Extending descriptive studies of animal-associated microorganisms (microbiota) to define causal mechanistic bases for their influence on animal traits is an emerging imperative. In this study, we reveal that D. melanogaster starvation resistance (SR), a model quantitative trait in animal genetics, responds to the presence and identity of the microbiota. Using a predictive analysis, we reveal that the amino acid methionine has a key influence on D. melanogaster SR and show that bacterial methionine metabolism mutants alter normal patterns of SR in flies bearing the bacteria. Our data further suggest that these effects are additive, and we propose the untested hypothesis that, similar to bacterial effects on fruit fly triacylglyceride deposition, the bacterial influence may be through dietary modification. Together, these findings expand our understanding of the bacterial genetic basis for influence on a nutritionally relevant trait of a model animal host.
KEYWORDS: Acetobacter, Lactobacillus, Drosophila melanogaster, microbiota, metagenome-wide association, MGWA, starvation resistance, symbiosis
ABSTRACT
Animal-associated microorganisms (microbiota) dramatically influence the nutritional and physiological traits of their hosts. To expand our understanding of such influences, we predicted bacterial genes that influence a quantitative animal trait by a comparative genomic approach, and we extended these predictions via mutant analysis. We focused on Drosophila melanogaster starvation resistance (SR). We first confirmed that D. melanogaster SR responds to the microbiota by demonstrating that bacterium-free flies have greater SR than flies bearing a standard 5-species microbial community, and we extended this analysis by revealing the species-specific influences of 38 genome-sequenced bacterial species on D. melanogaster SR. A subsequent metagenome-wide association analysis predicted bacterial genes with potential influence on D. melanogaster SR, among which were significant enrichments in bacterial genes for the metabolism of sulfur-containing amino acids and B vitamins. Dietary supplementation experiments established that the addition of methionine, but not B vitamins, to the diets significantly lowered D. melanogaster SR in a way that was additive, but not interactive, with the microbiota. A direct role for bacterial methionine metabolism genes in D. melanogaster SR was subsequently confirmed by analysis of flies that were reared individually with distinct methionine cycle Escherichia coli mutants. The correlated responses of D. melanogaster SR to bacterial methionine metabolism mutants and dietary modification are consistent with the established finding that bacteria can influence fly phenotypes through dietary modification, although we do not provide explicit evidence of this conclusion. Taken together, this work reveals that D. melanogaster SR is a microbiota-responsive trait, and specific bacterial genes underlie these influences.
IMPORTANCE Extending descriptive studies of animal-associated microorganisms (microbiota) to define causal mechanistic bases for their influence on animal traits is an emerging imperative. In this study, we reveal that D. melanogaster starvation resistance (SR), a model quantitative trait in animal genetics, responds to the presence and identity of the microbiota. Using a predictive analysis, we reveal that the amino acid methionine has a key influence on D. melanogaster SR and show that bacterial methionine metabolism mutants alter normal patterns of SR in flies bearing the bacteria. Our data further suggest that these effects are additive, and we propose the untested hypothesis that, similar to bacterial effects on fruit fly triacylglyceride deposition, the bacterial influence may be through dietary modification. Together, these findings expand our understanding of the bacterial genetic basis for influence on a nutritionally relevant trait of a model animal host.
INTRODUCTION
The study of resident microorganisms (microbiota), including the ability of these microorganisms to modulate organismal phenotypes, is a rapidly developing field in animal biology (1–6). For example, the microbiota influence on nutrient metabolism suggests a multiorganismal basis for these varied effects (7–13). An ongoing concern in such studies is extending our understanding from description to mechanism. Here, we use statistical genomic modeling to predict host-influencing bacterial genetic factors, and we verify some of these predictions by mutant analysis. We focus on the association between Drosophila melanogaster and its microbiota using D. melanogaster starvation resistance (SR) as a representative quantitative trait.
D. melanogaster SR is a model quantitative trait. SR is rapidly scored and can be applied to large population sizes (14–16). Insights from high-throughput (e.g., genome-wide association studies [GWAS]) and more mechanistic studies have identified numerous host genes that contribute to D. melanogaster SR. These primarily include genes in central and fatty acid metabolism and have been identified by classic genetic approaches, GWAS, and experimental evolution (15–21). For example, the gluconeogenesis enzyme pepck gene is upregulated during D. melanogaster starvation (19). Because it is closely linked to host energy storage and metabolism, SR can also be used as an indicator or predictor of other host phenotypes. Variation in life history traits, such as development time, body weight and fat content, and life span (17, 22–25), are correlated with SR. SR has also been linked to other traits, including locomotion (26), heat shock response (27), cardiac function (28), and sleep patterns (29). The relative ease of using SR as a model phenotype has therefore enabled the dissection of its own genetic mechanisms and provided insight into mechanisms underlying other animal traits.
In recent years, the D. melanogaster microbiota has emerged as a model for host-microbiota interactions. The Drosophila microbiota is of low diversity, usually dominated by readily cultured Lactobacillales, Acetobacteraceae, and Gammaproteobacteria in either wild or laboratory-reared Drosophila species. As in mammals, there is no “core” microbiota; instead, the microbiota is inconstant with high interindividual variability in terms of bacterial identity and localization (30–34). The D. melanogaster microbiota also requires frequent dietary replenishment to maintain the normally observed bacterial loads, although there is also evidence that some bacteria persist even after the bulk flow of food has passed through the gut (35, 36). Drosophila-associated bacterial communities are readily eliminated by bleach treatment (2, 37), and bacterium-free flies display developmental and health traits similar to those of conventionally reared flies on nutrient-rich diets (38). The influence of bacterial communities or individual bacteria on different traits has shown that, among other traits, bacterium-associated flies have lower fat contents and shorter development times (3, 5, 39–41). Because both of these traits are positively correlated with SR, we speculated the microbiota might also decrease fruit fly SR. The ready manipulation of the Drosophila microbiota enabled this work.
Here, we investigated the influence of the microbiota on D. melanogaster SR. Using a metagenome-wide association (MGWA) approach, we predicted that microbial B vitamin and methionine metabolism genes influence SR. Employing dietary supplementation and mutant analysis experiments, we confirmed a role for dietary methionine and bacterial methionine metabolism in D. melanogaster SR, including a noninteractive effect between the two. Together, these findings confirm that bacterial methionine metabolism genes can influence the SR of D. melanogaster reared on a nutrient-rich diet.
RESULTS
Associated bacteria influence D. melanogaster SR.
To discern if associated bacteria influence D. melanogaster SR, we compared the period of survival under starvation conditions between bacterium-associated and bacterium-free Canton-S flies. Consistent with the known influence of the microbiota to reduce the triacylglyceride content of flies, D. melanogaster flies reared in the presence of a defined 5-species microbial community lived nearly 2 fewer days than flies reared free of associated microbes (Fig. 1; see also Table S1 in the supplemental material). This work demonstrates that associated microbes can contribute to the SR of laboratory-reared D. melanogaster flies and raises questions if these effects are shared by all or a subset of the associated microbes, what the genetic mechanisms for these effects are, and how they may be related to known mechanisms for lipid storage in the flies.
FIG 1.
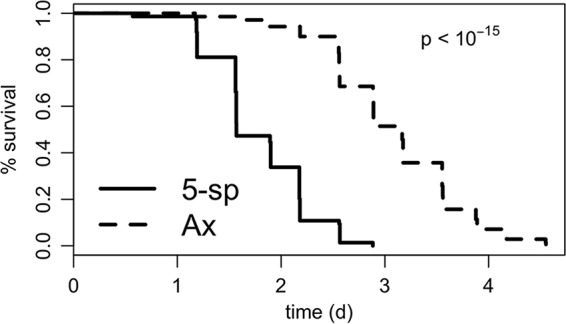
The microbiota influences D. melanogaster SR. To test if the presence of associated microbes significantly influenced SR in D. melanogaster flies, Canton-S flies were reared bacterium-free (Ax) or with a defined 5-species microbiota (5-sp). SR was measured as the number of surviving flies from vials of 10 flies each in 8-h intervals. The difference between treatments was tested using a Cox mixed-effects survival model (96, 97). n = 9 vials of 10 flies per treatment (triplicate vials in each of 3 separate experiments).
There are species-specific bacterial effects on D. melanogaster SR.
