Abstract
Although autacoids primarily derived from the cyclooxygenase-2 and 5-lipoxygenase (LOX) pathways are essential mediators of inflammation, endogenous specialized proresolving mediators (SPMs) act as robust agonists of resolution. SPM biosynthesis is initiated by the conversion of arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid primarily via the 12/15-LOX pathway. Although 12/15-LOX activity is prominent in the cornea, the role of SPM pathway activation during infection remains largely unknown and is the focus of the current study. Pseudomonas keratitis was induced in resistant BALB/c and susceptible C57BL/6 (B6) mice. Biosynthetic pathways for proinflammatory autacoids and SPMs were assessed. Divergent lipid mediator profiles demonstrate the importance of 15-LOX pathways in the pathogenesis of ocular infectious disease. Results indicate that an imbalance of LOX enzymatic pathways contributes to susceptibility observed in B6 mice where deficient activation of SPM circuits, as indicated by reduced 15-hydroxy-eicosatetraenoic acid and 17-hydroxydocosahexaenoic acid levels, prevented transition toward resolution and led to chronic inflammation. In sharp contrast, BALB/c mice demonstrated a well-balanced axis of 5-LOX/12-LOX/15-LOX pathways, resulting in sufficient proresolving bioactive metabolite formation and immune homeostasis. Furthermore, a novel immunoregulatory role for 15-LOX was revealed in inflammatory cells (polymorphonuclear leukocytes and macrophages), which influenced phagocytic activity. These data provide evidence that SPM circuits are essential for host defense during bacterial keratitis.—Carion, T. W., Greenwood, M., Ebrahim, A. S., Jerome, A., Suvas, S., Gronert, K., Berger, E. A. Immunoregulatory role of 15-lipoxygenase in the pathogenesis of bacterial keratitis.
Keywords: lipid mediators, resolution, ocular infection, inflammation
Bacterial keratitis is a sight-threatening infection of the cornea that features pathophysiologic events caused by the invading microbe as well as the host response itself. Pseudomonas aeruginosa is a common, opportunistic, gram-negative pathogen that is commonly associated with the induction of bacterial keratitis. Risk factors for keratitis include contact lens wear, refractive corneal surgery, and immunocompromised conditions (1, 2). Corneal infections caused by Pseudomonas typically present as a rapidly progressing, suppurative stromal infiltrate with a marked mucopurulent exudate. Necrosis surrounded by inflammatory epithelial edema and stromal ulceration are characteristic of this disease, which culminates in significant destruction and loss of stromal tissue (3). In addition to structural alterations, secondary glaucoma and cataract may occur (4). Although antibiotic treatment, for the most part, effectively controls the pathogen, multidrug resistance for Pseudomonas is on the rise (5, 6). Furthermore, antibiotic treatment does not guarantee good visual outcome because proper resolution of inflammation within the host tissue remains an issue. Restoration of immune homeostasis is integral to ocular function. Treatment involving corticosteroids addresses the host response to an extent; however, there remains indecision regarding use of this class of hormones. Therefore, there remains a need for therapeutic modalities that are anti-inflammatory as well as immunoregulatory and proresolving.
Animal models of ocular infectious disease have been developed by means of topical application of bacteria after wounding the corneal epithelium, by intrastromal inoculation, or by placement of a contaminated contact lens or suture on the cornea. These paradigms have broadened our understanding of the mechanisms of corneal inflammation during bacterial keratitis. The current study uses well-characterized murine models of inflammatory disease whereby corneal infection induced experimentally by P. aeruginosa causes corneal perforation in C57BL/6 (B6) mice, a dominant Th1 responder (7). BALB/c mice, characterized as a Th2 dominant strain (7), effectively resolve the infection over time and are classified as resistant (7). Extensive studies using the susceptible/resistant models have provided invaluable information regarding the roles of inflammatory cells, cytokines, chemokines, and neuropeptides in modulating inflammation, innate immunity, and Th1- vs. Th2-like responses to P. aeruginosa in the eye (8–13). However, the role of lipid mediator pathways in corneal infection and inflammation has yet to be comparably characterized.
Inflammation, including initiation and resolution, is an active process. Eicosanoids such as arachidonic acid (AA)-derived prostaglandins, leukotrienes, and thromboxanes play key roles in the initiation of inflammation. Subsequently, in a time-dependent manner, initial proinflammatory cascades are counter-regulated by proresolving lipid signals or “specialized proresolving lipid mediators” (SPMs). SPMs are locally acting lipid mediators that play a pivotal role as endogenous agonists stimulating the resolution of inflammation (14). Lipoxins, resolvins, and protectins can be biosynthesized by lipoxygenases (LOXs), which comprise the main pathways for SPM biosynthesis. Studies have demonstrated LOX activity in the cornea (14–18), with 15-LOX appearing to be largely protective because it is a key enzyme for the generation of SPMs, which are essential for the resolution of inflammation (19). Proresolving actions include limiting polymorphonuclear leukocyte (PMN) infiltration, regulating the cytokine profile (20, 21), pain reduction (22), and noninflammatory macrophage functions such as efferocytosis (23–25). Although major strides have been made regarding lipid mediator research, the role of lipid mediators in the pathogenesis of bacterial keratitis has yet to be investigated. Therefore, in the current study, we characterize lipid mediator pathways in resistant and susceptible phenotypes using a murine model of Pseudomonas corneal infection. More importantly, we show an essential role for 15-LOX in inflammatory cell function and the resolution of ocular infectious disease.
MATERIALS AND METHODS
Experimental animal protocol
The left central corneas of 8-wk-old C57BL/6 (B6), BALB/c, and B6.129S2-Alox15tm1Fun/J (15-LOX−/−) female mice (The Jackson Laboratory, Bar Harbor, ME, USA) were harvested as previously described (9). A 5-µl aliquot of bacterial suspension containing 106 CFU/ml of P. aeruginosa strain [American Type Culture Collection (ATCC) 19666; Manassas, VA, USA] was applied topically to the wounded corneal surface. All animals were treated in a manner authorized by the Wayne State University Institutional Animal Care and Use Committee, and animal treatment conformed to the Association for Research in Vision and Ophthalmology’s statement on the Use of Animals in Ophthalmic and Vision Research.
Ocular response to bacterial infection
Infected eyes were observed daily, and disease response was graded using an established grading scale (26) for statistical comparison. A 4-point scale was used, where 0 = clear or slight opacity, partially or fully covering the pupil; +1 = slight opacity, fully covering the anterior segment; +2 = dense opacity, partially or fully covering the pupil; +3 = dense opacity, covering the entire anterior segment; and +4 = corneal perforation or phthisis. Mice were examined, and a clinical score was calculated for each group to express disease severity. Photographs were taken by slit-lamp to illustrate disease response at select time points postinfection (p.i.).
Lipidomic analyses
Comprehensive lipidomic analyses were carried out on whole corneas (1.8–2.1 mg each) from B6 and BALB/c mice (n = 5/group). SPMs, eicosanoids, and polyunsaturated fatty acids (PUFAs) were identified and quantified according to our published protocols (27, 28) Lipid autacoids from homogenized corneas were extracted by solid phase using Accubond ODS-C18 cartridges (Agilent Technologies, Santa Clara, CA, USA). Class-specific deuterated internal standards [PGE2-d4, LTB4-d4, 15-hydroxyeicosatetraenoic acid (HETE)-d8, lipoxin (LX)A4-d5, docosahexaenoic acid (DHA)-d5, AA-d8] were used to calculate extraction recovery. Samples were analyzed on an LC-MS/MS system consisting of a 1200 Series HPLC (Agilent Technologies), a Kinetex C18 Minibore Column (Phenomenex, Torrance, CA, USA), and an AB Sciex QTrap 3200 (Sciex, Redwood City, CA, USA). Analysis was carried out in negative ion mode, and SPMs, eicosanoids, and PUFAs were quantified using scheduled multiple reaction monitoring using 4–6 specific transition ions for each analyte.
