Although the direct effects of dietary soluble fiber on gut microbiota have been extensively studied, the more indirect effects of maternal nutrition solely during pregnancy on the development of the offspring's intestine are until now largely unexplored. Our data show that a maternal soluble fiber diet during pregnancy is independently associated with changes in the intestinal microbiota composition and metabolism of suckling piglets. These findings have direct implications for refining dietary recommendations in pregnancy. Moreover, a maternal soluble fiber diet reduces intestinal permeability and prevents intestinal inflammation and an excessive systemic immune response of suckling piglets. Therefore, the suckling piglets' resistance to disease was enhanced, diarrhea was reduced, and weight gain was raised. Additionally, the changes in gut microbiota in response to a maternal soluble fiber diet may also be directly correlated with the offspring's growth and gut development.
KEYWORDS: growth performance, intestinal permeability, maternal diet, microbiota composition, suckling piglets
ABSTRACT
Increasing evidence suggests that maternal diet during pregnancy modifies an offspring's microbiota composition and intestinal development in a long-term manner. However, the effects of maternal soluble fiber diet during pregnancy on growth traits and the developing intestine are still underexplored. Sows were allocated to either a control or 2.0% pregelatinized waxy maize starch plus guar gum (SF) dietary treatment during gestation. Growth performance, diarrhea incidence, gut microbiota composition and metabolism, and gut permeability and inflammation status of 14-day-old suckling piglets were analyzed. The maternal SF diet improved the growth rate and decreased the incidence of diarrhea in the piglets. Next-generation sequencing analysis revealed that the intestinal microbiota composition was altered by a maternal SF diet. The fecal and plasma levels of acetate and butyrate were also increased. Furthermore, a maternal SF diet reduced the levels of plasma zonulin and fecal lipocalin-2 but increased the plasma concentrations of interleukin 10 (IL-10) and transforming growth factor β (TGF-β). Additionally, the increased relative abundances of Lactobacillus spp. in SF piglets were positively correlated with growth rate, while the decreased abundances of Bilophila spp. were positively correlated with fecal lipocalin-2 levels. Our data reveal that a maternal SF diet during pregnancy has remarkable effects on an offspring's growth traits and intestinal permeability and inflammation, perhaps by modulating the composition and metabolism of gut microbiota.
IMPORTANCE Although the direct effects of dietary soluble fiber on gut microbiota have been extensively studied, the more indirect effects of maternal nutrition solely during pregnancy on the development of the offspring's intestine are until now largely unexplored. Our data show that a maternal soluble fiber diet during pregnancy is independently associated with changes in the intestinal microbiota composition and metabolism of suckling piglets. These findings have direct implications for refining dietary recommendations in pregnancy. Moreover, a maternal soluble fiber diet reduces intestinal permeability and prevents intestinal inflammation and an excessive systemic immune response of suckling piglets. Therefore, the suckling piglets' resistance to disease was enhanced, diarrhea was reduced, and weight gain was raised. Additionally, the changes in gut microbiota in response to a maternal soluble fiber diet may also be directly correlated with the offspring's growth and gut development.
INTRODUCTION
The adverse health effects of maternal malnutrition during pregnancy on the offspring are increasingly acknowledged (1). According to the Developmental Origin of Health and Disease (DOHaD) theory, undernutrition during gestation may disrupt normal development and contribute to adult disease by having permanent effects on the structure, function, and metabolism of the developing offspring (2). Evidence in rodents and humans supports the scientific premise that exposure to maternal high-fat or high-protein diets during pregnancy creates a long-lasting metabolic signature on the infant innate immune system and the juvenile gut microbiota, which predisposes the offspring to obesity and metabolic diseases (3, 4), whereas maternal dietary fiber intake protects offspring against high-fat diet-induced obesity and other metabolic diseases (5, 6). However, the effects of maternal soluble fiber diet during pregnancy on growth traits and the developing intestine are still underexplored.
The intestine is known to be of crucial importance for whole-body health but still is a relatively unexplored organ with respect to early life programming. The intestinal microbiota is acquired early in life and plays multiple roles in a host's growth and health, including energy extraction from the diet, gut barrier function and immune system maturation, and growth performance (7, 8). Increasing evidence suggests that early alterations in the succession of the gut microbiota have permanent metabolic consequences (9). Therefore, the development of an optimal microbiota composition during the early life period is of crucial importance for growth and metabolic health in neonates.
Emerging data also highlight the importance of the intestinal epithelium as a regulator of adult and neonate health (10). Increased intestinal permeability is involved in several disorders associated with low-grade inflammation, including obesity and irritable bowel syndrome in children (11, 12). The proposed mechanism is presumed to involve the increased passage of gut microbiota components, such as bacterial lipopolysaccharide (LPS), through the intestinal barrier into the circulation. Subsequently, increased circulating concentrations of bacterial LPS can lead to metabolic endotoxemia, a potential mediator of inflammation (13). Zonulin is a mediator known to regulate intestinal permeability by modulating intracellular tight junctions. Circulating zonulin has been used as a potential marker of intestinal permeability (14). Maternal nutrients, such as n-3 polyunsaturated fatty acids, have been associated with alterations in the intestinal permeability of offspring (15). In addition, dysbiosis in the gut microbiota has been shown to increase intestinal permeability (16).
Interest is rapidly growing around the beneficial effects of soluble dietary fiber in regulating gut microbiota and intestinal health. Soluble fibers are recognized prebiotics able to enrich probiotic bacteria, most notably Lactobacillus spp., that are beneficial for digestive function and intestinal barrier function (17). In addition, short-chain fatty acids (SCFAs), which are primary fermentation products of soluble fiber, play an important role in intestinal health. For example, acetate has been shown to be an anti-inflammatory metabolite to maintain gut homeostasis, and butyrate is beneficial for modulating intestinal permeability (18, 19). Our previous results showed that the combined soluble fiber materials (pregelatinized waxy maize starch plus guar gum) had a high water-binding capacity and swelling capacity (20). Pregelatinized waxy maize starch, a fermentation-resistant starch composed mainly of highly branched amorphous amylopectin, has many specific food attributes (21). Guar gum, a galactomannan extracted from the endosperm of leguminous guar, with high viscosity and hydrating properties, is used as a stabilizer and thickener in various food products (22). Moreover, the highly fermentable dietary fiber guar gum can escape digestion in the small intestine and is thought to act exclusively via bacterial fermentation and concomitant alterations in the gut microbiota (23).
Although the direct effects of dietary fiber on gut microbiota composition have been extensively studied, the more indirect effects of maternal nutrition solely during pregnancy on the development of the offspring's intestine are until now largely unexplored. Therefore, we hypothesized that the inclusion of combined soluble fiber materials of pregelatinized waxy maize starch and guar gum (SF) in a pregnancy diet may have remarkable effects on an offspring's growth traits, gut microbiota, and the development of gut function. Accordingly, in the present study, we investigated the effects of a maternal soluble fiber diet during pregnancy on growth performance, diarrhea incidence, gut microbiota composition and metabolism, and gut permeability and inflammation status of suckling piglets.