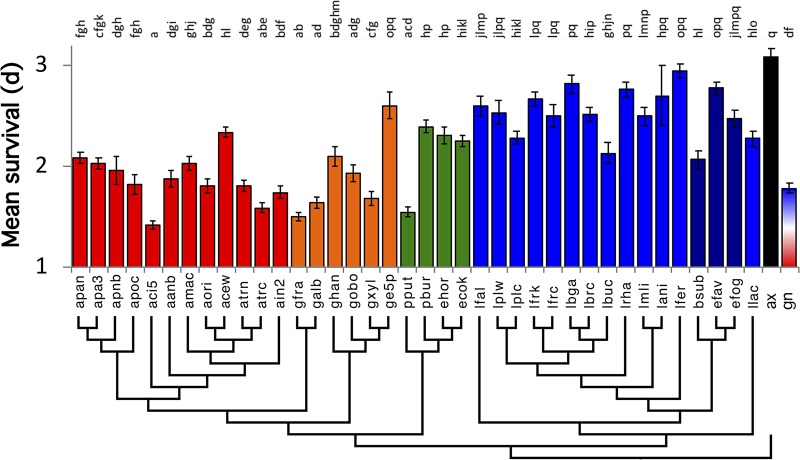
Because the bacteria influenced D. melanogaster SR, we reasoned that individual microbes might confer species-specific effects similar to the effects detected in previous studies of other microbiota-responsive Drosophila traits (3, 5, 39, 40). We measured SR in a single Canton-S line that was monoassociated with 38 different bacterial species, with one species colonizing each Canton-S test group (Fig. 2 and Table S1). In general, flies that bore lactic acid bacteria survived longer under starvation conditions than did flies that were monoassociated with acetic acid bacteria or gammaproteobacteria. There were congeneric exceptions to these trends, including Acetobacter cerevisiae, which conferred greater SR than some Acetobacteraceae, and Bacillus subtilis and Lactobacillus buchneri, which each shortened SR to a greater extent than other Firmicutes. Gluconobacter species tended to confer the lowest average SR. No bacteria extended SR above the level observed in axenic flies. While we cannot rule out that untested strains could further enhance D. melanogaster SR, these findings suggest that, on a nutrient-rich diet, individual members of the common Drosophila microbiota are generally antagonistic or neutral to but do not promote D. melanogaster SR above levels observed in bacterium-free flies.
FIG 2.
Species-specific bacterial influence on D. melanogaster SR. To determine the influence of different bacterial strains on D. melanogaster SR, Canton-S fly SR was measured when this fly line was monoassociated with 38 different strains of gammaproteobacteria, alphaproteobacteria, or Firmicutes (4-letter codes from Table 2). Different letters over the bars represent statistically significant differences between treatments, as determined by a Cox mixed-effects survival model (for each treatment, n = triplicate vials of 10 flies in each of three separate experiments, unless a vial was discarded with contamination). Results are color-coded by taxonomic groups: red, Acetobacter; orange, non-Acetobacter acetic acid bacteria; green, gammaproteobacteria; blue, lactic acid bacteria; purple, non-lactic acid bacteria Firmicutes; black, axenic; red-blue, 5-species gnotobiotic (containing both Acetobacter and Lactobacillus isolates). The phylogeny was constructed from 16S sequences that were extracted from publicly available whole-genome sequences of these strains.
Bacterial B vitamins and amino acids are predicted to decrease D. melanogaster SR.
To predict bacterial genes that contribute to the varied influences of the different species on D. melanogaster SR, we performed MGWA. We clustered amino acid sequences derived from whole-genome sequences available for each of the species we tested, identifying 14,225 orthologous groups (OGs) that contained more than 1 amino acid sequence present in 5,855 phylogenetic distribution groups (PDGs; Table S2). A phylogenetic distribution group is defined as a unique set of taxa in which an OG is present. A total of 4,822 (82%) of the OGs contained amino acid sequences that were present in just one PDG (median, 1 OG · PDG−1; mean ± standard error of the mean [SEM], 2.4 ± 0.15 OG · PDG−1), suggesting that genotype-phenotype associations could be readily attributed to one or a few genes in each PDG (Fig. S1). We associated each gene presence-absence pattern with D. melanogaster SR for 4,297 PDGs and identified 82 PDGs, collectively bearing 432 OGs (median, 1 OG · PDG−1; mean ± SEM, 5.3 ± 2.2 OG · PDG−1), below a nominal Bonferroni-corrected threshold of a P value of <1 × 10−4 (Table S3).
To focus on a subset of these top hits, we identified pathways with multiple genes in the top predicted MGWA hits for functional analysis. A KEGG enrichment analysis of 108 KEGG pathways containing at least one OG from the top hits list revealed that 7 KEGG pathways bore significantly more OGs than expected by chance, including genes in vitamin B7 and vitamin B12, methionine, and glutathione metabolism (Table 1). No pathways bore fewer genes than expected by chance. Additionally, many genes involved in B vitamin metabolism were among the most significant hits, including vitamins B3, B5, B6, and B9 (Table S4). Together, these results predict that bacterial vitamin B and methionine metabolism, of which methionine metabolism is linked to glutathione metabolism through the transsulfuration pathway, involves vitamin B12 as a cofactor and is related to vitamin B6 metabolism, may influence D. melanogaster SR. To test this prediction, we adopted a 2-fold approach. First, we identified the nutrients with the most dramatic influence on D. melanogaster by a dietary supplementation screen. Then, by bacterial mutant analysis, we tested the prediction that bacterial genes involved in the metabolism of the most impactful metabolites would also influence D. melanogaster SR.
TABLE 1.
KEGG enrichment analysis of significant MGWA predictionsa
| KEGG pathway IDb | KEGG pathway name | No. of top genesc | Total no. of genesd | P value |
|---|---|---|---|---|
| ko00860 | Porphyrin and chlorophyll metabolism | 14 | 42 | 0.002 |
| ko00900 | Terpenoid backbone biosynthesis | 8 | 17 | 0.004 |
| ko00780 | Biotin metabolism | 6 | 10 | 0.004 |
| ko02020 | Two-component system | 7 | 157 | 0.01 |
| ko01120 | Microbial metabolism in diverse environments | 20 | 291 | 0.02 |
| ko00270 | Cysteine and methionine metabolism | 11 | 43 | 0.03 |
| ko00480 | Glutathione metabolism | 5 | 13 | 0.03 |
Using a survival model that accounted for experimental block and the monoassociated bacterial strain as random effects, we detected the association between gene presence or absence for 13,343 clusters of orthologous groups (OGs) and D. melanogaster SR. KEGG enrichment analysis of OGs identified bacterial functions that were enriched in the MGWA predictions, resulting in the categories listed above. KEGG identification number and pathway type are identified on the left, with significant P values on the right. The genes listed in each category were among the top-ranked MGWA predictions; methionine metabolism is among these top hits.
ID, identification.
Number of KEGG pathway genes in the 432 most significant OGs.
Number of KEGG pathway genes in all 13,343 clustered OGs.
Dietary methionine decreases D. melanogaster SR.
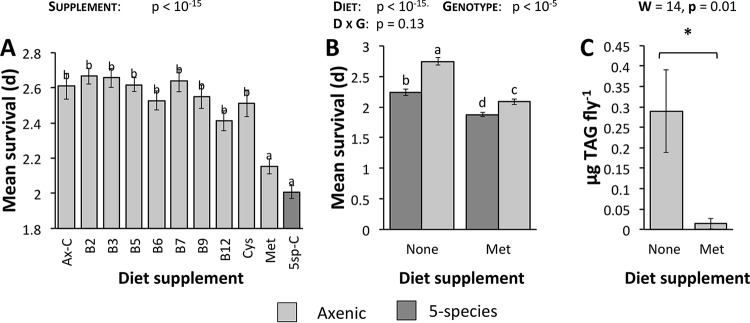
As a test of the prediction that MGWA-predicted B vitamins or amino acids influence D. melanogaster SR, we compared SR in bacterium-free flies reared on supplemented diets. We assayed bacterium-free flies so that any differences in SR could be attributed exclusively to the supplement and not to interactions with or an influence on the microbiota (e.g., nutrients promoting bacterial growth or bacteria catabolizing the nutrients). Nutrients were supplemented at molarities similar to those used on previous D. melanogaster diet studies (38, 42, 100). Similar to our inoculations with the 38-strain panel of bacteria, no supplements extended fly SR above the levels observed in bacterium-free flies, but diets supplemented with methionine substantially shortened fruit fly SR (Fig. 3A and S2 and Table S5). Initially, these findings suggested a role for methionine, but not other nutrients, in D. melanogaster SR. In a subsequent experiment, we confirmed the specificity of the methionine effect and ruled out that the effect was only due to its higher level of supplementation. When we inoculated the diets with 10 mM vitamin B6, vitamin B12, or cysteine (the three nutrients that conferred the next-lowest SR), the B vitamins did not confer comparable SR to methionine, but cysteine did (Fig. S3 and Table S6). The effects of methionine and cysteine were not specific to amino acids since supplementation with glycine, an amino acid with an auxiliary relationship to the methionine cycle, conferred SR more similar to SR with supplementation of vitamins B6 and B12 than to SR with supplementation of methionine (Fig. S3). Thus, while we cannot rule out that the B vitamins would influence SR at other concentrations, these experiments emphasize that increased dietary methionine has a specific effect to shorten D. melanogaster SR.