Real-time RT-PCR
Total RNA was isolated from individual whole corneas (n = 5/group) for in vivo analysis or collected from cultured cells for in vitro analysis using RNA-STAT 60 (Tel-Test, Friendswood, TX, USA) according to the manufacturer’s recommendations and quantified by spectrophotometric determination (260 nm). Total RNA (100 ng) was reverse transcribed and used to produce a cDNA template. Next, cDNA was amplified using SYBR Green Master Mix (Thermo Fisher Scientific, Ann Arbor, MI, USA) as suggested by the manufacturer. A 10-µl reaction mixture contained 5 µl 2x SYBR Green PCR Master Mix, 1 µl diethylpyrocarbonate-water, 1 µl forward and reverse primers, and 2 µl cDNA (diluted 1:10). All primers for the PCR were designed using Primer3 (http://primer3.ut.ee/). Semiquantitative real-time RT-PCR was performed using the CFX Connect Real-Time RT-PCR Detection System (Bio-Rad, Hercules, CA, USA). PCR amplification conditions were determined using routine methods (29). Relative transcript levels were determined using the relative standard curve method comparing the amount of target normalized to an endogenous reference (β-actin). Data are shown as the means ± sd for relative transcript levels.
Bacterial load
Corneas from B6, BALB/c, and 15-LOX−/− mice were collected at 2 d p.i., and the number of viable bacteria was determined. Individual corneas were homogenized in sterile 0.9% saline containing 0.25% bovine serum albumin. Serial 10-fold dilutions of the samples were plated on Pseudomonas isolation agar in triplicate, and plates were incubated overnight at 37°C. Results are reported as CFU/cornea ± sem.
Flow cytometric analyses and detection of activated caspase-3/7
Individual corneas from B6, BALB/c, and 15-LOX knockout (KO) mice (n = 4/group) were harvested at 2 d p.i. Individual corneas were kept in sterile tubes containing 250 μl RPMI 1640 without serum. Liberase TL (2.50 mg/ml; Roche, Indianapolis, IN, USA) was added to each tube, followed by incubation at 37°C for 45 min on a Disruptor Genie (Scientific Industries, Bohemia, NY, USA). At the end of the incubation period, samples were triturated using a 3-ml syringe plunger and passed through a 70-μm cell strainer. Finally, the single-cell suspension was washed with 5 ml RPMI 1640 containing 10% fetal bovine serum (FBS) and pelleted at 315 g for 8 min in a refrigerated centrifuge.
Caspase-3/7 activation was detected in the subsets of CD45+ immune cells using a FAM-FLICA Caspase-3/7 assay kit (Immunochemistry Technologies, Bloomington, MN, USA) per the manufacturer’s instructions. Briefly, single-cell suspensions of infected corneas from all 3 groups of mice were incubated with FAM-FLICA caspase-3/7 at 37°C and 5% CO2 for 45 min. At the end of the incubation period, cells were washed twice with 1× wash buffer provided in the kit followed by blocking of the FCRs with an anti-mouse CD16/32 antibody on ice. Cell surface staining for CD45, CD11b, Ly6G, and F4/80 molecules was carried out, and samples were immediately acquired using a flow cytometer (LSRFortessa; Beckton Dickinson, San Jose, CA, USA). Data were analyzed using FlowJo software. The following antibodies were used for cell surface staining: PerCP-Cy5.5–conjugated rat anti-mouse CD11b (M1/70, 1:200), PE-Cy7–conjugated rat anti-mouse Ly6G (1 A8, 1:200), APC-conjugated rat anti-mouse macrophage (F4/80, 1:200), and Alexa 700–conjugated CD45 (30-F11, 1:200). All antibodies were purchased from BD Biosciences (San Jose, CA, USA).
TUNEL assay
Whole eyes from infected B6, BALB/c, and 15-LOX KO mice were removed at 2 d p.i. for TUNEL staining with a terminal deoxynucleotidyl transferase kit (TACS; R&D Systems, Minneapolis MN, USA) according to the manufacturer’s protocol (11). In brief, eyes were embedded in Tissue-Tek (Sakura Finetek, Torrence, CA, USA) optimal cutting temperature compound and flash-frozen in liquid nitrogen. Eyes were cut into 10-μM thick sections for TUNEL staining. Control sections were treated similarly but without the terminal deoxynucleotidyl transferase enzyme. Sections were photographed under a microscope (Axiophot; Carl Zeiss, Oberkochen, Baden-Württemberg, Germany) with digital imagery (Axiocam; Carl Zeiss).
Cell isolation and culture
Peritoneal PMNs were isolated from B6, BALB/c, and 15-LOX−/− mice as previously described (8, 30). In brief, mice received an i.p. injection of a 9% casein solution (1.0 ml) at 27 and 3 h before cell harvest. PMNs were lavaged from the peritoneal cavity 3 h after the second injection. Cells were collected with harvest solution (0.02% EDTA in 1× PBS), washed, and isolated using a 90% Percoll gradient. Viable cells (>95%) were counted and resuspended in RPMI 1640 + 10% FBS. Density was determined by in vitro assays.
Macrophages were isolated from the peritoneal cavity of B6 and BALB/c mice that had received a single i.p. injection of 3% thioglycollate solution (1.0 ml) 5 d before cell harvest (8, 31). Peritoneal exudate cells were collected by lavage with harvest solution (DMEM + 5% FBS) and stained with trypan blue (1:1), and viable cells (>95%) were enumerated. After a differential cell count, cells were seeded in 12-well plates at specific densities detailed below for each in vitro assay. Nonadherent cells were removed 24 h later, and isolated macrophages were used for phagocytosis assays.
In vitro stimulation assay
PMNs (1 × 106 cells/well) were stimulated with PA 19660 (100 µl of 106 CFU/µl) with or without the 15-LOX inhibitor PD-146176 (2 µM; Santa Cruz Biotechnology, Dallas, TX, USA). Cells were stimulated for 3 h (37°C, 5% CO2) and treated with gentamicin (200 µg/ml) for an additional 15 min to kill extracellular bacteria. Cells were processed for RNA extraction, or supernatants were collected for protein analysis.
Phagocytosis assay
Phagocytic uptake of B6- and BALB/c-derived PMNs and macrophages was measured using a CytoSelect 96-well phagocytosis assay (Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s protocol. Enzyme-labeled Escherichia coli particles or Zymosan particles were used as phagocytosis-inducing pathogens for PMNs and macrophages, respectively. Briefly, cells were plated at a density of 2.5 × 104 cells/well and incubated with 15-LOX small interfering RNA (siRNA) (On-TargetPlus Mouse Alox15; GE Healthcare Dharmacon, Lafayette, CO, USA) at 37.5 nM for 24–48 h before addition of the phagocytosis pathogen. siRNA (On-TargetPlus Non-Targeting Pool; GE Healthcare Dharmacon) was used as a negative control for both cell types. Internalized particles were detected by absorbance measurements obtained at 405 nm using an automated plate reader.
Statistical analysis
Samples sizes were determined statistically prior to experimentation. The difference in clinical score between 2 groups at each time point was tested by the Mann-Whitney U test (GraphPad Prism; GraphPad Software, La Jolla, CA, USA). For all other experiments, a 1-way ANOVA followed by Bonferroni’s multiple comparison test (GraphPad Prism) was used for analysis of 3 or more groups. An unpaired Student’s t test was used for comparison between 2 groups. Data were considered significant at P ≤ 0.05. For all in vivo experiments, a minimum of 5 animals/group/time point were used unless otherwise noted. All experiments were carried out in triplicate, and representative data from a typical experiment are shown.
RESULTS
Lipidomic profile in B6 vs. BALB/c corneas
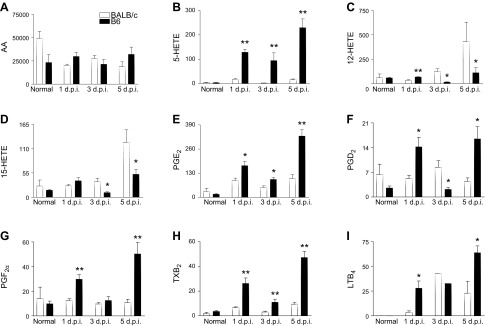
To investigate signature lipid metabolite profiles correlating with disease outcome in susceptible B6 vs. resistant BALB/c mice, analysis was carried out on corneas at 3 and 5 d p.i. Levels of the ω-6 fatty acid AA were comparable between B6 and BALB/c mice before and after infection (Fig. 1A), indicating there is no underlying imbalance in the availability of initial substrate between the 2 mouse strains. However, significant differences were detected in downstream metabolic intermediates. 5-HETE (Fig. 1B), an intermediate metabolite of leukotrienes and a marker of leukocyte 5-LOX activity, was significantly elevated after infection in B6 compared with BALB/c corneas. In contrast, 12-HETE (Fig. 1C) and 15-HETE (Fig. 1D), respective 12/15-LOX pathway markers, were elevated in corneas of BALB/c vs. B6 at both time points. Increased inflammatory eicosanoid pathway activity was supported by increased levels of prostaglandins that have established roles in corneal inflammatory and chronic injury responses (18) and are known to be produced by cyclooxygenase (COX)-2. PGE2 levels (Fig. 1E) were increased significantly in B6 mice compared with BALB/c mice at both time points after infection. PGD2 (Fig. 1F) and PGF2α (Fig. 1G) were similar between the 2 mouse strains at 3 d but were elevated significantly at 5 d p.i. in B6 vs. BALB/c corneas. Thromboxane B2 (Fig. 1H), another proinflammatory prostanoid, was significantly up-regulated at 3 and 5 d p.i. in B6 corneas. In addition, the potent neutrophil chemoattractant LTB4 (Fig. 1I) was similarly elevated in both strains at d 3 but was significantly higher at 5 d p.i. in B6 vs. BALB/c mice. There were no significant differences for any of the aforementioned intermediates or lipid mediators in the normal corneas of B6 and BALB/c mice.