RESULTS
Growth rate was increased and diarrhea was reduced in suckling piglets from SF-fed sows.
The weight gain and the occurrence of diarrhea of piglets were assessed during the whole lactation period (21 days). Although there was no significant difference (P > 0.05) in initial body weight (BW), we found the final BW was higher (P < 0.05) in suckling piglets from SF-fed sows (Table 1). The average daily gain (ADG) during week 3 and the whole suckling period was higher (P < 0.05) for piglets in the SF treatment than that in the control (CON) group (Table 1). Given that postnatal growth of mammals is mainly controlled by the activity of the somatotropic axis (24), we examined whether a maternal SF diet can enhance growth hormone (GH) sensitivity. The results presented in Table 2 show that the plasma concentrations of GH and insulin-like growth factor 1 (IGF-1) were significantly increased in suckling piglets from SF-fed sows. Furthermore, the diarrhea rates and diarrhea index during the whole suckling period were lower (P < 0.05) in suckling piglets from SF-fed sows than those from CON-fed sows (Table 1). In summary, the results support the idea that a maternal SF diet during pregnancy improves growth rate and resistance to disease in the suckling offspring.
TABLE 1.
Effect of maternal SF diet during pregnancy on growth performance and diarrhea rate of suckling piglets
| Parametera | Mean (n = 12)b |
SEM | P value | |
|---|---|---|---|---|
| CON | SF | |||
| Initial BW (kg) | 1.58 | 1.61 | 0.04 | 0.95 |
| Final BW (kg) | 6.49 B | 7.09 A | 0.16 | 0.04 |
| ADG (g/day) | ||||
| Wk 1 | 188.42 | 229.76 | 13.32 | 0.12 |
| Wk 2 | 261.32 | 252.57 | 8.51 | 0.62 |
| Wk 3 | 251.23 B | 301.27 A | 9.10 | <0.01 |
| Wk 1 to 3 | 233.66 B | 261.20 A | 6.74 | 0.04 |
| Diarrhea rate (%) | 13.69 A | 10.35 B | 0.70 | 0.04 |
| Diarrhea index | 0.54 A | 0.19 B | 0.04 | <0.01 |
BW, body weight; ADG, average daily gain.
Values within a row with different uppercase letters differ significantly at a P value of <0.05. CON, control diet group; SF, 2.0% pregelatinized waxy maize starch and guar gum diet group.
TABLE 2.
Effect of maternal SF diet during pregnancy on plasma hormone of 14-day-old suckling piglets
| Concna | Mean (n = 12)b |
SEM | P value | |
|---|---|---|---|---|
| CON | SF | |||
| GH (pg/ml) | 587.65 B | 657.49 A | 16.89 | 0.04 |
| IGF-1 (ng/ml) | 309.04 B | 374.63 A | 11.09 | <0.01 |
GH, growth hormone; IGF-1, insulin-like growth factor 1.
Values within a row with different uppercase letters differ significantly at a P value of <0.05.
The intestinal microbiota composition and metabolism of the suckling piglets are significantly altered by a maternal SF-style diet.
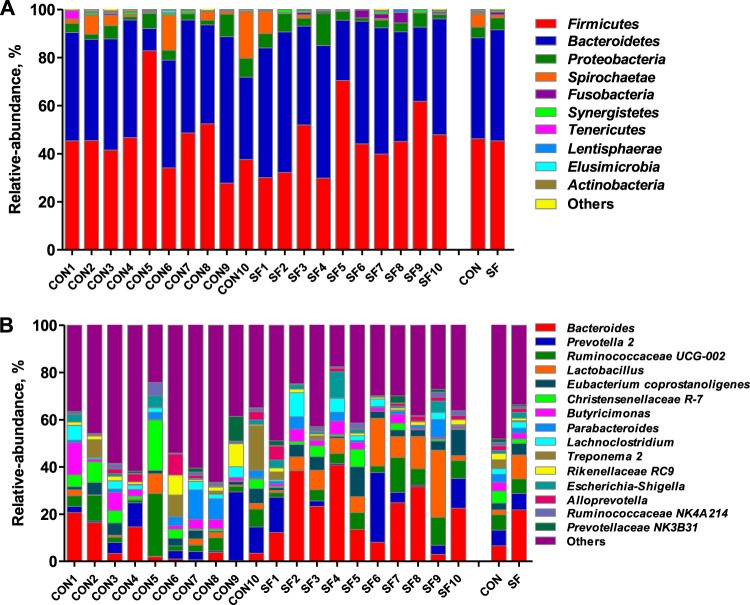
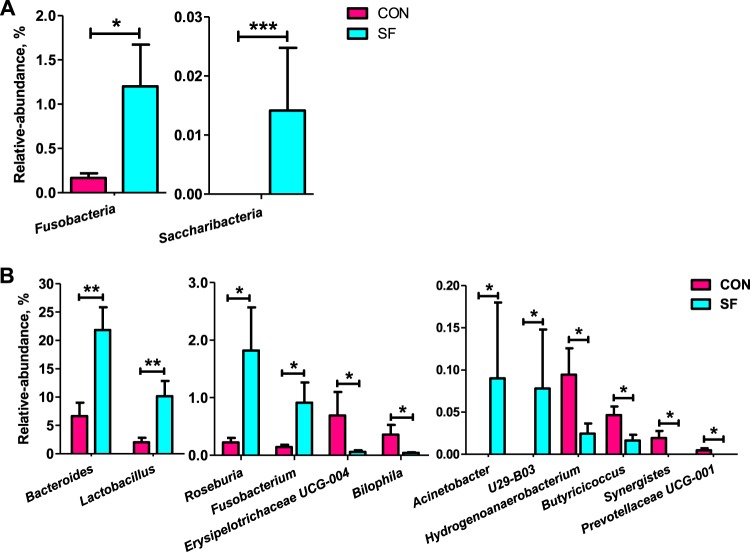
The microbiota composition and diversity of the fecal samples in 2-week-old piglets were assessed by deep sequencing of the V3–V4 region of the 16S rRNA genes. The top 10 phyla and the top 15 genera in relative abundance of the fecal microbiota that present in suckling piglets are displayed in Fig. 1. Bacteroidetes and Firmicutes were the most dominant phyla in both CON and SF piglets, followed by Proteobacteria, Spirochaetae, and Fusobacteria (Fig. 1A). These five phyla accounted for 98.23 and 98.94% of the reads for suckling piglets from the CON- and SF-fed sows, respectively. Other phyla (Synergistetes, Tenericutes, Lentisphaerae, Elusimicrobia, and Actinobacteria) were present at very low relative abundances. At the genus level, Bacteroides was the most dominant in both treatments. Other major genera included Prevotella 2, Ruminococcaceae UCG-002, Lactobacillus, Eubacterium coprostanoligenes, Christensenellaceae R-7, Butyricimonas, Parabacteroides, Lachnoclostridium, and Treponema 2; these genera accounted for more than 50% of the total sequences (Fig. 1B). Significant differences at the levels of phylum and genus of fecal microbiota between CON and SF piglets were further identified using Metastats analysis (Fig. 2). The relative abundances of the phyla Fusobacteria and Saccharibacteria were significantly elevated in suckling piglets from SF-fed sows compared with those from CON-fed sows (Fig. 2A). At