Abstract
Parental stress is a universal experience for parents who have children diagnosed with congenital heart disease and has been studied within the context of the child’s illness but not through a broader health disparity lens. This paper provides a thorough synthesis of the current literature on parental stress addressing disparities in parents of children with CHD. Several theories and models from within this literature are described and a new comprehensive framework, the Parental Stress and Resilience in CHD Model is presented. Future research and clinical implications are discussed.
Keywords: Parent, stress, resilience, congenital heart disease, mental health, health disparities
Introduction
Health disparities research has received much attention in the last two decades1. Variables of race, ethnicity, gender, sex, and socioeconomic status have emerged as key influencers of environmental resources, relative risk, human capital, and social status2. Ethnic, racial, and socioeconomic disparities exist related to the incidence and outcomes for children with Congenital Heart Disease (CHD)3–6. Yet the extent to which these disparities impact the experience of stress for parents of children with CHD is unknown. Parents of children born with CHD experience profound stress, from the time of diagnosis, through the infant’s hospitalization for cardiac surgery, and in the months and years that follow7–11. While survival for neonatal cardiac surgery has dramatically improved, children remain at risk for neurodevelopmental delay, including fine and gross motor, speech, cognitive, behavioral, and academic difficulties12. Many children must return for additional surgeries or interventions throughout childhood, compounding the stress experience for parents9. While these procedures are considered either corrective or palliative, the underlying CHD leaves these children with a chronic illness. The stress of parents across the lifespan of the child with CHD will impact overall family functioning and the child’s home environment, influencing the neurodevelopment of the infant throughout childhood13. Parental stress can also affect the parent’s own physical health, mental health, overall well-being, and quality of life14,15. Furthermore, studies have shown that parents of children with CHD experience more intense stress than parents of other pediatric populations, and that stress may be of particular concern when the child is less than one year of age10,11,16–18. This finding highlights the imperative for health care providers to pay focused attention to this issue and to address parental stress early and systematically. But the question remains: do additional disparities impact parental stress and ultimately parent and child health outcomes?
Currently, no model exists describing the experience of stress for parents of children with CHD and its full impact on the family across the child’s lifespan. Parental stress in CHD has been studied within the context of the child’s illness, around the times of diagnosis, surgery, hospitalization, and after discharge home7–9,16,18,19. Most of these studies on parental stress were framed within the context of the child’s disease and few had strong theoretical foundations. The disease-specific focus on parental stress research in CHD has not comprehensively elucidated factors related to health disparities. While some studies in CHD acknowledge the influence of specific types of health disparity variables on parent stress, such as parental education or socioeconomic status, the lack of research examining constructs within a broad parental stress framework is apparent. Studies on parental stress for parents of children with CHD have largely reported health disparity variables as incidental or secondary findings, or have included them as a covariate in their analysis and not expanded on their implications20. A comprehensive framework for parental stress in CHD would provide a broader lens with which to understand the full experience of stress for this population of parents.
The lack of theoretical underpinnings in parental stress research in CHD has also resulted in the use of a variety of constructs to study the stress of parents, including but not limited to parental stress, parenting stress, distress, psychological adjustment, trauma, and mental health. The transactional theory of stress originally described by Lazarus and Folkman21 has provided a foundation for many stress studies and frameworks for parental stress research. Lazarus and Folkman define stress as an overarching term, encompassing a stressor, or something perceived and appraised by the individual as taxing, which stimulates a stress response. Parental stress refers to any stress that is perceived by an individual while in the role of parent for a child. Parenting stress refers to the specific stressors arising from the parenting role, whereas parental stress is more broad, encompassing all potential stress experienced by a parent. For this review, distress and psychological adjustment will be considered specific types of responses to parental stress. It is of utmost importance that research utilize a theoretical foundation to define these constructs, and that variables be organized within a specific model to guide the research from conception to design and from data analysis to interpretation.
This paper will brief overview of the parental stress literature addressing disparities in parents of children with CHD. Several theories and models will be presented from within the CHD literature as well as from other pediatric populations. Based on the synthesis of the literature, a new comprehensive model for parental stress in CHD will be proposed. Future research and clinical implications will be discussed.
Review of Literature
To date, research on parental stress in CHD has focused on the influence of the child’s severity of cardiac disease, caregiving burden, or coping to identify predictors of stress or stress response in parents. Some studies, however, have uncovered disparities that exist. As opposed to providing a systematic literature review on stress and coping of parents with CHD and their mental health outcomes, which has already been recently presented 20,22,23, this review will focus on the health disparity outcomes found in the cardiac literature and compare and contrast it with parent stress literature from other populations.
Socioeconomic Status and Social Status
Education status may influence the stress of parents of children with CHD24. Education status has been frequently used in research as a proxy for socioeconomic status and/or social status in family research25. Many studies on parents of children from various populations (CHD, general surgery, pediatric cancer, irritable bowel syndrome, and trauma) have found that education status influences stress for parents, with lower education associated with an increased risk for stress and post-traumatic stress symptoms14,26–31. Mothers with higher education who had infants in neonatal intensive care were shown in one study to experience significantly higher stress response, as measured by state anxiety32. This was also found to be true in one study from the cardiac population that examined mothers of children with CHD33. More research is needed to determine the influence of education on parental stress.