Figure 1.
LC-MS/MS–based lipidomics of AA-derived intermediates and lipid mediator signals as detected in corneas of normal and 3-d and 5-d P. aeruginosa–infected B6 and BALB/c animals. Levels are provided for AA (A), 5-HETE (B), 12-HETE (C), 15-HETE (D), PGE2 (E), PGD2 (F), PGF2α (G), thromboxane B2 (TXB2) (H), and LTB4 (I). Results are represented as pg/sample ± sem (n = 5 corneas/group/time point). *P < 0.05 B6 vs. BALB/c.
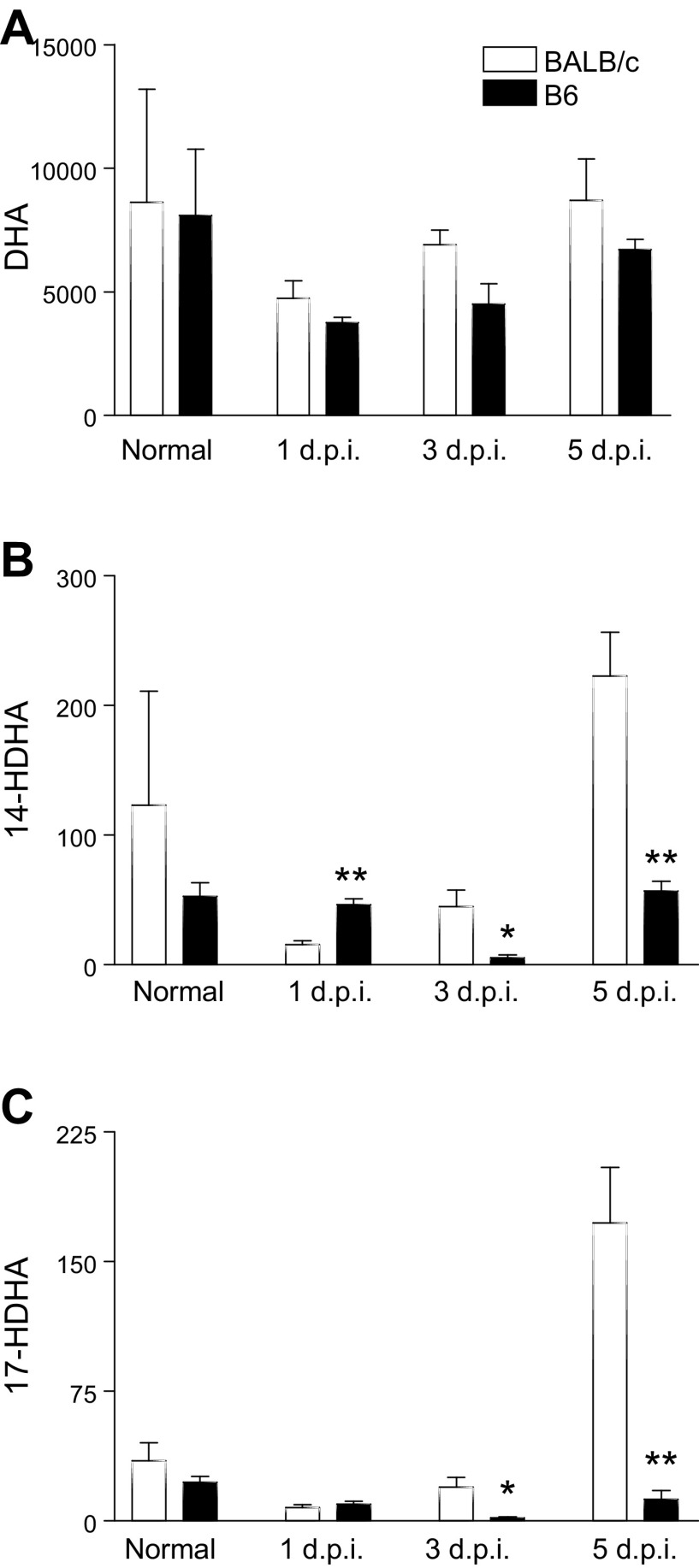
Levels of DHA-derived lipid mediators were assessed in corneas of B6 and BALB/c mice after infection. Despite similar levels of DHA (Fig. 2A), significant differences were observed in the downstream DHA metabolites 14-hydroxydocosahexaenoic acid (HDHA) and 17-HDHA, which are pathway markers for DHA-derived SPMs (32, 33) (Fig. 2B, C, respectively). BALB/c corneas demonstrated highly elevated levels of 14- and 17-HDHA compared with B6 at both 3 and 5 d p.i. Intrastrain trends were observed in B6 mice that were not evident in BALB/c mice; metabolite intermediates and lipid metabolite end products consistently decreased at 3 d p.i. (compared with 1 and 5 d), with the highest levels observed at 5 d p.i. In contrast, BALB/c mice appeared to trend upward with time or to peak at 3 d p.i. instead.
Figure 2.
LC-MS/MS–based lipidomics of DHA-derived intermediates as detected in corneas of normal and 3-d and 5-d P. aeruginosa–infected B6 and BALB/c animals. Levels of DHA (A) and the intermediate metabolites 14-HDHA (B) and 17-HDHA (C) are shown. Results are represented as pg/sample ± sem (n = 5 corneas/group/time point). *P < 0.05 B6 vs. BALB/c.
In view of the marked strain-specific differences in the activities of proinflammatory eicosanoid and SPM pathways that correlate with susceptible vs. resistant phenotypes, we assessed expression of LOX and COX enzymes. COX-2, an enzyme that is rapidly induced in most inflammatory responses, was significantly up-regulated in B6 compared with BALB/c corneas at 3 and 5 d p.i. (Fig. 3A). Consistent with an amplified inflammatory response, 5-LOX (Fig. 3B), a marker of inflammatory leukocytes, was significantly increased in B6 mice at 5 d p.i. On the other hand, platelet 12-LOX (Alox12) (Fig. 3C), an enzyme associated with SPM production, including lipoxins and maresins, was significantly decreased in B6 corneas at 3 d p.i. Most importantly, 12/15-LOX (Fig. 3D), a key marker of epithelial and mucosal proresolving pathway activity (i.e., biosynthesis of lipoxins, resolvins, and protectins), was reduced significantly at both 3 and 5 d p.i. in B6 compared with BALB/c mice. Protein levels of COX-2 (Fig. 3E), 5-LOX (Fig. 3F), and 12/15-LOX (Fig. 3G) followed similar trends observed at the mRNA level. Taken together, these data suggest that a fundamental difference in the response of B6 and BALB/c mice to bacterial keratitis is that the susceptible B6 strain has a phenotype of an amplified proinflammatory eicosanoid and impaired SPM response compared with BALB/c.
Figure 3.
Levels of lipid mediator biosynthetic enzymes. mRNA expression levels are reported as relative fold change of the gene of interest compared with β-actin ± sem. A–D) Transcript levels of COX-2 (A), 5-LOX (B), 12-LOX (C), and 15-LOX (D) are provided at 3 and 5 d after P. aeruginosa–induced infection in BALB/c and B6 corneas. E–G) Protein levels of COX-2 (E), 5-LOX (F), and 12/15-LOX (G) were detected by Western blot in BALB/c and B6 corneas at the same time points (n = 5 corneas/group/time point). *P < 0.05 B6 vs. BALB/c.