the genus level, Bacteroides, Lactobacillus, Roseburia, Fusobacterium, Acinetobacter, and U29-B03 exhibited increased relative abundances in piglets from SF-fed sows (Fig. 2B). In contrast, there was a remarkable decrease in the relative abundances of the genera Erysipelotrichaceae UCG-004, Bilophila, Hydrogenoanaerobacterium, Butyricicoccus, Synergistes, and Prevotellaceae UCG-001 in piglets from SF-fed sows (Fig. 2B). Microbial compositions between CON and SF piglets were further analyzed using the linear discriminant analysis coupled with effect size (LEfSe). At the phylum level, our LEfSe analysis revealed that the phylum Spirochaetae was significantly enriched in fecal samples of suckling piglets from CON-fed sows (Fig. 3). At the genus level, five known genera (Bacteroides, Lactobacillus, Anaerotruncus, Eisenbergiella, and Enterococcus) were significantly enriched in piglets from SF-fed sows, and six genera (Bilophila, Hydrogenoanaerobacterium, Butyricicoccus, Erysipelotrichaceae UCG-004, Synergistes, and Prevotellaceae UCG-001) were significantly enriched in suckling piglets from CON-fed sows (Fig. 3). We also observed that the class Bacilli and the two orders Lactobacillales and Selenomonadales were enriched in SF piglets, whereas the two classes Spirochaetes and Clostridia and the two orders Spirochaetales and Bradymonadales were enriched in CON piglets. Similarly, Bacteroidaceae, Lactobacillaceae, and Enterococcaceae were highly enriched in SF piglets, while at the family level, Spirochaetaceae in CON piglets was enriched. Additionally, two species, Lactobacillus salivarius and Lactobacillus gasseri, were enriched in SF piglets (Fig. 3). Therefore, the above-mentioned results indicate that an SF diet during pregnancy significantly changes the intestinal microbiota composition of suckling piglets.
FIG 1.
Taxonomic profiles of the fecal bacteria in 14-day-old suckling piglets of the CON- and SF-fed sows from 16S rRNA gene sequencing. The relative abundance of the top 10 phyla (A) and the top 15 genera (B) of fecal bacteria present in suckling piglets both from the CON- and SF-fed sows. CON1, the first sample from the control diet group; SF1, the first sample from the 2.0% pregelatinized waxy maize starch and guar gum diet group.
FIG 2.
Comparison of the fecal microbial community in 14-day-old suckling piglets from CON- and SF-fed sows. The bacterial phyla (A) and genera (B) differed between suckling piglets from CON- and SF-fed sows. Values are expressed as means ± SEM. Significance is considered at a P value of <0.05 and q of <0.05. *, effect of treatment, (P < 0.05); **, P < 0.01; ***, P < 0.001.
FIG 3.
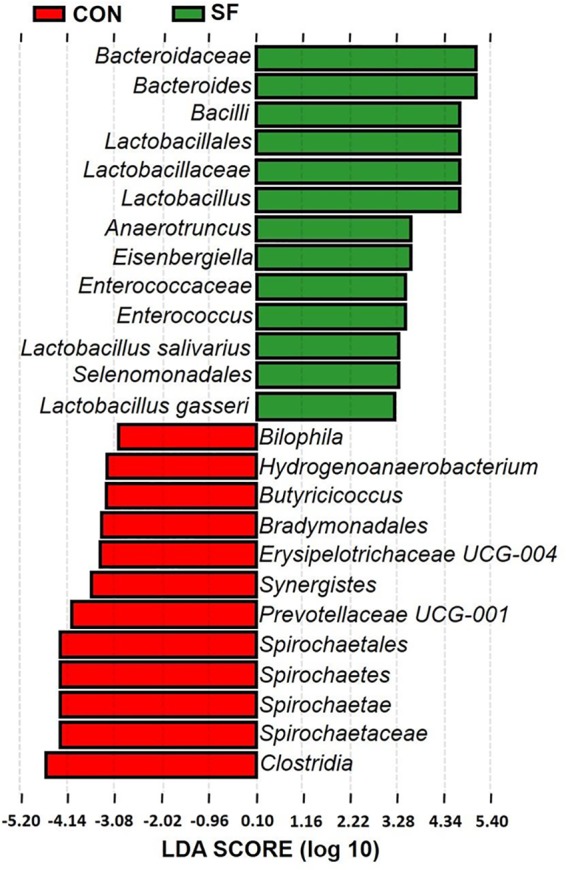
LEfSe Analysis of the fecal bacterial community of 14-day-old suckling piglets after maternal SF diet during pregnancy. CON, control diet group; SF, 2.0% pregelatinized waxy maize starch and guar gum diet group. LDA, linear discriminant analysis.
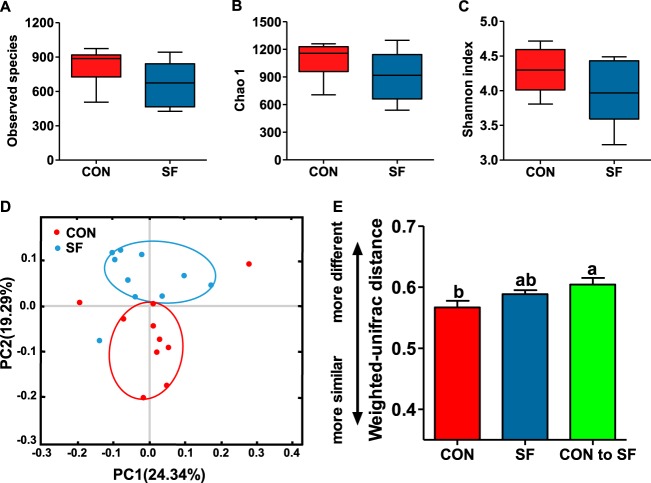
The remarkable changes in composition of the piglets gut microbiota across different treatments led us to assess the alpha- and beta-diversity measures of the fecal microbiota. Alpha-diversity measures (observed species, Chao1, and Shannon indices) of the fecal bacterial community showed negligible differences between suckling piglets of the CON- and SF-fed sows (Fig. 4A to C), while beta diversity, the principal-coordinate analysis (PCoA) based on weighted-UniFrac distance, revealed that the gut microbiota showed obvious segregation in suckling piglets from CON-fed to SF-fed sows (Fig. 4D). Furthermore, the interindividual variations were determined by average weighted-UniFrac distances between individuals in CON and SF suckling piglets, while intraindividual variations were determined by distances between paired CON and SF samples. Although there was no significant difference in the interindividual variations between CON and SF suckling piglets, we found that intraindividual variation from CON to SF was higher than the interindividual variation in CON piglets (Fig. 4E), which also manifested that the gut microbiota structures of suckling piglets were significantly altered by a maternal SF-style diet.
