Abstract
Objective:
Physical activity (PA) may improve the quality of life (QOL) of cancer survivors. However, the impact on patients with advanced cancer with high cachectic potential is unknown. We analyzed the feasibility of PA intervention using the multimodal program Nutrition and Exercise Treatment for Advanced Cancer (NEXTAC) and the impact on QOL in elderly patients with advanced cancer.
Methods:
We recruited 30 patients aged ≥70 years who were scheduled to receive the first-line chemotherapy for newly diagnosed advanced pancreatic or non-small-cell lung cancer. The QOL was assessed using the European Organization for Research and Treatment of Cancer QOL Questionnaire version 3.0, while the PA was measured using a pedometer/accelerometer. Instructors counseled patients to increase daily activity in an 8-week educational intervention. We assessed patient attendance, compliance, and intervention efficacy.
Results:
The median patients’ age was 75 years (range, 70–84 years). Twelve patients (40%) were cachectic at baseline. Twenty-eight (93%) patients attended all sessions. Six (21%) and 15 (52%) patients increased their indoor and outdoor activity, respectively. There were significant differences in measured PA, global QOL, and role and emotional functioning between the patients who increased outdoor activity and those who did not.
Conclusions:
The PA intervention of the NEXTAC program was feasible as the elderly patients with advanced cancer in this study were highly compliant. The majority of patients demonstrated behavioral changes that were associated with the improvement in global QOL. We conduct a randomized phase II study to measure the impact of the NEXTAC program on QOL and functional prognosis.
Keywords: Cancer cachexia, elderly, multimodal intervention, non-small-cell lung cancer, pancreatic cancer, physical activity

Introduction
Physical activity (PA) may improve the fitness status and quality of life (QOL) of cancer survivors.[1] Several reports suggest that PA can decrease the risk of cancers, may reduce recurrence, and may improve cancer-specific and overall mortality.[2] Detailed understanding of the metabolism in working muscle, myokines, and cross-talk among muscles, tumors, adipose tissue, and the immune system in clinical research may provide further evidence to support these findings.[3,4]
However, there is little evidence regarding the effectiveness of monomodal interventions with PA in patients with advanced cancer.[5,6] Underlying catabolism due to cancer cachexia may antagonize potential-positive effects of PA in this patient cohort.[7] Thus, PA is recommended for this patient population in the context of multimodal interventions.[8] The Nutrition and Exercise Treatment for Advanced Cancer (NEXTAC) program is a multimodal intervention designed to maintain or improve physical function in elderly patients with cancer at high risk of cancer cachexia. The NEXTAC program combines nutritional counseling, low-intensity resistance training, and PA intervention. Our PA intervention aimed to promote behavioral changes in daily activity, and goal setting was based on pedometer/accelerometer analysis.
This study aimed to test the feasibility of the PA intervention of the NEXTAC program and to explore the impact on exercise behavior as well as to determine the association between changes in QOL and behavioral changes possibly induced by the interventions.
Methods
Patient selection
The NEXTAC-ONE is a national, prospective, multicenter, single-arm study aimed to assess the feasibility and safety of the early introduction of nonpharmacological multimodal interventions for elderly patients with advanced cancer who are receiving chemotherapy. Patients were recruited from participating institutions using the following eligibility criteria: (1) histologically and/or cytologically proven advanced (locally advanced or metastatic) non-small-cell lung cancer (NSCLC) or pancreatic cancer; (2) age ≥70 years, with a scheduled first-line systemic chemotherapy course; (3) no previous systemic chemotherapy, except for adjuvant chemotherapy or chemoradiation completed >6 months before study entry; (4) Eastern Cooperative Oncology Group performance status of 0–1 and Barthel index of >90 points; (5) having at least one source of social support (family members or friends) who could monitor safety and compliance with the intervention throughout the 8-week study period; and (6) the ability to ambulate, read, and respond to questions without assistance.
Patients were excluded if there were any indications that radiotherapy or surgery could cure them, or if there were any indications of severe psychiatric disorder, an active infectious disease, unstable cardiac disease, or untreated symptomatic brain or bone metastases that prevented safe assessments or interventions. All patients provided written informed consent. The study was approved by the institutional review board of each institution and conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Assessment timing
The schedule of the NEXTAC program is summarized in Table 1. Assessments were planned on the same day of interventional sessions. Baseline assessments were performed between study entry and initiation of chemotherapy (T1 point). Subsequent assessments were planned at 4 ± 2 (T2 point) and 8 ± 2 (T3 point) weeks after the T1 point.