Disease response in 15-LOX−/− mice
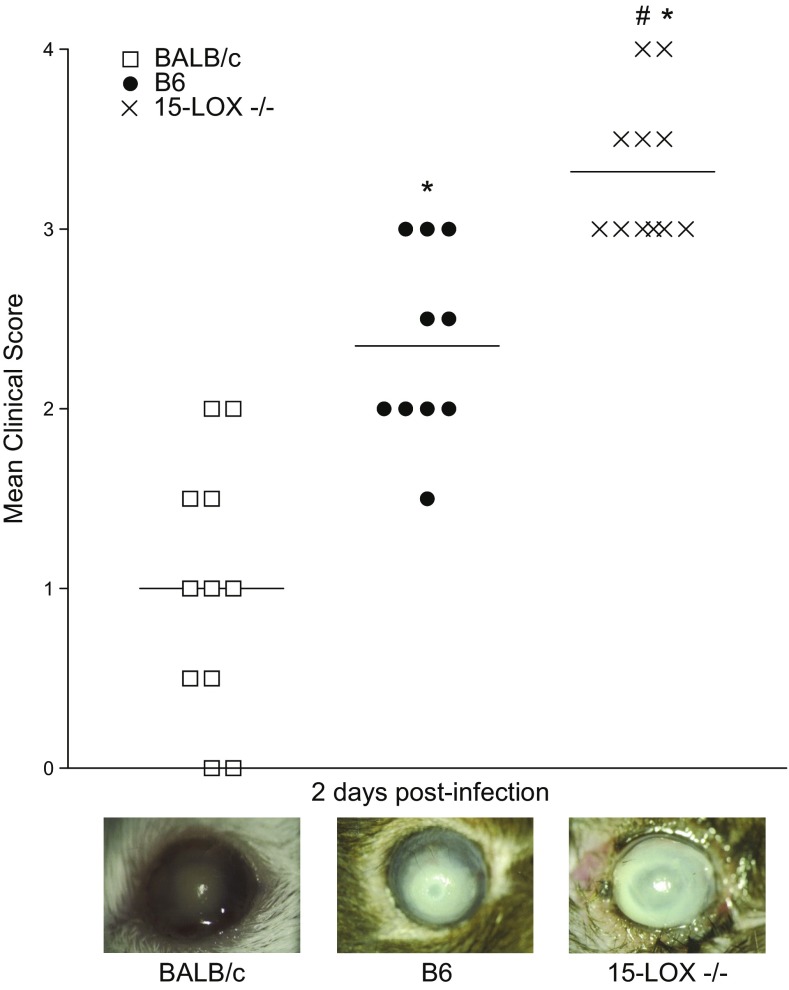
Given its abundant expression of 15-LOX in the cornea (16) and its role in the biosynthesis of SPMs, the immunoregulatory influence of this enzyme and its associated pathway in the host response to infection was investigated. Corneas of 15-LOX−/− animals fared significantly worse than corneas of BALB/c and B6 animals (Fig. 4). 15-LOX−/− corneas ranged from dense opacity covering the entire anterior segment (+3) to corneal perforation (+4) by 2 d p.i. Disease response was less severe in B6 and BALB/c animals. Seventy percent of B6 mice displayed dense opacity partially or fully covering the pupil (+2), and 30% displayed dense opacity covering the entire anterior segment (+3) but displayed no corneal perforation. Infected corneas of BALB/c mice ranged from slight opacity, fully covering the anterior segment (+1), to dense opacity, partially or fully covering the pupil (+2). Representative photographs taken at 2 d p.i. demonstrate the contrast in disease response between a (+3) 15-LOX−/− eye compared with B6 (+2) and BALB/c (+1) (Fig. 4). Due to the rapid progression and disease severity observed in the KO animals, subsequent analyses were carried out at 2 d p.i.
Figure 4.
Ocular disease response of P. aeruginosa–infected BALB/c, B6, and 15-LOX−/− mice. Corneal response was graded at 2 d p.i. (n = 10/group). Results are represented as median clinical scores ± sem. Photographs taken by slit-lamp provide further visualization of the differences in clinical scores between the different strains of mice. *P < 0.05 vs. BALB/c, #P < 0.05 vs. B6.
Bacterial load
Direct plate counts were used to determine the number of viable bacteria in infected corneas at 2 d p.i. Bacterial load in 15-LOX−/− corneas was significantly elevated compared with B6 and BALB/c mice (Fig. 5). Approximately 7.9 × 106 CFU/cornea were detected in infected 15-LOX−/− mice; this number is >2 times the bacterial burden detected in infected B6 corneas, which had ∼3.7 × 106 CFU/cornea. The fewest bacteria were detected in infected BALB/c corneas at 4.5 × 105 CFU/cornea, which was >17 times less than the rate detected in infected corneas of 15-LOX−/− mice.
Figure 5.
Quantification of viable bacteria. Bacterial counts were detected from corneas of BALB/c, B6, and 15-LOX−/− animals 2 d after P. aeruginosa–induced ocular infection. Results are reported as CFU/cornea ± sem (n = 6 corneas/group). *P < 0.05 vs. BALB/c, #P < 0.05 vs. B6.
Cytokine/chemokine expression in whole cornea
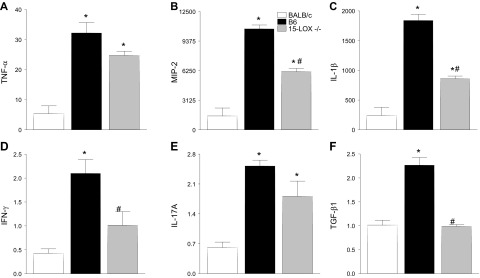
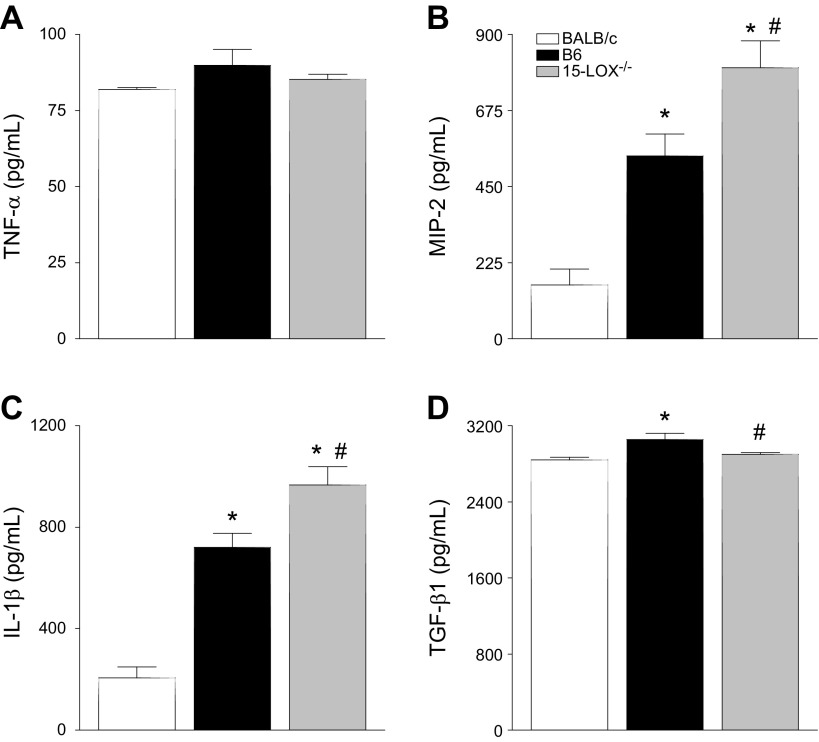
The cytokine/chemokine profile was investigated to determine if these inflammatory mediators were, in turn, dysregulated and thus contributing to the worsened disease outcome observed in 15-LOX−/− mice. Transcript levels of select pro- and anti-inflammatory cytokines/chemokines known to be differentially expressed in B6 and BALB/c P. aeruginosa–infected corneas (8) were analyzed in all 3 mouse strains at 2 d p.i. (Fig. 6). Results indicated that corneal mRNA expression of proinflammatory TNF-α (Fig. 6A), IL-1β (Fig. 6B), IL-17A (Fig. 6C), MIP-2 (Fig. 6D), and IFN-γ (Fig. 6E) was up-regulated in B6 compared with BALB/c at mice 2 d p.i. The anti-inflammatory molecule TGF-β1 (Fig. 6F) was increased in B6 over BALB/c mice at 2 d p.i.