FIG 4.
Comparison of the fecal microbial community diversity between 14-day-old suckling piglets from CON- and SF-fed sows during pregnancy. (A to C) Comparison of the number of observed OTUs (A), Chao1 (B), and Shannon index (C) between 14-day-old suckling piglets from CON- and SF-fed sows during pregnancy. (D) Trajectory of the gut microbiota structure of sows across the reproductive cycle based on weighted-UniFrac distance. (E) Interindividual variations were determined by average weighted-UniFrac distances between individuals in CON and SF piglets, while intraindividual variations were determined by distance-paired CON and SF piglets. Data are presented as means ± SEM (n = 10). (A to D) Significant effect of treatment at a P value of <0.05; values with different lowercase letters are significantly different. PC2, principal-component analysis 2.
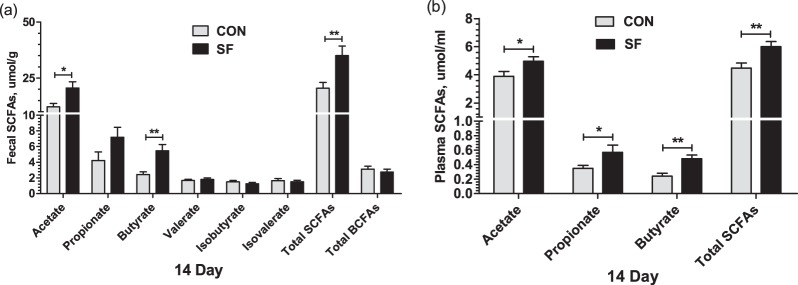
To analyze the effect of a maternal SF diet during pregnancy on intestinal microbiota metabolism, we then focused on the fecal and plasma SCFAs of suckling piglets from either CON- or SF-fed sows. The results showed that the levels of fecal (Fig. 5a) and plasma (Fig. 5b) acetate, butyrate, and total SCFAs in suckling piglets from SF-fed sows were higher (P < 0.05) than those from CON-fed sows at day 14 after birth. Additionally, the levels of plasma propionate were increased (P < 0.05) in suckling piglets from SF-fed sows. There was no significant difference (P > 0.05) in the fecal concentrations of valerate, isobutyrate, and isovalerate between the CON- and SF-fed treatments.
FIG 5.
Effect of maternal SF diet during pregnancy on short-chain fatty acids in fecal (a) and plasma (b) samples of 14-day-old suckling piglets. Data are presented as means ± SEM (n = 10). *, effect of treatment, (P < 0.05); **, P < 0.01.
Taken together, a maternal SF diet during pregnancy significantly altered the intestinal microbiota composition and metabolism of the 14-day-old suckling piglets.
A maternal SF diet reduces intestinal permeability and promotes gut health of suckling piglets.
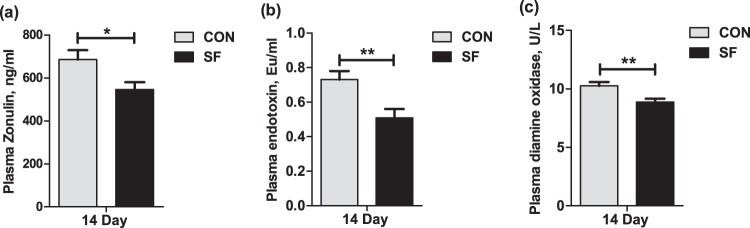
Considering recent evidence suggesting that microbiota-derived butyrate modulates intestinal barrier integrity via modulation of the expression of tight-junction proteins (25), we hypothesized that a maternal SF diet might reduce the gut permeability of suckling piglets, thus attenuating the intestinal or systemic inflammatory response. Three biomarkers related to intestinal permeability were measured. The results presented in Fig. 6 show that the plasma levels of zonulin, endotoxin, and diamine oxidase were significantly decreased in suckling piglets from SF-fed sows compared with those from CON-fed sows. Previous studies have suggested that the impaired intestinal mucosal integrity may enhance concentrations of zonulin and diamine oxidase and increase contents of endotoxin in blood (14, 26). This suggests that an SF diet during pregnancy reduces the intestinal permeability in suckling piglets.
FIG 6.
Effect of maternal SF diet during pregnancy on plasma zonulin (a), endotoxin (b), and diamine oxidase (c) of 14-day-old suckling piglets. Data are presented as means ± SEM (n = 12). *, effect of treatment, (P < 0.05); **, P < 0.01. Eu, endotoxin unit.
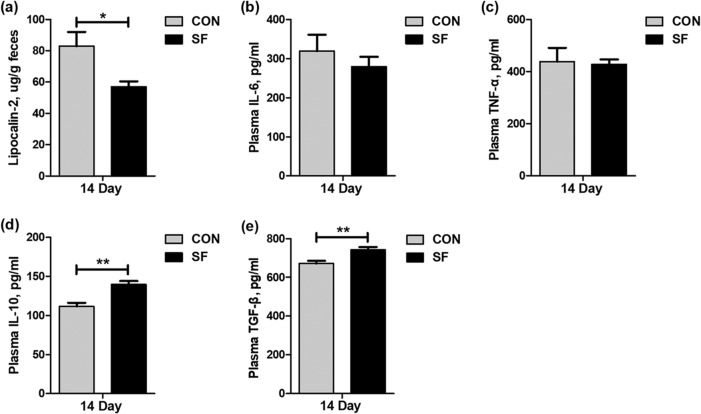
We next detected five biomarkers related to the gut health of suckling piglets. These biomarkers included fecal lipocalin-2 as a marker for intestinal inflammation (27) and a set of plasma cytokines (interleukin 6 [IL-6], interleukin 10 [IL-10], tumor necrosis factor α [TNF-α], and transforming growth factor β [TGF-β]) as markers for the immune system activation and systemic inflammatory response (28, 29). The levels of fecal lipocalin-2 were reduced (P < 0.05) in suckling piglets from SF-fed sows (Fig. 7a). The anti-inflammatory mediator IL-10 and immune tolerance mediator TGF-β were increased (P < 0.01) in the plasma of suckling piglets from SF-fed sows (Fig. 7d and e), although a change in systemic proinflammatory cytokines IL-6 and TNF-α was not observed.
FIG 7.
Effect of maternal SF diet during pregnancy on fecal lipocalin-2 (a) and plasma IL-6 (b), TNF-α (c), IL-10 (d), and TGF-β (e) levels of 14-day-old suckling piglets. Data are presented as means ± SEM (n = 12). *, effect of treatment, (P < 0.05); **, P < 0.01. IL-6, interleukin 6; TNF-α, tumor necrosis factor α; IL-10, interleukin 10; TGF-β, transforming growth factor β.
In conclusion, a maternal SF diet reduces intestinal permeability and prevents intestinal inflammation and excessive systemic immune response, thus promoting the intestinal health of suckling piglets.
Alterations in intestinal microbiota composition induced by a maternal SF diet were correlated with growth rate and gut health of suckling piglets.