Table 1.
Interventions in the Nutrition and Exercise Treatment for Advanced Cancer program
| Sessions (time allocation) | Interventions |
|---|---|
| T1 point (baseline) | |
| Nutritional session (30 min) | Nutritional advice ONS prescription* |
| Exercise session (30 min in each program) |
Home-based resistance training Prescription of exercise program Instruction of exercise procedures Education on self-modification Physical activity promotive counseling Prescription of target daily step Physical activity counseling Education on fall prevention |
| T2 point (4±2 weeks after baseline) | |
| Nutritional session (20 min) | Nutritional advice ONS prescription |
| Exercise session (20 min in each program) |
Home-based resistance training Modification of exercise program Education of self-modification Physical activity promotive counseling Modification of target daily step Physical activity counseling Education on fall prevention |
| T3 point (8±2 weeks after baseline) | |
| Nutritional session (20 min) | Nutritional advice |
| Exercise session (20 min in each program) |
Home-based resistance training Modification of exercise program Education on self-modification Physical activity promotive counseling Modification of target daily step Physical activity counseling Education on fall prevention |
*A branched-chain amino acid-rich ONS (Inner Power®, Otsuka Pharmaceutical Co., Ltd., Japan) was provided one pack daily for 8 weeks. ONS: Oral nutritional supplement
Physical activity measurement
PA was measured using a pedometer/accelerometer. After informed consent was obtained, the patients wore pedometers/accelerometers for >7 days before study entry to measure baseline PA (screening period). The average daily steps or the daily duration of PA during the screening period was set as the individual's baseline value. The average daily steps or daily duration of PA between the T1 and T2 points was set as the individual's value for the T2 point. The average daily steps or daily duration of PA between the T2 and T3 points was set as the individual's value for the T3 point. Patients were instructed to complete an exercise diary and record their daily steps.
An electronic pedometer/accelerometer with a storage capacity of 180 days (Kenz Lifecorder-GS, Suzuken Co., Ltd., Nagoya, Aichi, Japan) was attached on the side of the patient's waist.[9,10] Patients were instructed to wear the device for as long as possible in the daytime, starting from the time they changed clothes for daily activity in the morning to the time they changed into nightclothes for sleep. The device recorded the number of daily steps taken and the intensity of PA every 4 s throughout each day.
Participants were required to regularly visit the outpatient department of each institution for data collection. Collected data included the daily step count, daily duration of device wearing, and daily duration of PA rated ≥1.8 metabolic equivalents (METs). Wearing the pedometer/accelerometer for ≥5 h in a day was defined as a pedometer/accelerometer-wear day. Data collected on days when the device was worn for <5 h were excluded from the analysis.
Physical activity interview
At each time point, patients provided information about their indoor or outdoor activity by filling in the questionnaire. The questionnaire at the T1 point assessed the following seven items: (1) family structure, (2) typical daily schedule and routine, (3) occupational status, (4) performance of house chores (cleaning, washing clothes, preparing and cleaning up after meals, and shopping), (5) frequency of going out (number of days per week), (6) exercise habits (types and frequencies), and (7) number of falls during the prior month. The questionnaire at T2 and T3 points assessed only items (4), (5), (6), and (7).
During counseling, nurses, physiotherapists, or medical doctors who were registered and trained for this study collected further information according to a routine checklist. The checklist consisted of the following five items: (1) changes in indoor or outdoor activity (increase, no change, or decrease), (2) changes in social activity (increase, no change, or decrease), (3) risk factors for falls (history of falls in the past 4 weeks, environmental fall hazards, inappropriate shoes, and gait stability), (4) symptoms that possibly restricted the patient's PA (pains, dyspnea on exertion, hand or foot disorders, and cosmetic problems such as skin rash and numbness), and (5) activities of daily living assessed via the Barthel index.
