Abstract
Inflammatory bowel disease (IBD) is a chronic disease with gastrointestinal dysfunction as well as comorbidities such as inflammation-induced bone loss and impaired immune response. Current treatments for IBD all have negative, potentially severe side effects. We aimed to test whether exogenous treatment with irisin, a novel immunomodulatory adipomyokine, could ameliorate IBD-induced lymphatic and bone alterations. Irisin treatment improved both gut and bone outcomes by mitigating inflammation and restoring structure. In the gut, IBD caused colonic lymphatic hyperproliferation into the mucosal and submucosal compartments. This proliferation in the rodent model is akin to what is observed in IBD patient case studies. In bone, IBD increased osteoclast surface and decreased bone formation. Both gut and osteocytes in bone exhibited elevated levels of TNF-α and receptor activator of NF-κB ligand (RANKL) protein expression. Exogenous irisin treatment restored normal colonic lymphatic architecture and increased bone formation rate concurrent with decreased osteoclast surfaces. After irisin treatment, gut and osteocyte TNF-α and RANKL protein expression levels were no different from vehicle controls. Our data indicate that the systemic immunologic changes that occur in IBD are initiated by damage in the gut and likely linked through the lymphatic system. Additionally, irisin is a potential novel intervention mitigating both local inflammatory changes in the gut and distant changes in bone.—Narayanan, S. A., Metzger, C. E., Bloomfield, S. A., Zawieja, D. C. Inflammation-induced lymphatic architecture and bone turnover changes are ameliorated by irisin treatment in chronic inflammatory bowel disease.
Keywords: Crohn’s disease, immunolymph, osteoimmunology, therapeutic intervention
Inflammatory bowel disease (IBD) is a group of diseases including ulcerative colitis and Crohn’s disease that affects ∼1.6 million people in the United States and 2.2 million in Europe, and its incidence and prevalence is increasing worldwide (1, 2). Inflammation occurs along all or multiple sections of the gastrointestinal tract (GI), as in Crohn’s disease, or in specific sections, as in ulcerative colitis. Particularly of concern is the increasing incidence of pediatric onset IBD, especially Crohn’s disease (1, 3). Pediatric-onset IBD is characterized by more extensive disease and lifelong complications (1, 3). Although the exact etiology of IBD remains largely unknown, it is generally considered an autoimmune condition in which the immune system mounts an attack against tissues within the digestive tract leading to chronic, local and systemic inflammation (4–6). Indeed, patients with IBD suffer not only from the gut pathology but also from multiple extraintestinal inflammation-induced comorbidities (4–6). It is not fully understood how and which components of the immune system (cells and cytokines) selectively target the gut, or what roles the vascular systems play in transporting these components to and from the local gut sites to systemic circulation. Understanding this paradigm would not only allow for better characterization of the disease causation but also aid in the development of holistic treatments for systemic inflammatory conditions caused by IBD.
The lymphatic system is a component of the circulatory system that acts as the main transport path of fluid and elements from the parenchymal tissues to the lymph nodes via the afferent lymphatic vessels, and from the nodes to systemic circulation via the efferent lymphatic vessels (7). A functional lymphatic vascular network is necessary for delivery of lymph contents representing the immunologic state of the drained parenchyma to the lymph node to allow for appropriate immunologic responses. Inflammation-induced lymphangiogenesis, or the formation of new lymphatic structures, occurs in pathologies such as IBD, in which the intestinal lymphatic vessels proliferate from their normal topology in the submucosa to every layer of the inflamed small and large bowel (8–10). It is not fully understood what drives this uncontrolled lymphatic infiltration, whether it affects function, or how local cytokines (known to be significantly altered in IBD patients) are associated with GI lymphatic changes (11).
IBD causes comorbidities including high prevalence of osteopenia, osteoporosis, and, consequently, elevated fracture risk (12–14). This inflammation-induced bone loss is characterized by increased osteocyte protein expression of receptor activator of NF-κB ligand (RANKL), TNF-α, and IL-6 (all factors implicated in IBD pathology and known to stimulate bone resorption), increased osteoclast-covered bone surfaces, and decreased bone formation (15). However, it is unknown whether the immunologic processes driving bone loss in IBD are distinct from or parallel to those in the gut.
Furthermore, IBD is a chronic condition that has no cure; therefore, all current treatments aim to mitigate disease symptoms, but many have significant negative consequences. Corticosteroids further exacerbate bone loss and cause detrimental metabolic changes (16). Previously, immunosuppressant agents like azathioprine were a common therapy for IBD. However, azathioprine can cause leucopenia and bone marrow suppression or toxicity, leading to potentially lethal complications (17). Current treatments for IBD patients often include anticytokine treatments such as anti-TNF therapy. It has been shown that anti-TNF therapy can lead to increased risk of infection and autoantibody development (18, 19). Thus, the development of safe treatments to effectively mitigate inflammation in the gut and extraintestinal sites are needed for IBD patients.
Exercise induces peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) in muscle, which in turn increases the expression and secretion of the adipomyokine irisin (20). Muscle-specific knockout of PGC-1α results in local muscle inflammatory gene up-regulation, leading to our hypothesis that irisin, a circulating factor, could have systemic anti-inflammatory effects (21). Some studies have shown correlations between lower serum irisin and pathologies that involve some element of inflammation (22–26) In addition, recent studies have demonstrated that exogenous irisin treatment improved tissue function in lung, brain, and vascular endothelium in various rodent inflammatory pathologic models (25–27) Irisin has also recently emerged as a bone anabolic factor (28, 29). We know of no studies that have utilized irisin as a systemic anti-inflammatory agent, however, and little is known about effects of irisin on gut health. We hypothesized that the circulating factor irisin plays a holistic, beneficial role in modulating inflammation; therefore, treatment of IBD-like states in rodents with exogenous irisin would mitigate the inflammation-induced lymphatic and bone changes caused by the pathology.
This study aimed to characterize the lymphatic changes associated with GI inflammation that lead to immunologically driven pathologies in distant tissues (e.g., inflammation-induced alterations in bone turnover) and examine how exogenous irisin treatment during chronic IBD ameliorates the disease outcomes. We hypothesized that the immunologic mechanisms driving lymphatic changes in the gut would parallel immunologic changes influencing bone outcomes. Furthermore, we tested whether exogenous irisin treatment would holistically mitigate the T helper (Th)1 cell–driven gut and bone inflammatory pathology.
MATERIALS AND METHODS
Study design
Thirty-two 6-wk-old male Sprague-Dawley rats were ordered from Envigo (Huntingdon, United Kingdom) and housed individually in an institutionally approved animal facility on a 12-h light/dark cycle. At 2 mo of age, animals were randomly divided into 4 different groups (n = 8/group): vehicle (Veh), Veh with irisin (Veh+Ir), IBD induced with 2,4,6-trinitrobenzenesulfonic acid (TNBS), and IBD induced with TNBS with irisin (TNBS+Ir). Previous work demonstrated sufficient power for bone measures and colon histopathology in groups of ≥6 (15). Gut inflammation in a rodent model of IBD was induced by rectally instilling 1 μl/g body weight of TNBS (30 mg/kg; MilliporeSigma, Burlington, MA, USA) in 30% ethanol:DiH2O solution (30:70, v/v) (on d 1, 7, 14, 21 and 28) as previously described (15, 30, 31). TNBS-induced IBD rodent models are primarily likened to human Crohn’s disease because all areas of the bowel wall are affected (30, 31). Rectal instillations of TNBS in 30% ethanol or 30% ethanol alone (for Veh) began at 2 mo of age and continued for up to 4 wk. For instillation, animals were anesthetized and an 18-gauge catheter with a blunted end was inserted 7.5 cm into the rectum. The anus was held closed for 5 min after instillation to control the contact of the enema solution with the colon, after which animals were allowed to recover from anesthesia and excess enema solution was excreted. This process occurred once per week; the fifth and final instillation occurred 3–4 d before animals were euthanized. Fluorochrome calcein labels (MilliporeSigma) were injected intraperitoneally 8 and 3 d prior to euthanization to label mineralized surfaces on bone. All animal procedures were approved by the Texas A&M Institutional Animal Use and Care Committee and conform to National Institutes of Health (Bethesda, MD, USA) guidelines.