Other measures of socioeconomic status including income and occupation have been shown to influence parental stress. Parents with low income or financial difficulty experience an increase in both perceived stress, chronic stress, and stress response31,34,35. The financial burden arising from the child’s chronic illness has been noted in cardiac and non-cardiac populations36,37. Spijkerboer and colleagues found that low socioeconomic status classified by occupation type influenced greater psychologic distress in parents of children treated for CHD38. Lower scores on mental well-being, as measured by the General Health Questionnaire, were found to be significantly correlated with poverty in parents with a child undergoing cardiac surgery39. Another study examined parental stress levels in a pediatric cardiac ICU and step down unit and found that parental stress scores were negatively correlated with poverty level and that foreign born mothers had significantly higher stress during their child’s hospitalization16. They concluded that poverty, place of birth, and citizenship may all play a role in predicting parental stress levels. Other studies in both cardiac and non-cardiac populations have also proposed that foreign born parents or immigrants experience lower social status than parents living in their place of birth. These studies have shown that parents with this particular vulnerability are at risk for higher stress symptoms9,14,27,34,40.
Sex
Fathers are often overlooked in maternal and child health research34; however, the role of the father for child well-being should not be ignored. Studies from community samples confirm that the mental health of fathers influences the child’s behavioral and emotional development, as well as later psychopathology, independent of the mother’s mental health41,42. Unfortunately, much research on parental stress has focused on mothers, yet studies including fathers have found differences. Studies on parental stress in both cardiac and non-cardiac populations have shown that mothers and fathers perceive stress and react to stressors differently 26,32,37,43–49. The majority of research has shown that mothers score significantly higher on stress and anxiety measures9,17,26,46. For parents of children with CHD, mothers have been shown to have significantly higher distress, anxiety, depression, and somatization than fathers9,18,49–52. Mothers and fathers have also been shown to use different coping mechanisms to adjust to stressors as parents of children with CHD19,23. Furthermore, the literature is replete with examples of how sex differences exist in the physiology of the stress response between men and women across the life span53–58. Due to these clear differences, it is important to consider sex as a biomarker in research on parental stress.
Most research on parental stress tends to only include mothers or have increased participation by mothers, creating a bias in the sample7,20,59. This may create an unfortunate disparity in the literature and potential bias of interpretation on the experience of parental stress for fathers. In addition, no research has been published that specifically addresses parental stress in CHD for same sex couples, adoptive parents, or single parents. It has been shown in community samples that single parents living alone are at higher risk, bearing a greater burden of stress29,60, and that love between partners, regardless of sexual orientation, can mediate parental stress14,61. Furthermore, cohabitation seems particularly important34. One study concluded that cohabitation with a partner was “a major determinant of stress during pregnancy and the year following birth for parents, especially for mothers. What mattered most in this sample was living together at the time of birth, not whether the couple was legally married at that time”34 (p. 623).
Additional Health Disparity Variables
Several variables were not identified in the cardiac literature, but should be considered when examining health disparities, including race, language barriers, physical health, stigma, and social capital. General population studies on parenting stress have found an increased risk of high stress in parents who are Black, Hispanic, or non-English speaking14,34. Unfortunately, most research on parental stress in CHD has been conducted with mainly White samples or have not reported race as a demographic variable7,16,24,37,47,51,62–69. Additionally, study design for parental stress in CHD research in the United States, United Kingdom, Canada, and Australia often limits inclusion criteria to subjects who have the ability to read or write English, or the authors do not mention translation of research instruments7,24,37,47,51,62–65,67,68,70. Furthermore, a parent’s own health or special needs status can exacerbate the parental stress experience for parents14. Mothers of infants with CHD are especially vulnerable after giving birth and recovering from a vaginal delivery or cesarean section37. Stress arises when parents are not well, in pain, or limited in their ability to care for their child. Additional consideration can be given to the stress occurring from stigma associated with certain health issues like obesity, diabetes, and even stress and mental health itself71–73. Stigma can also be experienced by parents for a variety of reasons based on race, gender, and sexual identity, among others, and has been shown to greatly contribute to stress and impact health72,74,75. Finally, social capital, defined as social cohesion and community networks, is emerging as a variable contributing to health and mental health outcomes in disparities research76,77. While few studies have examined its influence on parent stress, one study in Vietnam has demonstrated the influence of social capital on the distress of mothers who have children with disabilities78.
Review of Theoretical Frameworks on Parental Stress
In 2006, Mussato provided a review of literature in Cardiology in the Young on theoretical frameworks on child and family adaptation and illness79. In her review, she highlighted constructs integral to the adjustment of families with a child who has a chronic illness and proposed an adapted framework (Figure 1). Mussato posited that prior experience with stress, the child’s illness-related factors, anxiety, and family developmental stage would all influence the parents’ perception of the initial stress of the child’s diagnosis with a chronic illness. In addition, she proposed that the ability of the parents to cope with the stress of the child’s chronic illness is impacted by their social support and resources, hardiness, problem solving, and communication skills.
Figure 1.