A Spearman correlation analysis was performed to evaluate the potential link between alterations in gut microbiota composition induced by a maternal SF diet and the growth and health parameters of suckling piglets (Fig. 8). The genus Lactobacillus was positively correlated with ADG (P < 0.01), plasma GH levels (P < 0.01), and plasma IGF-1 levels (P < 0.05) but negatively correlated with diarrhea rate (P < 0.05). The genus Bacteroides was positively correlated with plasma IL-10 levels (P < 0.05). Additionally, the genus Bilophila was positively correlated with fecal lipocalin-2 levels (P < 0.05).
FIG 8.
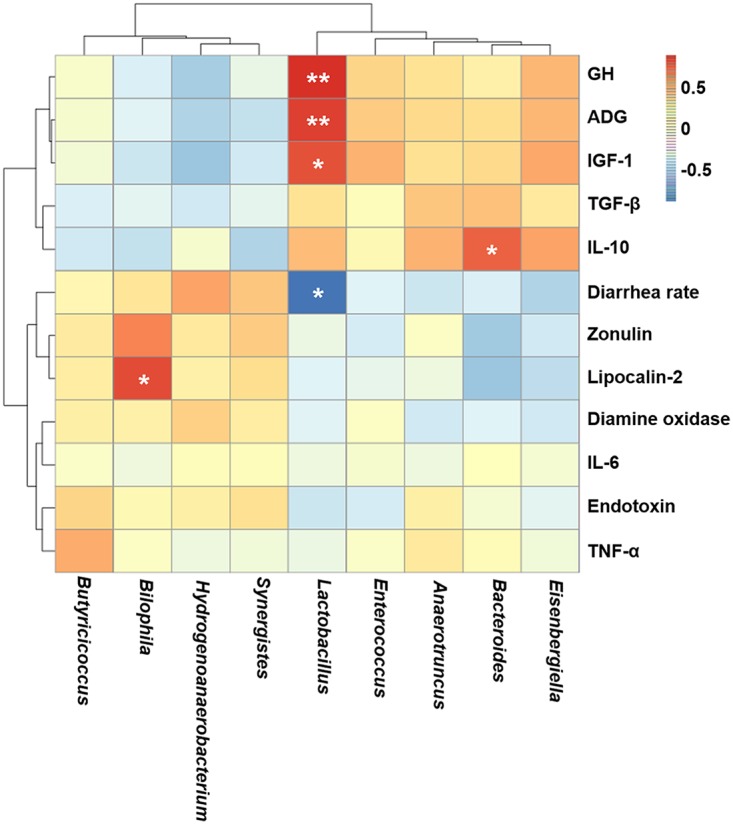
Heatmap of the Spearman r correlations between the gut microbiota significantly modified by maternal SF diet and growth and health parameters of 14-day-old suckling piglets. *, P < 0.05; **, P < 0.01 (following the Spearman correlation analysis). GH, growth hormone; ADG, average daily gain; IGF-1, insulin-like growth factor 1.
DISCUSSION
The impact of the maternal diet on the development of the offspring is generally accepted (30, 31), but the effects of a maternal SF diet during pregnancy on the growth traits and developing intestines were, until now, still largely unknown. The results obtained in this study clearly showed that maternal exposure to an SF diet during pregnancy significantly alters the intestinal microbiota composition of suckling 2-week-old piglets. Substantial colonization of the intestinal lumen in piglets starts at the moment of delivery, ultimately resulting in a microflora that is more or less stable in composition after 28 days (32). The pattern of microbial succession within the gastrointestinal tract has been suggested to be crucial in shaping growth traits and gut health in newborn individuals (8). The Firmicutes and Bacteroidetes phyla accounted for more than 90% of the total sequences, similarly to previous findings in the cecal and fecal microbiota of suckling piglets (33, 34). Moreover, the greatest enrichment being that of the Bacteroides genus in suckling piglets was in agreement with trends seen in humans (35). It has been suggested that Bacteroides spp. are abundant in the neonate gastrointestinal microbiota because they are adapted to use a wide range of both milk oligosaccharides and host-derived glycans (e.g., sulfomucin) as a unique carbon source (35). The strong increase in relative abundance of the Lactobacillus genus in the offspring of SF diet-fed sows is in line with the findings of previous studies in older humans after exposure to a high-fiber diet (17). Lactobacillus was one of the most dominant genera, accounting for approximately 15% of the 16S rRNA gene sequences from porcine intestinal samples, regardless of age (36). Moreover, Lactobacillus species are common probiotics, and they play important roles in pathogen defense and improve intestinal barrier function and immunity in piglets (37). Importantly, our results revealed that the growth rate and plasma concentrations of GH and IGF-1 were increased, but diarrhea rates were decreased, in piglets from SF-fed sows. Furthermore, the Spearman correlation analysis showed that the genus Lactobacillus was positively correlated with ADG, plasma GH levels, and plasma IGF-1 levels but negatively correlated with diarrhea rate. In mammals, postnatal growth is controlled by the activity of the somatotropic axis, in which GH instructs the liver and peripheral tissues to produce IGF-1 in order to promote organ and systemic growth (24). Schwarzer et al. reported that the gut microbiota is necessary to boost postnatal growth by enhancing GH sensitivity and thereby facilitating IGF-1 production and activity (38). Thus, these observations imply that Lactobacillus spp. increased by a maternal SF diet during pregnancy might be important for the improvement of growth rate and resistance to disease in the suckling offspring.