Irisin injections
Irisin-treated rats received 2 intraperitoneal injections of recombinant irisin (18 ng/ml; AdipoGen Life Sciences, San Diego, CA, USA) per week (3.5 d apart) for 3 wk.
Tissue processing and histologic analysis
The entire colon was removed from each animal, processed (∼3-cm-long portion was used), and stained with hematoxylin and eosin (in a “Swiss roll” orientation). Samples were scored on a scale of 0–4 (0, no damage; 4, severe damage) based on epithelial structure (1–2: loss of goblet cells, structural modifications, or both; 3–4: damage to epithelium in terms of missing cells, gaps, complete erosion, or fibrosis), crypt structure (1: minimal gaps between crypts, basal 1/3 damaged, intact epithelium; 2: inflammatory infiltrate, basal 2/3 damaged; 3: loss of crypt, beginning of fibrosis; 4: complete loss of crypt and epithelium), cellularity (1: mucosal infiltrate; 2: mucosal + submucosal; 3: pregranuloma; and 4: granuloma or swelling into epithelial layer from crypt), and edema (separation of muscularis mucosa with epithelial layer, thickness of submucosa and muscularis externa). Furthermore, skip lesions typically occurred in TNBS colons; overall scores were adjusted to account for the area of tissue affected because the entire ∼3 cm section was assessed. We accounted for undamaged sites by calculating the scores based on the percentage of the area that was damaged (32, 33). Sites were assessed at both ×20 and 40 magnification. All tissue scoring was conducted blindly with an experienced pathologist who verified and validated the scoring system.
Colonic immunofluorescence
Colon tissues were collected, flushed, and processed for embedding in paraffin or optimal cutting temperature compound. Some tissues were fixed in 4% paraformaldehyde overnight and embedded in paraffin. The paraffin sections (10 μm) were then deparaffinized, blocked for 30 min with 2.5% goat serum:PBS (1:40, v/v) at room temperature, and incubated overnight at 4°C with primary antibodies: anti-podoplanin (Novus Biologicals, Littleton, CO, USA), anti–TNF-α (LifeSpan Biosciences, Seattle, WA, USA), and anti-RANKL (Abcam, Cambridge, United Kingdom). Frozen tissue sections (10 μm) were fixed in 4% paraformaldehyde for 45 min at room temperature and incubated overnight at 4°C with primary antibodies: anti-podoplanin, anti–IL-4 (Abcam), anti–IL-10 (Abcam), and anti–IFN-γ (Abcam). Sections were incubated with corresponding secondary antibodies for mouse IgG1 Alexa Fluor-488 and rabbit IgG Alexa-Fluor 633 (Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at room temperature in the dark. Sections were then mounted in ProLong Gold Antifade (Thermo Fisher Scientific) with DAPI and viewed through a confocal microscope (Olympus Fluoview 300; Tokyo, Japan). Images (1024 × 1024 pixels) were acquired from 5 randomly selected fields using a ×20 objective lens with 2-μm-thick z-stack slices. Z stacks were imported into ImageJ v.1.51 and quantified consistently across each group [fluorescence integrated density (ID) = region of interest area × the mean fluorescence intensity].
Dynamic and static bone histomorphometry
Undemineralized right proximal tibia and 4th lumbar vertebra (L4) were fixed in 4% phosphate buffered formalin and then subjected to serial dehydration and embedded in J. T. Baker methyl methacrylate (Avantor, Center Valley, PA, USA). Serial frontal sections (8 µm thick) were left unstained for analysis of fluorochrome labels (6 d apart in final week) using dynamic histomorphometry, with measures including relative mineralized bone surface, mineral apposition rate, and bone formation rate (BFR) as previously described (15). Additional frontal sections of the proximal tibia and L4 (4 µm thick) were treated with a Von Kossa stain and tetrachrome counterstain and imaged at ×40 magnification to identify relative osteoclast surface and osteoid surface as previously described (15). All analyses were completed on an OsteoMeasure Analysis System version 3.3 (Osteometrics, Decatur, GA, USA) by the same individual to ensure consistency in all measures. All nomenclature for cancellous histomorphometry follows standard usage (34).
Immunohistochemistry of osteocyte proteins
Left distal femurs were fixed in 4% phosphate-buffered formalin and then stored in 70% ethanol prior to decalcification in a formic acid:sodium citrate solution (36:10, v/w). Tissues were then paraffinized and 8-µm sections were immunostained with the following primary antibodies as previously described: anti–TNF-α, anti-IL–6 (Abcam), anti–IL-10 (Abcam), anti–IL-4 (Abcam), anti–annexin V (Abcam), anti-sclerostin (R&D Systems, Minneapolis, MN, USA), anti-RANKL, and anti-osteoprotegerin (OPG; Biorbyt, Cambridge, United Kingdom) (15). All sections were counterstained with methyl green (see Supplemental Fig. 1 for representative images). Sections were analyzed as the percentage of osteocytes stained positively for the protein within a 4-mm2 region of interest within the distal femur cancellous bone as previously described (15). All analyses were completed by the same individual.
Statistical analyses
Data were analyzed using a 2 × 2 factorial ANOVA (TNBS by irisin). If the model 2 × 2 ANOVA was statistically significant (P < 0.05), main effects for TNBS, irisin, and TNBS * Ir interaction were recorded. Post hoc all-groups analyses were conducted to determine which groups were different when the model was statistically significant. Data are represented as means ± sd.
RESULTS
All animals, regardless of treatment received, maintained body weight and normal eating behaviors
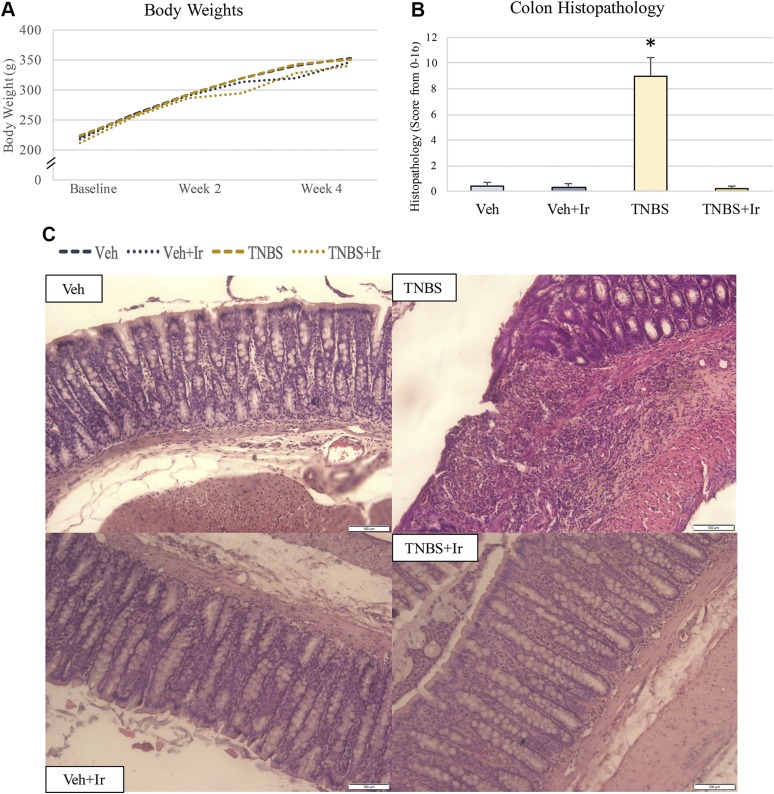
There were no differences in body weight (P = 0.465) or food intake during the study (Fig. 1A). No animals exhibited overt signs of sickness or distress during the entire experimental period, and no differences in cage activity as a result of treatment were observed.
Figure 1.
Body weights and colon histopathology. A) Body weights did not change during the study. B) Aggregated colon histopathology scores validate gut inflammation and damage in the TNBS rats. Histopathology in TNBS+Ir was no different from that of the 2 Veh-treated groups. *P < 0.05. C) Representative images of colon histopathology. All error bars indicate sd. Scale bars, 100 μm.
Irisin treatment during IBD restored GI structural integrity
TNBS animals had a disrupted intestinal epithelial lining, with an associated increase in lamina propria cellularity breaching from the submucosa into the mucosal space (Fig. 1B, C). Associated with this breach was disruption to the crypt structures. TNBS animals also developed mild edema. Veh+Ir and TNBS+Ir colon histology were comparable to Veh (Fig. 1B, C).
