Abstract
Objective:
New or worsening disability can develop in elderly patients in just 1 week of hospitalization for acute illness. Elderly patients with cancer, particularly those with cancer cachexia, are vulnerable to disability. This study aimed to explore the impact of hospitalization and cachexia on physical activity (PA) in elderly patients during chemotherapy.
Methods:
We prospectively enrolled 18 patients aged ≥70 years with newly-diagnosed, advanced non-small-cell lung cancer scheduled to initiate first-line chemotherapy. PA was measured using an accelerometer (Lifecorder®, Suzuken Co., Ltd., Japan). Mean daily steps at baseline, during hospitalization, and subsequent weeks (1st, 2nd, and 3rd week after discharge) were compared.
Results:
A total of 30 hospitalizations for chemotherapy were evaluated in 18 patients with a median age of 74.5 years. The median number of baseline daily steps was 3756. Fifteen cases (50%) showed fewer daily steps during hospitalization and no recovery to baseline level during the 1st week after discharge. Long hospitalizations (≥8 days) and the presence of cachexia were associated with persistent physical inactivity. One patient developed disability within 30 days after hospitalization.
Conclusions:
Physical inactivity was frequently seen after hospitalization for chemotherapy in elderly patients with advanced lung cancer. Longer in-hospital days and the presence of cancer cachexia caused slow recovery from physical inactivity. Individualized hospitalization planning based on careful consideration of patient age and the presence of cancer cachexia may be needed to prevent physical inactivity and disability.
Keywords: Cancer cachexia, chemotherapy, elderly, lung cancer, physical activity

Introduction
Bed rest can cause physical dysfunction regardless of age.[1] However, older age enhances the harmful effects of bed rest on cardiopulmonary fitness,[2] muscle mass, and strength.[3] McGavock et al. reported that the rate of decline in physical fitness accelerated after the age of 50 years.[4] This decline in physical fitness accelerates further in elderly individuals aged ≥70 years. Just 1 week of hospitalization for acute illness can lead to the development of new or worsened disability. This is known as hospitalization-associated disability (HAD).[5] The most important risk factor for HAD is physical inactivity during hospitalization.[6]
Elderly patients living with advanced cancer are particularly vulnerable to physical dysfunction. We previously reported that cancer cachexia and muscle depletion were frequently observed in elderly patients with newly-diagnosed, advanced non-small-cell lung cancer (NSCLC).[7] The majority of these patients lost their walking capacity shortly after chemotherapy. As a result, they developed disability and stayed longer in the hospital throughout the course of cancer. This was especially true for those who had cancer cachexia at baseline.[8]
Following recent progress in supportive care provision and less toxic chemotherapy, the majority of systemic chemotherapy is performed in outpatient clinics. However, some patients require multiple hospitalizations for chemotherapy, for the following possible reasons: Clinical trial schedule (e.g., blood sampling for pharmacokinetic analysis), difficulty in making frequent hospital visits due to the distance of travel from home to the hospital, patient concern about adverse events, or patient and caregiver preferences. These hospitalizations for chemotherapy may have harmful effects on physical activity (PA) in elderly patients with cancer.
The aims of this study were to observe changes in PA before, during, and after hospitalization, and to determine the presence of persistent decline in PA after hospitalization in elderly patients with advanced NSCLC who were receiving systemic chemotherapy. In addition, we aimed to explore the impact of cancer cachexia on the recovery of PA.