In addition, we also found that a maternal SF diet resulted in a higher proportion of Bacteroides and Roseburia spp. in suckling piglets. In contrast, it was found that maternal high-fat diet during pregnancy may affect bacteria present in the infant gut after at least 6 weeks of age, resulting in a relative depletion of Bacteroides spp. (3). Bacteroides and Roseburia participate in energy extraction by the degradation of polysaccharides into SCFAs, which are of importance for the rapidly growing infants (39–41). Thus, these results led us to analyze intestinal microbiota metabolism in piglets from SF-fed sows, and we focused on the fecal and plasma SCFAs of suckling piglets. Consequently, we found that SCFAs, especially butyrate, were increased in the feces and plasma of piglets. Considering recent evidence suggesting that microbiota-derived butyrate can modulate intestinal barrier integrity and the immunology tolerance of newborn individuals (19, 25, 42), we then assessed the gut permeability and intestinal or systemic inflammatory response of piglets from SF-fed sows. We found that three biomarkers (plasma zonulin, endotoxin, and diamine oxidase) positively related to intestinal permeability and a marker (fecal lipocalin-2) for intestinal inflammation were reduced in suckling piglets from SF-fed sows, indicating that a maternal SF diet reduces intestinal permeability and prevents intestinal inflammation. Interestingly, the Spearman correlation analysis showed that the decreased relative abundances of Bilophila spp. in piglets from SF-fed sows were positively correlated with fecal lipocalin-2 levels. Bacteria within the genus Bilophila are strictly anaerobes and contribute less than 0.01% of the normal gut microbiota in human and pigs (43). It was shown that the increasing abundance of Bilophila-containing clusters is positively associated with fat feeding and gut inflammation in mice and humans (44, 45). The possible mechanism by which Bilophila spp. cause gut inflammation is the production of sulfide that breaks the mucus barrier, thereby allowing the close proximity of bacteria to the epithelium, leading to epithelial damage and inflammation (46). Thus, these observations imply that decreased Bilophila spp. might be one of the important causes of a maternal SF diet during pregnancy to improve gut barrier function and relieve gut inflammation in offspring. Moreover, we also found that the anti-inflammatory mediator IL-10 and immune tolerance mediator TGF-β were increased in the plasma of suckling piglets from SF-fed sows. Therefore, a maternal SF diet during gestation may prevent the overactivation of immune responses, thus allowing a greater proportion of the nutrients contained in milk to be directed toward growth rather than the immune response. Notably, the Spearman correlation analysis showed that the genus Bacteroides was positively correlated with plasma IL-10 concentration. Recent reports have suggested that polysaccharides and butyrate generated by Bacteroides spp. promote healthy gut immunity by stimulating CD4 expansion and the production of the anti-inflammatory cytokine IL-10 (47, 48). Our findings support the hypothesis that the maternal consumption of SF during pregnancy could shape the microbiota community of offspring to a healthier pattern. However, whether these changed genera directly contribute to the phenotype needs further investigation. Additionally, this study does not allow us to determine the mechanism through which maternal gestational diet alters the offspring gut microbiome. Alterations to the maternal microbiome in gut and milk associated with diet are thought to impact bacterial transmission to the neonate, but when and how this occurs are uncertain. Given the overall importance of maternal diet during gestation to offspring health, further studies are warranted to clarify these potential mechanisms.
In conclusion, our data show that a maternal SF diet during pregnancy is independently associated with significant changes in intestinal microbiota composition and metabolism of the 14-day-old suckling piglets. These findings have direct implications for refining dietary recommendations in pregnancy. Moreover, a maternal SF diet reduces intestinal permeability and prevents intestinal inflammation and excessive systemic immune response of the suckling piglets. Therefore, the suckling piglets' resistance to disease was enhanced, diarrhea was reduced, and weight gain was raised. Additionally, putative correlations were observed between genera and parameters on growth traits and gut health, implying that the changes in the gut microbiota in response to a maternal SF diet may also be directly correlated with the offspring's growth and gut development, but further research is required to address these potential mechanisms.
MATERIALS AND METHODS
Ethical approval.
All procedures involving animals were carried out in accordance with guidelines for animal studies issued by the Institutional Animal Care and Use Committee of Huazhong Agricultural University, and its protocol was approved by this committee (permit number HZAUSW-2016-009).
Animals, diets, and housing.
A total of 24 multiparous Landrace sows with an average parity of 4.74 ± 1.10 were selected to this study. Sows did not have diarrhea and had never received antibiotics before the study. Sows were inseminated with semen from the same Duroc boar. After insemination, sows were then allocated to one of two treatment groups (12 sows/treatment) based on their parity, back fat thickness, and body weight (BW). During gestation, the sows were fed with two different diets consisting of a control gestation diet (CON) and the same control diet supplemented with 2.0% pregelatinized waxy maize starch (Hangzhou, China) plus guar gum (Yunzhou, China) to replace 2% rice bran meal (SF) (85.7% pregelatinized waxy maize starch and 14.3% guar gum). Each sow was fed the same amount of feed during the whole gestation. In detail, sows were fed 2.0 kg/day of the corresponding diet from days 0 to 30 of gestation, 2.4 kg/day diet from days 31 to 80 of gestation, 2.6 kg/day diet from days 81 to 95 of gestation, and 3.0 kg/day diet from day 96 to parturition. Sows were fed twice per day at 0700 and 1400 h. All sows were allowed to consume the same lactation diets ad libitum. All diets based on corn-soybean meal were formulated to meet or exceed the nutrient requirements of gestating sows as recommended by the National Research Council (49) and to contain the same content for all nutrients other than soluble fiber level. Details are provided in the supplemental material describing sow diet ingredients and nutrient composition (Table S1). On day 107 of pregnancy, sows were moved to individual farrowing pens with crates, slatted floors, and heat pads for the piglets. At parturition, the numbers of stillborn and born-alive piglets within the litter were recorded. In the 12 h following farrowing, the litter size and the individual piglet birth weights were measured. When possible, the litter size was adjusted to 11 to 12 piglets by adding or removing piglets within each sows' dietary group without changing the mean litter birth weight. Piglets had no access to creep feed during the experiment and therefore relied on sow's milk as their sole source of nutrients. The piglets had no access to probiotics and antibiotics. Both sows and piglets had free access to water.
Determination of growth performance and diarrhea rate.
The body weights of the piglets were individually measured on days 1, 7, 14, and 21 to determine the ADG. The health status and mortality of each piglet were recorded, and the occurrence of diarrhea was visually assessed and evaluated by individual scoring the consistency of the feces at 0900 and 1600 h each day by trained observers blinded to the treatments according to the method of Marquardt et al. (50). The diarrhea rate (%) was calculated as ([the total number of piglets with diarrhea within a treatment]/[total number of experimental piglets × total observational days]) × 100. The diarrhea index was calculated as sum of feces score/(total number of experimental piglets × total observational days).
Sample collection.
On day 14, one median-weight piglet from each litter (total of 12 piglets/treatment) was selected to collect blood and fecal samples. Blood samples were collected from piglets by vena jugularis, with a minimum amount of stress, into heparinized tubes (5 ml). Plasma samples were then obtained by centrifuging the blood samples at 3,000 × g and 4°C for 10 min and were stored at −80°C until analysis. Fresh fecal samples were individually collected using sterile 20-ml centrifuge tubes (without any treatment) from the piglets and then stored at −80°C until analysis.
Analysis of fecal and plasma short-chain fatty acids.
The short-chain fatty acid (acetate, propionate, butyrate, and valerate) concentrations and branched-chain fatty acid (BCFA) (isobutyrate and isovalerate) concentrations in feces and plasma of the sows were analyzed by a gas chromatographic (GC) method, as described by Chen et al. (51), with minor modifications. Briefly, approximately 1.5 g of feces was first homogenized in 1.5 ml of deionized water. The samples were centrifuged at 12,000 × g and 4°C for 10 min. Thereafter, supernatants of feces and plasma samples were acidified with 25% meta-phosphoric acid at a 1:5 ratio. After 30 min at 4°C, samples were then centrifuged at 12,000 × g and 4°C for 10 min. An aliquot of the supernatant (1 μl) was separated and analyzed using a gas chromatograph (GC 2010; Shimadzu, Japan) equipped with a CP-Wax 52 CB column of 30.0 m by 0.53 mm internal diameter (i.d.) (Chrompack, The Netherlands). SCFAs were quantified using external standard curves from 0.5 to 100 μmol/ml the respective authentic organic acids (Fluka, Switzerland).
Determination of hormones, zonulin, endotoxin, diamine oxidase, and cytokines in plasma and lipocalin-2 in feces.
