Abstract
Fibulin-7 (Fbln7) has been identified as the latest member of the fibulin family of secreted glycoproteins in developing teeth, functioning as a cell adhesion molecule and interacting with other matrix proteins, receptors, and growth factors. More recently, we have shown that the C-terminal Fbln7 fragment (Fbln7-C) has antiangiogenic activity in vitro. Fbln7 is also expressed in immune-privileged tissues, such as eye and placenta, but its functional significance is unknown. In the current study, we show that human monocytes adhere to both full-length Fbln7 (Fbln7-FL) and Fbln7-C, in part, via integrins α5β1 and α2β1. Morphologic studies and surface expression analyses of CD14, mannose receptor (CD206), major histocompatibility complex II, and CD11b receptors revealed that both Fbln7-FL and Fbln7-C inhibit M-CSF–induced monocyte differentiation. Fbln7-C had significantly greater negative effects on cell spreading and stress fiber formation, including the production of IL-6 and metalloproteinase-1/-9 compared with Fbln7-FL. Furthermore, in an LPS-induced systemic inflammation model, Fbln7-C and Fbln7-FL reduced the infiltration of immune cells, such as neutrophils and macrophages, to the inflamed peritoneum. Thus, these results suggest that Fbln7 and Fbln7-C could modulate the activity of immune cells and have therapeutic potential for inflammatory diseases.—Sarangi, P. P., Chakraborty, P., Dash, S. P., Ikeuchi, T., de Vega, S., Ambatipudi, K., Wahl, L., Yamada, Y. Cell adhesion protein fibulin-7 and its C-terminal fragment negatively regulate monocyte and macrophage migration and functions in vitro and in vivo.
Keywords: extracellular matrix, immunomodulation, inflammation
Extracellular matrix (ECM) proteins play important roles in the activation and migration of inflammatory and immune cells (1). During an inflammatory response and after extravasation from blood vessels, leukocytes interact with ECM proteins, such as collagen, fibronectin, vitronectin, and laminin, via cell-surface integrins (1). These interactions, through integrins, induce cell differentiation and activation and modulate cellular functions (2). During an ongoing inflammatory process, inflammatory mediators, such as proteases and matrix metalloproteinases (MMPs), degrade ECM proteins and release their bioactive fragments, which can modulate the functions of immune cells, such as cytokine and chemokine production, phagocytosis, and their activation (1, 3). For example, fragments from collagen, laminin, and elastin have chemotactic effects on neutrophils, and elastin fragments inhibit the response of monocytes to LPS stimulation. Similarly, fibronectin, laminin, and hyaluronan fragments increase MMP production from monocytes and macrophages (3).
Fibulins are a group of secreted glycoproteins that are structurally characterized by the presence of a series of epidermal growth factor–like modules in the middle and a fibulin-type module at the C terminus (4). Fibulins are often associated with elastic fibers, basement membranes, and other matrices, and can participate in protein–protein interactions, form intramolecular bridges, and stabilize supramolecular structures. Fibulins have also been demonstrated to influence many cellular functions, including cell morphology, motility, growth, and adhesion, and they are implicated in physiologic and pathologic conditions, such as angiogenesis and tumor formation (4, 5). More recent findings have suggested their possible roles in inflammatory and autoimmune diseases (6–8).
The fibulin family consists of 7 members, of which the latest member, fibulin-7 (Fbln7), was identified in developing teeth. Unlike other members, Fbln7 possesses a sushi domain at the N terminus, which is involved in protein–protein interactions (9). Fbln7 functions as a cell adhesion molecule for the dental mesenchyme and odontoblasts, and interacts with other matrices, such as heparin, Fbln1, and fibronectin (9). Fbln7 is also expressed constitutively in blood vessels by endothelial cells. More recently, we have shown that a recombinant C-terminal Fbln7 fragment (Fbln7-C) binds to HUVECs via integrin α5β1 and that it inhibits HUVEC tube formation and vessel sprouting in aortic ring assays, which suggests potential antiangiogenic activity of the Fbln7 C-terminal region (10). Fbln7 is highly expressed in such tissues as the eye and placenta (unpublished data), which have specialized microenvironments for the regulation of angiogenesis as well as for immune cell activation and functions (9); therefore, Fbln7 or its fragments, such as Fbln7-C, could also be involved in immunomodulation in such tissues.
In the current study, by using human monocytes and an LPS-induced systemic inflammation mouse model, our results suggest that full-length Fbln7 (Fbln7-FL) and Fbln7-C are negative regulators of inflammation and immune cell function and that they may be useful as therapeutic reagents for inflammatory diseases.
MATERIALS AND METHODS
Expression and purification of Fbln7 recombinant proteins
As described by Hozumi et al. (11), near Fbln7-FL (75–440 aa, 42 kDa) and Fbln7-C (135–440 aa, 33 kDa) cDNA were cloned into the pCEP-MulPuroD vector, which contained a BM-40 signal peptide, a His-tag, and a multicloning site under the control of the CMV promoter and enhancer. Recombinant Fbln7-FL and Fbln7-C proteins were produced in Freestyle 293F cells by using a 293Fectin reagent (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were purified and concentrated as described by de Vega et al. (9).
In vivo inflammation model
LPS-induced endotoxemia was performed in 8- to 12-wk-old C57BL/6 (Harlan, Indianapolis, IN, USA) male mice, according to a protocol approved by the Animal Care and Use Committee, National Institute of Dental and Craniofacial Research, National Institutes of Health, and the Institute Animal Ethics Committee, Indian Institute of Technology Roorkee. For endotoxemia assays, an LPS from Escherichia coli O55:B (MilliporeSigma, Burlington, MA, USA) was administered by injection (36.7 mg/kg body weight, i.p.) as per titration of the received lot of LPS. Recombinant proteins (10 μg/dose, i.v.) were administered in a mouse tail vein and the retro-orbital venous plexus after 1 and 4 h after LPS injection as used for other therapeutic approaches (12). Lungs were isolated at 18 h postinjection and digested by using collagenase IV (MilliporeSigma) to prepare a single-cell suspension for flow cytometric analysis. Peritoneal cells and bone marrow cells were isolated at 18 h postinjection, and flow cytometric analysis was performed. We used BD Horizon Fixable Viability Stain 450 to gate live cells (FVS450; BD Biosciences, San Jose, CA, USA). For surface marker staining, purified anti-mouse Fc receptor (CD16/CD32), F4/80–Alexa Fluor 488, Ly6G-PE, major histocompatibility complex (MHC)-II–PerCP, and CD11b–APC Abs were used (BioLegend, San Diego, CA, USA). All samples were fixed with 3.7% formaldehyde and collected on a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed by using FlowJo software (FlowJo, Ashland, OR, USA).
Monocyte isolation and activation
Human peripheral blood cells were isolated via leukapheresis of healthy volunteers at the Department of Transfusion Medicine at the National Institutes of Health. The protocol was designed to protect participants from research risks, as defined in 45CFR46, and to abide by all National Institutes of Health guidelines for human participants research (IRB number: 99-CC-0168). Blood cells were diluted in Ca2+- and Mg2+-containing PBS, and monocytes were isolated via counterflow centrifugal elutriation, as described by Zhou et al. and Wahl et al. (13, 14). For cell adhesion and migration assays, monocytes were stimulated with TNF-α (50 ng/ml) and granulocyte M-CSF (GM-CSF; 50 ng/ml) for 3 h at 37°C and 48 h for the MMP assay.