Methods
Patient selection
This prospective longitudinal observational study was performed in our center from February 2014 to June 2016. The eligibility criteria for the study participants were as follows: (1) histologically and/or cytologically proven Stage III or IV NSCLC including postoperative recurrence; (2) age ≥ 70 years, with planned first-line systemic chemotherapy; (3) no previous systemic chemotherapy or thoracic radiotherapy (adjuvant chemotherapy was not counted as prior chemotherapy); (4) Eastern Cooperative Oncology Group performance status (PS) of 0–1; (5) ability to ambulate, read, and respond to questions without assistance; and (6) expected survival of >12 weeks. Patients were excluded if they had a severe psychiatric disorder, active infectious disease, unstable cardiac disease, or untreated symptomatic brain or bone metastases that prevented safe assessment. All patients provided written informed consent. The study was approved by the institutional review board and conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Assessments for physical activity and activities of daily living
PA was measured using an electronic accelerometer (Kenz Lifecorder-GS, Suzuken Co., Ltd., Nagoya, Aichi, Japan). After informed consent was obtained, the patients wore the device on the waist at the side of the body.[9,10] Patients were instructed to wear the device for as long as possible in the daytime, starting from the time they changed clothes for daily activity in the morning to the time they changed into nightclothes for sleep. The device provided a record of the number of daily steps taken and the intensity of PA every 4 s throughout each day. Regular visits to the outpatient department of each institution allowed for data collection. Collected data included the number of daily steps and the daily duration of device-wearing. Data on days with a duration of device-wearing <5 h/day were excluded from the analysis.
PA was monitored throughout the data cutoff date (December 31, 2016). The values for each component of hospitalization were calculated as follows. The average number of daily steps during the prior 7 days of each hospitalization was set as the baseline value. The average number of daily steps between the 2nd day of hospitalization and the day before discharge was set as the value during hospitalization. The average number of daily steps between the day after discharge and the 8th day after discharge was set as the value for the 1st week after discharge. The average number of daily steps between the 9th day after discharge and the 15th day after discharge was set as the value for the 2nd week after discharge. The average number of daily steps between the 16th day after discharge and the 22nd day after discharge was set as the value for the 3rd week after discharge. No prescription for PA or walking was provided to the patients during the study period.
For the assessment of activities of daily living, the attending physician or physiotherapist at each hospital visit estimated the Barthel index. A disabling event was defined as a decrease in the Barthel index from the baseline value by >10 points. HAD was defined as having a disabling event within 30 days from the first day of hospitalization. Hospitalization-associated physical inactivity (HAPI) was defined as having decreased mean daily steps both during hospitalization and during the 1st week as compared with mean daily steps at baseline.
Diagnosis of muscle depletion and cancer cachexia
Cancer cachexia was defined as unintentional weight loss of >5% during the preceding 6 months, or >2% in patients with a body mass index (BMI) <20 kg/m2, or the presence of muscle depletion according to consensus criteria.[11] The cross-sectional area of skeletal muscle mass at the third lumbar vertebra level was measured[7,12] at baseline using SYNAPSE VINCENT software version 3 (FUJIFILM Medical Systems, Japan). Muscle depletion was defined based on the lumbar skeletal muscle index cutoffs of <43.0 cm2/m2 for men with a BMI <25.0 kg/m2, <53.0 cm2/m2 for men with a BMI ≥25.0 kg/m2, and <41.0 cm2/m2 for women.[13]
Statistical analysis
The Wilcoxon signed-rank test was used for the pair-wise comparison of measurement changes between study visits, whereas the Wilcoxon rank-sum test was used for comparisons between two independent groups. For all analyses, P < 0.05 was considered significant. All analyses were performed using JMP version 13.0 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Of 26 screened patients, we recruited 25 between February 2014 and June 2014 [Figure 1]. Six patients did not receive chemotherapy. One male was excluded from analysis because his baseline data were lost due to the initial failure of the accelerometer. The remaining 18 patients were analyzed in this study. Patients were followed up until the cutoff date (December 31, 2016). Median age was 74.5 (range, 70–82) years. The majority of patients had PS 1 and Stage IV disease. Eleven patients (61%) met the criteria for cancer cachexia. The first-line chemotherapy regimen included platinum-based regimens, single-arm cytotoxic regimens, and epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI). Adverse events related to chemotherapy were generally mild, including Grade 1 or 2 hematological or nonhematological toxicities according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. No severe adverse events were reported during the study period. Patients who had received platinum-based chemotherapy required multiple hospitalizations a maximum of 4 times. The median number of hospitalizations was 1 time (range, 1–4) [Table 1]. There was no significant difference in patient characteristics between cachectic and noncachectic patients.
Figure 1.