TNBS resulted in infiltration of podoplanin+ structures into the mucosal compartment that was resolved with irisin treatment
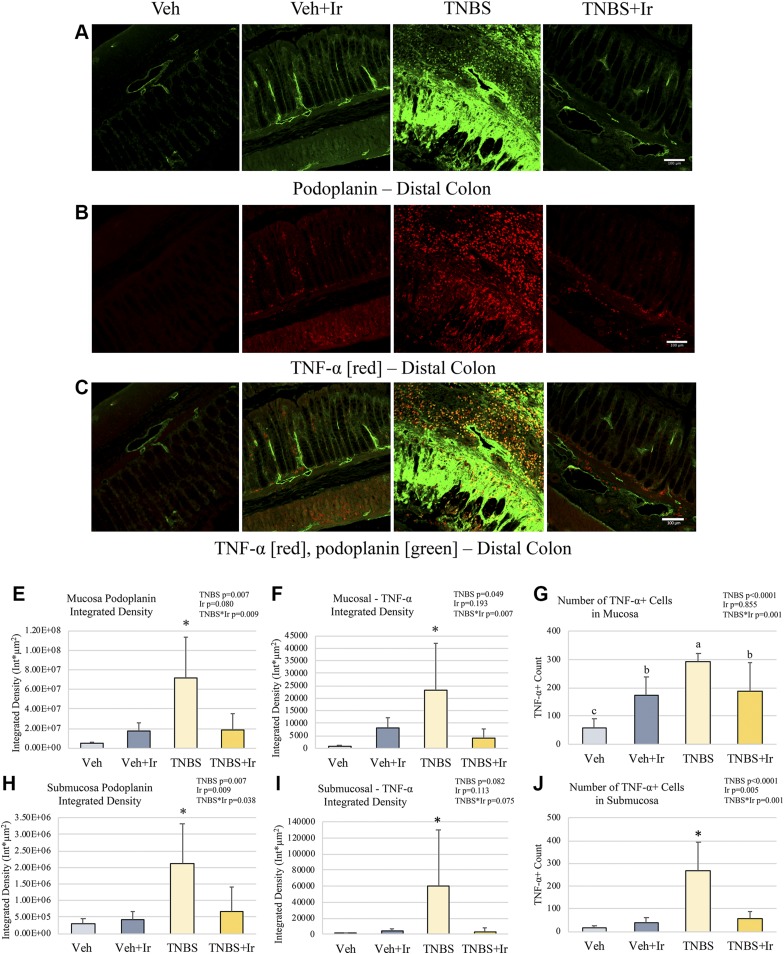
Colonic sections were characterized via immunofluorescence staining for the canonical lymphatic endothelial marker, podoplanin. In TNBS rats, there was a stark increase in surface area and expression of podoplaninhi regions compared with Veh animals, evident in both colonic mucosal and submucosal compartments (Fig. 2A, C, D, G and see Supplemental Fig. 2 for higher magnification representative images). These podoplaninhi regions lacked the distinct traversing lymphatic network observed in the Veh mucosal compartment and instead became amorphous, unorganized structures in TNBS rodents. We quantified this change, accounting for both the increase in area and in podoplanin expression intensity, noting a mucosal 14× ID increase and submucosal 7.5× ID increase in TNBS rodents compared with Veh (Fig. 2D, G). In the TNBS submucosa, the lymphatic marker podoplaninhi was present and had a vascular morphology, with an overall increase in vessel diameters (data not shown). TNBS+Ir animals showed a lymphatic architecture comparable to Veh, with a remarkable restoration of lymphatic architecture in both mucosal and submucosal compartments (Fig. 2A, C). Podoplanin+ ID values for both the mucosal and submucosal compartments were notably lower in TNBS+Ir animals that accompanied the restoration of typical lymphatic topology in these regions (Fig. 2D, G). Although the effects of irisin on lymphatic vessels are unknown, we did observe a mild increase in podoplanin ID directly by irisin (Fig. 2D, G, at the mucosa effect size = 0.191, at the submucosa effect size = 0.189).
Figure 2.
Podoplanin and TNF-α immunofluorescence. A–C) Representative images of podoplanin (A), TNF-α (B), and overlay (C) for each group in the distal colon; original magnification, ×20. D) Podoplanin ID was higher in the mucosa of TNBS than in that of all other groups. E) ID of TNF-α was higher in the TNBS mucosa that in that of all other groups. F) The number of TNF-α+ cells was highest in TNBS followed by both irisin groups; Veh had the lowest number. G) Podoplanin ID was higher in the TNBS submucosal compartment than in that of all other groups. H) TNF-α ID was higher in the TNBS submucosal area than in that of all other groups. I) In the submucosa, the number of TNF-α+ cells was elevated in TNBS compared with all other groups. *P < 0.05. All error bars indicate sd. Scale bars, 100 μm.
The increased podoplanin+ density in TNBS is associated with elevated TNF-α, with irisin ameliorating the proinflammatory cytokine milieu in the colon
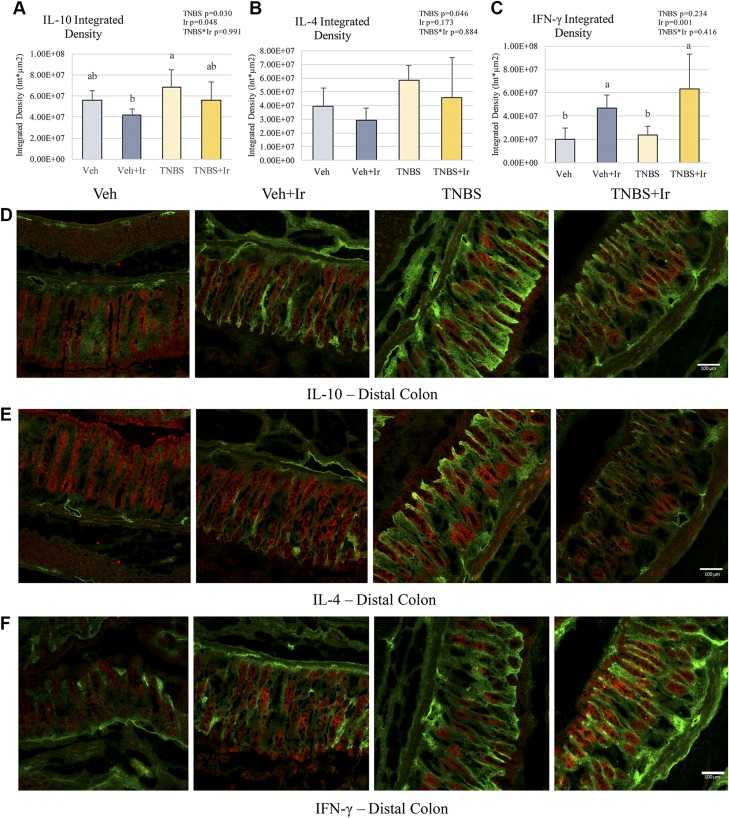
Various proteins involved in inflammatory signaling (including such cytokines as TNF-α, IFN-γ, IL-10, and IL-4) were assessed in the colonic compartments in association with lymphatic structural changes (Figs. 2–4). Notably TNF-α+ cells were significantly elevated in TNBS animals, with regards to number of cells, area of expression covered per region of interest, and protein expression per cell in both the mucosal and submucosal compartments (Fig. 2B, C, E–I). Veh animals had minimal TNF-α expression and cell counts, with any TNF-α+ cells localized to the colonic mucosal compartment (Fig. 2B, C, E–I). TNBS animals had a 6-fold increase in TNF-α+ mucosal cells (effect size = 0.550) and 30-fold increase in TNF-α+ submucosal cells (effect size = 0.556), with respective 31.9-fold mucosal and 76.2-fold submucosal increase in ID (effect size = 0.566 and 0.177, respectively) (Fig. 3E–I). Here we show TNBS+Ir animals also had significantly reduced TNF-α+ ID in both the mucosal submucosal compartments (effect size of TNBS * Ir = 0.486 and 0.495, respectively) compared with TNBS alone in association with a restored lymphatic architecture (Fig. 3E, H). In addition, we observed a reduction in TNF-α+ cells after irisin treatment. We also characterized the impact of additional Th1/2 cytokines, notably IFN-γ, IL-10, and IL-4 (Fig. 4). Expression of these cytokines was restricted to the mucosal space. IL-10 expression was elevated, but not statistically different, in TNBS compared with Veh, with IL-4 also showing an increase in TNBS rats, though not statistically elevated; irisin ameliorated these increases in the TNBS+Ir animals (Fig. 4A, B). Interestingly there was an increase in IFN-γ due to irisin treatment (Fig. 4C).