Cell adhesion assay
Assays were performed in 96-well flat-bottom plates (Dynex Technologies, Chantilly, VA, USA). Wells were coated overnight at 4°C with 50 µl of various amounts of Fbln7-FL, Fbln7-C, and fibronectin (MilliporeSigma), then blocked with 2% bovine serum albumin (BSA) for 1 h at 37°C. TNF-α–activated and unstimulated monocytes (5 × 104 cells in 100 µl/well) were plated and incubated for 2 h at 37°C. Attached cells were measured by using the CCK8 cell counting kit (Dojindo, Rockville, MD, USA). For cell adhesion inhibition experiments, cells were incubated with blocking Abs (10 µg/ml) against integrin α5β1, α2β1, and αvβ3 (MilliporeSigma) for 30 min before plating.
In vitro monocyte differentiation, activation, and flow cytometry
Blood was collected from healthy donors according to the Institute Human Ethics Committee, Indian Institute of Technology Roorkee–approved protocol, and peripheral blood mononuclear cells (PBMCs) were isolated by using Histopaque 1077 (MilliporeSigma). Monocytes were separated from PBMCs by using the plastic adherence method, as described by Cassol et al. (15), with modifications. In brief, 5 × 105 PBMCs were seeded in a 48-well flat-bottom plate in 400 µl RPMI 1640 medium without FBS (Thermo Fisher Scientific) for 1 h. Tightly adhered monocytes were further cultured in RPMI 1640 (10% FBS) with or without M-CSF (25 ng/ml; Thermo Fisher Scientific). Fbln7-FL (10 µg/ml) and Fbln7-C (10 µg/ml) were added to M-CSF–treated wells. After d 3 and d 5, surface staining was performed by using anti-human CD206–PE, CD11b–APC, MHC-II–FITC, and CD14–PerCP Abs (BioLegend). We used BD Horizon Fixable Viability Stain 450 to gate on live cells (FVS450; BD Biosciences). All samples were acquired on a FACS Verse flow cytometer (BD Biosciences). Data were analyzed by using BD FACSuite software (BD Biosciences).
For integrin expression, monocytes were stimulated with TNF-α (50 ng/ml) or LPS (100 ng/ml) for 3 h in DMEM at 37°C, and surface staining was performed by using purified mouse anti-human integrins α3, α5, and αv (MilliporeSigma) primary Abs and Alexa Fluor 488–labeled goat anti-mouse (Thermo Fisher Scientific) secondary Abs.
Reactive oxygen species detection assay
A cell-permeable, oxidation-sensitive dye, 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; MilliporeSigma), was used to detect intracellular reactive oxygen species (ROS) production. Monocytes were isolated and differentiated in the presence of M-CSF (25 ng/ml), Fbln7-FL (10 µg/ml), and Fbln7-C (10 µg/ml). After 72 h, cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA; Abcam, Cambridge, MA, USA) for 15 min followed by PBS wash. Cells were incubated for 15 min at 37°C, 5% CO2, with 10 µM DCF-DA, which, upon oxidation, converts to the highly fluorescent 2′,7′-dichlorofluorescein. Samples were acquired by using a FACS Verse flow cytometer and analyzed by using BD FACSuite software.
Reduction, alkylation, and trypsin digestion of monocyte lysates
Two hundred micrograms of protein from a 14-h monocyte culture was precipitated with trichloroacetic acid (MilliporeSigma) at 4°C overnight. Centrifugation was subsequently performed to pellet proteins, then they were washed with ice-cold acetone and the air-dried pellets were dissolved in 6 M urea/100 mM Tris-HCl (pH 8.5). Proteins were then reduced with 200 mM DTT (MilliporeSigma) and alkylated with the addition of 200 mM iodoacetamide (MilliporeSigma), and subsequently digested overnight at 37°C with trypsin (1:25 enzyme to protein ratio by weight; Promega, Madison, WI, USA). Peptides were then desalted by using the Oasis HLB-1 (1 mg) reverse-phase cartridge (Waters, Milford, MA, USA) and vacuum concentrated until dry.
Protein identification by mass spectrometry
Tryptic peptide mixtures from each condition were loaded in technical duplicates onto a Zorbax C18 trap column (Agilent Technologies, Santa Clara, CA, USA) for additional desalting of the peptide mixture. Peptides were then subjected to nano-LC and eluted onto an analytical column (New Objective, Woburn, MA, USA) by using a 120-min acetonitrile gradient (5–35%) at a flow rate of 250 nl/min, and peptides were ionized by using electrospray ionization in the positive ion mode and detected by using a LTQ-Orbitrap Velos (Thermo Electron Corp, Beverly, MA, USA). The 6 most intense ions were measured in the Orbitrap with the resolution set at 30,000 (m/z 400), and fragment ions were measured in the LTQ. We used the spectral count method to detect fold changes in the expression or abundance of the proteins identified by mass spectrometry for a comparative analysis between the fibronectin, Fbln7-FL, and Fbln7-C samples.
Cytokine ELISA analysis
Monocytes were cultured for 36 h, as previously described, and supernatants were analyzed for IL-6 levels by using the sandwich ELISA protocol. Capture and detection Abs and recombinant standards were purchased from PeproTech (Rocky Hill, NJ, USA). The color reaction was developed by using the TMB liquid substrate system (MilliporeSigma) and measured by using a Tekan Safire microplate reader at 655 nm. Quantification was performed with Prism software (GraphPad Software, La Jolla, CA, USA). For serum cytokine analysis, samples were isolated and analyzed for IL-6 levels by using the sandwich ELISA protocol. Capture and detection Abs were purchased from BioLegend. The color reaction was developed by using the TMB liquid substrate system (BioLegend). The reaction was stopped by using 2 N H2SO4 (Himedia, Mumbai, Maharashtra) and measured at 450 nm by using an EPOCH2 plate reader (BioTek, Winooski, VT, USA).
Quantitative real-time RT-PCR
Total RNA was isolated from adhered monocytes at different time points by using the RNeasy kit (Qiagen, Germantown, MD, USA). RNA was reverse transcribed by using iScript reverse-transcription supermix for quantitative RT-PCR (Bio-Rad, Hercules, CA, USA), and real-time RT-PCR was performed by using the CFX96 real-time RT-PCR detection system (Bio-Rad) and the StepOne Plus real-time RT-PCR system (Applied Biosystems, Foster city, CA, USA). RT-PCR was performed using the SYBR green PCR master mix (Bio-Rad), and RT-PCR amplification of a housekeeping gene (human GAPDH) was performed for each sample as a control for sample loading and to allow normalization between samples. Primers used were as follows: human GAPDH: forward: 5′-TCCTCTGACTTCAACAGCGACAC-3′, reverse: 5′-TCTCTCTTCCTCTTGTGCTCTTGC-3′; human IL-6: forward: 5′-GAAAGCAGCAAAGAGGCACT-3′, reverse: 5′-TTTCACCAGGCAAGTCTCCT-3′; human IL-1β: forward: 5′-AGGGACAGGATATGGAGCAA-3′, reverse: 5′-ACGCAGGACAGGTACAGATT-3′; and human IL-12: forward: 5′-GCCCTGTGCCTTAGTAGTATTT-3′, reverse: 5′-GCTCATCAATAACTGCCAGCAT-3′.
