Abstract
The objective of this article is to introduce the Clinical Framework for Quality Improvement of Cancer Cachexia (Cachexia Care Framework) as a tool to demonstrate the relevance of integrating the clinical components of cancer cachexia and the organizational strategies of a cancer institution on the quality of patient care and delivery of services throughout the cancer cachexia continuum. The data sources included peer-reviewed literature relevant to cancer cachexia and quality cancer care, and the authors’ expertise. The Cachexia Care Framework results from a combination of the international consensus definition of cancer cachexia, the Institute of Medicine report Ensuring Quality Cancer Care, and the authors’ experience with a cancer cachexia clinic. This framework is proposed as a guidance for oncology nurses and other healthcare providers to improve the quality of care of cancer cachexia patients. Specifically, the framework can be used by oncology nurses involved in the care of patients diagnosed with cancer cachexia either in direct patient care, administration, research, or education. Nurses can use the framework in clinical practice to identify specific assessments and interventions based on the cachexia stage of the patient; in nursing administration, the framework offers a wide view of potential errors that can happen and the opportunity to prevent them; in nursing research, the framework illustrates the several factors and processes that can impact patient outcomes; and in nursing education, the framework outlines the elements necessary to develop and implement a continuum education curriculum to educate the workforce of oncology nurses, and in the academic setting, an interprofessional curriculum to educate nurses and many other healthcare disciplines.
Keywords: Cancer cachexia, oncology nutrition, quality cancer care frameworks

Introduction
Cancer cachexia is a multidimensional syndrome that affects up to half of all cancer patients.[1,2,3] Involuntary weight loss in patients with cancer is often overlooked and rarely managed actively; it is an unmet need. Cancer cachexia can lead to significant functional impairment in patients as well as psychological distress and poor quality of life in patients and families if it is not addressed.[4,5] It can also impact cancer treatment, linked to the risk of poor response to treatment and shortened survival.[1] Team approaches and holistic care to the management of cancer cachexia have been proposed as a model of improving patient care and outcomes.[6,7,8,9]
The purpose of this article is to propose a clinical framework for quality improvement of cancer cachexia by focusing on potential gaps in care that prevents the patient from accessing high-quality care, throughout the stages of cancer cachexia. The framework integrates new definitions of cancer cachexia, its stages, and opportunities for improvement. The authors have implemented a cancer cachexia clinic over the past 10 years[9] and will translate this experience into a framework for quality improvement, using the international classification system developed by Fearon et al.[2] and the Institute of Medicine (IOM) report Ensuring Quality Cancer Care.[10] It is the authors’ hope to define a novel approach to improve quality of care for patients affected by cancer cachexia.
Cancer Cachexia
Cachexia is a serious and debilitating condition that affects patients with cancer. Cachexia originates from the Greek words kakos hexis meaning “bad condition” and affects patients with cancer and other chronic diseases.[11] A most recent international consensus was reached for the definition of cancer cachexia: “a multifactorial syndrome characterized by an ongoing loss of muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment.”[2] Cachexia remains a major challenge in cancer because it affects 50%–80% of cancer patients[12] and causes between 20% and 60% of cancer deaths.[13] Cancer associated-weight loss is present at the time of diagnosis in 34% of patients with lung cancer and gastrointestinal cancer including patients in Stage I (18%) and Stage II (26%) disease and is also associated with decreased survival.[14] The prevalence of cancer cachexia ranges from 12% to 85% depending on the definition used.[15] Based on the most recent consensus definition, the prevalence of cancer cachexia is strongly related to cancer type, being highest in lung cancer (83%), and gastrointestinal cancers (62%) and lowest in hematologic cancers (13%).[16]
Pathophysiology