Anthropometric measurements and quality of life
At each time point, body weight (kg) was measured to the nearest 0.1 kg, and the body mass index (BMI; kg/m2) was subsequently calculated. The cross-sectional area of skeletal muscle mass at the third lumbar vertebra level was measured at baseline using sliceOmatic software (version 5.0, Tomovision, Montreal, Quebec, Canada).[11] The lumbar skeletal muscle index (cm2/m2) was reported. QOL was assessed at each time point using the European Organization for Research and Treatment of Cancer QOL Questionnaire version 3.0 (EORTC-QLQ-C30). The scores of global QOL and five functional scales (physical, role, emotional, cognitive, and social) were calculated according to the EORTC-QLQ-C30 guideline.[12]
Diagnosis of muscle depletion and cancer cachexia
Muscle depletion was defined based on lumbar skeletal muscle index cutoffs of <43.0 cm2/m2 for men with a BMI of <25.0 kg/m2, <53.0 cm2/m2 for men with a BMI of ≥25.0 kg/m2, and < 41.0 cm2/m2 for women.[13] Cancer cachexia was defined as unintentional weight loss of >5% during the preceding 6 months or >2% in patients with a BMI <20 kg/m2 or the occurrence of muscle depletion according to consensus criteria.[14]
Interventional sessions
The schedule and contents of interventions are summarized in Table 1. The PA intervention of the NEXTAC comprised three sessions in an 8-week intervention period. The intervention was an educational program that promoted self-support. In the first session at the T1 point, the program was presented to the patient; this took approximately 30 min. Follow-up interventions were given at the T2 or T3 points, and approximately 20 min was required to review compliance and modify and optimize the programs. Physicians advised patients to attend each session with their caregivers or supporters to maximize the efficacy and safety of the intervention.
Prescription algorithm of target daily steps
In each exercise session, the nurses, physiotherapists, or occupational therapists assessed the patient's PA and prescribed individual target steps. The initial target step count was determined according to the average daily steps during the screening period as follows:
If the patient's average steps were 2000 or less, the target step count was 2000 steps. Instructors educated patients to go outside at least once daily
If the patient's average daily steps were 2001–7999 steps, the target step count was the average steps plus 2000 steps to a maximum of 8000 steps
If the patient's average daily steps were ≥8000 steps, the patients were educated to maintain their current level of PA.
At follow-up in T2 or T3, instructors modified the target step according to the average steps taken during T1 to T2 or during T2 to T3 using the same prescription algorithm.
Physical activity promotive counseling
At baseline, instructors used handouts to discuss the relationship between cancer cachexia, cancer treatment, weight loss, functional loss, and disability [upper half of Figure 1]. During counseling, instructors explained the relationship between the patient's actual indoor or outdoor activity and the measured PA by showing the patients a summary report obtained from the analysis of the pedometer/accelerometer software (Lifelyzer-05 coach, Suzuken, Japan). They discussed methods for increasing promotive factors and decreasing inhibitory factors of PA in the patient's daily life [lower half of Figure 1]. If active symptom control or social support was needed to improve PA, the information was shared with the nurses, medical doctors, psychotherapists, or/and medical social workers and the countermeasures were discussed. Instructors explained the importance of fall prevention[15] for safety during PA as follows:
Figure 1.
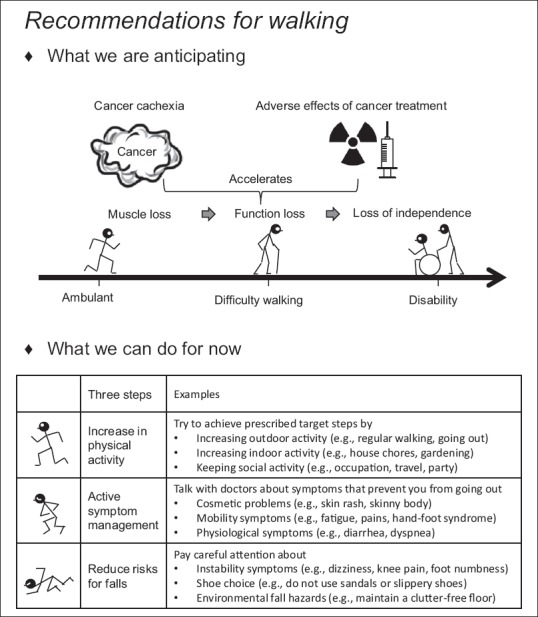
Patient's handout (translated from Japanese). “What we are anticipating” explains the future risks for difficulty walking and disability. “What we can do for now” explains the countermeasures for future risks for difficulty walking and disability
When you feel dizzy or lightheaded, do not force yourself to walk further
When you feel pain in the knee or foot, numbness in the toe, or need a walking stick/aid, pay careful attention to the possibility of fall
Choose well-fitting shoes. Do not use sandals or slippery shoes when walking
Review hazards that could cause falls in your home, such as obstacles on the floor or difference in floor heights.