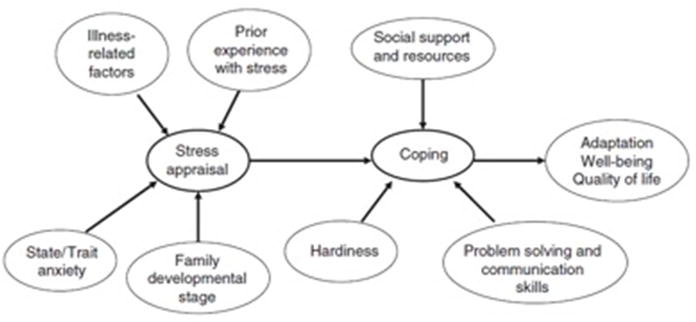
Mussato’s model includes essential components influencing the stress experience for parents of children with CHD, such as stress appraisal, coping, and adaptation, mirroring similar frameworks cited in the literature including Thompson’s Transactional Stress and Coping model of adjustment to chronic illness80 and McCubbin and Patterson’s Double ABCX Model of Adjustment and Adaptation81. She also highlighted the importance of social support on parent and child well-being, which has been proposed and validated by other frameworks, such as the Family Stress Model30. From a broader perspective, however, none of the constructs directly address issues regarding health disparities. Some, however, can be inferred. Prior experience with stress, for example, is important when considering how individuals perceive stress, cope, and react to stress, which in turn can influence long-term physical and mental health outcomes. Prior stress, when chronic, has been referred to in the literature as allostatic load, which occurs when physiologic systems adapting to stress are overworked, do not shut off after a stressful event, or under-respond to stress causing other systems to overwork82. Like Lazarus and Folkman’s definition of stress in which a stressor elicits a stress response21, the allostatic load model proposes that:
“the perception of threat and mobilization of these allostatic mechanisms are fundamentally shaped by individual differences in constitutional (genetics, development, experience), behavioral (coping and health habits), and historical (trauma/abuse, major life events, stressful environments) factors that ultimately determine one’s resiliency to stress”83(p.3).
Studies have demonstrated the physical and biopsychosocial impact of allostatic load across the lifespan83. The accumulation of allostatic load can overexpose the body to pro-inflammatory mediators of stress, resulting in adverse effects on body organs and systems, ultimately leading to disease onset and/or progression84. Both acute stress and chronic stress can contribute to allostatic load. What is wholly unknown is the impact of past chronic stress on parental stress and on parent and child health outcomes in CHD. Furthermore, parental stress in CHD could perhaps be considered its own form of chronic stress, contributing to the allostatic load on parents. Consideration for both acute and chronic stress is paramount in the examination of allostatic load, the overall stress experience, and its impact on parent health and well-being.
Examining both chronic and acute stress for parents of children with CHD will also elucidate important health disparity variables impacting overall stress and allostatic load34. Chronic stress is a wide category of stressors defined as the “ongoing demands that threaten to exceed the resources of an individual in areas of life such as family, marriage, parenting, work, health, housing, and finances” 34(p. 616). Chronic stressors are more prevalent for parents of racial/ethnic minorities, who tend to live in more impoverished settings with crime, pollution, and fewer resources34. Acute stressors are those conditions or events that occur with a clear beginning and ending, such as the death of a loved one, divorce, injury, or catastrophic event. Within the context of CHD, the child’s diagnosis and day of surgery could be considered acute stressors for parents, but parents are not isolated from exposure to other acute stressors as well. The examination of different forms of stress can more fully describe the influences of stress on parent health disparities34. These forms include financial stress, pregnancy stress, life events, chronic life stress, perceived stress, interpersonal violence, perceived racism, and parenting stress. A more accurate conceptualization of parental stress in CHD must include all of these forms of stress that individuals can experience while in the role of a parent for a child born with CHD.
Another model worth review is The Preconception Stress and Resiliency Pathways Model, which was developed through an interdisciplinary initiative of the National Institutes of Health and the National Institute of Child Health and Human Development to address health disparities in pregnancy and child developmental outcomes85. Major elements within the model include the
“centrality of the preconception/inter-conception period, role of fathers and the parental relationship, maternal allostatic load (a composite biomarker index of cumulative wear-and-tear of stress), resilience resources of parents, and local neighborhood and community level influences (e.g., employment, housing, education, health care, and stability of basic necessities)” 85(p. 707).
The model also highlights the inextricable link between parent stress and parent mental health with child neurodevelopment, health, and behavior outcomes. For parents of children with CHD, this link cannot be understated, yet remains under researched. However there is growing recognition to explore parental stress and its influence on child development in CHD13.
Finally, The Preconception Stress and Resiliency Pathways Model addresses an important concept related to stress: resilience. Historically, resilience has been defined in varying ways, as a response to stress, a final outcome, a protective factor, or as a return to baseline after a stress-inducing experience86. Scholars who seek to apply resilience to chronic stress have criticized these definitions of resilience and argue that a more broad conceptualization is needed. More recently, resilience has been defined as “a state of adaptation to a lifetime of stress and strain”(p.13)83. The Preconception Stress and Resiliency Pathways Model defines the concept as an overarching process and capacity of an individual, not an isolated factor or outcome, using resilience resources such as perceived social support, spirituality and religious practices, cognitive and coping skills, and tangible resources to resist the effects of long term stress86. Resilience is then a “process involving an ability to withstand and cope with ongoing or repeated demands and maintain healthy functioning in different domains of life such as work and family”86(p.637). Within the context of parental stress in CHD, resilience can be defined similarly as the process and capacity of individuals to positively adapt and cope to stressors while in the role of parent for a child with CHD. This process of resilience will influence the stress response of the parent and the overall health and wellbeing of parent and child.