Figure 6.
Real-time RT-PCR of select cytokine and chemokine transcript levels in corneas of BALB/c, B6, and 15-LOX−/− animals at 2 d p.i. mRNA expression levels for TNF-α (A), IL-1β (B), IL-17A (C), MIP-2 (D), IFN-γ (E), and TGF-β1 (F) are represented as relative fold change for the gene of interest compared with β-actin ± sem (n = 6 corneas/group). *P < 0.05 vs. BALB/c, #P < 0.05 vs. B6.
Regarding 15-LOX−/− mice, mRNA levels of the aforementioned proinflammatory molecules fell between those observed in B6 and BALB/c animals (Fig. 6). Furthermore, TGF-β1 mRNA was similar between 15-LOX−/− and BALB/c mice. When confirmed at the protein level (Fig. 7), TNF-α (Fig. 7A) and TGF-β1 (Fig. 7D) followed similar trends as mRNA expression. However, MIP-2 (Fig. 7B) and IL-1β (Fig. 7C) were significantly up-regulated in corneas of 15-LOX−/− mice. Overall though, it appears that the worsened disease pathogenesis in 15-LOX−/− mice is not particularly due to an imbalance in the cytokine/chemokine profile.
Figure 7.
Protein levels of TNF-α (A), MIP-2 (B), IL-1β (C), and TGF-β1 (D) in corneas of BALB/c, B6, and 15-LOX−/− animals at 2 d p.i. Results are reported as means ± sem (n = 5 corneas/group). *P < 0.05 vs. BALB/c, #P < 0.05 vs. B6.
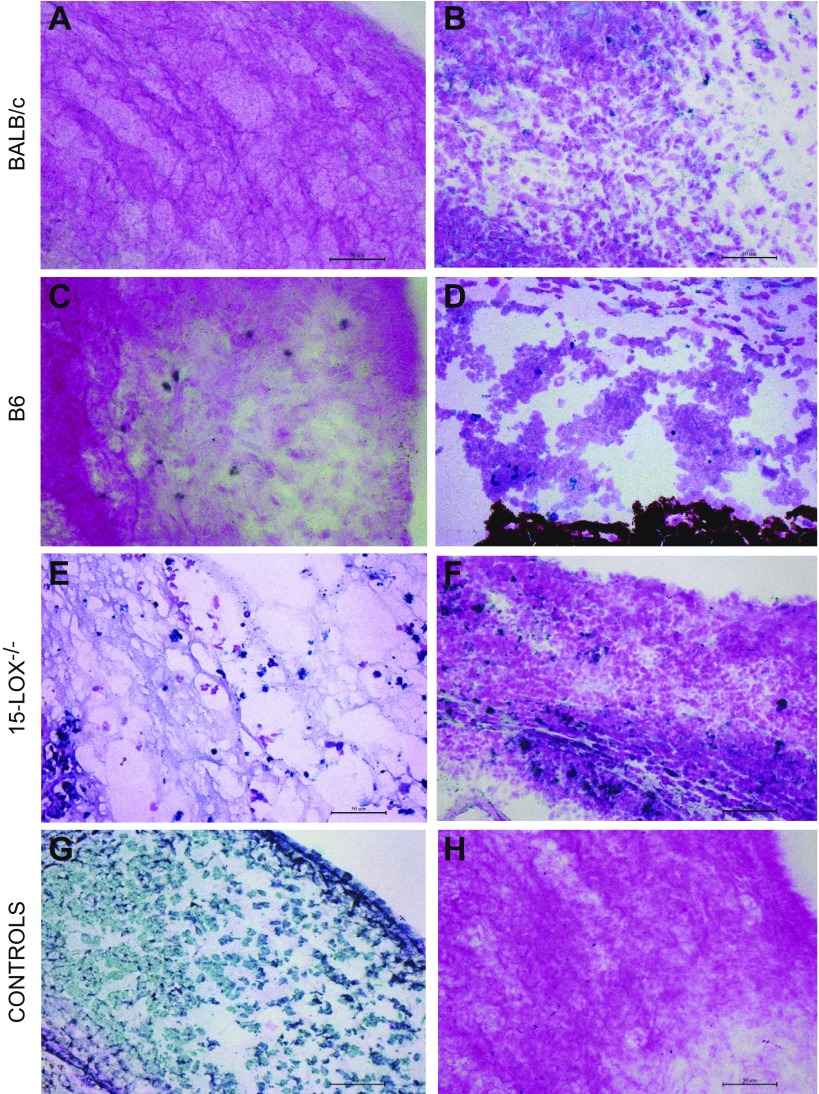
TUNEL staining
TUNEL staining was carried out to detect corneal damage associated with apoptosis and/or necrosis (Fig. 8). At 2 d p.i., infected BALB/c eyes revealed no positive staining in the central cornea (Fig. 8A) and revealed slight staining in the peripheral cornea (Fig. 8B), whereas B6 mice showed more TUNEL-positive stained cells centrally (Fig. 8C) and peripherally (Fig. 8D). 15-LOX −/− mice displayed substantially more intense TUNEL-positive staining throughout the central (Fig. 8E) and peripheral (Fig. 8F) cornea compared with B6 and BALB/c mice, which is further indicative of the extensive corneal damage in these animals after P. aeruginosa infection. Positive and negative controls are provided in Fig. 8G and H, respectively.
Figure 8.
Apoptosis and necrosis as detected by TUNEL staining in the infected cornea 2 d after P. aerguinosa infection. Sections of the central cornea (A, C, E) and peripheral cornea (B, D, F) are provided for BALB/c, B6, and 15-LOX−/− mice, respectively. Positive (G) and negative (H) controls are included. Scale bars, 50 μm.
Inflammatory cell infiltrate
Given the exacerbated disease response observed in 15-LOX−/− mice, flow cytometry was used to analyze 2 major inflammatory cell infiltrates associated with corneal infection, neutrophils (PMNs), and macrophages (Fig. 9). At 2 d p.i., there were no significant differences in absolute numbers of CD11b+CD45+Ly6G+ cells, identified as PMNs, between infected corneas in BALB/c and B6 mice (Fig. 9A), whereas significantly fewer PMNs were detected in corneas of 15-LOX−/− mice. No differences were observed, however, in the percentage of apoptotic PMNs between 15-LOX−/− compared with B6 or BALB/c corneas (Fig. 9C), although significantly more apoptotic PMNs were detected in BALB/c mice than in B6 mice.
Figure 9.
Representative flow cytometry plots denoting the gating strategies used to identify PMNs (A), macrophages (B), percentage of apoptotic PMNs (C), and percentage of apoptotic macrophages (D). Cell staining was performed on infected corneas of BALB/c, C57BL/6, and 15-LOX−/− mice at 2 d p.i. FAM-FLICA staining was used to detect activated caspase-3/7 in the infected corneas. Results are reported as mean ± sd for each cornea (n = 4 corneas/group). *P < 0.05, **P < 0.01 (where indicated).
No significant differences were observed in absolute numbers of macrophages (CD11b+CD45+Ly6G−F4/80+) between the 3 groups (Fig. 9B), although macrophages comprised a much smaller percentage of the total number of CD11b+ CD45+ cells compared with PMNs. Despite similar cell numbers quantitated in infected corneas, there was a significant increase in apoptotic macrophages (Fig. 9D) detected in 15-LOX−/− mice, whereas no differences were observed between B6 and BALB/c.