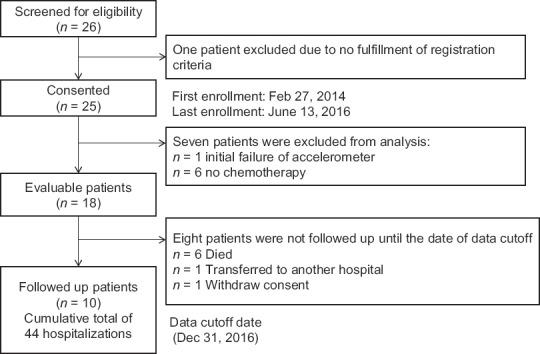
Patient flowchart
Table 1.
Patient characteristics
| Characteristics | All | Cachexia* | Noncachexia |
|---|---|---|---|
| Number of patients | 18 | 11 | 7 |
| Median age (range) | 74.5 (70-82) | 74 (70-82) | 76 (70-81) |
| Gender (women:men) | 9:9 | 4:7 | 5:2 |
| ECOG-PS, n (%) | |||
| 0 | 6 (33) | 3 (27) | 3 (43) |
| 1 | 12 (67) | 8 (73) | 4 (57) |
| Stage, n (%) | |||
| III | 1 (6) | 1 (100) | 0 |
| IV | 17 (94) | 10 (59) | 7 (41) |
| Chemotherapy regimen | |||
| Cisplatin + pemetrexed | 3 | 2 | 1 |
| Cisplatin + etoposide | 1 | 1 | 0 |
| Docetaxel | 5 | 3 | 2 |
| Vinorelbine | 2 | 2 | 0 |
| Gefitinib | 4 | 1 | 3 |
| Erlotinib | 3 | 2 | 1 |
| Number of hospitalizations, median (range) | 1 (1-4) | 1 (1-4) | 1 (1-4) |
*Diagnosis of cancer cachexia was based on the International Consensus Criteria.ECOG-PS: Eastern Cooperative Oncology Group-performance status
Hospitalizations
The cumulative total number of hospitalizations was 44 during the study period [Figure 2]. A final total of 30 hospitalizations were included for analysis after excluding 14 hospitalizations, in which the purpose of hospitalization was not for chemotherapy. All of the patients received systemic chemotherapy after hospitalization [Table 2]. Chemotherapy regimens included cytotoxic regimens (n = 23) and EGFR-TKIs (n = 7). A total of 13 of 30 hospitalizations (43%) required ≥8 hospital days. The reasons for prolonged hospitalization (≥8 hospital days) included blood sampling schedule for clinical trial (n = 5), delay in starting chemotherapy due to nonmedical reasons (n = 3, e.g., informed consent) or patient condition (n = 2), extension of hospital stay due to patient's concern about side effects (n = 3), and waiting for family's pick-up (n = 1). There were no complications or chemotherapy-related adverse events that necessitated an extension of hospital stay. There was no significant difference in length of hospital days between cachectic and noncachectic patients. The median number of baseline daily steps in the week before each hospitalization was 3756 (range, 885–19,957) per day. Baseline daily steps in cachectic patients were significantly less than in noncachectic patients (3212 steps versus 5809 steps per day, P < 0.05). The majority of noncachectic patients (60%) had mean daily steps of > 5000 per day.
Figure 2.
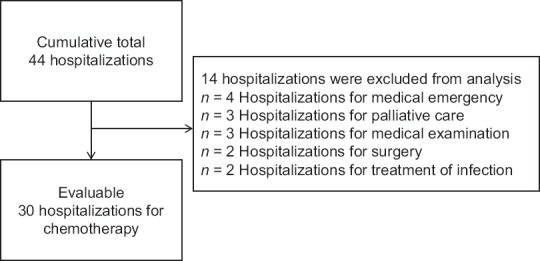
Selection of evaluated hospitalization
Table 2.