Western blot
For the detection of MMP-1 and MMP-9,conditioned media from the monocyte cultures (5 × 106/ml of DMEM) were harvested at 48 h after activation. BSA (50 μg/ml) was added to the culture supernatants before the precipitation of the proteins with ice-cold ethanol (final concentration, 60%) at 70°C for 15 min. Pelleted proteins were lyophilized by rotary evaporation. Lyophilized proteins were resuspended in an SDS Laemmli loading buffer, and electrophoresis was performed as described by Zhou et al. (13). For detection, blots were incubated with primary Abs against MMP-1 and MMP-9 (Thermo Fisher Scientific). Blots were analyzed by the addition of Alexa Fluor 680 secondary Abs (Thermo Fisher Scientific), and infrared fluorescence was detected with the Odyssey infrared imaging system from LI-COR Biosciences (Lincoln, NE, USA). Band densities in the Western blot were determined with ImageJ (NIH, Besthesda, MD, USA). For Erk1/2 analysis, cell lysates were collected at different time points from the monocytes cultured on Fbln7-FL or Fbln7-C. For Western blot analysis, primary Abs against Erk1/2, phospho-Erk1/2 (Cell Signaling Technology, Danvers, MA, USA), and secondary anti-mouse IgG horseradish peroxidase were used. We used SuperSignal West Dura Chemiluminescent Substrate (Thermo Fisher Scientific) to detect the proteins.
Time-lapse video microscopy
Cell migration on fibronectin (5 μg/ml) substrates was examined by using time-lapse video microscopy. Glass-bottom culture dishes were coated with fibronectin and blocked with 2% BSA. After activation with TNF-α for 2 h, monocytes were incubated for 30 min with anti-α5β1 (10 µg/ml) blocking Ab or recombinant proteins (20 μg/ml) and 5 × 104 cells in 200 μl F-12 medium that contained monocyte chemoattractant protein-1 (100 ng/ml). Dishes were placed in a built-in CO2 incubator on the stage of a Zeiss Axiovert 25 microscope (Thornwood, NY, USA) and subjected to time-lapse video microscopy for 1 h. Images of the cells were acquired every 10 s. Cell migration videos were analyzed by using ImageJ.
Immunofluorescence staining of actin
Glass chamber slides (MilliporeSigma) were coated with recombinant proteins, fibronectin (5 μg/ml) or Fbln7-FL and Fbln7-C (20 μg/ml), overnight at 4°C. Wells were washed and blocked with 2% BSA. After washing, monocytes (1 × 104 in 200 μl/well) were plated in the presence of TNF-α and allowed to attach for 3 h. Once the cells were attached, they were washed with prewarmed PBS, fixed with prewarmed 4% paraformaldehyde for 10 min, and permeabilized with 0.1% Triton X-100 for 5 min. Actin staining was performed using phalloidin-rhodamine (Thermo Fisher Scientific) according to the manufacturer’s instructions, and images were acquired by using a Zeiss LSM 510 NLO META confocal microscope.
Data analysis
All values are expressed as means ± sem, and the differences between all groups were analyzed by using paired nonparametric t test. All statistics were performed using Prism software, and values of P < 0.05 were considered significant. All liquid chromatography–tandem mass spectrometry data were searched using the MASCOT algorithm within Proteome Discoverer 1.3 (Thermo Electron Corp) against the human SwissProt protein database to obtain the peptide and protein identifications. For all searches, trypsin was specified as the enzyme for protein cleavage, allowing up to 2 missed cleavages. Oxidation (M) and carbamidomethylation (C) were set as dynamic and fixed modifications, respectively, and mass tolerance levels of 20 ppm and 0.8 Da were set for the precursor and fragment ions, respectively. For tandem mass spectrometry data visualization, MASCOT results were imported into Scaffold 3Q+ (Proteome Software).
RESULTS
Fbln7-FL and Fbln7-C bind to human monocytes via integrins α5β1 and α2β1
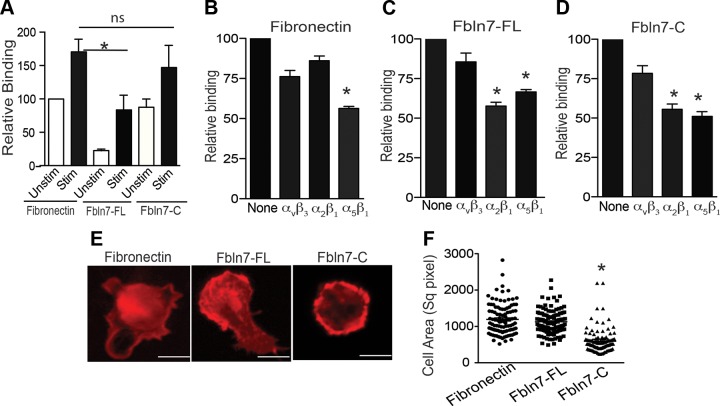
We hypothesized that recombinant nearly Fbln7-FL and/or Fbln7-C may exhibit immunomodulatory activity. To test this hypothesis, we first examined the cell binding activity of these proteins for human peripheral blood monocytes with and without treatment of TNF-α as a proinflammatory cytokine. In the cell binding assay, untreated and TNF-α–stimulated monocytes were added to 96-well plates that were coated with Fbln7-FL, Fbln7-C, and fibronectin as a positive control, and the number of cells that were attached to the wells was measured (Fig. 1A). Similar to fibronectin, untreated monocytes attached to both Fbln7-FL and Fbln7-C. The relative binding of monocytes to Fbln7-FL was lower than that of Fbln7-C and fibronectin. The binding of TNF-α–stimulated monocytes to all 3 proteins was increased compared with that of untreated monocytes (Fig. 1A). As shown in Supplemental Fig. 1B, TNF-α–stimulated monocytes attached to Fbln7-C in a dose-dependent manner. Immune cells interact with ECM proteins primarily via integrins (16). For example, monocyte attachment to fibronectin is mediated via integrins α4β1 and α5β1. (17) Increased cell binding to TNF-α– and LPS-stimulated monocytes may be caused by an increase in the expression levels of integrins by TNF-α and LPS treatment (Supplemental Fig. 1C). Recent findings by de Vega et al. (10) have demonstrated that HUVEC binding to Fbln7-C is mediated via integrin α5β1. To identify possible integrin receptors that are involved in the binding of Fbln7-FL and Fbln7-C to monocytes, cell attachment inhibition assays were performed by using blocking Abs against the integrins α5β1, α2β1, and αvβ3 (Fig. 1B). The blocking Ab to integrin α5β1 reduced monocyte attachment to fibronectin by 40% (Fig. 1B), as reported (17). The blocking Abs against integrins α5β1 and α2β1 inhibited the attachment of monocytes to Fbln7-FL (Fig. 1C) and Fbln7-C (Fig. 1D), which suggests that these integrins are potential receptors for Fbln7-FL and Fbln7-C on monocytes.