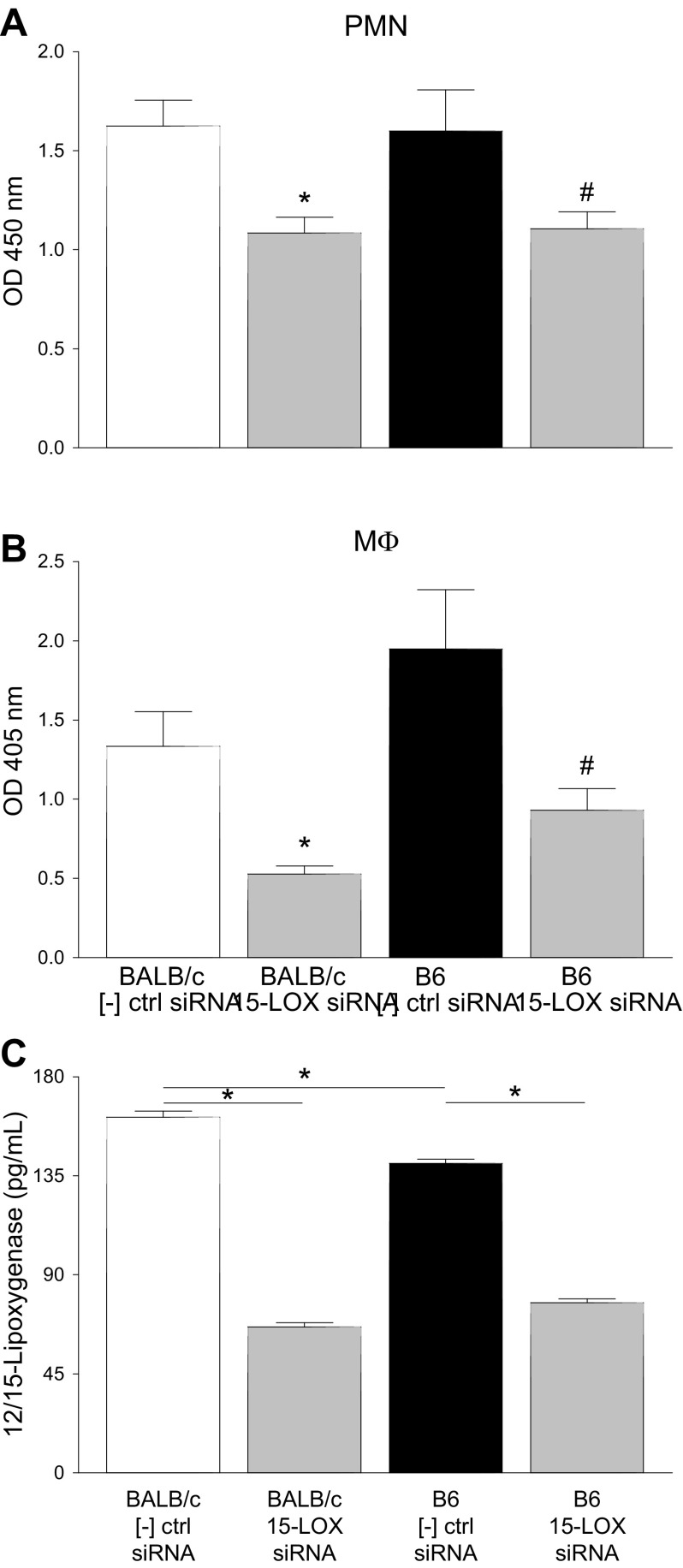
Phagocytosis detection
Taken together, our previous results suggest that differences in disease outcome between B6 and BALB/c mice were not due to insufficient numbers of key inflammatory cells but rather were an issue of cell function. Without 15-LOX, essential innate inflammatory functions are not carried out by PMNs and macrophages. Therefore, we next investigated whether 15-LOX regulated phagocytic functions (Fig. 10). Enzyme-labeled E. coli and zymosan were used as the phagocytosis pathogens for PMNs and macrophages, respectively. Cells were either treated with scrambled siRNA as a negative control or with 15-LOX siRNA prior to exposure to the phagocytosis pathogen. PMNs (Fig. 10A) and macrophages (Fig. 10B) derived from BALB/c and B6 mice showed similar levels of phagocytosis under normal conditions (scrambled siRNA controls). However, treatment with 15-LOX siRNA significantly impaired the phagocytic ability of PMNs and macrophages derived from both strains compared with the control groups, indicating a role for 15-LOX regarding inflammatory cell function. Efficiency of 15-LOX siRNA is shown for PMNs (Fig. 10C); similar results were obtained in macrophages (data not shown). Further PMN viability was maintained at >75% at 24 h and at 65–70% after 48 h of siRNA treatment.
Figure 10.
PMN and macrophage phagocytosis detection. A, B) After 15-LOX siRNA treatment, peritoneal-derived PMNs (A) and macrophages (B) from BALB/c and B6 mice were exposed to an enzyme-labeled phagocytosis pathogen (E. coli for PMNs; zymosan for macrophages). Scrambled siRNA was used as a negative control. C) Efficiency of knockdown as measured by ELISA for 12/15-LOX as represented in PMNs. Data are reported as means ± sd. *P < 0.05 vs. BALB/c control, #P < 0.05 vs. B6 control (unless otherwise noted).
DISCUSSION
Lipid mediators play an essential role in the initiation, inhibition, and resolution of inflammation. Although several diseases have been investigated in great detail, these key signaling molecules have yet to be elucidated to the same extent in ocular infectious disease. The modeling system used in the current study allows us to compare and contrast 2 distinct disease outcomes: susceptibility, as demonstrated by corneal thinning and perforation in B6 mice, and resistance, as exhibited by resolution in BALB/c mice. As such, this study characterized the lipid mediator profiles of Pseudomonas-induced keratitis, specifically regarding the development of susceptibility vs. resistance, and highlights the regulatory features of 15-LOX in influencing inflammatory cell function.
Despite similarities in initial PUFA substrates, as indicated by AA and DHA levels, a considerable divergence was revealed between infected B6 and BALB/c mice by way of disparate enzyme levels, metabolic intermediates, and bioactive lipid mediator signals. BALB/c mice displayed a tailored and well-balanced lipid mediator response after infection. Early proinflammatory signals were abundant initially but were temporally counter-balanced by the activation of SPM circuits. This balance correlated with the resolution of disease and with the resultant resistant phenotype. Although B6 animals also displayed an early release of initiating mediators, it was much greater in amplitude compared with BALB/c mice. Levels of prostaglandins, leukotrienes, and thromboxane never decreased and, in fact, increased over time. Furthermore, changes in 15-HETE and 17-HDHA suggest differential activation of SPM circuits in B6 mice that were significantly lower than in BALB/c mice and appeared to be insufficient to counterbalance robust leukotriene and prostanoid production, which likely is a key factor in sustaining a state of chronic inflammation and susceptibility. This latter profile demonstrated trends similar to those observed in localized aggressive periodontitis (34) and polymicrobial systemic sepsis (35).
Despite the presence of prostaglandins, which are largely proinflammatory in nature, studies have shown that PGE2 and PGD2 play key roles in lipid mediator class switching. More specifically, PGE2 has been shown to induce LXA4 formation in PMNs (36). Thus, it appears that this class switching occurs more efficiently in BALB/c animals yet is deficient in susceptible B6 mice due to the overwhelming persistence of proinflammatory signals and insufficient activation of SPM circuits and related enzymatic activity. The mouse corneal epithelium highly expresses Alox15, which is a 12/15-LOX with primary 12-LOX activity because it generates 12-HETE and 15-HETE in an approximate ratio of 3:1. Functional expression of 5-LOX (Alox5) and 12/15-LOX (Alox15) in mouse corneas and mouse corneal epithelium has been established by Gronert et al. (16). Alox15 in mice is the most abundant lipoxygenase in the healthy cornea (37, 38). Alox12 and Aloxe mRNA is expressed in mouse corneas. However, Chen et al. (39) have reported that platelet-type 12-LOX (Alox12) is not expressed at the protein level in mouse eyes. The contribution of Aloxe to 12-HETE formation in the cornea has not been investigated. However, it has been established that in the healthy and inflamed cornea, 12/15-LOX (Alox15) from epithelial cells and/or PMNs is the predominant source of 12-HETE and 15-HETE (16). Although limitations of lipidomic analysis preclude us from ruling out whether CYP450 formation contributes to overall endogenous HETE levels in healthy or inflamed corneas, results from LOX enzyme expression and inhibitor studies combined with the 15-LOX−/− mouse strongly support our findings.