Characteristics of evaluated hospitalizations
| Characteristics | All | Patient’s status at baseline | P | |
|---|---|---|---|---|
| Cachexia | Noncachexia | |||
| Number of hospitalizations | 30 | 20 | 10 | |
| Chemotherapy regimen, n (%) | ||||
| Cytotoxic regimen | 23 (77) | 17 (85) | 6 (60) | NS |
| EGFR-TKI | 7 (23) | 3 (15) | 4 (40) | |
| Hospital days, n (%) | ||||
| ≤7 days | 17 (57) | 13 (65) | 4 (40) | NS |
| ≥8 days | 13 (43) | 7 (35) | 6 (60) | |
| Baseline daily steps in prior week of hospitalization, median (SPD, range) | 3756 (885-19,957) | 3212 (1298-6294) | 5809 (885-19,957) | <0.05 |
| Classification of daily steps, n (%) | ||||
| <2000 SPD | 6 (20) | 4 (20) | 2 (20) | <0.05 |
| 2000-4999 SPD | 15 (50) | 13 (65) | 2 (20) | |
| ≥5000 SPD | 9 (30) | 3 (15) | 6 (60) | |
| Hospitalization-associated physical inactivity, n (%) | 15 (50) | 8 (40) | 7 (70) | NS |
EGFR-TKI: Epidermal growth factor receptor-tyrosine kinase inhibitor, SPD: Steps per day, NS: Not significant
Hospitalization-associated physical inactivity
HAPI was seen in 15 hospitalizations [50%, Table 2]. A greater proportion of HAPI was observed in noncachectic patients (7 hospitalizations, 70%) than in cachectic patients (8 hospitalizations, 40%), without a statistically significant difference. No patient developed disability during hospitalization. Time series changes in daily steps among all hospitalizations are shown in Figure 3. Mean daily steps decreased by – 848 ± 331 during hospitalization (P < 0.05 in Wilcoxon signed-rank test). During the 1st week after discharge, the number of daily steps had not recovered, and the difference was – 670 ± 205 (P < 0.05). In general, the number of daily steps recovered to the baseline level in the 2nd and 3rd week following hospitalization. One hospitalization (3%) with HAPI developed HAD after discharge.
Figure 3.
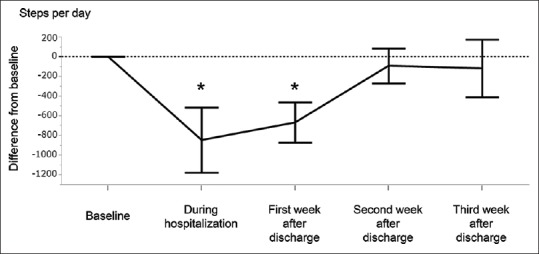
Hospitalization-associated physical inactivity. The mean difference from baseline daily steps is shown. *Wilcoxon signed-rank test P < 0.05
Length of hospital days, cachexia, and hospitalization-associated physical inactivity
Longer hospitalizations (≥8 hospital days) showed sustained physical inactivity until the 3rd week after discharge. Shorter hospitalizations (≤7 hospital days) showed recovery in the 2nd week [Figure 4]. This tendency was further emphasized by the presence of cancer cachexia at baseline [Figure 5]. Cachectic patients with prolonged hospitalization (n = 7) showed a sustained decrease in PA until the 3rd week after discharge. Of the cachectic patients, three patients did not show recovery after the 4th week after discharge, and continued chemotherapy, while four patients discontinued chemotherapy due to deterioration of PS.
Figure 4.
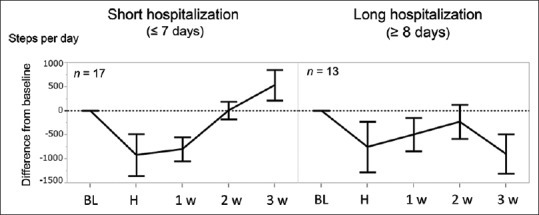
Length of hospitalization and hospitalization-associated physical inactivity. The mean difference from baseline daily steps is shown in short (≤7 days) and long (≥8 days) hospitalizations. BL: Baseline, H: During hospitalization, 1w: First week after discharge, 2w: Second week after discharge, 3w: Third week after discharge
Figure 5.