Revised Conceptual Model
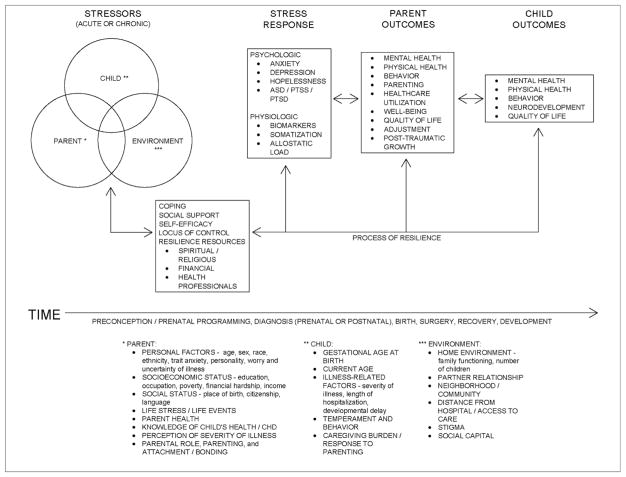
Using the themes and constructs from the theoretical models reviewed above as well as synthesis of literature from the pediatric cardiac population, community samples, other pediatric populations, and vulnerable populations, a new conceptual model, the Parental Stress and Resilience in Congenital Heart Disease Model, was created to provide a comprehensive perspective on parental stress (Figure 2). This new model will provide a broader lens through which researchers and clinicians can examine and support parental stress in CHD. A more comprehensive understanding of parental stress through this model will help to identify health disparity variables influencing parental stress and inform the development of interventions to support all parents and their children with CHD.
Figure 2.
The Parent Stress and Resilience in CHD Model acknowledges that prenatal factors occurring in the preconception period will have lifelong impact on the parent as well as the child with CHD85. Evidence is growing suggesting that the biologic stress response of mothers, specifically that of the hypothalamic-pituitary-adrenal axis dysregulation, before and during pregnancy, can have profound impact on the future mental and physical health of the mother’s offspring34,85,87. Past chronic stress from poverty, racism, residential segregation, community violence, and stigma will also contribute to the biologic impact of stress on the mother and child in utero34,74,85,88. In addition, this model posits that both acute and chronic stressors34 arise from three categories7,37: infant/child with CHD, environment, and parent7,37. Child related factors include the child’s gestational age at birth89, current age16,29, illness related factors9,11,60,66,68,89–91 (severity of illness, length of hospitalization, developmental delay, other genetic issues), length of hospitalization after cardiac surgery60, child temperament and behavior10,64,66, and caregiving burden18,92–94. Environmental stressors can occur from the home environment85 (such as family functioning23 and number of children7) as well as the parent partner/marital relationship85. Neighborhoods or communities with violence, crime, poor resources, and pollution generate an added challenge for parents88. Parents living in neighborhoods located a far distance from a children’s hospital or who have poor access to care may also experience greater stress37. In addition, social stigma experienced by parents in their environment as well as social capital will influence parent stress and overall health74,77,78. Stressors arising from the parent include a myriad of personal factors including age95, sex50,52,96, race and ethnicity14,34, trait anxiety7,96, personality37, worry66 and uncertainty of illness60,97. The parent’s socioeconomic status and social status 18,24,30,96,98, any past or current life stress or life events95, as well as the parent’s own health29 contribute to the stress faced by parents. In addition, the perception of the child’s severity of illness37,69 as well as the parent’s knowledge or lack of knowledge about the child’s health and CHD 99,100 influence parental stress. Finally, parental role7, parenting10, and attachment/bonding101 impact the stress experience for parents.
Coping46,60,97, social support18,26,30,44,46,63,96,102,103, caregiving self-efficacy104, locus of control26, and other resilience resources85,86 (spiritual and religious practices and beliefs70,99, financial and tangible resources, and support from health professionals70,97) mediate between the perception of stressors and stress response for the parent of a child with CHD. Both psychologic and physiologic forms of stress response can occur7,37. Psychologic forms of stress response manifest as feelings of anxiety7, depression93, and/or hopelessness98, as well as clinical symptoms of acute stress disorder46,51, post-traumatic stress symptoms60, or post-traumatic stress disorder40 in some. Physiologic stress responses include parent biomarkers85, somatization18, and allostatic load34,82,85.
Over time, the cumulative impact of the stress/coping/response cycle in parents and the process of resilience will influence parental mental and physical health85, behavior85, parenting24, well-being105, health care utilization14, quality of life15,69,89, and adjustment15,24. Parents may also experience Post-Traumatic Growth, a positive change through the process of resilience that includes improved self-perception, self-esteem, confidence, and personal power, increased compassion and authenticity with others, and an enhanced philosophy of life that brings meaning and maturity from past difficult circumstances106. These parental outcomes will ultimately influence the child’s mental and physical health107, behavior and neurodevelopment11,108, and quality of life15.