FIG 3.
Dietary methionine influences D. melanogaster SR and triglyceride content. (A) To test MGWA predictions that B vitamins and sulfur amino acids influence fruit fly SR, SR was measured in bacterium-free Canton-S flies reared on a YG diet supplemented with B vitamins, cysteine (Cys), or methionine (Met). As controls, bacterium-free flies (Ax-C) and 5-species gnotobiotic flies (5sp-C) were reared on an unsupplemented YG diet. (B) To test for interactive effects between dietary methionine supplementation and the microbiota, SR was measured in flies reared in a factorial design to compare bacterial treatment and methionine supplementation (none = no supplement; Met = 10 mM supplemented methionine). D, diet; G, genotype. (C) Triacylglyceride (TAG) contents of axenic flies reared on methionine-supplemented versus normal YG diets. Light-gray bars, axenic flies; dark-gray bars, 5-species gnotobiotic flies. Different letters over the bars represent statistically significant differences between treatments, as determined by a Cox mixed-effects survival model (A and B; for each treatment, n = triplicate vials of 10 flies in each of three separate experiments, unless a vial was discarded with contamination) or a Wilcoxon test (C, n = 4 to 5 replicates in each of 2 experiments per treatment). W, Wilcoxon test statistic.
Bacterial influence on D. melanogaster SR is additive, but not interactive, with dietary methionine supplementation.
The dietary supplementation experiments confirmed that increasing the methionine content of the fly diet shortens D. melanogaster SR. Because the MGWA predicted that bacterial methionine metabolism influences D. melanogaster SR, we sought to determine if the methionine effect on SR was dependent upon the presence of associated microorganisms. In a factorial design, we measured SR in axenic and gnotobiotic flies that were reared on a normal or methionine-supplemented diet. Both bacterial presence and diet supplementation influenced fly SR, but the interaction term was not significant, suggesting additive but not interactive effects of the microbiota and diet (Fig. 3B and S4 and Table S7). Further, similar to the established effect of bacteria to lower the fat content of flies (5, 9, 39), adding methionine to the fly diet substantially lowered D. melanogaster fat content (Fig. 3C and Table S8). Thus, bacteria do not potentiate or buffer the SR-shortening effect of methionine on D. melanogaster, which is correlated with diet-dependent changes in fly fat storage.
Bacterial methionine metabolism genes decrease D. melanogaster SR.
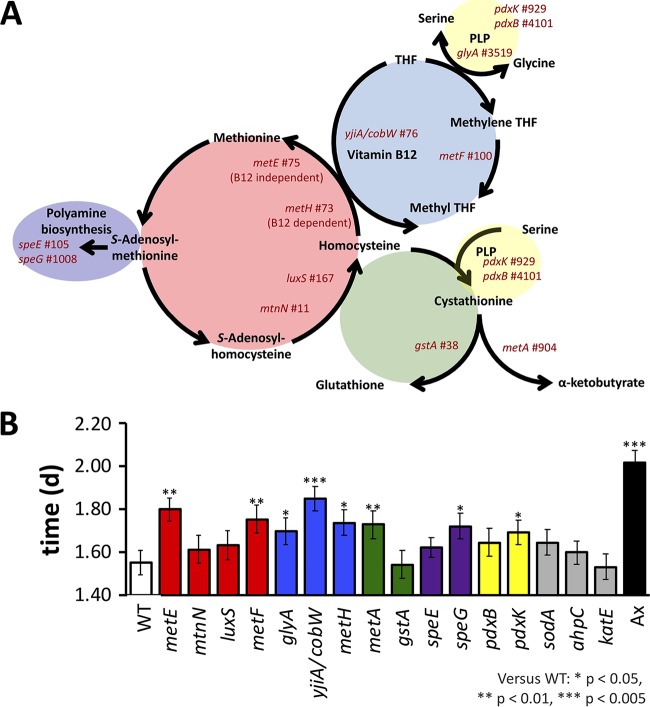
To test if bacterial methionine metabolism influences D. melanogaster SR, we individually associated a Canton-S fly line with bacterial mutants bearing transposon insertion mutations in methionine cycle genes. Because genes in cysteine and methionine metabolism were enriched in our top MGWA hits and because methionine metabolism influences SR, we selected genes based on their relationship to methionine metabolism, including genes in the transsulfuration pathway, one-carbon metabolism, or polyamine biosynthesis (Fig. 4A). All mutants were obtained from the KEIO collection, a library of kanamycin-marked Escherichia coli transposon insertion mutants (43, 44), and were individually associated with D. melanogaster Canton-S. Each of the mutants associated with the flies, but bacterial load was not included as a covariate in the analysis since it did not vary in the mutants or correlate with the starvation influence of the different bacteria (Fig. S5 and Table S9). The SR of the flies associated with the different E. coli mutants was compared to that of flies bearing wild-type E. coli strains. Consistent with the idea that bacterial methionine metabolism influences D. melanogaster SR, several of the mutants bearing lesions in different methionine- or related-pathway genes led to longer SR in the flies (Fig. 4B and S6 and Table S10). Conversely, mutations in three genes that were not involved in methionine metabolism or predicted as top hits by our MGWA analysis did not alter the SR of the flies relative to the wild-type E. coli strain. Taken together, these results confirm a role for some, but not all, bacterial methionine metabolism genes in D. melanogaster SR.
FIG 4.
Methionine cycle mutants decrease SR. SR of D. melanogaster that was monoassociated with E. coli methionine cycle mutants was compared to SR of D. melanogaster bearing a wild-type (WT) E. coli strain (4-letter codes from Table 2). (A) A simplified overview of the bacterial methionine biosynthesis pathway and related contributing pathways. Each mutant tested is listed near its pathway location, and mutants in the same subcycle are arranged by color. Gene name and MGWA PDG ranking (number to the right) are shown. The schematic is based on the work of Selhub (101). (B) Bacterial mutations that significantly influenced SR relative to the background E. coli control (WT) are indicated by asterisks. Ax, axenic. *, P < 0.05; **, P < 0.005; ***, P < 0.0005 (calculated by a Cox mixed-effects survival model). THF, tetrahydrofolate; PLP, pyridoxal 5′-phosphate.
DISCUSSION
The results of our study indicate a correlation between the presence of Drosophila-associated bacteria and host SR. By investigating the genetic influences of Drosophila-associated bacteria using MGWA, we found that the variability of SR is related to the identity of associated bacterial species. Our computational analysis predicted that bacterial B vitamin and amino acid biosynthesis genes influence SR, especially genes involved in methionine metabolism. Through dietary supplementation experiments, we determined that methionine decreases Drosophila SR. A nonsignificant interaction between methionine supplementation in the diet and microbiota composition suggested that the effect of dietary methionine supplementation was not suppressed or potentiated by associated microbes. Additionally, a role for bacterial methionine metabolism was implicated rearing the flies with bacteria bearing lesions in methionine cycle genes. Together, these findings reveal that the microbial influence on D. melanogaster SR can be attributed, at least in part, to the activities of methionine metabolism genes in the associated microorganisms.