Figure 1.
Human monocytes bind to Fbln7-FL and Fbln7-C via integrins and inhibit cell spreading. A) Binding of unstimulated and TNF-α–stimulated monocytes to 96-well plates that were coated with fibronectin (5 µg/ml), Fbln7-FL (10 µg/ml), and Fbln7-C (10 µg/ml). Bar graph shows the quantification of relative binding compared with binding of unstimulated monocytes on a fibronectin substrate as 100. B–D). Binding inhibition of TNF-α–stimulated monocytes plated on fibronectin (B), Fbln7-FL (C), and Fbln7-C (D) substrates by blocking Abs to integrins αvβ3, α2β1, and α5β1. Cell binding activity on each substrate without the Ab was set as 100 and compared with cell binding activity with the Ab. Data represent the average of 3 independent experiments performed in triplicate. E) Actin stress fiber formation of TNF-α–stimulated monocytes on fibronectin, Fbln7-FL, and Fbln7-C substrates by phalloidin staining. Images were taken with a ×63 objective. Scale bars, 5 µm. F). Quantification of the cell area of the attached cells (100 cells) counted from the representative snapshots (×20) taken 30 min after the attachment of activated monocytes on each substrate by using ImageJ. ns, not significant. Data represent the results from 2 independent experiments. *P < 0.05.
Fbln7-C inhibits actin stress fiber formation in human monocytes
ECM proteins can influence immune cell spreading and migration (1). De Vega et al. (10), demonstrated that Fbln7-C, not Fbln7-FL, inhibits cell spreading and actin fiber formation in HUVECs; therefore, we examined the effects of Fbln7-FL, Fbln7-C, and fibronectin as a control on cell spreading and actin stress fiber formation of TNF-α–stimulated monocytes. Stress fiber formation in monocytes that were plated on these substrates was analyzed by staining F-actin with phalloidin using confocal microscopy. Fibronectin promoted stress fiber formation and spreading in activated monocytes (Fig. 1E). In contrast, Fbln7-C inhibited stress fiber formation and did not promote spreading, whereas Fbln7-FL had less inhibitory activity (Fig. 1E). Second, in a monocyte attachment assay on 96-well plates that were coated with different substrates, cell spreading areas were analyzed by counting the total cell areas of the monocytes from 3 separate field images taken with a ×20 objective. A significant reduction of the cell areas in Fbln7-C–coated plates was found compared with Fbln7-FL– and fibronectin-coated plates (Fig. 1F), which confirms an inhibitory effect of Fbln7-C on monocyte spreading and actin fiber formation with less of an inhibitory effect of Fbln7-FL.
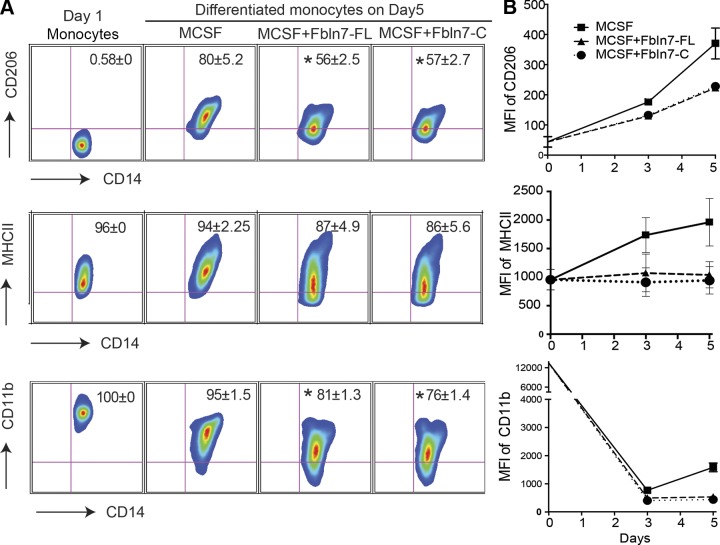
Fbln7-FL and Fbln7-C inhibit the differentiation of human monocytes into macrophages
Monocytes differentiate into tissue macrophages after exiting blood vessels for their physiologic and inflammatory functions. Monocytes can be induced to differentiate into macrophages in cell cultures by treating them with CSFs, such as the M-CSF and GM-CSF, of which M-CSF is often used to generate monocyte-derived macrophages that mimic tissue macrophages (18). During monocyte differentiation by M-CSF, the sizes of differentiating cells were increased (Supplemental Fig. 2A); however, both Fbln7-FL and Fbln7-C reduced the size of cells with higher reduction levels by Fbln7-C, which suggests that these proteins inhibited M-CSF–mediated monocyte differentiation (Supplemental Fig. 2A). We next performed a flow cytometric analysis of the macrophage differentiation markers, CD11b, MHC-II, CD14, and CD206, at d 3 and d 5 after differentiation. Both Fbln7-FL and Fbln7-C significantly inhibited the expression of CD11b, MHC-II, and CD14 (Fig. 2A, B), which further indicated a negative regulatory effect of Fbln7-FL and Fbln7-C on monocyte differentiation. As demonstrated in Fig. 2B, the kinetics analysis of the above-mentioned surface markers reveled that, although the presence of Fbln7-FL and Fbln7C had a strong inhibitory effect on CD11b and MHC-II, monocytes still retained the ability to slowly polarize toward the M-CSF–driven M2 phenotype, as indicated from an increase in the mean fluorescence intensity of CD206 expression on monocytes at d 5 compared with d 3.
Figure 2.
Fbln7-FL and Fbln7-C inhibit the differentiation of monocytes. Monocytes were cultured for 5 d in the presence of M-CSF (25 ng/ml), M-CSF + Fbln7-FL (10 μg/ml), and M-CSF+Fbln7-C (10 μg/ml), as described in Materials and Methods. A) Representative fluorescence-activated cell sorting plots from 3 independent experiments show the surface expression of CD206, MHC-II, and CD11b on FVS450−CD14+ macrophages at d 5. B) Line diagram represents mean fluorescence intensities (MFIs) of respective surface markers on monocytes that were cultured in the presence of M-CSF (solid line with square), M-CSF + Fbln7-FL (triangle broken line with triangle), and Fbln7-C (dotted line with circle) at d 3 (average of 4 separate experiments) and d 5 (average of 3 separate experiments). Results are expressed as means ± sem. *P < 0.05.