Implications
The newly created Parental Stress and Resilience in CHD Model will provide a helpful foundation for clinicians and researchers to create interventions to reduce stress and the subsequent impact of stress for parents of children with CHD and improve overall family well-being, quality of life, and child development. The model reveals that a one-size-fits-all approach will not be successful in adequately addressing the many facets influencing the stress experience to support the process of resilience for parents of children with CHD. However, there are interventions that all centers caring for children with CHD and their parents should implement as a standard of care.
First, substantial evidence exists that the support of parental and child mental health should become a priority for the care of patients with CHD and their families. Interdisciplinary teams should address this priority in all centers caring for patients with CHD and should include mental health professionals such as psychologists. Mental health screening for issues of stress, anxiety and depression need to be initiated for parents at diagnosis49 and during the child’s hospital stay for cardiac surgery24,109. Early and regular screening for mental health problems should continue at outpatient clinic visits throughout the child’s first year of life 22. Routine screening for mental health problems will assist health care providers in identifying parents who need early psychological support by social work or psychology. In addition, routine screening should include assessment of social risk, prior trauma, and other risk factors for parental stress and negative mental health outcomes identified by the Parental Stress and Resilience in CHD Model. Until routine screening becomes the standard of care for all centers, it will be difficult for centers to systematically address the specific needs regarding health disparities of parents and their children with CHD.
Psychological support interventions have been proposed and studied in parents of children with CHD. Prenatal counseling and support can reduce anxiety, especially for fathers49. In-hospital preoperative education also reduces anxiety for parents whose child is undergoing cardiac surgery110. Psychoeducation tailored to parents of infants with CHD that includes coaching for improved coping abilities, caregiving self-efficacy, and parenting skills (such as reading the infant cues and activities to promote development) has also shown promise111. Most recently, mindful meditation has been proposed as a potential strategy for coping while the child is in the hospital for cardiac surgery112. Future research should determine whether these interventions mitigate parental stress for parents who are experiencing disparities as well as ensure that the interventions do not widen any disparities by disproportionately benefiting parents at lower risk113. Integrating routine mental health screening by mental health professionals coupled with standard educational and psychoeducational interdisciplinary programs at targeted, at-risk periods (prenatal diagnosis, infant birth and hospitalization for cardiac surgery, at discharge and in the early months following) only represent the first set of interventions we must provide to all parents. Community-based participatory research including parents who may be at risk for disparities would be a useful methodological approach in designing future interventions that target at-risk populations.
Additional attention should also be given to family-centered care and family-based interventions as an essential strategy to reduce the mutual influences of stress between parent and child11. Some studies have specifically suggested ways to enhance parental role beginning in the pediatric cardiac ICU by providing opportunities to increase bonding and attachment between parent and child7,22, which is uniquely threatened when a child is diagnosed with CHD65,114–117. It is well established that early attachment behaviors between parent and child independently influences parent mental health and child physical, behavioral, and emotional development107,118,119, and this relationship has been confirmed in one study of mothers and children with CHD120. More attention must be given to this area of research and intervention. Other family-based interventions such as a family liaison nurse or a home visiting nurse would provide an added layer of support to families in parenting and caring for their child. These types of home visits have also been successful to support particularly vulnerable populations such as mothers with low SES121.
Social support has already shown to be effective in reducing some of the psychological and family outcomes of stress for parents of children with CHD18,44,90,100, in parents of critically ill children102, and in parents of children with other chronic illnesses36. More attention is needed, however, by both researchers and health care providers to assess parents’ current social support systems and provide ways to enhance their social network. One study by Lawoko and Soares found that significant predictors existed that placed parents at particular risk for limited social support: increased caregiving time, foreign-born parents, unemployment, financial burden of CHD, and increased levels of distress and hopelessness94. While social support is needed by all parents and is critical for parent–child health and well-being30, a sobering reality exists: the very parents who are the most vulnerable also have the highest risk for being the most isolated. Innovative strategies are needed to foster social support networks for these vulnerable groups. Furthermore, social networks differ based on culture and race. One example is that Black/African American parents often rely on grandparents or other family members to help care for their children, whereas White/Caucasian parents use non-family networks, such as friends or neighbors122. Social support interventions must be tailored based on individual need, resources, and cultural considerations. In addition, disparities have been identified in low-income families with respect to positive parenting practices that support child development. Parents from low-income households are less likely to participate in frequent, high quality positive parenting practices, including reading, singing, and interactive play, which are associated with increased risk of child developmental delay123. Higher perceived social support has been correlated with increased positive interactions between parent and child30. Social support is a promising target for intervention to reduce parent stress while also promoting child development. The development of social support beyond the neighborhood and community context should also be considered. In the last decade, social networks have been created through organizations and social media124. Parents of children with CHD are interested in joining organizations to meet other parents103 or using social media sites to enhance their support network125. Research has begun to emerge revealing how social media sites such as Facebook can be used to provide social support to individuals during health-related events126. Much more research is needed to understand the extent to which social media communities and other organizations support parents of children with CHD and how social risk may impact access to and utilization of these supports.