Our analysis demonstrates that the gut microbiota has strain-specific influence on D. melanogaster SR. For example, in our study, flies that were monoassociated with Lactobacillus species tended to be more starvation resistant than flies bearing individual Acetobacter strains, but several strains, such as Acetobacter cerevisiae and Gluconacetobacter europaeus, conferred SR on flies at levels that were more consistent with the levels observed in Lactobacillus-associated flies. We previously observed similar trends in bacterial influence on D. melanogaster fat content (3), and strain-specific effects have been observed in other studies of monoassociated flies (5, 39–41) and mice (45–47), as well as well-documented examples in mono- or oligospecific animal-microbe associations (48–50). Findings from the MGWA and follow-up validation experiments suggest that the bacterium-dependent differences can be attributed in part to specific metabolic functions of the bacteria, underscoring the limitations of attributing phenotype-conferring characteristics based on taxonomic or limited genomic information (e.g., full or partial 16S rRNA gene sequences).
Our current findings combined with previous work lead us to hypothesize that associated bacteria may be able to influence host SR through dietary modification. It has previously been shown that microbial catabolism of dietary glucose causally lowers the triacylglyceride content of the flies, an effect that could be recapitulated by rearing bacterium-free flies on diets of reduced glucose content (3, 51). In this work, we reveal that increasing the methionine content of the diet decreases triacylglyceride levels and SR in flies reared on those diets, in an additive relationship with the presence or absence of associated microbes. The impact of methionine supplementation on fat storage in other organisms varies (52–56), suggesting that more work is needed to understand the basis for the varied effects; one factor may be how nutritionally complete the original diet is, but this idea has not been tested explicitly. The correlated influence of triacylglyceride content and SR, together with the responsiveness of fruit fly triacylglyceride content to bacterium-dependent nutrient acquisition (3, 51), suggests the possibility that methionine production by associated microbes may influence the flies through its accumulation in the diet (nutrient acquisition). Alternatively or additionally, bacterial methionine metabolism may influence nutrient allocation, as has been shown for other bacterial influences on D. melanogaster nutrition (5, 39), or its effects may be correlated with but not causal of SR influence. For example, methionine may not be the key metabolite of interest. A recent analysis of the D. melanogaster life span, a trait that is positively correlated with SR in many natural fruit fly populations and is negatively influenced by dietary methionine (57), showed that levels of methionine cycle intermediates S-(5′-adenosyl)-l-homocysteine (SAH) and S-(5′-adenosyl)-l-methionine (SAM), but not methionine, were more directly associated with negative life span influence. Metabolomic and/or pulse-chase experiments are necessary to further test and develop this proposed hypothesis.
A role for methionine in D. melanogaster SR is not surprising given the influence of methionine metabolism on organismal life history traits (58). Life history traits influence an animal's fitness and reproduction, including organismal longevity, fecundity, time to development, energy storage, and survival under stress (including SR in Drosophila flies). A hallmark relationship between these traits is the trade-off between organismal resource investment in somatic maintenance (i.e., life span) and reproduction-related traits (59–62). In. D. melanogaster, SR is commonly positively associated with somatic maintenance but negatively associated with early fecundity traits (63–67), although this correlation is not apparent in all fly populations (68–71) and can be disrupted by genetic manipulation or selection (71–74). Methionine restriction is an established method for extending organismal life span, and increasing or decreasing the dietary methionine content of an isogenic host line can influence its adoption of somatic maintenance or reproduction-related traits (75–78). Therefore, the established relationships between life span and SR, and of methionine on the D. melanogaster life span, are consistent with the negative impact of methionine on SR reported in this study. Additionally, we observed that Acetobacteraceae tended to confer a shorter period of SR on Drosophila flies than the Lactobacillales strains we tested, an effect correlated with a trend toward Acetobacteraceae also shortening the period of fly development and lowering fly triacylglyceride content, as reported previously (3). Taken together with an established relationship between methionine restriction and D. melanogaster life span extension, our data confirm the influences of methionine on life span-correlated life history traits. Interesting directions for future research include testing the hypothesis that microbial methionine metabolism influences organismal life span. We note that this idea is speculative, as it has not been supported by screens for bacterial influence on Caenorhabditis elegans longevity (79, 80), and the manipulation of dietary methionine or methionine metabolism genes does not necessarily lead to tradeoffs between longevity and reproduction (58, 81, 82). Regardless, these data provide key hypotheses to pursue and support a previous assertion that future work investigating the relationship between Drosophila flies, the microbiota, and life history tradeoffs is of interest (83). Our data suggest that bacterial methionine metabolism is a possible mechanism for such influences.
In addition to methionine metabolism, other bacterial functions were predicted to influence D. melanogaster SR. We focused on methionine metabolism, since methionine had the most significant effect on SR in our dietary supplementation experiments. Conversely, supplementation with other B vitamins and amino acids did not influence D. melanogaster SR, possibly suggesting a high false-positive rate among MGWA predictions. Alternatively, we cannot rule out that other experiments that vary nutrient concentrations, e.g., by supplementing at different concentrations, removing the nutrients in defined diets, or using a different background diet, would lead us to detect an effect of these predicted genes. Additionally, there were many highly ranked individual genes on which we did not focus. For example, the top ranked genes were 2 genes involved in pyrroloquinoline quinone biosynthesis and utilization, pqqE and qdbA. Pyrroloquinoline quinone (PQQ) plays a key role in microbiota-dependent nutrient allocation and development rate in D. melanogaster, two traits that are positively associated with SR in natural D. melanogaster populations (22), supporting the idea that bacterial PQQ biosynthesis and utilization may influence D. melanogaster SR. The most significant result was for a phosphoglycerate mutase, suggesting a possible role of bacterial central carbon metabolism on D. melanogaster SR. Several genes with links to polyamine biosynthesis were also predicted to influence fruit fly SR. One significant MGWA prediction was a bacterial spermidine synthase. The presence of ornithine decarboxylase, the first and rate-limiting enzyme in polyamine biosynthesis, was associated with SR. Also, atoS, encoding a spermidine-responsive two-component system sensor kinase that regulates the expression of the fatty acid degradation operon, atoDAEB (84, 85), was associated with lower SR. Dietary intervention or genetic manipulation of D. melanogaster spermidine biosynthesis genes has shown that spermidine levels are positively associated with an increase in D. melanogaster life span (86–88), stress resistance (89, 90), and triacylglyceride content (90). Our data confirm a role for bacterial speG, an acetyltransferase gene that provides resistance to potentially toxic polyamines, in D. melanogaster SR (91, 92). Although the mechanism of such effects is unknown, if bacterial spermidine supplements Drosophila spermidine levels, it would be consistent with the established idea that bacterial machinery can complement or be redundant with host enzymatic functions. Taken together, these findings suggest a rich reservoir of additional bacterial functions that can be interrogated for influence on D. melanogaster SR.
In summary, our findings support a multiorganismal basis for D. melanogaster SR. While the primary focus of this work has been to demonstrate a role for methionine in D. melanogaster SR, which to our knowledge has not been previously demonstrated, our association analysis predicted that bacteria are likely to influence D. melanogaster SR through multiple functions. The methionine influence on SR is consistent with the life span-extending influence of methionine restriction on D. melanogaster and the positively correlated relationship of SR and life span as life history traits in D. melanogaster. We suspect that bacterial methionine metabolism influences D. melanogaster SR through dietary modification and nutrient acquisition, but this prediction comes with the caveat that we have not performed the necessary metabolomic experiments to test this idea. As a quantitative trait that has expanded our understanding of how a model animal responds to nutrient storage, we expect that better understanding the interactions between the microbiota and D. melanogaster SR has the potential to improve our understanding of the multiorganismal basis for animal traits.
MATERIALS AND METHODS
Bacterial and fly cultures.
All experiments were performed using a Wolbachia-free stock of Drosophila melanogaster Canton-S, obtained from Mariana Wolfner, Cornell University. The standard fly culture was a 12-h light-dark cycle at 25°C on a yeast-glucose (YG) diet (1 liter H2O, 100 g glucose [catalog no. 158968; Sigma], 100 g inactive brewer's yeast [catalog no. 02903312; MP Biomedicals], 1.2% agar [catalog no. A2530; Apex], 0.84% propionic acid, and 0.08% phosphoric acid).