Statistical analysis
Compliance was assessed by the proportion of days the patients completed their exercise diaries or wore pedometer/accelerometers for ≥5 h during the intervention period. Behavioral change was assessed by the proportion of patients who increased indoor or outdoor activity, increased total PA time (≥1.8 METs), or achieved their target step count. The Wilcoxon signed-rank test was used for pairwise comparison of measurement changes between study visits, whereas the Wilcoxon rank-sum test was used for comparisons between two independent groups. All analyses were performed using JMP version 13.0 for Windows (SAS Institute Inc., Cary, NC, USA), and P < 0.05 was considered statistically significant.
Results
Patients
We recruited 30 of the 46 patients screened [Figure 2]. The main reasons for ineligibility were failure to meet the registration criteria or ineligibility for systemic chemotherapy due to poor health. Twenty-four patients had NSCLC and six had pancreatic cancer. The median age was 75 years [range, 70–84 years, Table 2]. Cancer cachexia and muscle depletion were seen in 12 (40%) and 21 (70%) patients, respectively. Major comorbidities included chronic lung disease, type 2 diabetes, and cardiovascular disease. More than 50% of the patients were unemployed and had no regular exercise habit. A total of 10%–20% of patients were living alone, stayed at home most days of the week, rarely did house chores, and had a recent history of falls.
Figure 2.
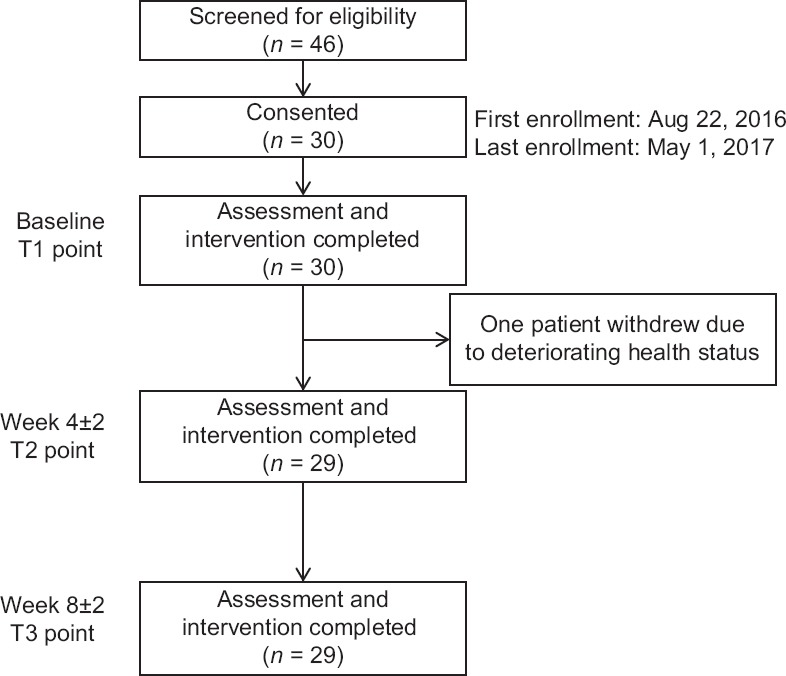
Patient flowchart
Table 2.