Many more interventions could be created to address issues proposed by the Parent Stress and Resilience in CHD Model and a comprehensive listing of potential innovations is beyond the scope of this review. However, the model elucidates certain vulnerabilities for parents that can be targeted in future research or clinical projects. Efforts must be given to support parents with low social status, directing particular attention to parents who are foreign born, non-English-speaking, single parent, low-income, of a minority race or ethnicity, or from an at-risk community with fewer resources.
Conclusion
This comprehensive review of literature on parent stress synthesized with research from other pediatric populations, uniquely conceptualized parent stress through the lens of health disparities. A new conceptual framework, the Parental Stress and Resilience in CHD Model, describes a broad view of parent stress with health, well-being, and quality of life implications for both parents and their children with CHD. Health care providers and researchers alike can utilize this model to design clinical projects, research studies, and innovative interventions to mitigate stress and promote resilience for all families, regardless of their risk for health disparities. The reality of allostatic load, arising from acute and chronic stress, and its biopsychosocial impact on families with a child diagnosed with CHD must be a focus of future study. Furthermore, the link between parental stress and child emotional, behavioral, and neurodevelopmental outcomes should become a priority for research in the next decade.
Acknowledgments
The author would like to thank several colleagues for their review and thoughtful feedback on this manuscript: Dr. Barbara Medoff-Cooper, Dr. José A. Bauermeister, Dr. Lisa M. Lewis, Dr. Kaitlin M. Best, and Dr. Abigail C. Demianczyk.
Financial Support
This work was supported by the National Institute of Nursing Research (NINR T32NR007100).
Footnotes
Conflicts of Interest
None
References
- 1.Berkowitz B, McCubbin M. Advancement of health disparities research: a conceptual approach. Nurs Outlook. 2005;53(3):153–159. doi: 10.1016/j.outlook.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Flaskerud JH, Winslow BJ. Conceptualizing vulnerable populations health-related research. Nurs Res. 1998;47(2):69–78. doi: 10.1097/00006199-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Knowles RL, Ridout D, Crowe S, et al. Ethnic and socioeconomic variation in incidence of congenital heart defects. Archives of disease in childhood. 2017;102(6):496–502. doi: 10.1136/archdischild-2016-311143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kucik JE, Cassell CH, Alverson CJ, et al. Role of health insurance on the survival of infants with congenital heart defects. American journal of public health. 2014;104(9):e62–70. doi: 10.2105/AJPH.2014.301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucik JE, Nembhard WN, Donohue P, et al. Community socioeconomic disadvantage and the survival of infants with congenital heart defects. American journal of public health. 2014;104(11):e150–157. doi: 10.2105/AJPH.2014.302099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Liu G, Druschel CM, Kirby RS. Maternal race/ethnicity and survival experience of children with congenital heart disease. J Pediatr. 2013;163(5):1437–1442. e1431–1432. doi: 10.1016/j.jpeds.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 7.Lisanti AJ, Allen LR, Kelly L, Medoff-Cooper B. Maternal Stress and Anxiety in the Pediatric Cardiac Intensive Care Unit. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2017;26(2):118–125. doi: 10.4037/ajcc2017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rychik J, Donaghue DD, Levy S, et al. Maternal psychological stress after prenatal diagnosis of congenital heart disease. J Pediatr. 2013;162(2):302–307. e301. doi: 10.1016/j.jpeds.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Vrijmoet-Wiersma CM, Ottenkamp J, van Roozendaal M, Grootenhuis MA, Koopman HM. A multicentric study of disease-related stress, and perceived vulnerability, in parents of children with congenital cardiac disease. Cardiol Young. 2009;19(6):608–614. doi: 10.1017/S1047951109991831. [DOI] [PubMed] [Google Scholar]
- 10.Golfenshtein N, Hanlon AL, Deatrick JA, Medoff-Cooper B. Parenting Stress in Parents of Infants With Congenital Heart Disease and Parents of Healthy Infants: The First Year of Life. Comprehensive child and adolescent nursing. 2017:1–21. doi: 10.1080/24694193.2017.1372532. [DOI] [PubMed] [Google Scholar]
- 11.Landolt MA, Ystrom E, Stene-Larsen K, Holmstrom H, Vollrath ME. Exploring causal pathways of child behavior and maternal mental health in families with a child with congenital heart disease: a longitudinal study. Psychol Med. 2014;44(16):3421–3433. doi: 10.1017/S0033291713002894. [DOI] [PubMed] [Google Scholar]