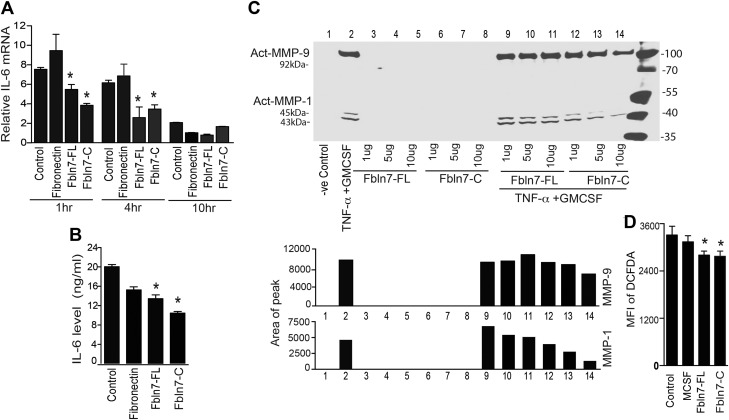
Fbln7-FL and Fbln7-C reduce proinflammatory cytokine, MMP1/9, and ROS production in activated monocytes in vitro
Recent studies have demonstrated that ECM proteins and their bioactive fragments that are released during inflammation can influence the ability of immune cells to respond to inflammatory stimuli (1). Consequently, we investigated the effects of Fbln7 proteins on the production of inflammatory mediators by TNF-α–activated monocytes. For these experiments, monocytes were cultured on plates that were coated with fibronectin (5 μg/ml), Fbln7-FL (20 μg/ml), Fbln7-C (20 μg/ml), or without any protein coating in the presence of TNF-α and GM-CSF. As shown in Fig. 3A, Fbln7-FL and Fbln7-C significantly inhibited IL-6 mRNA expression in monocytes at 1 and 4 h after activation compared with that of fibronectin and the control plate. As shown in Supplemental Fig. 2B, expression of IL-12 mRNA was also reduced in the presence of Fbln7-C at 1 h compared with fibronectin-coated plates, and at 4 h in the presence of both Fbln7-FL and Fbln7-C compared with both fibronectin-coated and control plates. The amount of IL-6 protein that is secreted from monocytes at 36 h after activation was also measured. Fbln7-FL and Fbln7-C significantly reduced IL-6 protein production levels (Fig. 3B). Compared with Fbln7-FL, the inhibitory effect of Fbln7-C was more profound on IL-6 mRNA expression and protein production by monocytes. Activated monocytes also release MMP1/9 during inflammation (13). We examined the effects of Fbln7-FL and Fbln7-C on MMP1/9 production by Western blot analysis in TNF-α– and GM-CSF–activated monocytes using various amounts of soluble Fbln7-FL and Fbln7-C. Only the active form of MMP-1 was detected in monocyte cultures. Ab against MMP-1 detects both pro–MMP-1 and active MMP-1, and, as previously reported, MMP-1 released by activated human monocytes is detected in the active form (13). We found that only Fbln7-C, not Fbln7-FL, reduced MMP1/9 production in activated monocytes in a dose-dependent manner (Fig. 3C). In addition, we investigated the roles of Fbln7-FL and Fbln7-C in the regulation of macrophage ROS production. For this, monocytes that were cultured in the presence of RPMI 1640 medium, M-CSF, M-CSF with Fbln7-FL, or M-CSF with Fbln7-C for 72 h were stimulated with PMA (50 ng/ml) for 15 min to induce ROS production. PMA-stimulated monocytes were then incubated with a cell-permeable, oxidation-sensitive dye, DCF-DA, for 15 min, followed by flow cytometry analysis of the cells that contained oxidized DCF-DA. As shown in Fig. 3D, the mean fluorescence intensity of DCF-DA was significantly lower in monocytes that were cultured in the presence of Fbln7-FL and Fbln7-C compared with monocytes that were cultured in RPMI 1640 medium. Taken together, these results indicate that Fbln7-C and Fbln7-FL with a moderate level have regulatory effects on monocyte cytokine, MMP, and ROS production.
Figure 3.
Effects of Fbln7-FL and Fbln7-C on the expression of proinflammatory and anti-inflammatory mediators in activated monocytes. A) Time course of IL-6 mRNA expression in monocytes that were plated on fibronectin, Fbln7-FL, Fbln7-C substrates, and uncoated controls that were treated with TNF-α in the presence of GM-CSF at various time points. Bar graphs show fold changes compared with unstimulated monocytes before culture. B) Expression of the IL-6 protein in the supernatant produced by monocytes that were cultured on the different substrates in the presence of TNF-α for 36 h in the presence of GM-CSF. IL-6 protein was quantitated by using sandwich ELISAs. C) Production of MMP1 and MMP9 of monocytes activated with TNF-α and GM-CSF for 48 h in the presence of various amounts of soluble Fbln7-FL and Fbln7-C. For this experiment, monocytes were plated in equal numbers per well, and identical amounts of supernatants from each well were evaluated for MMP1/9 protein levels. Proteins were analyzed by gel electrophoresis as described in Materials and Methods, and the individual band densities are shown below the Western blot gel. D) Differentiated cells in the presence or absence of Fbln7-FL and Fbln7-C were stimulated with 50 ng/ml PMA for 15 min followed by a 15-min incubation with DCF-DA. ROS production was compared by measuring oxidized DCF-DA by using flow cytometry. Bar graphs represent the average mean fluorescence intensity (MFI) of DCF-DAhi monocytes. IL-6 mRNA and protein expression data represent the average of 3 independent experiments performed in triplicate. DCF-DA staining data represent the average of 5 independent experiments. Results are expressed as means ± sem. *P < 0.05.
To further confirm the inhibitory effect of Fbln7 recombinant proteins on monocyte functions, we performed a proteomic analysis of the proteins that were isolated from monocytes that were cultured for 14 h on different protein substrates, which led to the identification of 622 proteins (Supplemental Fig. 3A and Supplemental Table 1). Of these proteins, 70% (n = 434) were common among all 3 groups, whereas ∼7.3% (n = 46), 6.9% (n = 43), and 5.7% (n = 36) were identified only in fibronectin, Fbln7-FL, and Fbln7-C, respectively (Supplemental Fig. 3A). A careful analysis of the spectral counts of common proteins confirmed a regulatory effect of Fbln7-C on the inflammatory protein expression of TNF-α–activated monocytes. A similar effect at a moderate level was also noted with Fbln7-FL. For example, as shown in Supplemental Fig. 3B, high mobility group box 1, MAPK, and galecin-3 were reduced in the Fbln7-C and Fbln7-FL groups, respectively, whereas the IL-1β receptor antagonist, an important anti-inflammatory protein, was specifically increased in both the Fbln7-FL and Fbln7-C groups. The abundance of CD14 and the monocyte number differentiation antigen—necessary for monocyte activation and differentiation—were also reduced in monocytes that were cultured on Fbln7-FL and Fbln7-C substrates. Furthermore, we analyzed the effect of Fbln7-FL and Fbln7-C on the activation of the MAPK/Erk1/2 signaling cascade that is involved in monocyte proliferation and differentiation during inflammation by using Western blot analysis of Erk1/2 phosphorylation in lysates that were isolated from TNF-α–activated monocytes that were plated on Fbln7-FL and Fbln7-C. As shown in Supplemental Fig. 3C, both Fbln7-FL, and Fbln7-FL reduced the phosphorylation level of Erk1/2 in activated monocytes before the addition on protein-coated plates, which confirmed an inhibitory effect on this pathway. In addition, our differentiation experiments also confirmed the inhibition of CD14 protein on the surface of monocytes that were cultured in presence of Fbln7-FL and Fbln7-C (Supplemental Fig. 3D), which further validated the findings of mass spectrometry analysis.