Fecal homogenates were prepared by solubilizing feces in 10% (wt/vol) phosphate-buffered saline (PBS) and were stored at −80°C. The plasma GH, IGF-1, IL-6, IL-10, TNF-α, TGF-β, zonulin, and endotoxin and the fecal total lipocalin-2 concentrations were determined by using porcine enzyme-linked immunosorbent assay kits (Bio-Swamp Life Science, Wuhan, China), according to the manufacturer's instructions. The plasma diamine oxidase activity was determined using an enzymatic kinetic spectrophotometry method with the diamine oxidase assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All procedures were performed in duplicate.
DNA extraction, 16S rRNA gene amplification, and Illumina MiSeq sequencing.
Microbial DNA was extracted from 200 mg of each fecal sample with a QIAamp Fast DNA stool minikit (Qiagen, Germany), according to the manufacturer's instructions. Successful DNA isolation was separated by agarose gel electrophoresis. The forward primer 341F (5′-ACT CCT ACG GGA GGC AGC AG-3′) and the reverse primer 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′) were used for amplification of the V3–V4 hypervariable region of a 16S rRNA gene. The PCR conditions were a predenaturation cycle at 94°C for 4 min, 25 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 45 s, elongation at 72°C for 30 s, and a final postelongation cycle at 72°C for 5 min. The PCR products were purified with AMPure XP beads (Agencourt). After purification, the PCR products were used for the construction of the libraries and then paired-end sequenced (2 × 250) on a MiSeq platform (Illumina, USA) at the Beijing Genomics Institute (BGI, China).
Sequence filtering, OTU clustering, and sequence analyses.
Sequences with an average Phred score lower than 30, ambiguous bases, homopolymer runs exceeding 6 bp, and sequence lengths shorter than 100 bp were removed. Only sequences with overlaps longer than 10 bp and without any mismatch were assembled according to their overlap sequences. Barcode and sequencing primers were trimmed from the assembled sequence. To avoid the effect of the sequencing depth on the composition of microbiota (52), we rarefied the library size to 27,346 tag-depth per sample by using the rarefy function in the R package. High-quality tags were clustered into operational taxonomic units (OTUs) at the 97% similarity level with USEARCH (53). Taxonomy assignments for 16S rRNA gene sequences were made with the RDP Classifier program (version 2.2) (54) and the Silva 16S sequence database. A Venn diagram was generated for comparisons among the OTUs of the groups. The alpha-diversity values of each sample were assessed on the basis of the observed OTUs, Chao1, and Shannon index. Beta-diversity measures dependent on weighted-UniFrac distance were calculated using mothur. LEfSe was conducted to identify bacterial taxa differentially represented between different groups at the genus or higher taxonomy level (biomarkers) (55).
Statistical analysis.
The litter was the experimental unit for growth performance and diarrhea score data (BW, ADG, diarrhea rate, and diarrhea index), and the piglet was the experimental unit for other indices. The normal distribution of data was verified by a Kolmogorov-Smirnov test before analysis. Differences for the data, including growth performance, SCFAs, plasma growth-related hormone, and biomarkers related to intestinal permeability and inflammation were examined by a Student t test (Statistical Analysis System 8.1; Cary, NC). The χ2 test was used to test for the diarrhea rate. Significance is reported at a P value of <0.05. The comparisons of taxonomic composition were performed using Metastats with Benjamini-Hochberg correction. Significance was considered at a P value of <0.05 and q of <0.05. All data are expressed as means ± standard error of the mean (SEM). Correlations were analyzed by using Spearman's correlation in R 3.0.2 (the R Foundation) with the RStudio 0.97.310 package and gplots for the heat map. Correlation results were corrected by false-discovery rate (FDR) analysis according to the Benjamini-Hochberg procedure, with an α of <0.05.
Accession number(s).
The 16S rRNA gene sequence data were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the accession number SRP149389.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Yangxiang Joint Stock Company for providing sow feeding facilities.
S.J. and J.P. designed the experiments, C.C. performed the experiments and wrote the manuscript, and H.W. and C.X. analyzed the data. All authors approved the final version to be published. All authors approved the final manuscript.
This research was supported by the National Key Research and Development Project of China (grant 2017YFD0502004), the China Agriculture Research System (grant CARS-36), Hubei Provincial Creative Team Project of Agricultural Science and Technology (grant 2007-620), and Fundamental Research Funds for the Central Universities of China (grant 2662017PY017).
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01047-18.
REFERENCES
- 1.Hanson MA, Gluckman PD. 2014. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94:1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJP. 2004. The developmental origins of adult disease. J Am Coll Nutr 23:588–595. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 3.Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, Aagaard KM. 2016. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 8:77. doi: 10.1186/s13073-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai M, Jellyman JK, Han G, Beall M, Lane RH, Ross MG. 2014. Rat maternal obesity and high fat diet program offspring metabolic syndrome. Am J Obstet Gynecol 211:237.e1–237.e13. doi: 10.1016/j.ajog.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallam MC, Barile D, Meyrand M, German JB, Reimer RA. 2014. Maternal high-protein or high-prebiotic-fiber diets affect maternal milk composition and gut microbiota in rat dams and their offspring. Obesity 22:2344–2351. doi: 10.1002/oby.20849. [DOI] [PubMed] [Google Scholar]
- 6.Hallam MC, Reimer RA. 2013. A maternal high-protein diet predisposes female offspring to increased fat mass in adulthood whereas a prebiotic fibre diet decreases fat mass in rats. Br J Nutr 110:1732–1741. doi: 10.1017/S0007114513000998. [DOI] [PubMed] [Google Scholar]
- 7.Tamburini S, Shen N, Wu HC, Clemente JC. 2016. The microbiome in early life: implications for health outcomes. Nat Med 22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 8.Mach N, Berri M, Estellé J, Leveneza F, Lemonnier G, Denis Leplat JJ, Chevaleyre C, Billon Y, Dore J, Rogel-Gaillard C, Lepage P. 2015. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ Microbiol Rep 7:554–569. doi: 10.1111/1758-2229.12285. [DOI] [PubMed] [Google Scholar]
- 9.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Rodriguez JGZ, Rogers AB, Robine N, Loke P, Blaser MJ. 2014. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. 2016. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7:196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rainone V, Schneider L, Saulle I, Ricci C, Biasin M, Al-Daghri NM, Giani E, Zuccotti GV, Clerici M, Trabattoni D. 2016. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int J Obes (Lond) 40:1026. doi: 10.1038/ijo.2016.26. [DOI] [PubMed] [Google Scholar]