The bacterial strains used are listed in Table 2, with accompanying growth media, temperature, and oxygen conditions. The media used included brain heart infusion medium (BHI; catalog no. 101480172; Sigma), lysogeny broth (LB; 1% tryptone, 0.5% yeast extract, 0.5% sodium chloride [93]), modified MRS medium (mMRS; 1.25% peptone, 0.75% yeast extract, 2% glucose, 0.5% sodium acetate, 0.2% dipotassium hydrogen phosphate, 0.2% triammonium citrate, 0.02% magnesium sulfate heptahydrate, 0.005% manganese sulfate tetrahydrate, 1.2% agar [40]), and potato medium (catalog no. P6685; Sigma-Aldrich). E. coli transposon insertion mutants obtained from the KEIO collection were cultured in the presence of 50 μg/ml kanamycin. Auxotroph identity was confirmed on either 22.7 μM pantothenate-supplemented or 10 mM methionine-supplemented M9 medium. Microoxic conditions were achieved in liquid culture by static incubation and in solid culture by flooding an airtight container with CO2 before sealing the container. Oxic strains were grown with shaking (liquid) or ambient atmosphere (solid).
TABLE 2.
Metagenome-wide association and KEGG enrichment analysis show that bacterial pathways significantly influence D. melanogaster SR
| Identifier | Relevant characteristics (strain name; resistance; PMID or accession no.)a | Preferred mediumb | Oxygen conditionsc |
|---|---|---|---|
| 7636 | Escherichia coli BW25113 (CGSC wild type); 10829079, 16738554 | LB | Oxic |
| 8399 | CGSC 7636 ΔspeE739::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 8422 | CGSC 7636 Δpfs-773::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 8713 | CGSC 7636 ΔahpC744::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 9346 | CGSC 7636 ΔspeG732::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 9386 | CGSC 7636 ΔgstA785::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 9453 | CGSC 7636 ΔkatE731::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 9859 | CGSC 7636 ΔpdxB729::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 9920 | CGSC 7636 ΔpdxK747::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 10018 | CGSC 7636 ΔglyA725::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 10100 | CGSC 7636 ΔluxS768::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 10758 | CGSC 7636 ΔmetE774::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 10798 | CGSC 7636 ΔsodA768::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 10826 | CGSC 7636 ΔmetF728::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 10856 | CGSC 7636 ΔmetA780::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 10862 | CGSC 7636 ΔmetH786::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| 11994 | CGSC 7636 ΔyjiA750::kan; Kmr; 10829079, 16738554 | LB | Oxic |
| aace | Acetobacter aceti NBRC 14818; BABW00000000 | mMRS | Oxic |
| acew | Acetobacter orientalis DmW_048; JOOY00000000 | mMRS | Oxic |
| aci5 | Acetobacter sp. strain DmW_043; JOMN00000000 | mMRS | Oxic |
| ain2 | Acetobacter indonesiensis DmW_046; JOMP00000000 | mMRS | Oxic |
| amac | Acetobacter malorum DmCS_005; JOJU00000000 | mMRS | Oxic |
| aori | Acetobacter orientalis DmW_045; JOMO00000000 | mMRS | Oxic |
| apa3 | Acetobacter pasteurianus 3P3; CADQ00000000 | mMRS | Oxic |
| apan | Acetobacter pasteurianus NBRC 101655; BACF00000000 | mMRS | Oxic |
| apnb | Acetobacter pasteurianus NBRC 106471 or LMG 1262; PRJDA65547 | mMRS | Oxic |
| apoc | Acetobacter pomorum DmCS_004; JOKL00000000 | mMRS | Oxic |
| atrc | Acetobacter tropicalis DmCS_006; JOKM00000000 | mMRS | Oxic |
| atrn | Acetobacter tropicalis NBRC 101654; BABS00000000 | mMRS | Oxic |
| bsub | Bacillus subtilis subsp. subtilis strain 168; NC_000964.3 | LB | Oxic |
| ecok | Escherichia coli strain K-12 substrain MG1655; NC_000913.3 | LB | Oxic |
| efav | Enterococcus faecalis V583; NC_004668.1 | BHI | Oxic |
| efog | Enterococcus faecalis OG1RF; NC_017316.1 | BHI | Oxic |
| ehor | Enterobacter hormaechei ATCC 49162; AFHR00000000 | LB | Oxic |
| galb | Gluconobacter sp. strain DsW_056; JOPF00000000 | Potato | Oxic |
| ge5p | Gluconacetobacter europaeus 5p3; CADS00000000 | Potato | Oxic |
| gfra | Gluconobacter frateurii NBRC 101659; BADZ00000000 | Potato | Oxic |
| ghan | Gluconacetobacter hansenii ATCC 23769; ADTV01000000 | Potato | Oxic |
| gobo | Gluconacetobacter oboediens 174Bp2; CADT00000000 | Potato | Oxic |
| gxyl | Gluconacetobacter xylinus NBRC 3288; NC_016037.1 | Potato | Oxic |
| lani | Lactobacillus animalis KCTC 3501; AEOF00000000 | mMRS | Microoxic |
| lbga | Lactobacillus brevis subsp. gravesensis ATCC 27305; NZ_ACGG01000000 | mMRS | Microoxic |
| lbrc | Lactobacillus brevis DmCS_003; JOKA00000000 | mMRS | Microoxic |
| lbuc | Lactobacillus buchneri NRRLB-30929; ACGG00000000 | mMRS | Microoxic |
| lfal | Leuconostoc fallax KCTC 3537; AEIZ00000000 | mMRS | Microoxic |
| lfer | Lactobacillus fermentum ATCC 14931; ACGI00000000 | mMRS | Microoxic |
| lfrc | Lactobacillus fructivorans DmCS_002; JOJZ00000000 | mMRS | Microoxic |
| lfrk | Lactobacillus fructivorans KCTC 3543; AEQY00000000 | mMRS | Microoxic |
| llac | Lactococcus lactis BPL1; JRFX00000000 | mMRS | Microoxic |
| lmli | Lactobacillus mali KCTC 3596 = DSM 20444; BACP00000000 | mMRS | Microoxic |
| lplc | Lactobacillus plantarum DmCS_001; JOJT00000000 | mMRS | Microoxic |
| lplw | Lactobacillus plantarum WCFS1; NC_004567.2 | mMRS | Microoxic |
| lrha | Lactobacillus rhamnosus GG; NC_013198.1 | mMRS | Microoxic |
| pbur | Providencia burhodogranariea DSM 19968; AKKL00000000 | LB | Oxic |
Kmr, kanamycin resistance.
Abbreviations and media are described in Materials and Methods.
Solid and liquid conditions for oxic and microoxic conditions are described in Materials and Methods.
Axenic and gnotobiotic flies.
Axenic and gnotobiotic fly cultures were derived after bleach sterilization of fly eggs (2). D. melanogaster Canton-S embryos <20 h post-egg deposition were collected from grape juice agar plates (YG diet plus 10% grape juice) by gentle scraping with a paintbrush, filtered through a 10-μm nylon mesh filter (catalog no. 57-102; Genesee Scientific), and dechorionated by rinsing the embryos twice for 150 s each in 0.6% sodium hypochlorite. Three rinses in sterile H2O concluded the dechorionation process. Thirty to 60 eggs were then transferred to a sterile YG diet (preservative omitted) in 50-ml centrifuge tubes. Axenic flies were left unmanipulated. Monoassociated flies were created by inoculating the sterile embryos with 50 μl of a single strain that, after 24 to 72 h of culture, was washed in phosphate-buffered saline (PBS) and normalized to an optical density at 600 nm (OD600) of 0.1. The 5-species gnotobiotic flies were reared by inoculating sterile eggs with 50 μl of a mixed culture (1:1:1:1:1 ratio) of OD600 of 0.1-normalized Acetobacter tropicalis DmCS_006, Acetobacter pomorum DmCS_004, Lactobacillus brevis DmCS_003, Lactobacillus fructivorans DmCS_002, and Lactobacillus plantarum DmCS_001 strains.