The pathophysiology of cancer cachexia results from both the tumor (primary cachexia) and the cancer treatments (secondary cachexia).[17,18] In primary cancer cachexia, the tumor causes a systemic inflammatory response that leads to multiple metabolic alterations originating from tumor cells and activated immune cells that release cytokines, chemokines, and other inflammatory mediators.[17,19] Cachexia, also understood as an energy-wasting syndrome, is characterized by a negative protein and energy balance led by a combination of decreased food intake and abnormal metabolism.[17,11] In secondary cancer cachexia, the cancer treatments (mainly surgery, chemotherapy, and radiation therapy) have a damaging impact on a patient's nutritional intake through the development of nutrition impact symptoms.[19,20] For example, surgical resection of gastrointestinal organs may cause dysphagia, gastroparesis, dumping syndrome, or malabsorption; and both chemotherapy and radiation therapy can induce anorexia, nausea, vomiting, mucositis, hypogeusia, xerostomia, or fatigue.[19,20,21]
Clinical classification
Cachexia is a clinical continuum of three stages: precachexia, cachexia, and refractory cachexia [Table 1].[2] In the precachexia stage, the patient experiences early clinical and metabolic manifestations (i.e., anorexia and impaired glucose tolerance) that may develop prior involuntary weight loss. The possibility of advancing to the next stage of cachexia depends on factors such as cancer type and stage, occurrence of systemic inflammation, minimum food intake, and poor response to cancer treatment.[2] In the cachexia stage, patients experience weight loss of more than 5% over the past 6 months; or a body mass index (BMI) of <20 kg/m2 and weight loss of more than 2%; or sarcopenia and weight loss of more than 2%.[2] Refractory cachexia results from preterminal advanced cancer or quickly growing cancer, unresponsive to treatments. This stage, related to increasing catabolism, is associated with low-performance status and a life expectancy of <3 months.[2] The severity of depletion can be categorized based on the speed of the continuous weight loss in combination with the concurrent degree of depletion of fat and body protein mass.[2] For example, a patient with a BMI of 28 and a history of weight loss is more at risk if he/she developed sarcopenia and less risk if his/her muscle mass had no change.
Table 1.
Classification of cancer cachexia
| Stages | Criteria |
|---|---|
| Precachexia[2] | Weight loss ≤5% Anorexia and metabolic change |
| Cachexia[2] | Weight loss >5% over the past 6 months (in the absence of simple starvation) or BMI <20 and any degree of weight loss >2% or Appendicular skeletal muscle index consistent with sarcopenia and any degree of weight loss >2% |
| Refractory cachexia[2] | Variable degree of cachexia Cancer disease both procatabolic and not responsive to anticancer treatment Low-performance score <3 months expected survival |
BMI: Body mass index
Management: Assessment and interventions
The management of cancer cachexia includes clinical assessment and multimodal interventions provided by an interdisciplinary team. There is not a consensus agreement about a specific tool to assess cachexia, but there is a consensus about the domains that should be involved in cancer cachexia assessment: anorexia or decreased food intake, catabolic drivers, muscle mass and strength, and functional and psychosocial effects.[20] Because of the lack of cancer cachexia assessment tools, the most common tools used are malnutrition assessment tools that help to determine the degree of malnourishment and when nutritional support should be initiated.[11]
Several authors have discussed the relevance of using a multimodality approach to treat cancer cachexia. Del Fabbro[20] proposed a multimodality treatment model that includes pharmacologic and nonpharmacologic interventions and nutrition support. Pharmacologic interventions target the various metabolic mechanisms contributing to cancer cachexia, for example, thalidomide, interleukin inhibitors, fish oil, and nonsteroidal anti-inflammatory drugs are used to manage pro-inflammatory cytokines; ghrelin, megestrol, corticosteroids, and ghrelin mimetics are used to control poor appetite; androgens and insulin address endocrine dysfunction; and beta-blockers manage the elevated resting energy expenditure and weight. Progress in the comprehension of the molecular biology of the brain, immune system, and skeletal muscle have provided novel targets for the treatment of cachexia.[19] There are a number of clinical trials investigating pharmacologic agents targeted to inflammatory processes during the precachexia and the cachexia stages.[22] The nonpharmacologic interventions include exercise, nutrition counseling, and control of nutritional impact symptoms (NIS). Bruggeman et al. recommend that oncologists make early referrals for timely management of cachexia to palliative care specialists.[22] Cancer cachexia is a complex syndrome and the goal of the treatment is to fix the various broken pieces using an interdisciplinary team approach.[6,23]