Baseline patient characteristics (n=30)
| Variables | n (%) |
|---|---|
| Age, median (range) | 75 (70-84) |
| Gender (female:male) | 10:20 |
| ECOG-PS | |
| 0 | 11 (37) |
| 1 | 19 (63) |
| Cancer type | |
| non-small-cell lung cancer | 24 (80) |
| Pancreatic cancer | 6 (20) |
| Stage | |
| III | 3 (10) |
| IV or postoperative recurrence | 27 (90) |
| Treatment | |
| Cytotoxic regimen | 20 (67) |
| Targeted regimen | 10 (33) |
| Comorbidities | |
| Chronic lung disease | 13 (43) |
| Type 2 diabetes | 9 (30) |
| Cardiovascular disease | 7 (23) |
| Double cancer | 3 (10) |
| Cerebrovascular disease | 1 (3) |
| Lifestyle | |
| Living alone | 4 (14) |
| Unemployed | 18 (62) |
| No exercise habit | 16 (53) |
| History of falls in prior 1 month | 3 (10) |
| Frequency of going out (≤2 days a week) | 4 (13) |
| No participation in house chores | 5 (17) |
| Nutritional status | |
| Percentage weight change in the past 6 months (mean±SD) | −3.0±6.8 |
| Cancer cachexia* | 12 (40) |
| Skeletal muscle depletion† | 21 (70) |
*Diagnosis was based on the international consensus criteria, †Skeletal muscle depletion was defined as lumbar skeletal muscle mass index of <43.0 cm2/m2 for men with a BMI <25.0 kg/m2, <53.0 cm2/m2 for men with a BMI ≥25.0, and <41.0 cm2/m2 in women. ECOG-PS: Eastern Cooperative Oncology Group Performance Status, SD: Standard deviation, BMI: Body mass index
Attendance and compliance
During the study period, one man withdrew consent due to deteriorating health status from a respiratory infection unrelated to the study procedures [Figure 2]. He missed two of the three PA sessions at T2 and T3 points. One woman accidentally missed one session at the T3 point. She attended a make-up session 9 days later, which exceeded the scheduled limit of the T3 point. The remaining 28 patients (93.3%) completed all sessions on time. The median proportion of days of completing the exercise diary and wearing a pedometer/accelerometer was 94% and 98%, respectively.
Safety
Safety was assessed for all 30 patients. Grade 1 adverse events possibly related to the PA intervention of the NEXTAC program were observed in five patients and included muscle pain (n = 2), arthralgia (n = 1), dyspnea on exertion (n = 1), and plantar aponeurosis (n = 1).
Assessments of behavioral change
Results of the measurement of behavioral changes between the baseline and T2 point are summarized in Table 3. The patients’ subjective assessments of their behavioral change were obtained through direct interviews by instructors. Six patients (21%) increased their indoor activity between baseline and T2 points, while 15 (52%) patients increased their outdoor activity. Twenty-two patients (76%) maintained their social activity between the baseline and T2 points.
Table 3.
Behavioral changes
| Parameters* | Increased or complete, n (%) | No change, n (%) | Decreased, n (%) |
|---|---|---|---|
| Patient’s subjective assessment† | |||
| Indoor activity | 6 (21) | 19 (66) | 4 (14) |
| Outdoor activity | 15 (52) | 5 (17) | 9 (31) |
| Social activity‡ | 0 | 22 (76) | 6 (21) |
| Questionnaire-based assessment§ | |||
| Participation in house chores | 12 (41) | 6 (21) | 11 (38) |
| Frequency of going out | 20 (69) | 3 (10) | 6 (21) |
| Accelerometer-based assessment | |||
| Daily steps|| | 16 (55) | 7 (24) | 6 (21) |
| Time spent in physical activity¶ | 17 (59) | 6 (21) | 6 (21) |
*Patients were classified with changes in parameters from baseline to T2 point, †Classification by patient’s answer to direct interview at T2 point, ‡Data in one patient were not obtained, §Classification according to the comparison of the results of questionnaire at baseline with those at T2 point. Patients who participated in house chores at T2 point were classified as increased or complete. Patients who were going out 7 days a week at T2 point were classified as increased or complete, ||Patients whose daily steps at T2 point were ≥500 steps higher or lower or others (classified as no change) as compared with that at baseline, ¶Patients whose time spent in physical activity (≥1.8 metabolic equivalent) at T2 point was ≥5 min higher or lower or others
According to the questionnaire-based qualitative assessment, between the baseline and T2 point, 12 patients (41%) increased their participation in completing house chores and 20 patients (69%) increased the frequency of going out at least once weekly. Daily steps and PA time increased in 20 (66%) and 19 (59%) patients, respectively. As a result, overall daily steps significantly increased at the T2 point by 571 ± 275 steps per day. Most patients maintained their daily steps through the T3 point [Figure 3]. The percentages of patients who achieved their prescribed target step count at the T2 and T3 points were 24% and 21%, respectively.
Figure 3.