- 12.Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. 2008. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr 153:646–650. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira APB, Texeira TFS, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. 2012. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 108:801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. 2012. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One 7:e37160. doi: 10.1371/journal.pone.0037160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Quelen F, Chevalier J, Rolli-Derkinderen M, Mourot J, Neunlist M, Boudry G. 2011. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol 589:4341–4352. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Lu L, Sun J, Petrof EO, Claud EC. 2016. Preterm infant gut microbiota affects intestinal epithelial development in a humanized microbiome gnotobiotic mouse model. Am J Physiol Gastrointest Liver Physiol 311:G521–G532. doi: 10.1152/ajpgi.00022.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawicki CM, Livingston KA, Obin M, Roberts SB, Chung M, McKeown NM. 2017. Dietary fiber and the human gut microbiota: application of evidence mapping methodology. Nutrients 9:125. doi: 10.3390/nu9020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Song P, Fan P, Hou C, Thacker P, Ma X. 2015. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr 145:2774–2780. doi: 10.3945/jn.115.217406. [DOI] [PubMed] [Google Scholar]
- 20.Tan C, Wei H, Zhao X, Xu C, Peng J. 2017. Effects of dietary fibers with high water-binding capacity and swelling capacity on gastrointestinal functions, food intake and body weight in male rats. Food Nutr Res 61:1308118. doi: 10.1080/16546628.2017.1308118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimotoyodome A, Suzuki J, Kameo Y, Hase T. 2011. Dietary supplementation with hydroxypropyl-distarch phosphate from waxy maize starch increases resting energy expenditure by lowering the postprandial glucose-dependent insulinotropic polypeptide response in human subjects. Br J Nutr 106:96–104. doi: 10.1017/S0007114510005854. [DOI] [PubMed] [Google Scholar]
- 22.Fuongfuchat A, Seetapan N, Makmoon T, Pongjaruwat W, Methacanon P, Gamonpilas C. 2012. Linear and non-linear viscoelastic behaviors of crosslinked tapioca starch/polysaccharide systems. J Food Eng 109:571–578. doi: 10.1016/j.jfoodeng.2011.10.022. [DOI] [Google Scholar]
- 23.Ohashi Y, Sumitani K, Tokunaga M, Ishihara N, Okubo T, Fujisawa T. 2015. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef Microbes 6:451–455. doi: 10.3920/BM2014.0118. [DOI] [PubMed] [Google Scholar]
- 24.Butler AA, Le Roith D. 2001. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles1. Annu Rev Physiol 63:141–164. doi: 10.1146/annurev.physiol.63.1.141. [DOI] [PubMed] [Google Scholar]
- 25.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. 2017. Regulation of inflammation by microbiota interactions with the host. Nat Immunol 18:851–860. doi: 10.1038/ni.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luk GD, Bayless TM, Baylin SB. 1983. Plasma postheparin diamine oxidase. Sensitive provocative test for quantitating length of acute intestinal mucosal injury in the rat. J Clin Invest 71:1308–1315. doi: 10.1172/JCI110881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. 2012. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca JE, Santos MJ, Canhão H, Choy E. 2009. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev 8:538–542. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Pang Y, Moses HL. 2010. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. 2017. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol 17:508. doi: 10.1038/nri.2017.58. [DOI] [PubMed] [Google Scholar]
- 31.Desai M, Jellyman JK, Ross MG. 2015. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes (Lond) 39:633–641. doi: 10.1038/ijo.2015.13. [DOI] [PubMed] [Google Scholar]
- 32.Slifierz MJ, Friendship RM, Weese JS. 2015. Longitudinal study of the early-life fecal and nasal microbiotas of the domestic pig. BMC Microbiol 15:1–12. doi: 10.1186/s12866-014-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, Isaacson RE. 2011. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet Microbiol 153:124–133. doi: 10.1016/j.vetmic.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Xu Y, Chen X, Fang C, Zhao L, Chen F. 2017. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front Microbiol 8:1688. doi: 10.3389/fmicb.2017.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, Sonnenburg JL. 2011. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10:507–514. doi: 10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu Q, Li P, Hao S, Zhang Y, Kim SW, Li H, Ma X, Gao S, He L, Wu WJ, Huang X, Hua J, Zhou B, Huang R. 2015. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci Rep 5:9938. doi: 10.1038/srep09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Qian K, Wang C, Wu Y. 2017. Roles of probiotic lactobacilli inclusion in helping piglets establish healthy intestinal inter-environment for pathogen defense. Probiotics Antimicrob Proteins 10:243–250. doi: 10.1007/s12602-017-9273-y. [DOI] [PubMed] [Google Scholar]
- 38.Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. 2017. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 39.Comstock LE. 2009. Importance of glycans to the host-Bacteroides mutualism in the mammalian intestine. Cell Host Microbe 5:522. doi: 10.1016/j.chom.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Chassard C, Goumy V, Leclerc M, Del'homme C, Bernalier-Donadille A. 2007. Characterization of the xylan-degrading microbial community from human faeces. FEMS Microbiol Ecol 61:121–131. doi: 10.1111/j.1574-6941.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 41.Fernández J, Redondo-Blanco S, Gutiérrez-del-Río I, Miguélez EM, Villar CJ, Lombó F. 2016. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: a review. J Funct Foods 25:511–522. doi: 10.1016/j.jff.2016.06.032. [DOI] [Google Scholar]
- 42.Nakajima A, Kaga N, Nakanishi Y, Ohno H, Miyamoto J, Kimura I, Hori S, Sasaki T, Hiramatsu K, Okumura K, Miyake S, Habu S, Watanabe S. 2017. Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J Immunol 199:3516. doi: 10.4049/jimmunol.1700248. [DOI] [PubMed] [Google Scholar]
- 43.Baron EJ. 1997. Bilophila wadsworthia: a unique Gram-negative anaerobic rod. Anaerobe 3:83–86. doi: 10.1006/anae.1997.0075. [DOI] [PubMed] [Google Scholar]
- 44.Shen W, Wolf PG, Carbonero F, Zhong W, Reid T, Gaskins HR, McIntosh MK. 2014. Intestinal and systemic inflammatory responses are positively associated with sulfidogenic bacteria abundance in high-fat-fed male c57bl/6j mice. J Nutr 144:1181–1187. doi: 10.3945/jn.114.194332. [DOI] [PubMed] [Google Scholar]
- 45.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. 2012. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ijssennagger N, van der Meer R, van Mil SW. 2016. Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol Med 22:190–199. doi: 10.1016/j.molmed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. 2010. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol 3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 48.Tanoue T, Atarashi K, Honda K. 2016. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 16:295–309. doi: 10.1038/nri.2016.36. [DOI] [PubMed] [Google Scholar]
- 49.National Research Council. 2012. Nutrient requirements of swine: 11th revised ed. National Academies Press, Washington, DC. [Google Scholar]
- 50.Marquardt RR, Jin LZ, Kim JW, Fang L, Frohlich AA, Baidoo SK. 1999. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol Med Microbiol 23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 51.Chen H, Chen D, Michiels J, De Smet S. 2013. Dietary fiber affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Commun Agric Appl Biol Sci 78:71–78. [PubMed] [Google Scholar]
- 52.Hughes JB, Hellmann JJ. 2005. The application of rarefaction techniques to molecular inventories of microbial diversity. Methods Enzymol 397:292–308. doi: 10.1016/S0076-6879(05)97017-1. [DOI] [PubMed] [Google Scholar]
- 53.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.