Clinical Framework for Quality Care in Cancer Cachexia: An Interdisciplinary Approach
Eight years ago, the authors proposed a cancer cachexia clinic model to assess and manage the multiple symptoms of cancer cachexia patients through an innovative all in one interdisciplinary clinic, the Cancer Appetite and Rehabilitation (CARE) Clinic model.[9] In the CARE clinic model, the patients would be assessed and managed by the entire team, all disciplines in one stop; instead of having multiple appointments and being evaluated by multiple disciplines at different visits. The clinic's interdisciplinary model included a physician, a nurse practitioner, a nutritionist, a physical therapist, a speech pathologist, and a clinic assistant. Although the CARE clinic continues to run and manage cancer patients with cachexia, the authors noticed that some patients were not referred to the clinic and some patients did not return to the clinic for follow-up; therefore, they did not receive the care they needed, in spite of having the cancer cachexia clinic available to them. Adhering to the IOM report Delivering High-Quality Cancer Care[24] that focuses on the relevance of high-quality care along the entire trajectory of the cancer care continuum, the authors decided to conduct a gap analysis of the services offered to head-and-neck cancer patients, including the CARE clinic, with the purpose of identifying why some of these patients were lost in the process of care. The gap analysis report was critical not only to recognizing some flaws in care but also to learning that the quality of care of cancer cachexia patients should go beyond the care provided at the CARE clinic; it should cover the total course of the cancer cachexia disease process.[25]
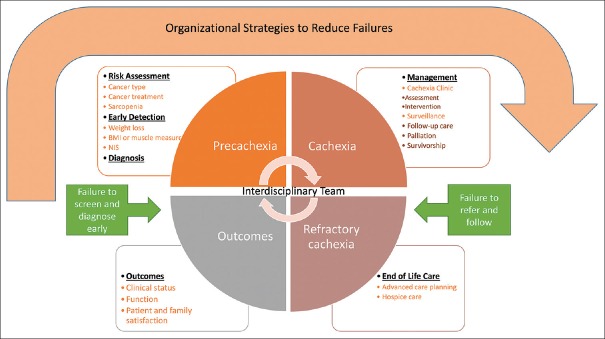
The authors propose a Clinical Framework for Quality Care in Cancer Cachexia (Cachexia Care Framework) to first, guide the care of cancer cachexia patients; and second, to identify any potential failures in cancer cachexia services that may hinder the quality of care offered to this patient population [Figure 1]. The proposed framework integrates the following: (a) the IOM report Ensuring Quality Cancer Care that focuses on both, the main points of cancer care (risk assessment, primary prevention, screening, detection, diagnosis, treatment, recurrence, surveillance, and end-of-life care), and on the identification of failures in care in the transitions between the points of care;[10] (b) the main elements of the cancer cachexia definition, classification, and recommendations described on the cancer cachexia international consensus;[2] and (c) the CARE clinic model.[9] Many factors influence cancer care environments and the performance of cancer care institutions. The Cachexia Care Framework has three main components, clinical, organizational, and outcomes. The clinical component consists of the continuum of care for cancer cachexia carried out by an interdisciplinary team at each stage of the disease and at each point of cancer care from risk assessment to end-of-life care. The organizational component refers to the organizational performance with regard to identifying failures in cachexia care and strategies to eradicate these failures that often happen during transitions of care. The outcomes component assists the interdisciplinary team and the cancer care institution to measure if the care provided is effective. The outcomes listed in Figure 1 are suggested, but more can be added depending on the cancer institution's needs.
Figure 1.
Clinical framework for quality care in cancer cachexia: an interdisciplinary approach
Clinical component
There is an obvious change in the management of cancer cachexia; while in the past, the focus was on refractory cachexia, currently, it is on early detection and management of cancer patients in the precachexia stage.[2] The interdisciplinary cancer cachexia team plays a crucial role in the evaluation of patients throughout the course of their disease; they assess and manage cancer patients who are at risk in developing cachexia or who are already diagnosed with cachexia.
Precachexia stage
The precachexia stage includes risk assessment, early detection, and diagnosis.