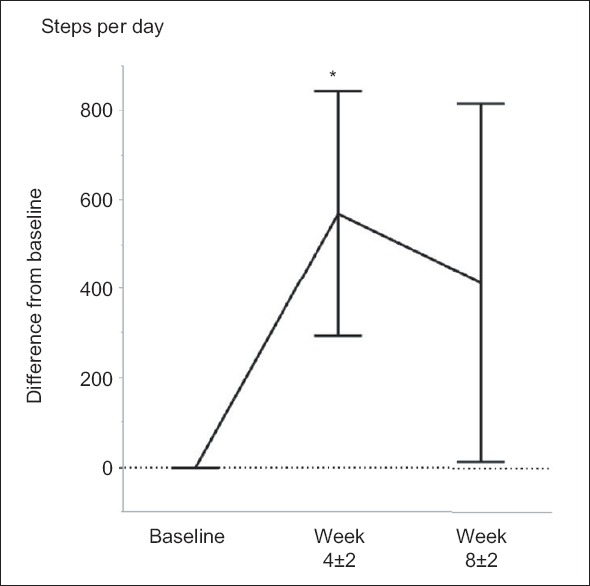
Change in daily steps. Difference from baseline daily steps was shown at T2 point (4 ± 2 weeks) and T3 point (8 ± 2 weeks). *Wilcoxon signed-rank test, P < 0.05
Relationship between behavioral change and outcomes
Associations between the patients’ subjective behavioral changes until the T2 point and outcome measures were exploratorily analyzed [Table 4]. Patients who increased outdoor activity had significant improvements from baseline in daily steps, PA time, global QOL, role scale, and emotional scale. Patients who maintained or decreased outdoor activity had a significant decrease in BMI from baseline.
Table 4.
Behavioral change and outcomes
| Patient’s subjective assessment | Baseline value (n=29) | Change in outdoor activity | Change in indoor activity | ||
|---|---|---|---|---|---|
| Increased (n=15) | Not increased (n=14) | Increased (n=6) | Not increased (n=23) | ||
| Nutrition | |||||
| BMI (kg/m2) | 21.8±0.6 | 0.2±0.2* | −0.6±0.2† | −0.1±0.2 | −0.2±0.2 |
| Physical activity | |||||
| Daily steps (steps/day) | 4253±463 | 1367±286*,† | −282±367 | 1131±405† | 425±327 |
| Time spent in PA (min/day) | 47.1±4.9 | 13.2±3.0*,† | −3.6±3.9 | 10.9±3.7 | 3.6±3.5 |
| EORTC-QLQ-C30 scale | |||||
| Global QOL score | 62.4±4.8 | 11.7±6.2*,† | −7.7±5.5 | 0.0±13.3 | 2.9±4.7 |
| Functional scales | |||||
| Physical functioning | 84.6±3.0 | 6.2±3.8 | 2.4±2.6 | 12.2±6.3 | 2.3±2.3 |
| Role functioning | 84.5±4.2 | 7.8±3.6*,† | −7.1±6.2 | 19.4±6.7* | −4.3±3.8 |
| Emotional functioning | 76.1±4.6 | 16.7±5.3*,† | 2.4±3.4 | 29.2±7.7*,† | 4.7±3.1 |
| Cognitive functioning | 73.0±4.9 | 5.6±4.2 | 2.4±3.4 | 13.9±6.7 | 1.4±2.8 |
| Social functioning | 77.6±4.6 | 11.1±6.4 | 0.0±4.9 | 5.6±3.5 | 5.8±5.2 |
Changes in parameters from baseline to T2 point were presented as mean±SE. *P<0.05 in Wilcoxon test in comparison with “not increased” group, †P<0.05 in Wilcoxon signed-rank test in comparison with baseline value in each subgroup. PA: Physical activity of ≥1.8 metabolic equivalent, BMI: Body mass index, EORTC-QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire version 3.0, QOL: Quality of life, SE: Standard error
There were significant differences in daily steps, global QOL, BMI, PA time, role scale score, and emotional scale score between groups. Differences in daily steps and global QOL between the groups were maintained through the T3 point [Figure 4]. Patients who increased indoor activity had significant improvement in daily steps and emotional scale score. The role scale and emotional scale scores were significantly different between the groups.
Figure 4.