- 12.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16:92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 13.Wernovsky G, Licht DJ. Neurodevelopmental Outcomes in Children With Congenital Heart Disease-What Can We Impact? Pediatr Crit Care Med. 2016;17(8 Suppl 1):S232–242. doi: 10.1097/PCC.0000000000000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raphael JL, Zhang Y, Liu H, Giardino AP. Parenting stress in US families: implications for paediatric healthcare utilization. Child: Care, Health & Development. 2010;36(2):216–224. doi: 10.1111/j.1365-2214.2009.01052.x. [DOI] [PubMed] [Google Scholar]
- 15.Ernst MM, Marino BS, Cassedy A, et al. Biopsychosocial Predictors of Quality of Life Outcomes in Pediatric Congenital Heart Disease. Pediatr Cardiol. 2017 doi: 10.1007/s00246-017-1730-6. [DOI] [PubMed] [Google Scholar]
- 16.Franck L, Mcquillen A, et al. Parent stress levels during children's hospital recovery after congenital heart surgery. Pediatric Cardiology. 2010;31:961–968. doi: 10.1007/s00246-010-9726-5. [DOI] [PubMed] [Google Scholar]
- 17.Dudek-Shriber L. Parent stress in the neonatal intensive care unit and the influence of parent and infant characteristics. Am J Occup Ther. 2004;58(5):509–520. doi: 10.5014/ajot.58.5.509. [DOI] [PubMed] [Google Scholar]
- 18.Lawoko S, Soares JJF. Psychosocial morbidity among parents of children with congenital heart disease: A prospective longitudinal study. Heart & Lung. 2006;35(5):301–314. doi: 10.1016/j.hrtlng.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Doherty N, McCusker CG, Molloy B, et al. Predictors of psychological functioning in mothers and fathers of infants born with severe congenital heart disease. Journal of Reproductive and Infant Psychology. 2009;27(4):390–400. [Google Scholar]
- 20.Woolf-King SE, Anger A, Arnold EA, Weiss SJ, Teitel D. Mental Health Among Parents of Children With Critical Congenital Heart Defects: A Systematic Review. Journal of the American Heart Association. 2017;6(2) doi: 10.1161/JAHA.116.004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazarus RS, Folkman S. Stress, Appaisal and Coping. New York, NY: Springer; 1984. [Google Scholar]
- 22.Kolaitis GA, Meentken MG, Utens E. Mental Health Problems in Parents of Children with Congenital Heart Disease. Frontiers in pediatrics. 2017;5:102. doi: 10.3389/fped.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson AC, Frydenberg E, Liang RP, Higgins RO, Murphy BM. Familial impact and coping with child heart disease: a systematic review. Pediatr Cardiol. 2015;36(4):695–712. doi: 10.1007/s00246-015-1121-9. [DOI] [PubMed] [Google Scholar]
- 24.Hearps S, et al. Psychosocial risk in families of infants undergoing surgery for a serious congenital heart disease. Cardiol Young. 2014;23(4):632–638. doi: 10.1017/S1047951113000760. [DOI] [PubMed] [Google Scholar]
- 25.Conger RD, Conger KJ, Martin MJ. Socioeconomic Status, Family Processes, and Individual Development. J Marriage Fam. 2010;72(3):685–704. doi: 10.1111/j.1741-3737.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scrimin S, Haynes M, Alto G, Bornstein MH, Axia G. Anxiety and stress in mothers and fathers in the 24 h after their child's surgery. Child: Care, Health & Development. 2009;35(2):227–233. doi: 10.1111/j.1365-2214.2008.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norberg AL, Lindblad F, Boman KK. Parental traumatic stress during and after paediatric cancer treatment. Acta Oncologica. 2005;44(4):382–388. doi: 10.1080/02841860510029789. [DOI] [PubMed] [Google Scholar]
- 28.Guilfoyle SM, Denson LA, Baldassano RN, Hommel KA. Paediatric parenting stress in inflammatory bowel disease: application of the Pediatric Inventory for Parents. Child: Care, Health & Development. 2012;38(2):273–279. doi: 10.1111/j.1365-2214.2010.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson LS. Predictors of parenting stress in a diverse sample of parents of early adolescents in high-risk communities. Nursing Research. 2008;57(5):340–350. doi: 10.1097/01.NNR.0000313502.92227.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConnell D, Breitkreuz R, Savage A. From financial hardship to child difficulties: main and moderating effects of perceived social support. Child: Care, Health & Development. 2011;37(5):679–691. doi: 10.1111/j.1365-2214.2010.01185.x. [DOI] [PubMed] [Google Scholar]
- 31.Sturms LM, van der Sluis CK, Stewart RE, Groothoff JW, ten Duis HJ, Eisma WH. A prospective study on paediatric traffic injuries: health-related quality of life and post-traumatic stress. Clinical Rehabilitation. 2005;19(3):312–322. doi: 10.1191/0269215505cr867oa. [DOI] [PubMed] [Google Scholar]
- 32.Shields-Poe D, Pinelli J. Variables associated with parental stress in neonatal intensive care units. Neonatal network : NN. 1997;16(1):29–37. [PubMed] [Google Scholar]
- 33.Davis CC, Brown RT, Bakeman R, Campbell R. Psychological adaptation and adjustment of mothers of children with congenital heart disease: stress, coping, and family functioning. J Pediatr Psychol. 1998;23(4):219–228. doi: 10.1093/jpepsy/23.4.219. [DOI] [PubMed] [Google Scholar]