Risk assessment
Risk assessment of cachexia can be conducted by either the nurse practitioner, the nurse navigator, or the nutritionist of the team. Although about one-third of cancer patients present with high nutritional risk and have the potential to develop cachexia, there are certain factors that may help narrow down the list of cancer patients who need to be assessed for cancer cachexia. Risk assessment consists of the determination of certain factors that may alert cancer care providers about the patients’ nutritional deterioration. These factors include cancer type, cancer treatment, performance status, and sarcopenia. Cancer patients diagnosed with gastrointestinal cancers (pancreatic cancer, gastric cancer, and esophageal cancer) and lung cancer are at highest risk of developing cachexia.[20,26,27] Patients undergoing cancer treatments that cause serious side effects affecting their dietary intake may experience malnutrition and cachexia, for example, patients with cancer of head and neck receiving concurrent chemotherapy and radiation therapy may develop severe stomatitis and dysgeusia that may lead to secondary cachexia.[28,29] Poor performance status can be identified in pretreatment stages; it is mostly associated with early weight loss and inferior cancer cachexia outcomes.[30] Sarcopenia, an age-related condition characterized by progressive loss of skeletal muscle mass and strength, is developed independently of any disease process.[31] It has a prevalence of 60% among adults 60-year-old and older and the muscle mass loss goes up to 15% per decade at 70 years of age and older.[32] While the majority of sarcopenic patients do not develop cachexia, the majority of cancer cachexia patients develop sarcopenia.[33] Elderly patients who already have sarcopenia as a result of their aging process and are diagnosed with cancer, are at a higher risk to developing cachexia. The overlapping of these both conditions can work synergistically to induce increasing muscle mass loss.[33]
Early detection
Early detection of cachexia or precachexia can be conducted by either one clinician or combination of clinicians such as a nutritionist, a nurse navigator, a nurse practitioner, and a physician. Early detection includes assessment of high-risk cancer patients by measuring weight loss, BMI or muscle mass, and NIS.[2] There is not a specific cancer cachexia assessment tool, but a few have been suggested including the Edmonton Symptom Assessment Scale, the abridged patient-generated subjective assessment, the malnutrition-screening tool, the mini-nutritional assessment, and the 12-item Anorexia/Cachexia Scale (A/CS-12) among others.[11,20,34,35] Diagnosis of precachexia is made based on the criteria described in the international consensus described in Table 1.
Cachexia stage
The cachexia stage includes management and surveillance. The management of cancer cachexia consists of assessment and intervention.
Management
The Cachexia Care Framework recommends providing the management of cancer cachexia through a specialized cancer cachexia clinic. An example is the CARE clinic described previously by the authors. The clinic is run by an interdisciplinary team composed of a nurse practitioner, a nutritionist, a physical therapist, a speech pathologist, and a physician. A social worker, a chaplain, and a psychologist may be called depending on the patient and family's needs; they may be available on the same day of the visit or on another day. A comprehensive assessment is conducted on those cancer patients who were previously screened and diagnosed with either precachexia or cachexia.
Assessment
The assessment, an extended version of the initial early detection screening, includes symptom assessment, catabolic, muscle mass and strength, and functional and psychosocial effects.[2] All symptoms are assessed, in particular, those impacting nutrition such as anorexia, nausea, vomiting, depression, gastroparesis, dysgeusia, pain, diarrhea, and constipation.[2,20] The catabolic drivers and systemic inflammation involved in cachexia can be measured with the C-reactive protein.[2] Furthermoe, other comorbid deficiencies such as thyroid dysfunction, hypogonadism, and Vitamin B12 and D deficiencies should be investigated as they may contribute to fatigue, muscle weakness, and anorexia.[2]
One of the main contributions of the international consensus with regard to assessment of cancer cachexia patients is the importance of measuring muscle mass and strength. Determining body composition in a patient with cachexia is a helpful tool because it facilitates distinguishing between skeletal muscle and adipose tissue.[33] Although there is not an agreement about the assessment methods, the recommendation is to use cross-sectional imaging (computerized tomography [CT] or magnetic resonance imaging [MRI]), dual-energy X-ray imaging, anthropometry (mid-arm muscle area), and bioimpedance analysis.[2] CT or MRI of the lumbar vertebral landmark (L3) has been validated as the best area because in this region, skeletal muscle and adipose tissue correspond to whole-body tissue quantities.[36] Muscle strength is usually assessed by the physical therapist using the upper limb handgrip dynamometry.
Functional and psychosocial effects are assessed by any member of the cachexia clinic. Psychosocial issues are further explored by a psychologist, social worker, and/or chaplain. The psychological effect of cachexia on cancer patients was identified in a qualitative study reporting about the many symbolic meanings of food on patients.[37] Eating is a social activity with psychological relevance to people; thus, anorexia and weight loss become distressing factors to patients and their families.