It has been shown that transgenic overexpression of 15-LOX is protective in rabbit models of periodontal disease (40). 15-LOX−/− mice have also been used to study corneal wound healing and angiogenic responses (16, 41, 42). These studies established that 15-LOX−/− mice display impaired wound healing and amplified inflammatory angiogenesis. More importantly, the observed exacerbated inflammatory response correlated with impaired 15-HETE and reduced LXA4 formation in the cornea, a phenotype that was rescued by LXA4 treatment. Given the abundant expression of 15-LOX in the cornea (16) and its role in the generation of SPMs, this led us to investigate this enzyme and related pathways in the pathogenesis of Pseudomonas-induced keratitis. Corneas of 15-LOX−/− animals perforated much earlier (2 d) compared with wild-type B6 animals, which typically perforate at 5–7 d p.i. This accelerated disease response was accompanied by excessive bacterial load. Initially, it was thought that the increased bacterial burden in the 15-LOX−/− animals was due to a dysregulated cytokine profile. This did not appear to be the case because the overall cytokine profile was comparable to BALB/c and B6 animals at 2 d p.i. These data are further supported by cytokine levels at 5 d p.i. (Supplemental Fig. 1). Results at this later time point are comparable between 15-LOX−/− and B6 mice; taken together, these results indicate that the cytokine/chemokine profile is not a key feature underlying exacerbated disease pathogenesis observed in 15-LOX−/− animals.
TUNEL staining provided additional evidence for a regulatory role of 15-LOX regarding cellular function. Results from 15-LOX−/− and B6 mice were further indicative of the extensive corneal damage in these animals after infection compared with BALB/c mice. As a result, we investigated 2 major inflammatory cells associated with ocular infections: PMNs and macrophages. These cells are needed for proper phagocytosis and clearance of invading pathogens. Additionally, it has been shown that SPMs counter-regulate mediators that trigger leukocyte trafficking (32) and enhance phagocytosis of apoptotic PMNs, cellular debris, and bacteria (24, 33, 43, 44). Although there were no differences in the absolute numbers of infiltrating macrophages between the 3 groups, there was a significant increase in apoptotic macrophages in the corneas of KO animals. PMNs were significantly decreased in 15-LOX−/− compared with BALB/c and B6 animals, but there were no differences in apoptotic PMNs. Without proper bacterial clearance by PMNs in 15-LOX−/− mice, the pathogen is able to persist and flourish in the cornea, contributing to early corneal perforation in these animals. In addition, removal of apoptotic PMNs by resolution-type macrophages is essential to the resolution of inflammation. Deficiencies of these cellular aspects may contribute to the exacerbated disease pathogenesis observed in 15-LOX−/− animals.
There were no differences in absolute numbers of PMNs or macrophages between infected corneas of BALB/c and B6 mice, suggesting that the observed susceptible phenotype in B6 mice is due to functional cellular deficits vs. reduced numbers of cellular infiltrate. It is known that PMNs and macrophages perform phagocytic functions during infection; PMNs are the main cell to remove pathogens through phagocytosis and intracellular killing and then undergo apoptosis (45). It has been shown that during the normal course of inflammation, apoptotic PMNs display a proresolving phenotype characterized by SPM biosynthesis (23). However, results from the current study suggest that PMNs from susceptible B6 mice are not undergoing apoptosis, as indicated by caspase-3 and caspase-7 levels detected by flow cytometry, but perhaps undergo a combination of prolonged activation and subsequent necrosis, which may further contribute to a sustained chronic state of inflammation observed in these animals.
Macrophages are responsible for phagocytosing apoptotic PMNs through efferocytosis in an effort to resolve inflammation. As a result, we questioned whether the 15-LOX pathway is involved in the regulation of phagocytic function in either of these inflammatory cells. Despite similar numbers of macrophages and PMNs in the infected corneas of BALB/c and B6 mice, active phagocytosis was shown to be significantly impaired upon 15-LOX siRNA treatment. In essence, without 15-LOX and activated downstream pathways, PMNs are most likely unable to perform their normal physiologic functions (reactive oxygen species production, bacterial phagocytosis, and killing), which are key components to effectively regulate invading pathogens. This is thought to be compounded by macrophages that are not able to perform their efferocytotic function to clear inflammatory PMNs. Macrophages have also been shown to require a pro- to anti-inflammatory switch as a prerequisite for efferocytosis, which leads to enhanced SPM production (23, 32). Knockdown of 15-LOX-1 has been shown to reduce phagocytotic activity of human macrophages as well (46). Without these proper inflammatory cell functions, cells persist and become necrotic as the pathogen thrives within the cornea, destroying tissue integrity and leading to corneal perforation. Although it has been indicated that proinflammatory mediators can delay PMN apoptosis and subsequent clearance by macrophages (47, 48), this is the first report linking 15-LOX and PMN/macrophage phagocytic function during corneal infection. Our findings suggest that 15-LOX plays a key role in host immune defense function, including control of inflammation, PMN clearance, and bacterial clearance. Hence, impaired 15-LOX expression leads to inflammatory effector cell dysregulation and exacerbated disease pathogenesis.
There are structural differences between of mouse and human corneas that may limit the transferability of our findings. Whereas the mouse cornea is ∼30% epithelium and 70% stroma, the human cornea is 10% epithelium and 90% stroma (49). Such differences in composition are reflected by an increase in epithelial layers due to more numerous squamous cells in the central cornea of the mouse (50). This variation could contribute to differences in wound healing, where the mouse may take longer to restore the epithelium and thus longer to heal. However, it has also been shown that both mouse (epithelial sheets, whole cornea) and human (corneal epithelial cells) express 5-LOX and high levels of 15-LOX (51), which are both capable of generating SPMs. Taken together, the results of this study underscore the importance of SPM circuits in balancing inflammation after corneal infection and reveal that impaired expression of 15-LOX at the ocular surface leads to failed resolution and exacerbated inflammatory disease.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Eye Institute Grants R01 EY023226 (to E.A.B.), P30EY004068 (Core Grant), and R01 EY026082 (to K.G.), and by Research to Prevent Blindness. The authors declare no conflicts of interest.
Glossary
- AA
arachidonic acid
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- FBS
fetal bovine serum
- HDHA
hydroxy-docosahexaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- KO
knockout
- LOX
lipoxygenase
- LX
lipoxin
- p.i.
postinfection
- PMN
polymorphonuclear leukocyte
- PUFA
polyunsaturated fatty acid
- siRNA
small interfering RNA
- SPM
specialized proresolving lipid mediator
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. W. Carion performed the research, analyzed the data, and wrote the paper; M. Greenwood performed the research; A. S. Ebrahim performed the research; A. Jerome performed the research; S. Suvas analyzed the data and contributed analytic tools; K. Gronert contributed analytic tools, analyzed the data, and edited the paper; and E. A. Berger designed the research, analyzed the data, and wrote the paper.