To confirm the identity of associated microbes and to test for bacterial contamination in the flies, pools of five female flies were homogenized in 125 μl of bacterium-specific growth medium and 125 μl Lysing Matrix D ceramic beads (catalog no. 11654034; MP Biomedicals) on a FastPrep-24 for 30 to 60 s at 4.0 M/s. The CFU load was determined by serial dilutions on the same preferred growth medium after 24 to 72 h. Strain identity was confirmed by visual inspection of the colonies. White opaque colonies were classified as lactobacilli (minor differences in color and texture aided in strain-specific identification), and tan semitransparent colonies were classified as Acetobacter species. Many Acetobacteraceae strains could not be distinguished visually, and we therefore cannot rule out cross-contamination between Acetobacter species. Any vials containing greater than 100 CFU · fly−1 of bacterial species not administered were discarded, and vials containing the appropriate load of administered bacteria were included in fly SR analysis. Significant differences in the bacterial loads of the different mutants were tested by a Kruskal-Wallis test, and multiple comparisons were performed using the PMCMR (94) and multcompView (95) packages in R by a Tukey honest significant difference (HSD) test.
SR assay.
The SR assay was conducted using pools of 10 5- to 7-day-old female flies. Flies were lightly anesthetized with CO2 and transferred to foam-capped vials containing 5 ml 1% agarose. Fly survival was monitored daily at 0, 4, 8, 12, and 16 h into the light/dark cycle, with the 16-h readings performed under a red light. At each time point, the number of dead flies was recorded, and the assay continued until all flies in a vial were dead. Treatment-dependent differences in D. melanogaster SR were analyzed using Cox mixed-survival models in R, with experimental replicate and, when it lowered the Akaike information criterion (AIC), vial included as random effects (96, 97). An R markdown file for the statistical outputs corresponding to each figure is available in File S2 in the supplemental material.
Metagenome-wide association.
To predict bacterial genes that influence D. melanogaster SR, we performed a metagenome-wide association (MGWA) analysis, as in our previous work (3). SR was measured in D. melanogaster flies that were individually reared with one of 38 genome-sequenced bacterial strains. The strains were selected to represent bacterial taxa commonly found in flies, together with a few neighboring taxa to provide genetic diversity (Table 2). Most of the taxa in this study were the same as in a previous MGWA analysis we conducted. Orthologous groups (OGs) of genes were identified in the strain panel using OrthoMCL, with an inflation factor of 1.5, as described previously (3). To perform the MGWA, OG presence-absence patterns were statistically associated with D. melanogaster SR using a Cox mixed-survival model with experimental block and bacterial strain as random effects in R (98). A nominal Bonferroni-corrected threshold of a P value of <1 × 10−4 was used to determine the significance of the predictions.
To test for bacterial functions that were enriched in the significant MGWA hits, the representation of KEGG categories in the top hits was determined by a chi-square test. KEGG functions were assigned to a representative sequence from each OG using BlastKOALA. KEGG pathway assignments for the total data set (13,343 OGs) and the top hits (432 OGs) were retrieved in KEGG Pathway (http://www.genome.jp/kegg/tool/map_pathway1.html). Pathway enrichment was determined by a significant false-discovery rate (FDR)-corrected chi-square test. Chi-square tests and FDR correction were performed in R (99).
Dietary supplementation.
Dietary supplementations were performed by rearing bacterium-free or bacterium-associated D. melanogaster on a YG diet inoculated with nutritional supplements. Supplement concentrations were modeled after previous work (38, 42, 100) (Table S11) and added at 1.86 μM (B2), 1.62 μM (B3), 22.7 μM (B5), 8.27 μM (B6), 0.409 μM (B7), 20.4 μM (B9), 7.38 μM (B12), 1 mM (l-cysteine), and 10 mM (l-methionine). In a second experiment, all nutrients were supplemented at 10 mM.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge members of the Chaston lab for assistance with SR assays, Allen Gibbs and Chris Hardy (UNLV) for helpful discussions, and 3 anonymous reviewers for feedback.
This work was supported by startup funds and a mentoring environment grant from Brigham Young University to J.M.C., and an ORCA award from BYU for undergraduate research to A.M.J.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00662-18.
REFERENCES
- 1.Smith K, McCoy KD, Macpherson AJ. 2007. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Koyle ML, Veloz M, Judd AM, Wong AC, Newell PD, Douglas AE, Chaston JM. 2016. Rearing the fruit fly Drosophila melanogaster under axenic and gnotobiotic conditions. J Vis Exp 113:e54219. doi: 10.3791/54219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaston JM, Newell PD, Douglas AE. 2014. Metagenome-wide association of microbial determinants of host phenotype in Drosophila melanogaster. mBio 5:e01631-. doi: 10.1128/mBio.01631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Loso T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, Hentschel U, King N, Kjelleberg S, Knoll AH, Kremer N, Mazmanian SK, Metcalf JL, Nealson K, Pierce NE, Rawls JF, Reid A, Ruby EG, Rumpho M, Sanders JG, Tautz D, Wernegreen JJ. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A 110:3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ. 2011. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov II, Honda K. 2012. Intestinal commensal microbes as immune modulators. Cell Host Microbe 12:496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greiner TU, Hyotylainen T, Knip M, Backhed F, Oresic M. 2014. The gut microbiota modulates glycaemic control and serum metabolite profiles in non-obese diabetic mice. PLoS One 9:e110359. doi: 10.1371/journal.pone.0110359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. 2014. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Ridley EV, Wong ACN, Westmiller S, Douglas AE. 2012. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS One 7:e36765. doi: 10.1371/journal.pone.0036765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tremaroli V, Bäckhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 13.Chaston JM, Dobson AJ, Newell PD, Douglas AE. 2015. Host genetic control of the microbiota mediates the Drosophila nutritional phenotype. Appl Environ Microbiol 82:671–679. doi: 10.1128/AEM.03301-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayroles JF, Carbone MA, Stone EA, Jordan KW, Lyman RF, Magwire MM, Rollmann SM, Duncan LH, Lawrence F, Anholt RR, Mackay TF. 2009. Systems genetics of complex traits in Drosophila melanogaster. Nat Genet 41:299–307. doi: 10.1038/ng.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay TF, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF, Anholt RR, Barron M, Bess C, Blankenburg KP, Carbone MA, Castellano D, Chaboub L, Duncan L, Harris Z, Javaid M, Jayaseelan JC, Jhangiani SN, Jordan KW, Lara F, Lawrence F, Lee SL, Librado P, Linheiro RS, Lyman RF, Mackey AJ, Munidasa M, Muzny DM, Nazareth L, Newsham I, Perales L, Pu LL, Qu C, Ramia M, Reid JG, Rollmann SM, Rozas J, Saada N, Turlapati L, Worley KC, Wu YQ, Yamamoto A, Zhu Y, Bergman CM, Thornton KR, et al. . 2012. The Drosophila melanogaster genetic reference panel. Nature 482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Massouras A, Inoue Y, Peiffer J, Ramia M, Tarone AM, Turlapati L, Zichner T, Zhu D, Lyman RF, Magwire MM, Blankenburg K, Carbone MA, Chang K, Ellis LL, Fernandez S, Han Y, Highnam G, Hjelmen CE, Jack JR, Javaid M, Jayaseelan J, Kalra D, Lee S, Lewis L, Munidasa M, Ongeri F, Patel S, Perales L, Perez A, Pu L, Rollmann SM, Ruth R, Saada N, Warner C, Williams A, Wu YQ, Yamamoto A, Zhang Y, Zhu Y, Anholt RR, Korbel JO, Mittelman D, Muzny DM, Gibbs RA, Barbadilla A, Johnston JS, Stone EA, Richards S, Deplancke B, et al. . 2014. Natural variation in genome architecture among 205 Drosophila melanogaster genetic reference panel lines. Genome Res 24:1193–1208. doi: 10.1101/gr.171546.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy CM, Burke MK, Everett LJ, Han MV, Lantz KM, Gibbs AG. 2018. Genome-wide analysis of starvation-selected Drosophila melanogaster–a genetic model of obesity. Mol Biol Evol 35:50–65. doi: 10.1093/molbev/msx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan X, Sato-Miyata Y, Tsuda M, Muramatsu K, Asano T, Takeo S, Aigaki T. 2017. Deficiency of succinyl-CoA synthetase alpha subunit delays development, impairs locomotor activity and reduces survival under starvation in Drosophila. Biochem Biophys Res Commun 483:566–571. doi: 10.1016/j.bbrc.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 19.Zinke I, Schutz CS, Katzenberger JD, Bauer M, Pankratz MJ. 2002. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J 21:6162–6173. doi: 10.1093/emboj/cdf600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paaby AB, Bergland AO, Behrman EL, Schmidt PS. 2014. A highly pleiotropic amino acid polymorphism in the Drosophila insulin receptor contributes to life-history adaptation. Evolution 68:3395–3409. doi: 10.1111/evo.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Penninger JM. 2010. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Chippindale AK, Chu TJF, Rose MR. 1996. Complex trade-offs and the evolution of starvation resistance in Drosophila melanogaster. Evolution 50:753–766. doi: 10.1111/j.1558-5646.1996.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 23.Bubliy OA, Loeschcke V. 2005. Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. J Evol Biol 18:789–803. doi: 10.1111/j.1420-9101.2005.00928.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann AA, Harshman LG. 1999. Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity (Edinb) 83:637–643. doi: 10.1046/j.1365-2540.1999.00649.x. [DOI] [PubMed] [Google Scholar]