Intervention
The therapeutic intervention for cancer cachexia is accomplished by the entire interdisciplinary team. This includes a wide-ranging approach using pharmacologic and nonpharmacologic therapies, as well as, nutritional support to manage symptoms, reduce weight loss and muscle wasting, and improve strength and stamina.[2,20] The addition of exercise interventions is recommended to increase lean muscle mass and improve functionality.[2,22] However, more research, especially randomized clinical trials are needed to support the relevance of exercise and safety in cancer cachexia patients.[38]
Surveillance
Follow-up care of patients is frequently done at the cachexia clinic by the interdisciplinary team, but most recently, the nurse navigator has played a significant role in providing a close watch of these patients beyond the cachexia clinic and during their transitions throughout different health-care settings.[39]
Refractory cachexia stage
Refractory cachexia is the result of a very advanced and progressive cancer that is not responding to cancer treatment. In this stage, patients’ performance status is poor and cachexia is not reversible.[2] In contrast to previous cachexia medication trials that allowed the participation of terminally ill cancer patients, the current consensus opposes these patients enrollment in this type of studies; instead, palliative care and hospice care are recommended in patients with refractory cachexia.[2] Artificial nutrition and hydration are not encouraged in cancer patients at the end of life, in their place, palliative care providing aggressive symptom management and emotional support to the patient and family should be offered. The reduction of oral intake in the context of terminal disease can become a source of distress to patients and their families, as they erroneously believe that increased eating would increase survival.[40] Educating patients and families that forcing the ingestion of food would actually cause indigestion, nausea, and other symptoms would help them understand the natural disease process. Futhermore, they can learn about providing comfort measures at the end of life.
Organizational component
According to the report Ensuring Quality Cancer Care, “quality care means providing patients with appropriate services in a technically competent manner, with good communication, shared decision-making, and cultural sensitivity.”[10] Excellent cancer cachexia care depends on multiple factors related to the patient experiencing the condition, the clinicians providing the care, and the cancer institution supplying the infrastructure. Yet, most of the accountability depends on the structure and the organized support systems of the cancer institution and the clinicians offering the care.[10] The Cachexia Care Framework's organizational strategies are discussed below.
Organizational strategies to reduce failures
There are numerous barriers to the implementation of the Cachexia Care Framework. Organizational support is essential to guarantee quality improvement and implementation of recommendations for improvement in cancer care. The role of nursing administration is crucial to the success of this conceptual model and clinical practice. Support from nursing leadership could include advocating for funding through the budget process or grant opportunities, to ensure that the clinic is sustainable and functional. Identifying cost saving opportunities by implementing early detection and a clinic model would demonstrate the economic benefit of this model. Nursing leaders within the cancer service line are in a unique position to advocate for supportive care positions such as nutritionists, speech therapists, physical therapists, and palliative care nurses as essential cancer care team members. Nursing informatics would be an important partner in developing a cachexia screen that could be embedded in the electronic medical record, ensuring that all cancer patients are screened on diagnosis and at regular intervals in their care. Nursing leadership could also advocate for expanded inclusion in tumor conferences so that all interdisciplinary team members are part of the discussion about the treatment care plan. This would include allowing those interdisciplinary team members time away from direct care to attend such meetings. Nursing education is also a key driver in ensuring that oncology nurses and other interdisciplinary team members are aware of cancer cachexia risk, diagnosis, screening, and treatment, allowing nurses to play a key role in the treatment of these patients.
Cancer care is complex and often fragmented, and although much progress has been made in developing multidisciplinary tumor conferences, these are largely focused on physicians rather than the entire interdisciplinary team. Most cancer centers require that the patient attend separate appointments for medical oncology, surgical oncology, and radiation oncology. If supportive care options are available, these are usually separate, additional appointments as well. For a debilitated patient experiencing cancer cachexia, this can be exhausting. Providing a physical space where all providers can see the patient as a team not only improves communication among the team by allowing real-time discussions and care planning but it also decreases the physical exertion of the patient by having the team come to him or her instead of the patient traveling to multiple offices.
One potential solution to some of the barriers identified is the role of the nurse navigator. According to the Oncology Nursing Society, “an oncology nurse navigator (ONN) (a) participates in the care of patients with a past, current, or potential diagnosis of cancer, (b) assists patients with cancer, families, and caregivers to overcome health-care system barriers, and (c) provides education and resources to facilitate informed decision-making and timely access to quality health and psychosocial care throughout all phases of the cancer continuum”.[41] A master's prepared ONN is in an ideal role to identify and implement screening for cancer cachexia, for referring patients who are at risk or in early stage cachexia for treatment and for following up with patients to identify their individual barriers to screening, treatment or follow-up care, such as transportation, lack of education, or cost. The nurse navigator can address those barriers or refer to supportive care team members, to ensure that patients at risk for or suffering from cancer cachexia can access quality care. Nurse navigation is effective in increasing patient satisfaction and decreasing barriers to care.[42]
There are also financial and billing issues associated with multidisciplinary care. Finding space can be challenging and ensuring that all disciplines can bill from that space can be impossible, due to Medicare and insurance guidelines that ensure that care is being provided in appropriate settings for that specialty. For example, in the CARE Clinic, colleagues from physical therapy and speech therapy are unable to bill for visits that occur outside their identified clinic. As a result, a process was developed to cover the hourly cost of the therapists through a grant and avoided billing the patient for those hours. While this allowed implementation of the CARE clinic, it is not sustainable.