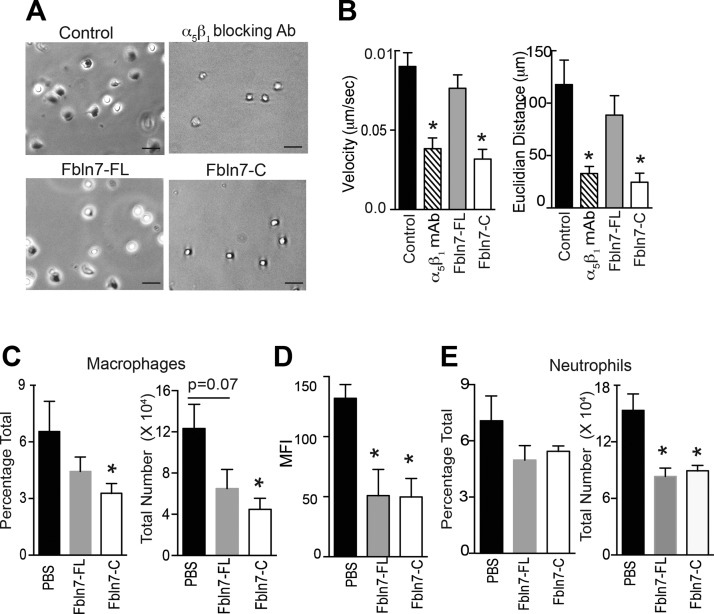
Fbln7-C inhibited the chemokine-mediated migration of activated monocytes in vitro and reduced macrophage infiltration in vivo
After confirming an inhibitory effect of Fbln7-C on monocyte spreading and inflammatory activity, we performed additional in vitro time-lapse cell migration assays to measure the effect of Fbln7-C and Fbln7-FL on the monocyte chemoattractant protein-1–mediated migration of TNF-α–activated monocytes on a fibronectin substrate. TNF-α–activated monocytes were preincubated with Fbln7-FL, Fbln7-C, integrin α5β1 blocking Ab, and a media-only control before cell migration assay. The presence of soluble Fbln7-C dramatically reduced the binding, spreading, velocity, and displacement (Euclidian distance) of monocytes on fibronectin, whereas Fbln7-FL exhibited moderate effects (Fig. 4A, B). Inhibitory effects of Fbln7-C were comparable with those observed with the blocking Ab against integrin α5β1 (Fig. 4A). These results indicate that Fbln7-FL binds to monocytes and exhibits a regulatory effect on inflammatory proteins without much interference with spreading and migration; however, Fbln7-C is able to inhibit both monocyte morphology and inflammatory pathways in addition to binding to other substrates. In addition, a stronger binding of Fbln7-C to integrin α5β1 in monocytes may induce the inhibition of monocyte attachment and spreading on a fibronectin substrate.
Figure 4.
Fbln7-C inhibits the monocyte chemoattractant protein-1 (MCP-1) –mediated migration of activated monocytes on fibronectin in vitro and reduces macrophage infiltration into inflamed tissues in vivo. Cell migration of TNF-α–stimulated monocytes on fibronectin in response to MCP-1 was performed by using time-lapse video microscopy. A) Snapshot from the live cell migration assay of TNF-α–activated human monocytes on fibronectin in response to MCP-1. Cells were pretreated with α5β1 blocking Ab (upper right), Fbln7-FL (20 µg/ml; lower left), and Fbln7-C (20 µg/ml; lower right) before plating cells on fibronectin-coated dishes. Scale bars, 50 µm. B) Velocity and Euclidian distance (length of straight line between the initial and final points) of the attached cells were quantified by using the manual tracking and chemotaxis tool in ImageJ (n = 11/group). C–E) Cells were isolated from peritoneal lavage and lungs of endotoxemic mice (18 h after LPS injection) that were administered PBS, Fbln7-FL (10 μg/dose i.v.), or Fbln7-C (10 μg/dose i.v.) in 2 doses at 1 and 4 h. Anti-F4/80, anti-CD11b, anti-Ly6G, and anti–MHC-II Abs were used for staining. FVS450− cells were gated (live cells) for analysis. C) Bar graph shows the frequencies and total number of macrophages (F4/80) in the peritoneal lavage. D) Bar graph shows the mean fluorescence intensities (MFIs) of MHC-II expression on macrophages in the peritoneal lavage. E) Bar graph shows the frequencies and total number of neutrophils (CD11bhiLy6Ghi) in the peritoneal lavage (n = 4/group for PBS and Fbln7-C groups; n = 3 for the Fbln7-FL group). Results are expressed as means ± sem. *P < 0.05.
Furthermore, we measured the immunomodulatory effects of Fbln7-FL and Fbln7-C in vivo by using an LPS -induced systemic inflammation mouse model (19). In this assay, LPS was intraperitonealy injected to induce an acute systemic inflammation. At 1 and 4 h after LPS injection, Fbln7-FL (10 µg/dose) and Fbln7-C (10 µg/dose) or PBS (control) were administered intravenously via the tail vein and the retro-orbital venous plexus, respectively. Peritoneal lavage and the lungs were analyzed at 18 h postinjection for the infiltration of inflammatory cells, such as F4/80-expressing macrophages, by using flow cytometry. As shown in Fig. 4C, the total number of F4/80+ macrophages in peritoneal lavage was significantly reduced in Fbln7-C–administered mice compared with control animals. A moderate, but not statistically significant, reduction in macrophage infiltration was also observed in Fbln7-FL–injected animals compared with controls. Of interest, as shown in Fig. 4D, infiltrating macrophages in peritoneal lavage demonstrated a significant reduction in the expression of MHC-II on their surface, which suggested an inhibitory effect of Fbln7-FL and Fbln7-C on monocyte migration and function. To further investigate the effect of Fbln7 protein administration, we analyzed CD11bhiLy6Ghi neutrophils, an important cell type that infiltrates after LPS administration (19). As shown in Fig. 4E, the frequency of neutrophils was also reduced in the peritoneum, with a significant reduction in the total number of infiltrating neutrophils compared with control animals. A moderate, but insignificant, reduction of macrophages was observed in the lungs of mice that were administered Fbln7-FL and Fbln7-C compared with PBS control (Supplemental Fig. 4A). To evaluate systemic inflammation, IL-6 levels in serum were analyzed and, as shown in Supplemental Fig. 4B, Fbln7-FL– and Fbln7-C–treated animals had moderately higher, but not significant, serum IL-6 levels compared with control animals.
DISCUSSION
Fbln7 was first identified in developing teeth and demonstrated to be involved in dental cell adhesion and interactions with matrix proteins. Fbln7 is expressed not only in teeth, but also in immunotolerant tissues, such as the eye and placenta; however, the physiologic relevance of Fbln7 expression in these tissues is not yet understood. In the current study, we found that recombinant Fbln7-FL and Fbln7-C proteins bind to human monocytes, in part, via integrins and act as immunomodulators in cell culture and in an acute inflammatory mouse model. Our data show that Fbln7-C inhibits monocyte and macrophage adhesion, spreading, differentiation, and inflammatory mediator production with a more potent inhibitory activity compared with that of Fbln7-FL. To the best of our knowledge, this is the first study that has demonstrated immunoregulatory functions for Fbln7 and its C-terminal fragment. These findings indicate that Fbln7 may be involved in the maintenance of specialized ECM microenvironments in tissues such as the eye, placenta, and cartilage (20).