- 25.Rion S, Kawecki TJ. 2007. Evolutionary biology of starvation resistance: what we have learned from Drosophila. J Evol Biol 20:1655–1664. doi: 10.1111/j.1420-9101.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee G, Park JH. 2004. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao X, Zhang S, Timakov B, Zhang P. 2007. The Hsp27 gene is not required for Drosophila development but its activity is associated with starvation resistance. Cell Stress Chaperones 12:364–372. doi: 10.1379/CSC-308.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy CM, Birse RT, Wolf MJ, Yu L, Bodmer R, Gibbs AG. 2015. Obesity-associated cardiac dysfunction in starvation-selected Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 309:R658–R667. doi: 10.1152/ajpregu.00160.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masek P, Reynolds LA, Bollinger WL, Moody C, Mehta A, Murakami K, Yoshizawa M, Gibbs AG, Keene AC. 2014. Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J Exp Biol 217:3122–3132. doi: 10.1242/jeb.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers GB, Kozlowska J, Keeble J, Metcalfe K, Fao M, Dowd SE, Mason AJ, McGuckin MA, Bruce KD. 2014. Functional divergence in gastrointestinal microbiota in physically-separated genetically identical mice. Sci Rep 4:5437. doi: 10.1038/srep05437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong AC, Chaston JM, Douglas AE. 2013. The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J 7:1922–1932. doi: 10.1038/ismej.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong AC, Luo Y, Jing X, Franzenburg S, Bost A, Douglas AE. 2015. The host as driver of the microbiota in the gut and external environment of Drosophila melanogaster. Appl Environ Microbiol 81:6232–6240. doi: 10.1128/AEM.01442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obadia B, Guvener ZT, Zhang V, Ceja-Navarro JA, Brodie EL, Ja WW, Ludington WB. 2017. Probabilistic invasion underlies natural gut microbiome stability. Curr Biol 27:1999.e8–2006.e8. doi: 10.1016/j.cub.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inamine H, Ellner SP, Newell PD, Luo Y, Buchon N, Douglas AE. 2018. Spatiotemporally heterogeneous population dynamics of gut bacteria inferred from fecal time series data. mBio 9:e01453-17. doi: 10.1128/mBio.01453-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blum JE, Fischer CN, Miles J, Handelsman J. 2013. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. mBio 4:e00860-. doi: 10.1128/mBio.00860-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakula M. 1969. The persistence of a microbial flora during postembryogenesis of Drosophila melanogaster. J Invertebr Pathol 14:365–374. doi: 10.1016/0022-2011(69)90163-3. [DOI] [PubMed] [Google Scholar]
- 38.Wong ACN, Dobson AJ, Douglas AE. 2014. Gut microbiota dictates the metabolic response of Drosophila to diet. J Exp Biol 217:1894–1901. doi: 10.1242/jeb.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. 2011. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Newell PD, Douglas AE. 2014. Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Appl Environ Microbiol 80:788–796. doi: 10.1128/AEM.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newell PD, Chaston JM, Wang YP, Winans NJ, Sannino DR, Wong ACN, Dobson AJ, Kagle J, Douglas AE. 2014. In vivo function and comparative genomic analyses of the Drosophila gut microbiota identify candidate symbiosis factors. Front Microbiol 5:576. doi: 10.3389/fmicb.2014.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sang JH. 1956. The quantitative nutritional requirements of Drosophila melanogaster. J Exp Biol 33:45–72. [Google Scholar]
- 43.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, Benoist C, Kasper DL. 2017. Mining the human gut microbiota for immunomodulatory organisms. Cell 168:928.e11–943.e11. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, Gordon JI. 2010. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem 285:22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. 2010. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell 141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez J, Tolosana I, Ok S, Smith S, Snoeck K, Day JP, Jiggins FM. 2017. Symbiont strain is the main determinant of variation in Wolbachia-mediated protection against viruses across Drosophila species. Mol Ecol 26:4072–4084. doi: 10.1111/mec.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murfin KE, Lee MM, Klassen JL, McDonald BR, Larget B, Forst S, Stock SP, Currie CR, Goodrich-Blair H. 2015. Xenorhabdus bovienii strain diversity impacts coevolution and symbiotic maintenance with Steinernema spp. nematode hosts. mBio 6:e00076-. doi: 10.1128/mBio.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graf J. 2016. Lessons from digestive-tract symbioses between bacteria and invertebrates. Annu Rev Microbiol 70:375–393. doi: 10.1146/annurev-micro-091014-104258. [DOI] [PubMed] [Google Scholar]
- 51.Huang JH, Douglas AE. 2015. Consumption of dietary sugar by gut bacteria determines Drosophila lipid content. Biol Lett 11:20150469. doi: 10.1098/rsbl.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espe M, Rathore RM, Du ZY, Liaset B, El-Mowafi A. 2010. Methionine limitation results in increased hepatic FAS activity, higher liver 18:1 to 18:0 fatty acid ratio and hepatic TAG accumulation in Atlantic salmon, Salmo salar. Amino Acids 39:449–460. doi: 10.1007/s00726-009-0461-2. [DOI] [PubMed] [Google Scholar]
- 53.Aissa AF, Tryndyak V, de Conti A, Melnyk S, Gomes TD, Bianchi ML, James SJ, Beland FA, Antunes LM, Pogribny IP. 2014. Effect of methionine-deficient and methionine-supplemented diets on the hepatic one-carbon and lipid metabolism in mice. Mol Nutr Food Res 58:1502–1512. doi: 10.1002/mnfr.201300726. [DOI] [PubMed] [Google Scholar]
- 54.Pérez-Jiménez A, Peres H, Rubio VC, Oliva-Teles A. 2013. Effects of diet supplementation with white tea and methionine on lipid metabolism of gilthead sea bream juveniles (Sparus aurata). Fish Physiol Biochem 39:661–670. doi: 10.1007/s10695-012-9728-8. [DOI] [PubMed] [Google Scholar]
- 55.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. 2006. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5:305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 56.Kawasaki M, Miura Y, Funabiki R, Yagasaki K. 2010. Comparison of the effects on lipid metabolism of dietary methionine and cystine between hepatoma-bearing and normal rats. Biosci Biotechnol Biochem 74:158–167. doi: 10.1271/bbb.90673. [DOI] [PubMed] [Google Scholar]
- 57.Parkhitko AA, Binari R, Zhang N, Asara JM, Demontis F, Perrimon N. 2016. Tissue-specific down-regulation of S-adenosyl-homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila. Genes Dev 30:1409–1422. doi: 10.1101/gad.282277.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shchedrina VA, Kabil H, Vorbruggen G, Lee BC, Turanov AA, Hirosawa-Takamori M, Kim HY, Harshman LG, Hatfield DL, Gladyshev VN. 2011. Analyses of fruit flies that do not express selenoproteins or express the mouse selenoprotein, methionine sulfoxide reductase B1, reveal a role of selenoproteins in stress resistance. J Biol Chem 286:29449–29461. doi: 10.1074/jbc.M111.257600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Travers LM, Garcia-Gonzalez F, Simmons LW. 2015. Live fast die young life history in females: evolutionary trade-off between early life mating and lifespan in female Drosophila melanogaster. Sci Rep 5:15469. doi: 10.1038/srep15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose MR. 1984. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38:1004–1010. doi: 10.2307/2408434. [DOI] [PubMed] [Google Scholar]