Additional barriers include lack of consensus on a cachexia screening tool that could be implemented for all high-risk patients at the start of treatment, the frequent understaffing of supportive care staff such as nutrition and palliative care in many cancer centers, the lack of provider and interdisciplinary team education about early diagnosis of cancer cachexia, and failure to address patients who miss appointments or are lost to follow-up.
Outcomes component
The outcomes include medical outcomes and patient-centered outcomes. Medical outcomes are cachexia status and mortality. Cachexia status should be determined at the time of cancer diagnosis and after cachexia interventions and cancer treatments have been implemented. The goal is to reverse cancer cachexia and to cure cancer or keep it in remission. Patient-centered outcomes are symptom management, patient's function, patient's quality of life, and patient satisfaction. Measurement of these outcomes helps the interdisciplinary team to evaluate the efficacy of cancer cachexia interventions and offers the possibility for improvement.
Nursing Implications
The Cachexia Care Framework, a knowledge translation framework, is useful for oncology practice because it helps organize and recognize the specific components, sequential stages, and contextual factors needed to implement cancer cachexia interventions effectively and attain the desired outcomes.[43] The framework is helpful for any oncology clinician, but for nurses, it highlights the nursing role diversely as it expands into the dimensions of nursing including clinical practice, administration, research, and education. For nursing practice, the framework establishes a congruent relationship between the cachexia stage and the specific assessments and interventions recommended at each clinical point in the process. For example, the roles of the oncology nurse and the nurse navigator are very important during risk assessment and early detection in the precachexia stage, while the nurse practitioner role is essential during management and surveillance of cancer cachexia patients. For nursing administration, the framework integrates the organizational nature of a cancer cachexia program and the multiple operational aspects necessary for the program to function efficiently. Nursing leadership is greatly involved in looking at the potential failures that can occur during the process of caring for the cancer cachexia population and the developing of strategies to either prevent errors or improve care in the health-care system. For nursing research, the framework provides the structure to examine how variations in the cancer cachexia components and the organizational characteristics of the cancer institution may result in improved patient outcomes and cancer cachexia services delivery. For nursing education, the framework enables nurse educators to have basic guidelines to construct a curriculum and determine what knowledge and skills are needed to ensure that the oncology nursing workforce is competent providing cancer cachexia care. From the academic point of view, the framework may assist the development and implementation of interprofessional education and collaborative practice curricula for nurses, medical students, speech-language pathologist students, and other health-care students.[44] Overall, the use of a framework for cancer cachexia care can have a positive impact on improving the quality of life of cancer patients and on the quality of the services delivered to these patients.