Figure 4.
Immunofluorescence of IL-10, IL-4, and IFN-γ (all red) colocalized with podoplanin (green) in the distal colon. A) IL-10 ID was slightly decreased after irisin treatment; TNBS showed a more significant change than Veh+Ir did. B) IL-4 ID was not significantly different in any of the 4 groups, but a main effect of TNBS was observed. C) IFN-γ ID was elevated after irisin treatment. D) Representative images of IL-10. E) Representative images of IL-4. F) Representative images of IFN-γ. *P < 0.05. Bars not sharing the same letter (a, b) are statistically different from each other (A, C). Error bars indicate sd. Scale bars, 100 μm.
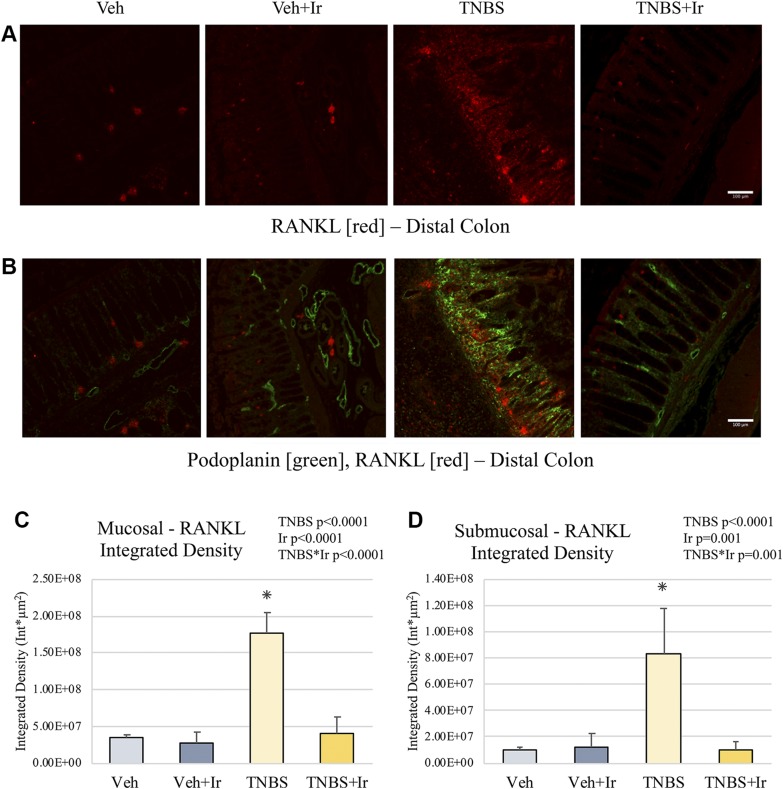
Figure 3.
Gut RANKL. A, B) Representative images of distal colon RANKL (A) and RANKL with podoplanin overlay (B). C) RANKL ID was higher in the TNBS mucosa than in that of all other groups. D) RANKL ID was elevated in the TNBS submucosal area compared with all other groups. *P < 0.05. Error bars indicate sd. Scale bars, 100 μm.
RANKL, a downstream target of TNF-α and a critical factor for lymphoid aggregate formation, is elevated in TNBS animals but ameliorated by irisin treatment
RANKL ID was significantly increased in TNBS animals, in both mucosal and submucosal compartments (effect size = 0.864 and 0.561, respectively; Fig. 3C, D and Supplemental Fig. 3 for representative images at a greater magnification). Significantly, irisin treatment in TNBS+Ir animals ameliorated the elevated RANKL expression to levels comparable to Veh animals (effect size of TNBS*Ir = 0.771 for mucosa; effect size = 0.595 for submucosa; Fig. 3C, D).
Bone turnover is altered due to TNBS favoring bone resorption, whereas irisin treatment improved bone formation
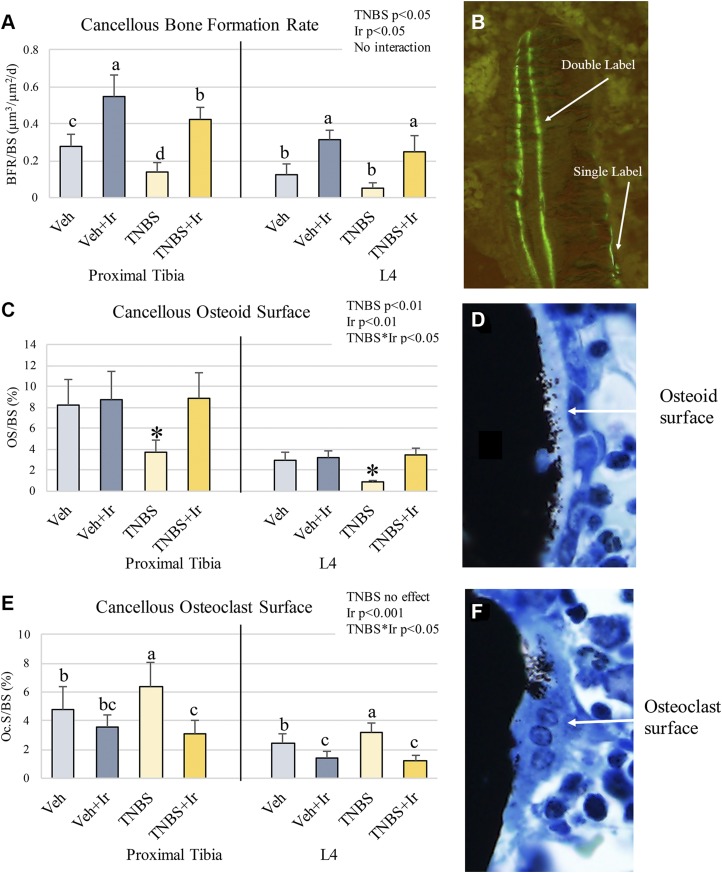
Chronic TNBS resulted in increased osteoclast surface at both the proximal tibia and L4 as well as lower osteoid surface and BFR (Fig. 5A–C).Exogenous treatment of irisin resulted in robust increase in BFR, 1.9–2.4-fold higher than groups that weren’t treated with irisin, regardless of TNBS (effect size = 0.789 for proximal tibia, 0.768 for L4; Fig. 5A). Increased BFR was due to increases in both mineralized surface and mineral apposition rate. Irisin treatment had significant main effects on all measures of bone turnover, resulting in a decrease in osteoclast surface and an increase in osteoid surface and BFR at both bone sites (Fig. 5B, C). Overall, the irisin treatment completely reversed the directionality of changes resulting from chronic TNBS treatment on measures of bone turnover.
Figure 5.
Bone histomorphometry. A) Cancellous BFR at the proximal tibia and L4 were depressed due to TNBS and elevated due to irisin treatment. B) Representative image of single- vs. double-labeled fluorochrome in cancellous bone. D) Representative image of osteoid surface. E) Osteoclast surface was increased in TNBS but decreased due to irisin. F) Representative image of osteoclast surface. *P < 0.05. Bars not sharing the same letter (a, b, c) are statistically different from each other (A, E). Error bars indicate sd.