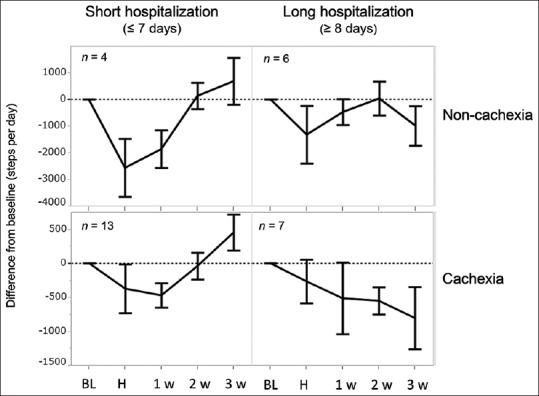
Cancer cachexia and hospitalization-associated physical inactivity. The mean difference from baseline daily steps is shown in short (≤7 days) and long (≥8 days) hospitalizations and presence or absence of cancer cachexia at baseline. BL: Baseline, H: During hospitalization, 1w: First week after discharge, 2w: Second week after discharge, 3w: Third week after discharge
Discussion
This is the first known study to observe changes in PA before and after hospitalization for chemotherapy in elderly patients with cancer. Our study has three major findings. First, we showed a high incidence of persistent physical inactivity after hospitalization in elderly patients with advanced NSCLC. Second, we showed that longer hospital days yielded a slower recovery from physical inactivity. Finally, we showed that the presence of cancer cachexia at baseline further impeded recovery from physical inactivity after a prolonged hospitalization.
Decreased PA due to restricted in-hospital mobility is known to precede the development of HAD.[6] We showed in this study that the majority of elderly cancer patients showed a sharp decline in PA during hospitalization and sustained physical inactivity after discharge. Only one of our study participants developed HAD, and this was attributed to the absence of acute illness in our patients. However, all patients had restricted daytime activity during hospitalization, which may lead to a persistent decline in PA and might result in an increased risk for future development of disability.
Longer hospital stay and prolonged bed rest are both important contributors to HAD in elderly individuals.[5,14] Hospitalized older adults spend 83% of the measured hospital stay lying in bed, with only 3% of their time spent standing or walking.[15] Accumulation of hospital days is inversely correlated with in-hospital physical inactivity.[16] Our patients who were fully ambulatory with good PS at baseline showed slow recovery from HAPI after prolonged hospitalization (≥8 days). It is difficult to differentiate the adverse effects of chemotherapy on PA from the adverse effects of hospitalization. However, the unfavorable impact of long hospitalization in our patients is assumed to be nonnegligible, as there were no chemotherapy-related severe adverse events or complications that necessitated extension of hospital stay.
Skeletal muscle depletion is associated with physical inactivity[17] and disability[18] in the community-dwelling elderly population. We previously reported that 63% of elderly patients with newly-diagnosed advanced NSCLC had muscle depletion at baseline.[7] The majority also had cancer cachexia and easily developed disability throughout the course of their illness.[8] In this study, 61% had cancer cachexia at baseline, and these may have had an increased sensitivity to physical inactivity caused by prolonged hospitalization.
Several lessons can be drawn from these results for future nursing care in the oncology setting. Sourdet et al.[19] reported that approximately 10% of HAD in patients was iatrogenic and that 80% of cases of HAD were potentially preventable. Common causes of HAD in that study included low in-hospital mobilization, overuse of diapers, and transurethral urinary catheterization. These factors restricted patient PA during hospitalization and may have led to the development of HAD. In our study, the reasons for a prolongation of hospitalization included scheduling of blood sampling for clinical trials, inadequate preparation of the patient for chemotherapy, patient anxiety, or the inability of family members to transport the patient to and from the hospital. These reasons could have been avoided in some cases by careful assessment of indications for in-hospital care, adequate preparations for chemotherapy, and patient education for self-management of adverse events after discharge.
Our study has several limitations. First, our study population was small and had heterogeneous treatment regimens. Second, our study included only Japanese patients. The medical environment varies among countries, and standards of care are rapidly changing. Thus, our results may not be directly transferable to different medical situations. However, reducing patients’ dependence on hospitalization and promoting self-management at home could be crucial steps to prevent HAPI and HAD in any context.