Conclusion
Cancer cachexia is a common syndrome, affecting up to half of all cancer patients and impacting function, response to treatment, mortality, and quality of life. This paper has proposed a clinical framework for quality improvement that integrates new definitions of cancer cachexia and focuses on gaps in care and opportunities for improvement. The Cachexia Care Framework guides the care of the cachexia patient and identifies any potential failures in services that may hinder the quality of cachexia care. The goal of this model is to improve care of the patient with cancer cachexia. Adding nurse navigation to the interdisciplinary team can address barriers to care, assess symptoms, provide patient education, and ensure an extra layer of support during transitions in care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fearon KC. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124–32. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 3.Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, et al. Cancer cachexia: A systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol. 2011;80:114–44. doi: 10.1016/j.critrevonc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Takayama K, Atagi S, Imamura F, Tanaka H, Minato K, Harada T, et al. Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients-Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support Care Cancer. 2016;24:3473–80. doi: 10.1007/s00520-016-3156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheelwright S, Darlington AS, Hopkinson JB, Fitzsimmons D, Johnson C. A systematic review and thematic synthesis of quality of life in the informal carers of cancer patients with cachexia. Palliat Med. 2016;30:149–60. doi: 10.1177/0269216315588743. [DOI] [PubMed] [Google Scholar]
- 6.Portman DG, Thirlwell S, Donovan KA, Alvero C, Gray JE, Holloway R, et al. Leveraging a team mental model to develop a cancer anorexia-cachexia syndrome team. J Oncol Pract. 2016;12:1046–52. doi: 10.1200/JOP.2016.013516. [DOI] [PubMed] [Google Scholar]
- 7.Scott D, Reid J, Hudson P, Martin P, Porter S. Health care professionals’ experience, understanding and perception of need of advanced cancer patients with cachexia and their families: The benefits of a dedicated clinic. BMC Palliat Care. 2016;15:100. doi: 10.1186/s12904-016-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millar C, Reid J, Porter S. Healthcare professionals’ response to cachexia in advanced cancer: A qualitative study. Oncol Nurs Forum. 2013;40:E393–402. doi: 10.1188/13.ONF.E393-E402. [DOI] [PubMed] [Google Scholar]
- 9.Granda-Cameron C, DeMille D, Lynch MP, Huntzinger C, Alcorn T, Levicoff J, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14:72–80. doi: 10.1188/10.CJON.72-80. [DOI] [PubMed] [Google Scholar]
- 10.Hewit M, Simone JV, editors. Washington, D.C: Institute of Medicine and National Research Council, National Academy Press; 1999. [Last accessed on 2018 Apr 17]. Ensuring Quality Cancer Care; p. 3. Available from: http://www.nap.edu/catalog/6467.html . [Google Scholar]
- 11.Blum D, Strasser F. Cachexia assessment tools. Curr Opin Support Palliat Care. 2011;5:350–5. doi: 10.1097/SPC.0b013e32834c4a05. [DOI] [PubMed] [Google Scholar]
- 12.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–71. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 13.von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: Facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261–3. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannavarapu BS, Lau SK, Carter K, Cannon NA, Gao A, Ahn C, et al. Prevalence and survival impact of pretreatment cancer-associated weight loss: A tool for guiding early palliative care. J Oncol Pract. 2018;14:JOP2017025221. doi: 10.1200/JOP.2017.025221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallengren O, Lundholm K, Bosaeus I. Diagnostic criteria of cancer cachexia: Relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer. 2013;21:1569–77. doi: 10.1007/s00520-012-1697-z. [DOI] [PubMed] [Google Scholar]
- 16.Vagnildhaug OM, Balstad TR, Almberg SS, Brunelli C, Knudsen AK, Kaasa S, et al. A cross-sectional study examining the prevalence of cachexia and areas of unmet need in patients with cancer. Support Care Cancer. 2018;26:1871–80. doi: 10.1007/s00520-017-4022-z. [DOI] [PubMed] [Google Scholar]
- 17.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: Understanding the molecular basis. Nat Rev Cancer. 2014;14:754–62. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 18.Strasser F, Bruera ED. Update on anorexia and cachexia. Hematol Oncol Clin North Am. 2002;16:589–617. doi: 10.1016/s0889-8588(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 19.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–9. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 20.Del Fabbro E. Current and future care of patients with the cancer anorexia-cachexia syndrome. Am Soc Clin Oncol Educ Book. 2015:e229–37. doi: 10.14694/EdBook_AM.2015.35.e229. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia – Pathophysiology and management. J Gastroenterol. 2013;48:574–94. doi: 10.1007/s00535-013-0787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ, et al. Cancer cachexia: Beyond weight loss. J Oncol Pract. 2016;12:1163–71. doi: 10.1200/JOP.2016.016832. [DOI] [PubMed] [Google Scholar]
- 23.Hui D. Cancer cachexia: It takes a team to fix the complex machinery. J Oncol Pract. 2016;12:1172–3. doi: 10.1200/JOP.2016.017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charting a New Course for a System in Crisis. Institute of Medicine. IOM. Delivering High Quality Cancer Care. 2013. [Last accessed on 2018 Apr 17]. Available from: http://www.iom.edu/qualitycancercare .