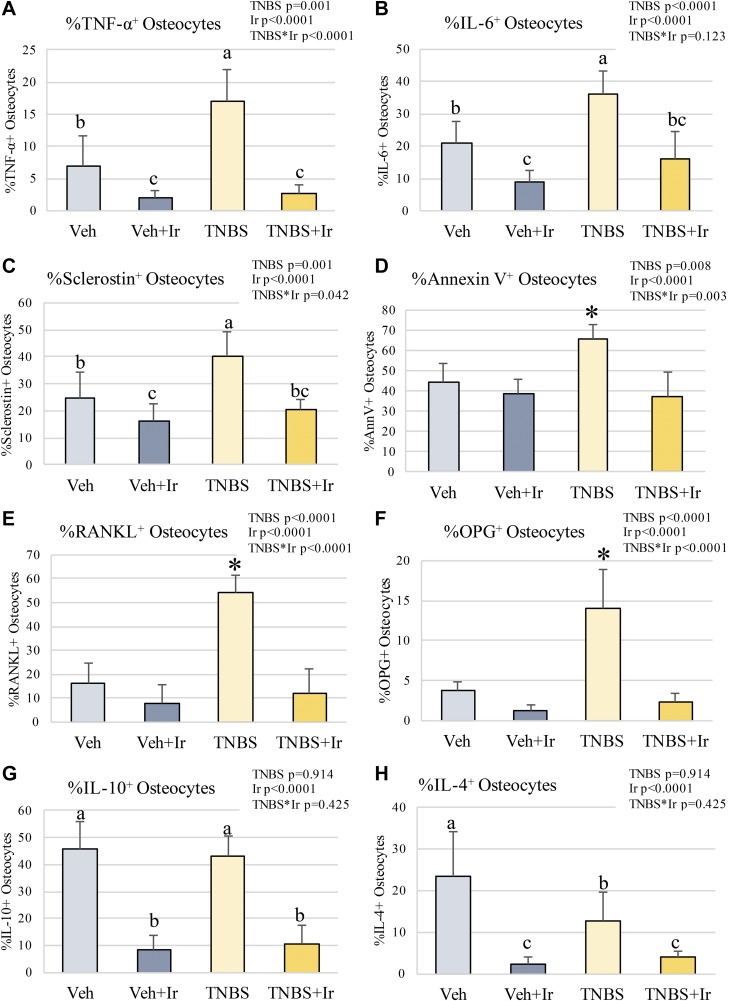
In IBD, osteocyte proteins reflect a proinflammatory state favoring bone resorption, but irisin treatment alters osteocyte proteins favoring an anabolic state in bone
TNBS-induced IBD caused an increase in osteocytes positive for TNF-α, IL-6, sclerostin (an inhibitor of bone formation), and osteoclastogenesis regulators RANKL and OPG (Fig. 6). Additionally, osteocyte apoptosis, as measured by annexin V, was elevated in IBD (Fig. 6D). Irisin treatment lowered these factors to levels at or lower than Veh, concurrent with significant reductions in osteoclast surface and increased BFR (Fig. 6). Other immunologic factors like IL-10 and IL-4 were lowered with irisin treatment, whereas TNBS had little impact on IL-10 and lowered IL-4 (Fig. 6G, H). These data indicate a Th1 inflammatory state in bone during IBD, which is reversed by irisin treatment.
Figure 6.
Osteocyte proteins. A) TNF-α–positive osteocytes were increased in TNBS but were reduced by irisin. B) IL-6–positive osteocytes were prevalent in TNBS but were reduced by irisin. C) TNBS had the greatest number of sclerostin-positive osteocytes; they were reduced in the presence of irisin. D) Annexin V–positive osteocytes were most prevalent in TNBS. E) RANKL-positive osteocytes were most prevalent in TNBS. F) OPG-positive osteocytes were most prevalent in TNBS. G) IL-10–positive osteocytes were reduced in irisin-treated animals. H) IL-4–positive osteocytes were reduced in irisin-treated animals. *P < 0.05. Bars not sharing the same letter (a, b, c) are statistically different from each other (A–C, G, H). Error bars indicate sd.
DISCUSSION
IBD leads to lifelong complications and poor quality of life in patients and all treatments to date also induce serious side effects. Here we report evidence of the anti-inflammatory impact of exogenous irisin treatment on gut and bone in a rodent model of chronic IBD. We discovered that parallel immunologic changes occurred in both the gut, the initial site of inflammation, and the bone, a site distant from the initial insult. Our data indicate a TNF-α/RANKL-driven pathology in both tissues likely leads to both lymphatic hyperproliferation and altered bone turnover. Importantly, treatment with irisin ameliorated the increase in these inflammatory markers during chronic IBD, restoring lymphatic structure and shifting bone turnover to favor formation. Here we demonstrated the beneficial effect of treatment with a novel exercise-associated myokine, irisin, in a holistic resolution of gut pathology, lymphatic proliferation, and negative bone turnover in a rodent model of IBD.
To date, the role of the lymphatic system in the context of the inflammatory changes associated with IBD has remained elusive. It has been previously reported in chronic IBD patient cases that the number of lymphatic vessels associated with the muscularis mucosae and submucosae increases, relative to normal intestines, in both ulcerative colitis and Crohn’s disease patients (8–10, 35). This phenomenon of increased lymphatic investment has been hypothesized as a mechanistic response to chronic, granulomatous inflammatory conditions to ensure quick delivery of leukocytes and infectious agents to the local lymph node (9, 10). The increased lymphatic investment in the mucosal and submucosal compartments even occurred in areas with low inflammatory infiltration scores in IBD patients’ guts, suggesting that lymphatic proliferation in the gut compartments precipitates increased gut cellularity (10). Whether these proliferative lymphatic vessels are functional, however, remains unclear; indeed, numerous studies, including the original paper by Crohn (36), have associated impaired lymphatic function with IBD. In our study, we show the physical, histologic features of chronic colonic inflammation are associated with significant infiltration of podoplaninhi lymphatic structures into the mucosal lamina propria, comparable to what is seen in IBD patients. Morphologically, these podoplaninhi lymphatic regions in TNBS animals do not form typical lymph–capillary networks or precollector or collecting vessel structures based on their lack of defined lumen and borders. The amorphous podoplaninhi regions appeared restricted to the mucosal compartment when present; however, it is unknown whether these regions are an expansion of the lymphatic capillaries in the lamina propria or a breach in the lymphatic endothelium below the mucosa into the lamina propria. Furthermore, podoplanin’s function is equivocal, but supporting evidence of its functional role includes interaction with galectin-8 in lymphatic endothelium to support cell adhesion to the surrounding extracellular matrix, lymphangiogenesis, and lymphocyte trafficking through podoplanin interactions with C-C motif chemokine ligand 21 (CCL21) (37–39). It is unknown whether this lymphatic proliferation is driven by local parenchymal tissue hydrodynamic alterations, leukocyte activation and migration, or production and transport of inflammatory cytokines (40). Characterizing these factors in association with the observed lymphatic infiltration in IBD would provide mechanistic elucidation of this abnormal hyperproliferation of lymphatic vessels in the colon.
We began the characterization of the immunologic responses for select Th1/2 cytokines at the sites of lymphatic proliferation. TNBS-induced colitis has been characterized as primarily a Th1-driven disorder, but these processes are spatiotemporally dependent. Comparisons between acute and chronic TNBS-induced IBD in mice support a strong Th1/17-driven response (11). However, it is not known how these cytokines are distributed in the local colonic compartment as well as in relation to lymphatic vasculature changes. TNF-α was an important elevated factor associated with lymphatic proliferation in IBD. TNF-α induces lymphangiogenesis via VEGF receptor 3–independent mechanisms (41). However, because podoplanin expression was elevated in TNBS animals and podoplanin enhances VEGF receptor 3 in pathologic lymphangiogenesis, it is unclear which lymphaniogenic axis is driving the increased lymphatic density in the colonic compartments (38). Furthermore, other cytokines—IL-4, IL-10, IFN-γ—are also known to shape lymphangiogenic responses. We did note faint linear staining of IL-10 and IL-4 along the intestinal smooth muscle of the colon (Fig. 4D, E)—observations that warrant future investigation. Th2 cytokines, including IL-4 and IL-13, have been shown to down-regulate lymphatic endothelial tube formation, whereas neutralization of IFN-γ increases lymphangiogenesis independent of VEGF-C (42–45). However, our data suggest a TNF-α–mediated lymphangiogenic response. We did not characterize transportation to the mesenteric lymph node via GI lymphatic vessels in this study; given the elevated colonic TNF-α, however, this may influence GI lymphatic collector pumping ability because TNF-α has been shown to reduce mesenteric lymphatic contractility (46). Investigation of lymphatic function during chronic IBD warrants further study.