ECM proteins and their fragments play important roles in the modulation of immune cell functions and the maintenance of the immune-privileged status of tissues (1). For example, Masli et al. (20) demonstrated that thrombospondin-1, an ECM protein, plays a critical role in maintaining the immune-privileged status in the eye by regulating the tolerance-promoting properties of TGF-β–treated antigen-presenting cells. Furthermore, the authors also demonstrated that the deficiency of thrombospondin-1 or its receptor CD36 inhibits the ability of antigen-presenting cells to prevent IL-12 production in the presence of TGF-β. Similarly, in the placenta, it has been suggested that the substratum of decidual stromal cells regulates macrophage function to prevent the rejection of fetoplacental allografts by the maternal immune system (21). As Fbln7 is highly expressed in the maternal decidua (data not shown), it is conceivable that Fbln7 could be involved in the maintenance of the microenvironment in the placenta. In support of this hypothesis, our in vitro and in vivo data show that Fbln7-C and Fbln7-FL at moderate levels inhibit immune cell functions, such as cell spreading and proinflammatory cytokine production, and migration on other ECM substrates. A number of inflammatory proteins (e.g., high-mobility group box 1, Hsp70, and galectin-3) and cell adhesion proteins (e.g., integrin β2, ICAM-1, tropomyosin α4 chain, and Rho GAP1) that were identified by mass spectrometric analysis from the lysate of TNF-α–stimulated monocytes were down-regulated when cultured on Fbln7-FL– or Fbln7-C–coated plates compared with monocytes that were cultured on fibronectin. CD206 is a member of the C-type lectin receptor family. This receptor plays a role in the recognition and endocytosis of mannosylated carbohydrate-containing pathogens. In addition, reports have shown that CD206 present on M2 macrophage plays an important role in collagen uptake and turnover (22). Data show that both Fbln7-FL and Fbln7-C significantly reduced M-CSF–induced monocyte differentiation as measured by surface expression of the CD206, CD14, MHC-II, and CD11b markers.
Fibronectin is expressed by various cell types, including macrophages, endothelial cells, and smooth muscle cells (23, 24), and it is implicated in the modulation of immune cell functions (23, 25, 26). The activation of immune cells, such as macrophages and T lymphocytes, increases their attachment to fibronectin, which suggests an important function of fibronectin in host defenses (24). Enzymatic digestion of Fbln7-FL with cathepsin B results in the formation of a smaller C-terminal fragment of Fbln7 (data not shown). A smaller Fbln7 fragment in serum from naïve mice assayed by Western blot with the Fbln7 Ab was also observed (data not shown). Although macrophages do not express Fbln7 mRNA or protein, functions are modulated by interactions with Fbln7-FL and Fbln7-C. During inflammatory responses, up-regulation of proteases could release Fbln7 from associated ECMs and degrade Fbln7 to create more bioactive Fbln7 fragments. Thus, Fbln7 or its fragments may bind to both circulating and infiltrating monocytes and macrophages and modulate their cellular functions.
Our results suggest that these Fbln7 proteins were bound to monocytes, in part, via integrins α5β1 and α2β1, and their binding efficiencies were increased upon stimulation with either LPS or TNF-α. It has been shown that fibronectin binding to integrin α5β1 is mediated via the RGD (Arg-Gly-Asp) sequence of fibronectin, and additional domains outside the RGD sequence are responsible for providing specificity (27); however, binding to Fbln7-FL and Fbln7-C is mediated in an RGD-independent manner as Fbln7 lacks the RGD motif (9). Our data show that Fbln7-C, but not Fbln7-FL, inhibited monocyte binding to fibronectin, possibly as a result of competing with integrins that are shared between fibronectin and Fbln7-C. Tenascin is another ECM protein that negatively regulates the functions of macrophages, and T and B lymphocytes were also shown to inhibit the binding of monocytes to fibronectin (28). Similar to the in vitro inhibitory effects on monocyte migration, administration of Fbln7-C significantly reduced the infiltration of activated macrophages compared with PBS-injected controls in a murine endotoxemia model. Of interest, in cell culture, Fbln7-FL did not abrogate the binding of monocytes to fibronectin, but was able to reduce macrophage infiltration into the inflamed peritoneum, which suggests a possible change in the conformation and fragmentation of the protein or interaction with other ECM proteins in the inflamed peritoneum. In addition to macrophages, significant reductions were also observed in both the percentages and total numbers of neutrophils in the inflamed peritoneum, which suggests that Fbln7 could bind to other immune cells that express integrins α5β1 and α2β1 and modulate their function. Systemic inflammation could alter the ECM protein distribution in various tissues (2). This could also modulate the integrin expression and the effect of Fbln7 and its fragments on the infiltration of macrophages and other inflammatory cells into various visceral organs. Additional investigation is required to evaluate the effects of Fbln7 and its fragments on other immune cell types and its possible implications for therapeutics in inflammatory diseases.
In summary, by using in vitro and in vivo models of inflammation, we have established the immunomodulatory activities of Fbln7-FL and Fbln-7-C. Our findings suggest that there is a negative regulatory role for Fbln7 in the functions of immune cells and that peptides that contain the bioactive domains of Fbln7 may serve as potential therapeutic reagents for inflammatory diseases.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. James Melvin, Dr. Marjan Gucek, and Dr. Steve Swatkoski [all of the U.S. National Institutes of Health (NIH)] for granting access to the NIH, National Heart, Lung, and Blood Institute Proteomics Core Facility. This work was supported by the Intramural Research Program of National Institute of Dental and Craniofacial Research (DE000485-26; to Y.Y.) and funds from the Department of Science and Technology, Government of India (YSS/2015/000451; to P.P.S.), the Department of Biotechnology, Government of India (102/IFD/SAN/1671/2014-2015; to P.P.S.), a Japan Society of Promotion of Science for Young Investigator (714175; to T.I. and S.d.V.), University Grants Commission, Government of India (Sr. No-2061430670; to S.P.D.), and the Ministry of Human Resource Development, Government of India (MHR 02-23-200-429; to P.C.). The authors declare no conflicts of interest.
Glossary
- BSA
bovine serum albumin
- DCF-DA
2′,7′-dichlorofluorescin diacetate
- ECM
extracellular matrix
- Fbln7
fibulin-7
- Fbln7-C
C-terminal fragment of fibulin-7
- Fbln7-FL
full-length fibulin-7
- GM-CSF
granulocyte M-CSF
- MHC
major histocompatibility complex
- MMP
metalloproteinase
- PBMC
peripheral blood mononuclear cell
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. P. Sarangi designed and performed the experiments and analyzed the data; P. Chakraborty designed and performed the monocyte differentiation studies and ROS production and in vivo experiments and analyzed the data; S. P. Dash performed RT-PCR and in vivo experiments and analyzed the data; K. Ambatipudi performed the mass spectrometric analyses; L. Wahl performed the MMP1/9 assay and provided guidance related to the monocytes; P. P. Sarangi, T. Ikeuchi, and S. de Vega designed the expression vectors and prepared the recombinant proteins; and P. P. Sarangi and Y. Yamada directed the study and wrote the manuscript.