Conclusion
HAPI is frequently seen in hospitalization for chemotherapy in elderly patients with advanced NSCLC. Our findings showed that longer hospital stays and the presence of cancer cachexia led to slow recovery from physical inactivity. Individualized hospitalization planning based on careful consideration of patient age and the presence of cancer cachexia may be needed to prevent physical inactivity and related disability.
Financial support and sponsorship
This work was supported by the 35th grant-in-aid from the Japanese Foundation for the Multidisciplinary Treatment of Cancer in 2014.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the patients and their families for taking part in this study, as well as the investigators and staff at all study sites. The authors would like to acknowledge Izumi Suishu, Yumi Oguma, Masayo Kaneko, Miyuki Sugiyama, Yumiko Iwamoto, Tamaki Ogasawara, Kana Kunitomo, Yumi Isogai, Noriko Yamada, Keiko Yamamoto, Madoka Kimura, Toshimi Inano and Keita Mori, for their instruction on designing this study.
References
- 1.Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–78. [PubMed] [Google Scholar]
- 2.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, et al. A 30-year follow-up of the Dallas bedrest and training study: I. Effect of age on the cardiovascular response to exercise. Circulation. 2001;104:1350–7. [PubMed] [Google Scholar]
- 3.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–4. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 4.McGavock JM, Hastings JL, Snell PG, McGuire DK, Pacini EL, Levine BD, et al. A forty-year follow-up of the Dallas bed rest and training study: The effect of age on the cardiovascular response to exercise in men. J Gerontol A Biol Sci Med Sci. 2009;64:293–9. doi: 10.1093/gerona/gln025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–93. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 6.Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H, et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–73. doi: 10.1111/j.1532-5415.2010.03276.x. [DOI] [PubMed] [Google Scholar]
- 7.Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer. 2017;17:571. doi: 10.1186/s12885-017-3562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naito T, Okayama T, Aoyama T, Ohashi T, Masuda Y, Kimura M, et al. Unfavorable impact of cancer cachexia on activity of daily living and need for inpatient care in elderly patients with advanced non-small-cell lung cancer in Japan: A prospective longitudinal observational study. BMC Cancer. 2017;17:800. doi: 10.1186/s12885-017-3795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasunaga A, Togo F, Watanabe E, Park H, Park S, Shephard RJ, et al. Sex, age, season, and habitual physical activity of older Japanese: The Nakanojo study. J Aging Phys Act. 2008;16:3–13. doi: 10.1123/japa.16.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: A validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–43. doi: 10.1079/BJN20031033. [DOI] [PubMed] [Google Scholar]
- 11.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 12.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 13.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 14.Kelley AS, Ettner SL, Morrison RS, Du Q, Sarkisian CA. Disability and decline in physical function associated with hospital use at end of life. J Gen Intern Med. 2012;27:794–800. doi: 10.1007/s11606-012-2013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–5. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 16.McCullagh R, Dillon C, Dahly D, Horgan NF, Timmons S. Walking in hospital is associated with a shorter length of stay in older medical inpatients. Physiol Meas. 2016;37:1872–84. doi: 10.1088/0967-3334/37/10/1872. [DOI] [PubMed] [Google Scholar]
- 17.Shephard RJ, Park H, Park S, Aoyagi Y. Objectively measured physical activity and progressive loss of lean tissue in older Japanese adults: Longitudinal data from the Nakanojo study. J Am Geriatr Soc. 2013;61:1887–93. doi: 10.1111/jgs.12505. [DOI] [PubMed] [Google Scholar]
- 18.Tanimoto Y, Watanabe M, Sun W, Hirota C, Sugiura Y, Kono R, et al. Association between muscle mass and disability in performing instrumental activities of daily living (IADL) in community-dwelling elderly in Japan. Arch Gerontol Geriatr. 2012;54:e230–3. doi: 10.1016/j.archger.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Sourdet S, Lafont C, Rolland Y, Nourhashemi F, Andrieu S, Vellas B, et al. Preventable iatrogenic disability in elderly patients during hospitalization. J Am Med Dir Assoc. 2015;16:674–81. doi: 10.1016/j.jamda.2015.03.011. [DOI] [PubMed] [Google Scholar]