Certain chronic inflammatory conditions result in comorbidities, the pathogenesis of which is not fully understood. For example, dextran sodium sulfate–induced colitis impairs dermal lymphatic drainage (47). Another study demonstrated GI-associated infection (i.e., Toxoplasma gondii) modulated the immunologic state of a distant site (i.e., bone marrow–derived myeloid cells) (48). In our case, we explored how the IBD pathology in the GI is characterized by specific inflammatory responses that drive analogous changes in bone. These inflammation-induced changes in bone turnover are characterized by increased osteocyte Th1 cytokines TNF-α and IL-6, with unchanged or lowered IL-10 and IL-4, respectively, similar to the response in the colon. Therefore, as in the gut, we hypothesis TNF-α is a major contributor to the change in bone. Because sclerostin is up-regulated by TNF-α, RANKL and OPG are both influenced by TNF-α and TNF-α can induce osteocyte apoptosis, a further signal to increase osteoclastic activity (49–53). These data support a paradigm in which inflammation in 1 organ bed can lead to similar adaptations at distant sites. Our speculative hypothesis for this process is that immunologic factors such as cytokines are carried via lymph to the draining node or are systemically transported via the local venous circulation, the response of which results in systemic effects at distant sites. Furthermore, modulating the local inflammatory changes via exogenous irisin improved lymphatic outcomes at the site of damage leading to a resolution of inflammation in both local (gut) and distant sites (bone). Understanding these processes further as well as identifying treatments that are capable of therapeutically impacting multiple tissues could lead to improved disease outcomes, fewer side effects, and better patient quality of life.
Proinflammatory cytokines TNF-α and IL-6 have been shown to increase RANKL, a critical factor in the final step of osteoclast formation. The role of RANKL in bone is well understood, but minimally explored in the context of driving IBD GI pathogenesis. RANKL and its decoy receptor OPG are known to be dysregulated in the serum of IBD patients and, therefore, hypothesized to be associated with IBD-induced bone loss (14, 54). Our bone-specific data indicates an increase in osteocyte RANKL and OPG associated with increases in osteoclast surface. It is not fully understood how RANKL or OPG influence the gut tissues; however, RANKL and TNF-α have been shown to be critical for the development and organization of secondary lymphoid tissues in both normal physiologic and chronic inflammatory pathologic conditions (55, 56). Furthermore, it is known that, in IBD, lymphoid aggregates reminiscent of lymph nodes develop at GI sites with high levels of inflammation (56, 57). It is unknown what drives these secondary mucosa–associated lymphoid tissues to appear; however, it has been speculated that the lymphatic hyperproliferation that typically occurs adjacent to the sites is a driver (58). We found elevated mucosal and submucosal RANKL in TNBS animals as well as elevated densities of TNF-α+ cells and podoplaninhi lymphatic areas, suggesting a mechanistic role of RANKL in colonic lymphoid aggregate development in IBD. It is unknown whether RANKL has a direct lymphatic effect because it has demonstrated modulation of node lymphatic endothelium but not peripheral lymphatic endothelium (59).
Irisin was first identified as a PGC1-α–derived myokine released from skeletal muscle during moderate-to-vigorous intensity exercise (20). Irisin has been shown to be a bone anabolic factor, with in vivo and cell culture models demonstrating increased osteoblastogenesis and BFR as well as decreased bone resorption and osteoclast formation (28, 29, 60). The exact mechanisms behind how irisin acts to increase bone formation and decrease resorption remain not fully understood although our data along with others indicate irisin modulating the RANKL-OPG pathway (29). Recently, several investigators have identified roles for irisin as a potential mediator of inflammatory processes in the lung, atherosclerotic lesions, and macrophages (25–27, 61). These include lowered infiltration of immune cells to inflammatory legions, reduced inflammatory adhesion markers [intracellular cell adhesion marker 1 (ICAM1), VCAM1], reduced expression of proinflammatory cytokines (TNF-α, IL-6, CCL2), and suppressed NF-κB activation (27, 62–64). We hypothesized that exogenous irisin treatment would modulate the local and distant inflammatory milieu in IBD holistically. Our investigation’s most novel finding is the complete amelioration of the TNF-α– and RANKL-driven pathogenesis of colonic lymphatic hyperproliferation at the originating site of inflammation, as well as a reduction in osteoclast function and increased bone formation at the distant site of inflammation in bone with irisin treatment. Irisin reduced both the elevated TNF-α+ in the gut of TNBS+Ir animals and the elevated TNF-α+ osteocytes. RANKL was similarly reduced in both gut and bone. Concurrent with these changes, irisin treatment restored colonic lymphatic architecture (as measured by podoplanin+ staining via immunofluorescence) as well as notably removing lymphoid aggregates. How irisin may be modulating podoplanin in our findings warrants further study, but our data suggest the interaction of irisin with podoplanin helps maintain and restore structural integrity, modulate lymphangiogenic processes, and influence immune cell chemotaxis based on changes in TNF-α+ cellularity. TNF-α has been shown to induce lymphatic proliferation (i.e., lymphangiogenesis), so it is plausible that the abnormal TNBS lymphatic morphology is due to this elevation in TNF-α+ cell types (41). Down-regulation of TNF-α may restore the lymphatic architecture, a response we observed with irisin treatment after the onset of TNBS. Irisin has been shown to suppress production of Th1 cytokines including TNF-α (25, 26). Although not tested here, we speculate irisin has direct effects on lymphatic vessels, potentially driving the amelioration of gut inflammatory processes as well as distant inflammatory processes. To our knowledge, this is the first investigation of the anti-inflammatory functions of irisin in gut and bone tissues. The direct role of irisin on lymphatic, immune, and bone systems in the context of physiology and pathophysiology is an area that should be explored further.
Using irisin as a novel therapeutic agent in inflammatory conditions is a promising clinical approach. Our data in a rodent model of chronic IBD matches observations of clinical patients in the context of both gut and bone outcomes and also characterizes the immunologic factors as potential drivers for the pathology development in the 2 tissue beds. Irisin here is observed to modulate the effects in the local tissue. Although measuring serum RANKL and TNF-α in patients with IBD provides valuable information, utilizing an animal model allowed us to measure tissue-specific changes in inflammatory factors, thus avoiding the high variability of circulating serum measures. However, characterizing the local changes in RANKL and TNF-α of patient samples would prove useful as comparators to our data. The fact that irisin’s sequence homology is nearly 100% identical across most mammalian species suggests there is great potential for it to prove effective in humans. There are no clinical trials utilizing irisin, but its use as a treatment has been considered in the context of therapeutic interventions for type 2 diabetes and metabolic syndrome due to irisin’s reported role in converting white adipose tissue to brown adipose tissue, thus causing increased energy expenditure (16).
A limitation of our study is that the pace at which the exogenous irisin treatment ameliorated the inflammation in both tissue sites is unclear; this warrants further study. Furthermore, the rats in our study experienced fairly mild gut inflammation without the weight loss, altered eating patterns, or diarrhea that can occur in more severe rodent models of IBD. In our animals with mild inflammation, we saw nearly a complete remediation of the inflammation status in both gut and bone due to the irisin treatment; however, more severe inflammation may require changing the dosage seen in this study to successfully mitigate the pathology. Although the Veh treatment (30% ethanol) is the standard control for TNBS, we do note that the Veh treatment itself may result in mild changes to the gut or bone that differ from what would be standard in a true control rat. Any possible Veh-induced alterations, however, do not alter the comparisons to TNBS data.
In summary, we demonstrated an association between lymphatic alterations and elevated colon TNF-α and RANKL levels in chronic TNBS-induced colitis. These changes in the gut were paralleled immunologically in the bone, leading to increased bone resorption and decreased bone formation. Importantly, we demonstrate for the first time that exogenous treatment with irisin blocked the gut inflammatory changes, improved lymphatic structure, and recovered bone turnover, likely by reducing TNF-α and RANKL. Therefore, we propose that irisin may be a holistic treatment that could mitigate inflammation in chronic inflammatory conditions.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the Texas A&M University Health Science Center Integrated Microscope and Imaging Laboratory (supported by the Department of Medical Physiology) in providing the resources utilized in this work for immunofluorescence imaging. This work was supported by Texas A&M University College of Education and Human Development Transforming Lives Seed Grant (to S.A.B.), and U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grant U01HL123420 (to D.C.Z.). Additional funds were provided by the Sydney and J. L. Huffines Institute for Sports Medicine and Human Performance (to C.E.M.). S.A.N. was supported, in part, by the National Space Biomedical Research Institute Space Life Sciences Fellowship (NCC 9-58). The authors declare no conflicts of interest.
