. Scand J Infect Dis. 2005;37:561–571. doi: 10.1080/00365540510038947. [DOI] [PubMed] [Google Scholar]
- 5.Popoola VO, Carroll KC, Ross T, Reich NG, Perl TM, Milstone AM. Impact of colonization pressure and strain type on methicillin-resistant Staphylococcus aureus transmission in children. Clin Infect Dis. 2013;57:1458–1460. doi: 10.1093/cid/cit542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortensen E, Trivedi KK, Rosenberg J, Cody SH, Long J, Jensen BJ, et al. Multidrug-resistant Acinetobacter baumannii infection, colonization, and transmission related to a long-term care facility providing subacute care. Infect Control Hosp Epidemiol. 2014;35:406–411. doi: 10.1086/675612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy P, Malczynski M, Obias A, Reiner S, Jin N, Huang J, et al. Screening for extended-spectrum beta-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bacteremia. Clin Infect Dis. 2007;45:846–852. doi: 10.1086/521260. [DOI] [PubMed] [Google Scholar]
- 8.Leung E, Weil DE, Raviglione M, Nakatani H. World Health Organization world health day antimicrobial resistance technical working G the WHO policy package to combat antimicrobial resistance. Bull World Health Organ. 2011;89:390–392. doi: 10.2471/BLT.11.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedmann R, Raveh D, Zartzer E, Rudensky B, Broide E, Attias D, et al. Prospective evaluation of colonization with extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae among patients at hospital admission and of subsequent colonization with ESBL-producing Enterobacteriaceae among patients during hospitalization. Infect Control Hosp Epidemiol. 2009;30:534–42. [DOI] [PubMed]
- 10.Luvsansharav UO, Hirai I, Nakata A, Imura K, Yamauchi K, Niki M, et al. Prevalence of and risk factors associated with faecal carriage of CTX-M beta-lactamase-producing Enterobacteriaceae in rural Thai communities. J Antimicrob Chemother. 2012;67:1769–1774. doi: 10.1093/jac/dks118. [DOI] [PubMed] [Google Scholar]
- 11.Khamsarn S, Nampoonsak Y, Busamaro S, Tangkoskul T, Seenama C, Rattanaumpawan P, et al. Epidemiology of antibiotic use and antimicrobial resistance in selected communities in Thailand. J Med Assoc Thail. 2016;99:270–275. [PubMed] [Google Scholar]
- 12.Harris AD, Jackson SS, Robinson G, Pineles L, Leekha S, Thom KA, et al. Pseudomonas aeruginosa colonization in the intensive care unit: prevalence, risk factors, and clinical outcomes. Infect Control Hosp Epidemiol. 2016;37:544–548. doi: 10.1017/ice.2015.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Zorrilla S, Camoez M, Tubau F, Periche E, Canizares R, Dominguez MA, et al. Antibiotic pressure is a major risk factor for rectal colonization by multidrug-resistant Pseudomonas aeruginosa in critically ill patients. Antimicrob Agents Chemother. 2014;58:5863–5870. doi: 10.1128/AAC.03419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An JH, Kim YH, Moon JE, Jeong JH, Kim SH, Kang SJ, et al. Active surveillance for carbapenem-resistant Acinetobacter baumannii in a medical intensive care unit: can it predict and reduce subsequent infections and the use of colistin? Am J Infect Control. 2017; 10.1016/j.ajic.2017.01.016. [DOI] [PubMed]
- 15.Latibeaudiere R, Rosa R, Laowansiri P, Arheart K, Namias N, Munoz-Price LS. Surveillance cultures growing carbapenem-resistant Acinetobacter baumannii predict the development of clinical infections: a retrospective cohort study. Clin Infect Dis. 2015;60:415–422. doi: 10.1093/cid/ciu847. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis WR, Jarvis AA, Chinn RY. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am J Infect Control. 2012;40:194–200. doi: 10.1016/j.ajic.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing; twentieth informational supplement: M100-S20. Wayne: CLSI; 2013. [Google Scholar]
- 18.Pasricha J, Koessler T, Harbarth S, Schrenzel J, Camus V, Cohen G, et al. Carriage of extended-spectrum beta-lactamase-producing enterobacteriacae among internal medicine patients in Switzerland. Antimicrob Resist Infect Control. 2013;2:20. doi: 10.1186/2047-2994-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oguz Mizrakci S, Arda B, Erdem HA, Uyar M, Tunger A, Sipahi OR, et al. Risk factors for gastrointestinal colonization by ESBL-producing Klebsiella pneumoniae and Escherichia coli in anaesthesiology and reanimation intensive care unit. Mikrobiyol Bul. 2013;47:223–229. doi: 10.5578/mb.4126. [DOI] [PubMed] [Google Scholar]
- 20.Dancer SJ. The problem with cephalosporins. J Antimicrob Chemother. 2001;48:463–478. doi: 10.1093/jac/48.4.463. [DOI] [PubMed] [Google Scholar]
- 21.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 22.Couderc C, Jolivet S, Thiebaut AC, Ligier C, Remy L, Alvarez AS, et al. Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case-control study. Clin Infect Dis. 2014;59:206–215. doi: 10.1093/cid/ciu236. [DOI] [PubMed] [Google Scholar]
- 23.Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother. 2002;49:999–1005. doi: 10.1093/jac/dkf009. [DOI] [PubMed] [Google Scholar]
- 24.Wang CY, Wu VC, Wang WJ, Lin YF, Lin YH, Chen YM, et al. Risk factors for nasal carriage of methicillin-resistant Staphylococcus aureus among patients with end-stage renal disease in Taiwan. J Formos Med Assoc. 2012;111:14–18. doi: 10.1016/j.jfma.2012.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.