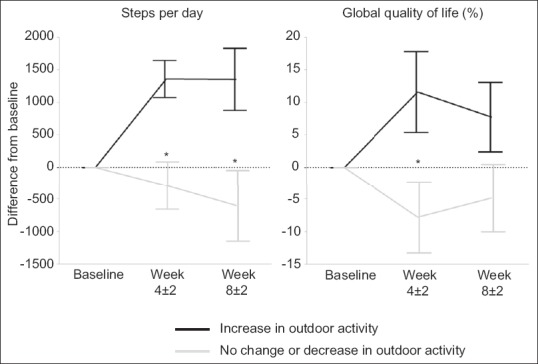
Impact of outdoor activity on daily steps and global quality of life. Difference from baseline daily steps or global quality of life in European Organization for Research and Treatment of Cancer Quality of Life Questionnaire version 3.0 were shown at T2 point (4 ± 2 weeks) and T3 point (8 ± 2 weeks). *Wilcoxon rank-sum test, P < 0.05
Discussion
This feasibility study offered three major findings. First, the PA intervention in the NEXTAC program was safe and feasible, with high compliance and low dropout rates for elderly patients with advanced cancer. Second, our intervention increased outdoor activity in more than 50% of patients. Finally, increased outdoor activity was associated with objectively measured PA and global QOL.
Changing health-related behavior is challenging in patients with advanced cancer. Quist et al.[16] conducted a feasibility study of a combined exercise intervention for patients with inoperable lung cancer. Only 8.7% of their patients adhered to the home training (walking and relaxation), while 73.3% adhered to the supervised gym-based exercise program. Patients were unmotivated due to lack of self-discipline and doubts about the efficacy of exercise.
There are also substantial difficulties involving recruitment and high attrition rate in exercise interventions for advanced cancer.[6] One possible reason may be the failure to incorporate behavioral change techniques (BCTs) along with the intervention.[17] Our PA intervention adopted several critical components of BCTs,[18] including goal setting, action planning and instruction, self-monitoring via diaries, and feedback from instructors. We also repeatedly enhanced patients’ risk perception about the possibility of developing cachexia, physical dysfunction, and disability in their cancer trajectory, which might have contributed to the low attrition rate and high compliance in our study.
A pedometer is a simple, motivational tool for self-monitoring of walking.[19] Although there is no standard pedometer-based intervention used in geriatric oncology, it is effective in promoting sustained increases in PA levels over 12 months in healthy adults.[20] Key predictors of a successful intervention included having a step goal,[21] self-record of daily steps,[22] and personalized feedback from instructors and caregivers.[23] In this study, the participants were asked to complete a diary of daily steps taken and to achieve individually prescribed target steps. In addition, participants received monthly feedback from instructors. These factors might have further enhanced the behavioral changes in this study.
A meta-analysis has shown that PA interventions can improve fitness and QOL in cancer survivors.[1] However, these results have not yet been reproduced in patients with advanced cancer.[24,25] Dhillon et al.[26] recently reported the results of their randomized controlled study of a pedometer-based PA intervention for advanced lung cancer. Although patient adherence to the intervention was acceptable, the intervention did not improve PA, fatigue, or QOL. They concluded that the minimal difference in PA was the main reason for the poor outcomes.
Our study showed that the majority of our patients increased their outdoor activity after the intervention. The presence of this behavioral change was confirmed by increased PA measured by the pedometer/accelerometer. An increase in outdoor activity was positively associated with global QOL. These results indicate that our PA intervention potentially improved the QOL of elderly patients with advanced cancer.
Our study has several limitations. First, our study population was heterogeneous in cancer type and treatment regimen. Second, behavioral change was assessed via direct interview or questionnaire, potentially carrying a risk of recall bias. Finally, our PA intervention was combined with home-based resistance training and nutritional intervention. Thus, outcome changes were not necessarily attributed to the PA intervention alone. However, behavioral changes in outdoor or indoor activity are likely to be influenced mainly by the PA intervention of the NEXTAC program.
Nonpharmacological multimodal intervention for patients with advanced cancer and high cachectic potential should be established. Based on the results of our study, we are currently conducting a prospective, multicenter, randomized phase II study of early exercise and nutritional interventions for elderly patients with advanced NSCLC and pancreatic cancer in Japan (Clinical Trial Registry No. UMIN000028801). We hypothesize that early induction of the NEXTAC program will help maintain physical function and prevent disability in elderly patients with advanced cancer who are at considerable risk of cancer cachexia.
Conclusion
The PA intervention of the NEXTAC program was feasible, demonstrating high compliance in elderly patients with advanced cancer. Behavioral changes in indoor or outdoor activity were observed in >50% of patients and were associated with improvement in global QOL. We now conduct a randomized phase II study to measure the impact of the NEXTAC program on QOL and functional prognosis.