Glossary
- BFR
bone formation rate
- GI
gastrointestinal tract
- IBD
inflammatory bowel disease
- ID
integrated density
- L4
4th lumbar vertebra
- OPG
osteoprotegerin
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- RANKL
receptor activator of NF-κB ligand
- Th
T helper
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- TNBS+Ir
2,4,6-trinitrobenzenesulfonic acid with irisin
- Veh
vehicle
- Veh+Ir
vehicle with irisin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. A. Narayanan and C. E. Metzger conceptualized the work, supervised the study, collected data, analyzed the data, and drafted and revised the manuscript; S. A. Bloomfield and D. C. Zawieja interpreted the data and revised the manuscript; and all authors approved the final version of the manuscript.
REFERENCES
- 1.Malmborg P., Hildebrand H. (2016) The emerging global epidemic of paediatric inflammatory bowel disease—causes and consequences. J. Intern. Med. 279, 241–258 10.1111/joim.12413 [DOI] [PubMed] [Google Scholar]
- 2.Molodecky N. A., Soon I. S., Rabi D. M., Ghali W. A., Ferris M., Chernoff G., Benchimol E. I., Panaccione R., Ghosh S., Barkema H. W., Kaplan G. G. (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42, quiz e30 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 3.Benchimol E. I., Fortinsky K. J., Gozdyra P., Van den Heuvel M., Van Limbergen J., Griffiths A. M. (2011) Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm. Bowel Dis. 17, 423–439 10.1002/ibd.21349 [DOI] [PubMed] [Google Scholar]
- 4.Danese S., Sans M., Fiocchi C. (2004) Inflammatory bowel disease: the role of environmental factors. Autoimmun. Rev. 3, 394–400 10.1016/j.autrev.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 5.Fakhoury M., Negrulj R., Mooranian A., Al-Salami H. (2014) Inflammatory bowel disease: clinical aspects and treatments. J. Inflamm. Res. 7, 113–120 10.2147/JIR.S65979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko J. K., Auyeung K. K. (2014) Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr. Pharm. Des. 20, 1082–1096 10.2174/13816128113199990416 [DOI] [PubMed] [Google Scholar]
- 7.Zawieja D. C. (2009) Contractile physiology of lymphatics. Lymphat. Res. Biol. 7, 87–96 10.1089/lrb.2009.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao S., von der Weid P. Y. (2014) Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis 17, 325–334 10.1007/s10456-014-9416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geleff S., Schoppmann S. F., Oberhuber G. (2003) Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch. 442, 231–237 [DOI] [PubMed] [Google Scholar]
- 10.Rahier J. F., De Beauce S., Dubuquoy L., Erdual E., Colombel J. F., Jouret-Mourin A., Geboes K., Desreumaux P. (2011) Increased lymphatic vessel density and lymphangiogenesis in inflammatory bowel disease. Aliment. Pharmacol. Ther. 34, 533–543 10.1111/j.1365-2036.2011.04759.x [DOI] [PubMed] [Google Scholar]
- 11.Alex P., Zachos N. C., Nguyen T., Gonzales L., Chen T. E., Conklin L. S., Centola M., Li X. (2009) Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 15, 341–352 10.1002/ibd.20753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjarnason I., Macpherson A., Mackintosh C., Buxton-Thomas M., Forgacs I., Moniz C. (1997) Reduced bone density in patients with inflammatory bowel disease. Gut 40, 228–233 10.1136/gut.40.2.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal M., Arora S., Li J., Rahmani R., Sun L., Steinlauf A. F., Mechanick J. I., Zaidi M. (2011) Bone, inflammation, and inflammatory bowel disease. Curr. Osteoporos. Rep. 9, 251–257 10.1007/s11914-011-0077-9 [DOI] [PubMed] [Google Scholar]
- 14.Ghishan F. K., Kiela P. R. (2011) Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G191–G201 10.1152/ajpgi.00496.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzger C. E., Narayanan A., Zawieja D. C., Bloomfield S. A. (2017) Inflammatory bowel disease in a rodent model alters osteocyte protein levels controlling bone turnover. J. Bone Miner. Res. 32, 802–813 10.1002/jbmr.3027 [DOI] [PubMed] [Google Scholar]
- 16.Seibel M. J., Cooper M. S., Zhou H. (2013) Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 1, 59–70 10.1016/S2213-8587(13)70045-7 [DOI] [PubMed] [Google Scholar]
- 17.Connell W. R., Kamm M. A., Ritchie J. K., Lennard-Jones J. E. (1993) Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 34, 1081–1085 10.1136/gut.34.8.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding T., Deighton C. (2007) Complications of anti-TNF therapies. Future Rheumatology 2, 587–597 [Google Scholar]
- 19.Atzeni F., Talotta R., Salaffi F., Cassinotti A., Varisco V., Battellino M., Ardizzone S., Pace F., Sarzi-Puttini P. (2013) Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun. Rev. 12, 703–708 10.1016/j.autrev.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 20.Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Boström E. A., Choi J. H., Long J. Z., Kajimura S., Zingaretti M. C., Vind B. F., Tu H., Cinti S., Højlund K., Gygi S. P., Spiegelman B. M. (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handschin C., Chin S., Li P., Liu F., Maratos-Flier E., Lebrasseur N. K., Yan Z., Spiegelman B. M. (2007) Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1α muscle-specific knock-out animals. J. Biol. Chem. 282, 30014–30021 10.1074/jbc.M704817200 [DOI] [PubMed] [Google Scholar]
- 22.Ebert T., Focke D., Petroff D., Wurst U., Richter J., Bachmann A., Lössner U., Kralisch S., Kratzsch J., Beige J., Bast I., Anders M., Blüher M., Stumvoll M., Fasshauer M. (2014) Serum levels of the myokine irisin in relation to metabolic and renal function. Eur. J. Endocrinol. 170, 501–506 10.1530/EJE-13-1053 [DOI] [PubMed] [Google Scholar]
- 23.Kurdiova T., Balaz M., Vician M., Maderova D., Vlcek M., Valkovic L., Srbecky M., Imrich R., Kyselovicova O., Belan V., Jelok I., Wolfrum C., Klimes I., Krssak M., Zemkova E., Gasperikova D., Ukropec J., Ukropcova B. (2014) Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J. Physiol. 592, 1091–1107 10.1113/jphysiol.2013.264655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo Y., Gleitsmann K., Mangner N., Werner S., Fischer T., Bowen T. S., Kricke A., Matsumoto Y., Kurabayashi M., Schuler G., Linke A., Adams V. (2015) Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J. Cachexia Sarcopenia Muscle 6, 62–72 10.1002/jcsm.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao L., Meng D., Yang F., Song H., Tang D. (2017) Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 487, 194–200 10.1016/j.bbrc.2017.04.020 [DOI] [PubMed] [Google Scholar]
- 26.Li D. J., Li Y. H., Yuan H. B., Qu L. F., Wang P. (2017) The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 68, 31–42 10.1016/j.metabol.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Mu Q., Zhou Z., Song H., Zhang Y., Wu F., Jiang M., Wang F., Zhang W., Li L., Shao L., Wang X., Li S., Yang L., Wu Q., Zhang M., Tang D. (2016) Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One 11, e0158038 10.1371/journal.pone.0158038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colaianni G., Cuscito C., Mongelli T., Pignataro P., Buccoliero C., Liu P., Lu P., Sartini L., Di Comite M., Mori G., Di Benedetto A., Brunetti G., Yuen T., Sun L., Reseland J. E., Colucci S., New M. I., Zaidi M., Cinti S., Grano M. (2015) The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 112, 12157–12162 10.1073/pnas.1516622112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J., Valverde P., Zhu X., Murray D., Wu Y., Yu L., Jiang H., Dard M. M., Huang J., Xu Z., Tu Q., Chen J. (2017) Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 5, 16056 10.1038/boneres.2016.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. (1989) Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96, 795–803 10.1016/S0016-5085(89)80079-4 [DOI] [PubMed] [Google Scholar]