REFERENCES
- 1.Carion T. W., McWhirter C. R., Grewal D. K., Berger E. A. (2015) Efficacy of VIP as treatment for bacteria-induced keratitis against multiple Pseudomonas aeruginosa strains. Invest. Ophthalmol. Vis. Sci. 56, 6932–6940 10.1167/iovs.15-17315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rattanatam T., Heng W. J., Rapuano C. J., Laibson P. R., Cohen E. J. (2001) Trends in contact lens-related corneal ulcers. Cornea 20, 290–294 10.1097/00003226-200104000-00010 [DOI] [PubMed] [Google Scholar]
- 3.Hazlett L. D. (2004) Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 23, 1–30 10.1016/j.preteyeres.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 4.Lotti R., Dart J. K. (1992) Cataract as a complication of severe microbial keratitis. Eye (Lond.) 6, 400–403 10.1038/eye.1992.82 [DOI] [PubMed] [Google Scholar]
- 5.Park S. H., Lim J. A., Choi J. S., Kim K. A., Joo C. K. (2009) The resistance patterns of normal ocular bacterial flora to 4 fluoroquinolone antibiotics. Cornea 28, 68–72 10.1097/ICO.0b013e318182259b [DOI] [PubMed] [Google Scholar]
- 6.Jhanji V., Sharma N., Satpathy G., Titiyal J. (2007) Fourth-generation fluoroquinolone-resistant bacterial keratitis. J. Cataract Refract. Surg. 33, 1488–1489 10.1016/j.jcrs.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 7.Gorham J. D., Güler M. L., Steen R. G., Mackey A. J., Daly M. J., Frederick K., Dietrich W. F., Murphy K. M. (1996) Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc. Natl. Acad. Sci. USA 93, 12467–12472 10.1073/pnas.93.22.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szliter E. A., Lighvani S., Barrett R. P., Hazlett L. D. (2007) Vasoactive intestinal peptide balances pro- and anti-inflammatory cytokines in the Pseudomonas aeruginosa-infected cornea and protects against corneal perforation. J. Immunol. 178, 1105–1114 10.4049/jimmunol.178.2.1105 [DOI] [PubMed] [Google Scholar]
- 9.Rudner X. L., Kernacki K. A., Barrett R. P., Hazlett L. D. (2000) Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J. Immunol. 164, 6576–6582 10.4049/jimmunol.164.12.6576 [DOI] [PubMed] [Google Scholar]
- 10.Berger E. A., Vistisen K. S., Barrett R. P., Hazlett L. D. (2012) Effects of VIP on corneal reconstitution and homeostasis following Pseudomonas aeruginosa induced keratitis. Invest. Ophthalmol. Vis. Sci. 53, 7432–7439 10.1167/iovs.12-9894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z., Barrett R. P., McClellan S. A., Zhang Y., Szliter E. A., van Rooijen N., Hazlett L. D. (2008) Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci. 49, 4458–4467 10.1167/iovs.08-1906 [DOI] [PubMed] [Google Scholar]
- 12.Huang X., Hazlett L. D. (2003) Analysis of Pseudomonas aeruginosa corneal infection using an oligonucleotide microarray. Invest. Ophthalmol. Vis. Sci. 44, 3409–3416 10.1167/iovs.03-0162 [DOI] [PubMed] [Google Scholar]
- 13.McClellan S. A., Huang X., Barrett R. P., van Rooijen N., Hazlett L. D. (2003) Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J. Immunol. 170, 5219–5227 10.4049/jimmunol.170.10.5219 [DOI] [PubMed] [Google Scholar]
- 14.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. (2000) Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192, 1197–1204 10.1084/jem.192.8.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J., Gronert K. (2017) The role of pro-resolving lipid mediators in ocular diseases. Mol. Aspects Med. 58, 37–43 10.1016/j.mam.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gronert K., Maheshwari N., Khan N., Hassan I. R., Dunn M., Laniado Schwartzman M. (2005) A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 280, 15267–15278 10.1074/jbc.M410638200 [DOI] [PubMed] [Google Scholar]
- 17.Li N., He J., Schwartz C. E., Gjorstrup P., Bazan H. E. (2010) Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. 26, 431–439 10.1089/jop.2010.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liclican E. L., Gronert K. (2010) Molecular circuits of resolution in the eye. Sci. World J. 10, 1029–1047 10.1100/tsw.2010.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan C. N. (2004) A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem. Cell Biol. 122, 305–321 10.1007/s00418-004-0695-8 [DOI] [PubMed] [Google Scholar]
- 20.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R. L. (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196, 1025–1037 10.1084/jem.20020760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S., Gronert K., Devchand P. R., Moussignac R. L., Serhan C. N. (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278, 14677–14687 10.1074/jbc.M300218200 [DOI] [PubMed] [Google Scholar]
- 22.Xu Z. Z., Zhang L., Liu T., Park J. Y., Berta T., Yang R., Serhan C. N., Ji R. R. (2010) Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 16, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalli J., Serhan C. N. (2012) Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 10.1182/blood-2012-04-423525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab J. M., Chiang N., Arita M., Serhan C. N. (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 10.1038/nature05877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang N., Fredman G., Bäckhed F., Oh S. F., Vickery T., Schmidt B. A., Serhan C. N. (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 10.1038/nature11042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazlett L. D., Moon M. M., Strejc M., Berk R. S. (1987) Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Invest. Ophthalmol. Vis. Sci. 28, 1978–1985 [PubMed] [Google Scholar]
- 27.Von Moltke J., Trinidad N. J., Moayeri M., Kintzer A. F., Wang S. B., van Rooijen N., Brown C. R., Krantz B. A., Leppla S. H., Gronert K., Vance R. E. (2012) Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490, 107–111 10.1038/nature11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y., Min K., Zhang Y., Su J., Greenwood M., Gronert K. (2015) Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J. Immunol. 195, 3086–3099 10.4049/jimmunol.1500610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux K. H. (1995) Optimization and troubleshooting in PCR. PCR Methods Appl. 4, S185–S194 10.1101/gr.4.5.S185 [DOI] [PubMed] [Google Scholar]
- 30.Luo Y., Dorf M. E. (2001) Isolation of mouse neutrophils. Curr. Protoc. Immunol. Chapter 3, Unit 3.20 10.1002/0471142735.im0320s22 [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Goncalves R., Mosser D. M. (2008) The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. Chapter 14, Unit 14.1 10.1002/0471142735.im1401s83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckley C. D., Gilroy D. W., Serhan C. N. (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 10.1016/j.immuni.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan C. N., Chiang N., Dalli J. (2017) New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. [E-pub ahead of print] Mol. Aspects Med. 10.1016/j.mam.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fredman G., Oh S. F., Ayilavarapu S., Hasturk H., Serhan C. N., Van Dyke T. E. (2011) Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS One 6, e24422 10.1371/journal.pone.0024422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang N., de la Rosa X., Libreros S., Serhan C. N. (2017) Novel Resolvin D2 receptor axis in infectious inflammation. J. Immunol. 198, 842–851 10.4049/jimmunol.1601650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 10.1038/89759 [DOI] [PubMed] [Google Scholar]
- 37.Gronert K. (2005) Lipoxins in the eye and their role in wound healing. Prostaglandins Leukot. Essent. Fatty Acids 73, 221–229 10.1016/j.plefa.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 38.Kenchegowda S., Bazan H. E. (2010) Significance of lipid mediators in corneal injury and repair. J. Lipid Res. 51, 879–891 10.1194/jlr.R001347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X. S., Kurre U., Jenkins N. A., Copeland N. G., Funk C. D. (1994) cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J. Biol. Chem. 269, 13979–13987 [PubMed] [Google Scholar]
- 40.Serhan C. N., Jain A., Marleau S., Clish C., Kantarci A., Behbehani B., Colgan S. P., Stahl G. L., Merched A., Petasis N. A., Chan L., Van Dyke T. E. (2003) Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 171, 6856–6865 10.4049/jimmunol.171.12.6856 [DOI] [PubMed] [Google Scholar]
- 41.Biteman B., Hassan I. R., Walker E., Leedom A. J., Dunn M., Seta F., Laniado-Schwartzman M., Gronert K. (2007) Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. FASEB J. 21, 2257–2266 10.1096/fj.06-7918com [DOI] [PubMed] [Google Scholar]
- 42.Leedom A. J., Sullivan A. B., Dong B., Lau D., Gronert K. (2010) Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 176, 74–84 10.2353/ajpath.2010.090678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka N., Irino Y., Shinohara M., Tsuda S., Mori T., Nagao M., Oshita T., Mori K., Hara T., Toh R., Ishida T., Hirata K. I. (2017) Eicosapentaenoic acid-enriched high-density lipoproteins exhibit anti-atherogenic properties. Circ. J. 82, 596–601 [DOI] [PubMed] [Google Scholar]
- 44.Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., Serhan C. N. (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178, 3912–3917 10.4049/jimmunol.178.6.3912 [DOI] [PubMed] [Google Scholar]
- 45.Berger E. A., McClellan S. A., Vistisen K. S., Hazlett L. D. (2013) HIF-1α is essential for effective PMN bacterial killing, antimicrobial peptide production and apoptosis in Pseudomonas aeruginosa keratitis. PLoS Pathog. 9, e1003457 10.1371/journal.ppat.1003457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiang N., Shinohara M., Dalli J., Mirakaj V., Kibi M., Choi A. M., Serhan C. N. (2013) Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J. Immunol. 190, 6378–6388 10.4049/jimmunol.1202969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. (1992) Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80, 2012–2020 [PubMed] [Google Scholar]
- 48.Lee A., Whyte M. K., Haslett C. (1993) Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54, 283–288 10.1002/jlb.54.4.283 [DOI] [PubMed] [Google Scholar]
- 49.Li H. F., Petroll W. M., Møller-Pedersen T., Maurer J. K., Cavanagh H. D., Jester J. V. (1997) Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF). Curr. Eye Res. 16, 214–221 10.1076/ceyr.16.3.214.15412 [DOI] [PubMed] [Google Scholar]
- 50.Henriksson J. T., McDermott A. M., Bergmanson J. P. (2009) Dimensions and morphology of the cornea in three strains of mice. Invest. Ophthalmol. Vis. Sci. 50, 3648–3654 10.1167/iovs.08-2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S. B., Hu K. M., Seamon K. J., Mani V., Chen Y., Gronert K. (2012) Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 26, 1506–1516 10.1096/fj.11-198036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.