Financial support and sponsorship
This study (UMIN000023207) was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP18ck0106212.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the patients and their families for taking part in this study, as well as the investigators and staff at all study sites. We would like to acknowledge Shuichi Mitsunaga, Toshimi Inano, Tetsuya Tsuji, Takashi Higashiguchi, Akio Inui, Teiko Yamaguchi, Naoharu Mori, Florian Strasser, Keita Mori, Kazuo Tamura, Hideki Aragane, and Yukitoshi Aoyagi, for their instruction on designing this study.
References
- 1.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J Natl Cancer Inst. 2012;104:815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz-Casado A, Martín-Ruiz A, Pérez LM, Provencio M, Fiuza-Luces C, Lucia A, et al. Exercise and the hallmarks of cancer. Trends Cancer. 2017;3:423–41. doi: 10.1016/j.trecan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–31. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddocks M, Murton AJ, Wilcock A. Therapeutic exercise in cancer cachexia. Crit Rev Oncog. 2012;17:285–92. doi: 10.1615/critrevoncog.v17.i3.60. [DOI] [PubMed] [Google Scholar]
- 6.Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: A systematic review. Prev Med. 2017;104:124–32. doi: 10.1016/j.ypmed.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–4. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Yasunaga A, Togo F, Watanabe E, Park H, Park S, Shephard RJ, et al. Sex, age, season, and habitual physical activity of older Japanese: The Nakanojo study. J Aging Phys Act. 2008;16:3–13. doi: 10.1123/japa.16.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: A validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–43. doi: 10.1079/BJN20031033. [DOI] [PubMed] [Google Scholar]
- 11.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 14.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 15.Moyer VA. U.S. Preventive Services Task Force. Prevention of falls in community-dwelling older adults: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2012;157:197–204. doi: 10.7326/0003-4819-157-3-201208070-00462. [DOI] [PubMed] [Google Scholar]
- 16.Quist M, Rørth M, Langer S, Jones LW, Laursen JH, Pappot H, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: A pilot study. Lung Cancer. 2012;75:203–8. doi: 10.1016/j.lungcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Bayly J, Wakefield D, Hepgul N, Wilcock A, Higginson IJ, Maddocks M, et al. Changing health behaviour with rehabilitation in thoracic cancer: A systematic review and synthesis. Psychooncology. 2018 doi: 10.1002/pon.4684. doi: 10.1002/pon.4684 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, et al. Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: A systematic review. Br J Cancer. 2014;110:831–41. doi: 10.1038/bjc.2013.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tudor-Locke C, Lutes L. Why do pedometers work? A reflection upon the factors related to successfully increasing physical activity. Sports Med. 2009;39:981–93. doi: 10.2165/11319600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Fitzsimons CF, Baker G, Gray SR, Nimmo MA, Mutrie N Scottish Physical Activity Research Collaboration (SPARColl) Does physical activity counselling enhance the effects of a pedometer-based intervention over the long-term: 12-month findings from the walking for wellbeing in the west study. BMC Public Health. 2012;12:206. doi: 10.1186/1471-2458-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: A systematic review. JAMA. 2007;298:2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 22.Heesch KC, Dinger MK, McClary KR, Rice KR. Experiences of women in a minimal contact pedometer-based intervention: A qualitative study. Women Health. 2005;41:97–116. doi: 10.1300/J013v41n02_07. [DOI] [PubMed] [Google Scholar]
- 23.Tudor-Locke CE, Myers AM, Rodger NW. Development of a theory-based daily activity intervention for individuals with type 2 diabetes. Diabetes Educ. 2001;27:85–93. doi: 10.1177/014572170102700110. [DOI] [PubMed] [Google Scholar]
- 24.Adamsen L, Quist M, Andersen C, Møller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uster A, Ruehlin M, Mey S, Gisi D, Knols R, Imoberdorf R, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin Nutr. 2017 doi: 10.1016/j.clnu.2017.05.027. pii: S0261-5614(17)30201-7. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon HM, Bell ML, van der Ploeg HP, Turner JD, Kabourakis M, Spencer L, et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann Oncol. 2017;28:1889–97. doi: 10.1093/annonc/mdx205. [DOI] [PubMed] [Google Scholar]