- 31.Antoniou E., Margonis G. A., Angelou A., Pikouli A., Argiri P., Karavokyros I., Papalois A., Pikoulis E. (2016) The TNBS-induced colitis animal model: an overview. Ann. Med. Surg. (Lond.) 11, 9–15 10.1016/j.amsu.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganta V. C., Cromer W., Mills G. L., Traylor J., Jennings M., Daley S., Clark B., Mathis J. M., Bernas M., Boktor M., Jordan P., Witte M., Alexander J. S. (2010) Angiopoietin-2 in experimental colitis. Inflamm. Bowel Dis. 16, 1029–1039 10.1002/ibd.21150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper H. S., Murthy S. N., Shah R. S., Sedergran D. J. (1993) Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238–249 [PubMed] [Google Scholar]
- 34.Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 10.1002/jbmr.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Alessio S., Correale C., Tacconi C., Gandelli A., Pietrogrande G., Vetrano S., Genua M., Arena V., Spinelli A., Peyrin-Biroulet L., Fiocchi C., Danese S. (2014) VEGF-C–dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Invest. 124, 3863–3878 10.1172/JCI72189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crohn B. B., Ginzburg L., Oppenheimer G. D. (1932) Regional Iletis: a pathologic and clinical entity. JAMA, 99, 1323–1329 [PubMed] [Google Scholar]
- 37.Cueni L. N., Detmar M. (2009) Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp. Cell Res. 315, 1715–1723 10.1016/j.yexcr.2009.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W. S., Cao Z., Sugaya S., Lopez M. J., Sendra V. G., Laver N., Leffler H., Nilsson U. J., Fu J., Song J., Xia L., Hamrah P., Panjwani N. (2016) Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat. Commun. 7, 11302 10.1038/ncomms11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerjaschki D., Regele H. M., Moosberger I., Nagy-Bojarski K., Watschinger B., Soleiman A., Birner P., Krieger S., Hovorka A., Silberhumer G., Laakkonen P., Petrova T., Langer B., Raab I. (2004) Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 15, 603–612 10.1097/01.ASN.0000113316.52371.2E [DOI] [PubMed] [Google Scholar]
- 40.Cromer W., Wang W., Zawieja S. D., von der Weid P. Y., Newell-Rogers M. K., Zawieja D. C. (2015) Colonic insult impairs lymph flow, increases cellular content of the lymph, alters local lymphatic microenvironment, and leads to sustained inflammation in the rat ileum. Inflamm. Bowel Dis. 21, 1553–1563 10.1097/MIB.0000000000000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji H., Cao R., Yang Y., Zhang Y., Iwamoto H., Lim S., Nakamura M., Andersson P., Wang J., Sun Y., Dissing S., He X., Yang X., Cao Y. (2014) TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat. Commun. 5, 4944 10.1038/ncomms5944 [DOI] [PubMed] [Google Scholar]
- 42.Shin K., Kataru R. P., Park H. J., Kwon B. I., Kim T. W., Hong Y. K., Lee S. H. (2015) TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat. Commun. 6, 6196 10.1038/ncomms7196 [DOI] [PubMed] [Google Scholar]
- 43.Kataru R. P., Kim H., Jang C., Choi D. K., Koh B. I., Kim M., Gollamudi S., Kim Y. K., Lee S. H., Koh G. Y. (2011) T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34, 96–107 10.1016/j.immuni.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 44.Zampell J. C., Avraham T., Yoder N., Fort N., Yan A., Weitman E. S., Mehrara B. J. (2012) Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am. J. Physiol. Cell Physiol. 302, C392–C404 10.1152/ajpcell.00306.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hos D., Bucher F., Regenfuss B., Dreisow M. L., Bock F., Heindl L. M., Eming S. A., Cursiefen C. (2016) IL-10 indirectly regulates corneal lymphangiogenesis and resolution of inflammation via macrophages. Am. J. Pathol. 186, 159–171 10.1016/j.ajpath.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 46.Chen Y., Rehal S., Roizes S., Zhu H. L., Cole W. C., von der Weid P. Y. (2017) The pro‐inflammatory cytokine TNF‐α inhibits lymphatic pumping via activation of the NF-κB‐iNOS signaling pathway. Microcirculation 24, e12364 10.1111/micc.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agollah G. D., Wu G., Peng H. L., Kwon S. (2015) Dextran sulfate sodium–induced acute colitis impairs dermal lymphatic function in mice. World J. Gastroenterol. 21, 12767–12777 10.3748/wjg.v21.i45.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Askenase M. H., Han S. J., Byrd A. L., Morais da Fonseca D., Bouladoux N., Wilhelm C., Konkel J. E., Hand T. W., Lacerda-Queiroz N., Su X. Z., Trinchieri G., Grainger J. R., Belkaid Y. (2015) Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity 42, 1130–1142 10.1016/j.immuni.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manolagas S. C., Parfitt A. M. (2013) For whom the bell tolls: distress signals from long-lived osteocytes and the pathogenesis of metabolic bone diseases. Bone 54, 272–278 10.1016/j.bone.2012.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baek K., Hwang H. R., Park H. J., Kwon A., Qadir A. S., Ko S. H., Woo K. M., Ryoo H. M., Kim G. S., Baek J. H. (2014) TNF-α upregulates sclerostin expression in obese mice fed a high-fat diet. J. Cell. Physiol. 229, 640–650 10.1002/jcp.24487 [DOI] [PubMed] [Google Scholar]
- 51.Kwan Tat S., Padrines M., Théoleyre S., Heymann D., Fortun Y. (2004) IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 15, 49–60 10.1016/j.cytogfr.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 52.Hofbauer L. C., Dunstan C. R., Spelsberg T. C., Riggs B. L., Khosla S. (1998) Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem. Biophys. Res. Commun. 250, 776–781 10.1006/bbrc.1998.9394 [DOI] [PubMed] [Google Scholar]
- 53.Tan S. D., Kuijpers-Jagtman A. M., Semeins C. M., Bronckers A. L. J. J., Maltha J. C., Von den Hoff J. W., Everts V., Klein-Nulend J. (2006) Fluid shear stress inhibits TNFα-induced osteocyte apoptosis. J. Dent. Res. 85, 905–909 10.1177/154405910608501006 [DOI] [PubMed] [Google Scholar]
- 54.Moschen A. R., Kaser A., Enrich B., Ludwiczek O., Gabriel M., Obrist P., Wolf A. M., Tilg H. (2005) The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 54, 479–487 10.1136/gut.2004.044370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 10.1038/16852 [DOI] [PubMed] [Google Scholar]
- 56.Aloisi F., Pujol-Borrell R. (2006) Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6, 205–217 10.1038/nri1786 [DOI] [PubMed] [Google Scholar]
- 57.Kaiserling E. (2001) Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology 34, 22–29 [PubMed] [Google Scholar]
- 58.Mooney E. E., Walker J., Hourihane D. O. (1995) Relation of granulomas to lymphatic vessels in Crohn’s disease. J. Clin. Pathol. 48, 335–338 10.1136/jcp.48.4.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cordeiro O. G., Chypre M., Brouard N., Rauber S., Alloush F., Romera-Hernandez M., Bénézech C., Li Z., Eckly A., Coles M. C., Rot A., Yagita H., Léon C., Ludewig B., Cupedo T., Lanza F., Mueller C. G. (2016) Integrin-alpha IIb identifies murine lymph node lymphatic endothelial cells responsive to RANKL. PLoS One 11, e0151848 10.1371/journal.pone.0151848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiao X., Nie Y., Ma Y., Chen Y., Cheng R., Yin W., Hu Y., Xu W., Xu L. (2016) Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 6, 18732 10.1038/srep18732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazur-Bialy A. I., Pocheć E., Zarawski M. (2017) Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int. J. Mol. Sci. 18, 701 10.3390/ijms18040701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong J., Dong Y., Dong Y., Chen F., Mitch W. E., Zhang L. (2016) Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. (Lond.) 40, 434–442 10.1038/ijo.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gannon N. P., Vaughan R. A., Garcia-Smith R., Bisoffi M., Trujillo K. A. (2015) Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 136, E197–E202 10.1002/ijc.29142 [DOI] [PubMed] [Google Scholar]
- 64.Lu J., Xiang G., Liu M., Mei W., Xiang L., Dong J. (2015) Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 243, 438–448 10.1016/j.atherosclerosis.2015.10.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.