REFERENCES
- 1.Sorokin L. (2010) The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 [DOI] [PubMed] [Google Scholar]
- 2.González A., Gómez B. L., Muñoz C., Aristizabal B. H., Restrepo A., Hamilton A. J., Cano L. E. (2008) Involvement of extracellular matrix proteins in the course of experimental paracoccidioidomycosis. FEMS Immunol. Med. Microbiol. 53, 114–125 [DOI] [PubMed] [Google Scholar]
- 3.Adair-Kirk T. L., Senior R. M. (2008) Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 40, 1101–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vega S., Iwamoto T., Yamada Y. (2009) Fibulins: multiple roles in matrix structures and tissue functions. Cell. Mol. Life Sci. 66, 1890–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timpl R., Sasaki T., Kostka G., Chu M. L. (2003) Fibulins: a versatile family of extracellular matrix proteins. Nat. Rev. Mol. Cell Biol. 4, 479–489 [DOI] [PubMed] [Google Scholar]
- 6.Wu J., Liu W., Bemis A., Wang E., Qiu Y., Morris E. A., Flannery C. R., Yang Z. (2007) Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 56, 3675–3684 [DOI] [PubMed] [Google Scholar]
- 7.Xiang Y., Sekine T., Nakamura H., Imajoh-Ohmi S., Fukuda H., Yudoh K., Masuko-Hongo K., Nishioka K., Kato T. (2006) Fibulin-4 is a target of autoimmunity predominantly in patients with osteoarthritis. J. Immunol. 176, 3196–3204 [DOI] [PubMed] [Google Scholar]
- 8.Lisi S., D’Amore M., Scagliusi P., Mitolo V., Sisto M. (2009) Anti-Ro/SSA autoantibody-mediated regulation of extracellular matrix fibulins in human epithelial cells of the salivary gland. Scand. J. Rheumatol. 38, 198–206 [DOI] [PubMed] [Google Scholar]
- 9.De Vega S., Iwamoto T., Nakamura T., Hozumi K., McKnight D. A., Fisher L. W., Fukumoto S., Yamada Y. (2007) TM14 is a new member of the fibulin family (fibulin-7) that interacts with extracellular matrix molecules and is active for cell binding. J. Biol. Chem. 282, 30878–30888 [DOI] [PubMed] [Google Scholar]
- 10.De Vega S., Suzuki N., Nonaka R., Sasaki T., Forcinito P., Arikawa-Hirasawa E., Yamada Y. (2014) A C-terminal fragment of fibulin-7 interacts with endothelial cells and inhibits their tube formation in culture. Arch. Biochem. Biophys. 545, 148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hozumi K., Suzuki N., Nielsen P. K., Nomizu M., Yamada Y. (2006) Laminin alpha1 chain LG4 module promotes cell attachment through syndecans and cell spreading through integrin alpha2beta1. J. Biol. Chem. 281, 32929–32940 [DOI] [PubMed] [Google Scholar]
- 12.Sarangi P. P., Lee H. W., Lerman Y. V., Trzeciak A., Harrower E. J., Rezaie A. R., Kim M. (2017) Activated protein C attenuates severe inflammation by targeting VLA-3highNeutrophil subpopulation in mice. J. Immunol. 199, 2930–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M., Zhang Y., Ardans J. A., Wahl L. M. (2003) Interferon-gamma differentially regulates monocyte matrix metalloproteinase-1 and -9 through tumor necrosis factor-alpha and caspase 8. J. Biol. Chem. 278, 45406–45413 [DOI] [PubMed] [Google Scholar]
- 14.Wahl S. M., Katona I. M., Stadler B. M., Wilder R. L., Helsel W. E., Wahl L. M. (1984) Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). II. Functional properties of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions. Cell. Immunol. 85, 384–395 [DOI] [PubMed] [Google Scholar]
- 15.Cassol E., Cassetta L., Rizzi C., Alfano M., Poli G. (2009) M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J. Immunol. 182, 6237–6246 [DOI] [PubMed] [Google Scholar]
- 16.Harburger D. S., Calderwood D. A. (2009) Integrin signalling at a glance. J. Cell Sci. 122, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber C., Alon R., Moser B., Springer T. A. (1996) Sequential regulation of alpha 4 beta 1 and alpha 5 beta 1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J. Cell Biol. 134, 1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacey D. C., Achuthan A., Fleetwood A. J., Dinh H., Roiniotis J., Scholz G. M., Chang M. W., Beckman S. K., Cook A. D., Hamilton J. A. (2012) Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 188, 5752–5765 [DOI] [PubMed] [Google Scholar]
- 19.Sarangi P. P., Hyun Y. M., Lerman Y. V., Pietropaoli A. P., Kim M. (2012) Role of β1 integrin in tissue homing of neutrophils during sepsis. Shock 38, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamiri P., Masli S., Kitaichi N., Taylor A. W., Streilein J. W. (2005) Thrombospondin plays a vital role in the immune privilege of the eye. Invest. Ophthalmol. Vis. Sci. 46, 908–919 [DOI] [PubMed] [Google Scholar]
- 21.Redline R. W., McKay D. B., Vazquez M. A., Papaioannou V. E., Lu C. Y. (1990) Macrophage functions are regulated by the substratum of murine decidual stromal cells. J. Clin. Invest. 85, 1951–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madsen D. H., Leonard D., Masedunskas A., Moyer A., Jürgensen H. J., Peters D. E., Amornphimoltham P., Selvaraj A., Yamada S. S., Brenner D. A., Burgdorf S., Engelholm L. H., Behrendt N., Holmbeck K., Weigert R., Bugge T. H. (2013) M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 202, 951–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura N., Nishinarita S., Takizawa T., Tomita Y., Horie T. (2000) Cultured human monocytes secrete fibronectin in response to activation by proinflammatory cytokines. Clin. Exp. Immunol. 120, 66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coito A. J., Onodera K., Kato H., Busuttil R. W., Kupiec-Weglinski J. W. (2000) Fibronectin-mononuclear cell interactions regulate type 1 helper T cell cytokine network in tolerant transplant recipients. Am. J. Pathol. 157, 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluftinger J. L., Kelly N. M., Hancock R. E. (1989) Stimulation by fibronectin of macrophage-mediated phagocytosis of Pseudomonas aeruginosa. Infect. Immun. 57, 817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis L. S., Oppenheimer-Marks N., Bednarczyk J. L., McIntyre B. W., Lipsky P. E. (1990) Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J. Immunol. 145, 785–793 [PubMed] [Google Scholar]
- 27.Takagi J., Strokovich K., Springer T. A., Walz T. (2003) Structure of integrin alpha5beta1 in complex with fibronectin. EMBO J. 22, 4607–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rüegg C. R., Chiquet-Ehrismann R., Alkan S. S. (1989) Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc. Natl. Acad. Sci. USA 86, 7437–7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.