- 25.Granda-Cameron C, Pauly M, DeMille D, Mante A, Null S, Malkowski J, et al. Gap analysis: A strategy to improve the quality of care of head and neck cancer patients. J Community Support Oncol. 2017;15:28–36. [Google Scholar]
- 26.Bozzetti F, Mariani L, Lo Vullo S, Amerio ML, Biffi R, et al. SCRINIO Working Group. Nutritional support of the oncology patient. Support Care Cancer. 2012;20:1919–28. doi: 10.1007/s00520-012-1387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Meij BS, Schoonbeek CP, Smit EF, Muscaritoli M, van Leeuwen PA, Langius JA, et al. Pre-cachexia and cachexia at diagnosis of stage III non-small-cell lung carcinoma: An exploratory study comparing two consensus-based frameworks. Br J Nutr. 2013;109:2231–9. doi: 10.1017/S0007114512004527. [DOI] [PubMed] [Google Scholar]
- 28.Muliawati Y, Haroen H, Rotty LW. Cancer anorexia-cachexia syndrome. Acta Med Indones. 2012;44:154–62. [PubMed] [Google Scholar]
- 29.Hopkinson JB, Wright DN, Foster C. Management of weight loss and anorexia. Ann Oncol. 2008;19(Suppl 7):vii289–93. doi: 10.1093/annonc/mdn452. [DOI] [PubMed] [Google Scholar]
- 30.Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, Herrstedt J, et al. Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Ann Oncol. 2014;25:1492–9. doi: 10.1093/annonc/mdu085. [DOI] [PubMed] [Google Scholar]
- 31.Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–7. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mislang AR, Di Donato S, Hubbard J, Krishna L, Mottino G, Bozzetti F, et al. Nutritional management of older adults with gastrointestinal cancers: An International Society of Geriatric Oncology (SIOG) Review Paper. J Geriatr Oncol. 2018 doi: 10.1016/j.jgo.2018.01.003. pii: S1879-4068(18)30014-6. [DOI] [PubMed] [Google Scholar]
- 33.Jacquelin-Ravel N, Pichard C. Clinical nutrition, body composition and oncology: A critical literature review of the synergies. Crit Rev Oncol Hematol. 2012;84:37–46. doi: 10.1016/j.critrevonc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Berry DL, Blonquist T, Nayak MM, Roper K, Hilton N, Lombard H, et al. Cancer anorexia and cachexia: Screening in an ambulatory infusion service and nutrition consultation. Clin J Oncol Nurs. 2018;22:63–8. doi: 10.1188/18.CJON.63-68. [DOI] [PubMed] [Google Scholar]
- 35.Gabrielson DK, Scaffidi D, Leung E, Stoyanoff L, Robinson J, Nisenbaum R, et al. Use of an abridged scored patient-generated subjective global assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer. 2013;65:234–9. doi: 10.1080/01635581.2013.755554. [DOI] [PubMed] [Google Scholar]
- 36.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 37.Hopkinson JB. Psychosocial impact of cancer cachexia. J Cachexia Sarcopenia Muscle. 2014;5:89–94. doi: 10.1007/s13539-014-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: Executive summary of a cochrane collaboration systematic review. J Cachexia Sarcopenia Muscle. 2015;6:208–11. doi: 10.1002/jcsm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordils-Perez J, Schneider SM, Gabel M, Trotter KJ. Oncology nurse navigation: Development and implementation of a program at a comprehensive cancer center. Clin J Oncol Nurs. 2017;21:581–8. doi: 10.1188/17.CJON.581-588. [DOI] [PubMed] [Google Scholar]
- 40.Del Río MI, Shand B, Bonati P, Palma A, Maldonado A, Taboada P, et al. Hydration and nutrition at the end of life: A systematic review of emotional impact, perceptions, and decision-making among patients, family, and health care staff. Psychooncology. 2012;21:913–21. doi: 10.1002/pon.2099. [DOI] [PubMed] [Google Scholar]
- 41.Oncology Nursing Society. Oncology Nurse Navigator Core Competencies. Pittsburg, PA: ONS; 2013. [Google Scholar]
- 42.Campbell C, Craig J, Eggert J, Bailey-Dorton C. Implementing and measuring the impact of patient navigation at a comprehensive community cancer center. Oncol Nurs Forum. 2010;37:61–8. doi: 10.1188/10.ONF.61-68. [DOI] [PubMed] [Google Scholar]
- 43.Hudon A, Gervais MJ, Hunt M. The contribution of conceptual frameworks to knowledge translation interventions in physical therapy. Phys Ther. 2015;95:630–9. doi: 10.2522/ptj.20130483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golom FD, Schreck JS. The journey to interprofessional collaborative practice: Are we there yet? Pediatr Clin North Am. 2018;65:1–2. doi: 10.1016/j.pcl.2017.08.017. [DOI] [PubMed] [Google Scholar]