- 61.Partridge L, Prowse N, Pignatelli P. 1999. Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc Biol Sci 266:255–261. doi: 10.1098/rspb.1999.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rose MR, Charlesworth B. 1981. Genetics of life history in Drosophila melanogaster. II. Exploratory selection experiments. Genetics 97:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wayne ML, Soundararajan U, Harshman LG. 2006. Environmental stress and reproduction in Drosophila melanogaster: starvation resistance, ovariole numbers and early age egg production. BMC Evol Biol 6:57. doi: 10.1186/1471-2148-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Service PM, Hutchinson EW, Rose MR. 1988. Multiple genetic mechanisms for the evolution of senescence in Drosophila melanogaster. Evolution 42:708–716. doi: 10.1111/j.1558-5646.1988.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 65.Chippindale AK, Hoang DT, Service PM, Rose MR. 1994. The evolution of development in Drosophila melanogaster selected for postponed senescence. Evolution 48:1880–1899. doi: 10.1111/j.1558-5646.1994.tb02221.x. [DOI] [PubMed] [Google Scholar]
- 66.Leroi AM, Chippindale AK, Rose MR. 1994. Long-term laboratory evolution of a genetic life-history trade-off in Drosophila melanogaster. 1. The role of genotype-by-environment interaction. Evolution 48:1244–1257. doi: 10.1111/j.1558-5646.1994.tb05309.x. [DOI] [PubMed] [Google Scholar]
- 67.Alic N, Partridge L. 2007. Antagonizing Methuselah to extend life span. Genome Biol 8:222. doi: 10.1186/gb-2007-8-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wit J, Sarup P, Lupsa N, Malte H, Frydenberg J, Loeschcke V. 2013. Longevity for free? Increased reproduction with limited trade-offs in Drosophila melanogaster selected for increased life span. Exp Gerontol 48:349–357. doi: 10.1016/j.exger.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Gasser M, Kaiser M, Berrigan D, Stearns SC. 2000. Life-history correlates of evolution under high and low adult mortality. Evolution 54:1260–1272. [DOI] [PubMed] [Google Scholar]
- 70.Phelan JP, Archer MA, Beckman KA, Chippindale AK, Nusbaum TJ, Rose MR. 2003. Breakdown in correlations during laboratory evolution. I. Comparative analyses of Drosophila populations. Evolution 57:527–535. [DOI] [PubMed] [Google Scholar]
- 71.Rose MR, Passananti HB, Chippindale AK, Phelan JP, Matos M, Teotonio H, Mueller LD. 2005. The effects of evolution are local: evidence from experimental evolution in Drosophila. Integr Comp Biol 45:486–491. doi: 10.1093/icb/45.3.486. [DOI] [PubMed] [Google Scholar]
- 72.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. 2001. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 73.Baldal EA, Brakefield PM, Zwaan BJ. 2006. Multitrait evolution in lines of Drosophila melanogaster selected for increased starvation resistance: the role of metabolic rate and implications for the evolution of longevity. Evolution 60:1435–1444. doi: 10.1111/j.0014-3820.2006.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 74.Wang PY, Neretti N, Whitaker R, Hosier S, Chang C, Lu D, Rogina B, Helfand SL. 2009. Long-lived Indy and calorie restriction interact to extend life span. Proc Natl Acad Sci U S A 106:9262–9267. doi: 10.1073/pnas.0904115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grandison RC, Piper MD, Partridge L. 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. 2014. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat Commun 5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. 1993. Low methionine ingestion by rats extends life span. J Nutr 123:269–274. [DOI] [PubMed] [Google Scholar]
- 78.Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, Lai CQ. 2007. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Dordr) 29:29–39. doi: 10.1007/s11357-006-9018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khanna A, Kumar J, Vargas MA, Barrett L, Katewa S, Li P, McCloskey T, Sharma A, Naude N, Nelson C, Brem R, Killilea DW, Mooney SD, Gill M, Kapahi P. 2016. A genome-wide screen of bacterial mutants that enhance Dauer formation in C. elegans. Sci Rep 6:38764. doi: 10.1038/srep38764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han B, Sivaramakrishnan P, Lin CJ, Neve IAA, He J, Tay LWR, Sowa JN, Sizovs A, Du G, Wang J, Herman C, Wang MC. 2017. Microbial genetic composition tunes host longevity. Cell 169:1249.e13–1262.e13. doi: 10.1016/j.cell.2017.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zajitschek F, Zajitschek SR, Friberg U, Maklakov AA. 2013. Interactive effects of sex, social environment, dietary restriction, and methionine on survival and reproduction in fruit flies. Age (Dordr) 35:1193–1204. doi: 10.1007/s11357-012-9445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee BC, Lee HM, Kim S, Avanesov AS, Lee A, Chun BH, Vorbruggen G, Gladyshev VN. 2018. Expression of the methionine sulfoxide reductase lost during evolution extends Drosophila lifespan in a methionine-dependent manner. Sci Rep 8:1010. doi: 10.1038/s41598-017-15090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fischer CN, Trautman EP, Crawford JM, Stabb EV, Handelsman J, Broderick NA. 2017. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. Elife 6:e18855. doi: 10.7554/eLife.18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lioliou EE, Mimitou EP, Grigoroudis AI, Panagiotidis CH, Panagiotidis CA, Kyriakidis DA. 2005. Phosphorylation activity of the response regulator of the two-component signal transduction system AtoS-AtoC in E. coli. Biochim Biophys Acta 1725:257–268. doi: 10.1016/j.bbagen.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 85.Jenkins LS, Nunn WD. 1987. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J Bacteriol 169:42–52. doi: 10.1128/jb.169.1.42-52.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minois N. 2014. Molecular basis of the ‘anti-aging’ effect of spermidine and other natural polyamines–a mini-review. Gerontology 60:319–326. doi: 10.1159/000356748. [DOI] [PubMed] [Google Scholar]
- 87.Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, Madeo F, Kroemer G. 2009. Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging (Albany NY) 1:961–970. doi: 10.18632/aging.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. 2009. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 89.Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, Sigrist SJ, Kroemer G, Madeo F. 2012. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis 3:e401. doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Minois N, Rockenfeller P, Smith TK, Carmona-Gutierrez D. 2014. Spermidine feeding decreases age-related locomotor activity loss and induces changes in lipid composition. PLoS One 9:e102435. doi: 10.1371/journal.pone.0102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fukuchi J, Kashiwagi K, Yamagishi M, Ishihama A, Igarashi K. 1995. Decrease in cell viability due to the accumulation of spermidine in spermidine acetyltransferase-deficient mutant of Escherichia coli. J Biol Chem 270:18831–18835. doi: 10.1074/jbc.270.32.18831. [DOI] [PubMed] [Google Scholar]
- 92.Limsuwun K, Jones PG. 2000. Spermidine acetyltransferase is required to prevent spermidine toxicity at low temperatures in Escherichia coli. J Bacteriol 182:5373–5380. doi: 10.1128/JB.182.19.5373-5380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pohlert T. 2014. The Pairwise Multiple Comparison of Mean Ranks package (PMCMR). https://cran.r-project.org/web/packages/PMCMR/vignettes/PMCMR.pdf.
- 95.Graves S, Piepho H-P, Selzer L, Dorai-Raj S. 2015. multcompView: visualizations of paired comparisons. R package version 0.1-7. https://cran.r-project.org/web/packages/multcompView/index.html.
- 96.Therneau T. 2012. Mixed effects Cox models, v2.2-3.
- 97.Therneau T. 2014. A package for survival analysis in S, v2.37-7. https://cran.r-project.org/web/packages/survival/index.html.
- 98.Sexton CE, Smith HZ, Newell PD, Douglas AE, Chaston JM. 2018. MAGNAMWAR: an R package for genome-wide association studies of bacterial orthologs. Bioinformatics 34:1951–1952. doi: 10.1093/bioinformatics/bty001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.R Core Team 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. [Google Scholar]
- 100.Blatch SA, Meyer KW, Harrison JF. 2010. Effects of dietary folic acid level and symbiotic folate production on fitness and development in the fruit fly Drosophila melanogaster. Fly (Austin) 4:312–319. doi: 10.4161/fly.4.4.13258. [DOI] [PubMed] [Google Scholar]
- 101.Selhub J. 1999. Homocysteine metabolism. Annu Rev Nutr 19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.