- 34.Dunkel Schetter C, Schafer P, Lanzi RG, et al. Shedding Light on the Mechanisms Underlying Health Disparities Through Community Participatory Methods: The Stress Pathway. Perspect Psychol Sci. 2013;8(6):613–633. doi: 10.1177/1745691613506016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yildiz A, Celebioglu A, Olgun H. Distress levels in Turkish parents of children with congenital heart disease. Australian Journal of Advanced Nursing. 2009;26(3):39–46. [Google Scholar]
- 36.Bonis S. Stress and Parents of Children with Autism: A Review of Literature. Issues in Mental Health Nursing. 2016;37(3):153–163. doi: 10.3109/01612840.2015.1116030. [DOI] [PubMed] [Google Scholar]
- 37.Lisanti AJ, Golfenshtein N, Medoff-Cooper B. The Pediatric Cardiac Intensive Care Unit Parental Stress Model: Refinement Using Directed Content Analysis. ANS Adv Nurs Sci. 2017 doi: 10.1097/ANS.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spijkerboer AW, Helbing WA, Bogers AJ, Van Domburg RT, Verhulst FC, Utens EM. Long-term psychological distress, and styles of coping, in parents of children and adolescents who underwent invasive treatment for congenital cardiac disease. Cardiol Young. 2007;17(6):638–645. doi: 10.1017/S1047951107001333. [DOI] [PubMed] [Google Scholar]
- 39.Lopez R, Frangini P, Ramirez M, et al. Well-Being and Agency in Parents of Children With Congenital Heart Disease: A Survey in Chile. World journal for pediatric & congenital heart surgery. 2016;7(2):139–145. doi: 10.1177/2150135115623284. [DOI] [PubMed] [Google Scholar]
- 40.Helfricht S, Latal B, Fischer JE, Tomaske M, Landolt MA. Surgery-related posttraumatic stress disorder in parents of children undergoing cardiopulmonary bypass surgery: a prospective cohort study. Pediatr Crit Care Med. 2008;9(2):217–223. doi: 10.1097/PCC.0b013e318166eec3. [DOI] [PubMed] [Google Scholar]
- 41.Ramchandani P, Stein A, Evans J, O'Connor TG team As. Paternal depression in the postnatal period and child development: a prospective population study. Lancet. 2005;365(9478):2201–2205. doi: 10.1016/S0140-6736(05)66778-5. [DOI] [PubMed] [Google Scholar]
- 42.Ramchandani PG, Stein A, O'Connor TG, Heron J, Murray L, Evans J. Depression in men in the postnatal period and later child psychopathology: a population cohort study. J Am Acad Child Adolesc Psychiatry. 2008;47(4):390–398. doi: 10.1097/CHI.0b013e31816429c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinelli J, Saigal S, Bill Wu Y-W, et al. Patterns of change in family functioning, resources, coping and parental depression in mothers and fathers of sick newborns over the first year of life. Journal of Neonatal Nursing. 2008;14(5):156–165. [Google Scholar]
- 44.Tak YR, McCubbin M. Family stress, perceived social support and coping following the diagnosis of a child's congenital heart disease. Journal of advanced nursing. 2002;39(2):190–198. doi: 10.1046/j.1365-2648.2002.02259.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoehn KS, Wernovsky G, Rychik J, et al. Parental decision-making in congenital heart disease. Cardiol Young. 2004;14(3):309–314. doi: 10.1017/S1047951104003099. [DOI] [PubMed] [Google Scholar]
- 46.Shaw RJ, Deblois T, Ikuta L, Ginzburg K, Fleisher B, Koopman C. Acute stress disorder among parents of infants in the neonatal intensive care nursery. Psychosomatics. 2006;47(3):206–212. doi: 10.1176/appi.psy.47.3.206. [DOI] [PubMed] [Google Scholar]
- 47.Wray J, Sensky T. Psychological functioning in parents of children undergoing elective cardiac surgery. Cardiol Young. 2004;14(2):131–139. doi: 10.1017/S1047951104002045. [DOI] [PubMed] [Google Scholar]
- 48.Haegen MV, Luminet O. Stress, Psychosocial Mediators, and Cognitive Mediators in Parents of Child Cancer Patients and Cancer Survivors: Attention and Working Memory Pathway Perspectives. Journal of Psychosocial Oncology. 2015;33(5):504–550. doi: 10.1080/07347332.2015.1067279. [DOI] [PubMed] [Google Scholar]
- 49.Pinto NM, Weng C, Sheng X, et al. Modifiers of stress related to timing of diagnosis in parents of children with complex congenital heart disease. J Matern Fetal Neonatal Med. 2016;29(20):3340–3346. doi: 10.3109/14767058.2015.1125465. [DOI] [PubMed] [Google Scholar]
- 50.Utens EM, Versluis-Den Bieman HJ, Verhulst FC, Witsenburg M, Bogers AJ, Hess J. Psychological distress and styles of coping in parents of children awaiting elective cardiac surgery. Cardiol Young. 2000;10(3):239–244. doi: 10.1017/s1047951100009173. [DOI] [PubMed] [Google Scholar]
- 51.Franich-Ray C, Bright MA, Anderson V, et al. Trauma reactions in mothers and fathers after their infant's cardiac surgery. J Pediatr Psychol. 2013;38(5):494–505. doi: 10.1093/jpepsy/jst015. [DOI] [PubMed] [Google Scholar]
- 52.Bevilacqua F, Palatta S, Mirante N, et al. Birth of a child with congenital heart disease: emotional reactions of mothers and fathers according to time of diagnosis. J Matern Fetal Neonatal Med. 2013;26(12):1249–1253. doi: 10.3109/14767058.2013.776536. [DOI] [PubMed] [Google Scholar]
- 53.Carpenter T, Grecian SM, Reynolds RM. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis. 2017;8(2):244–255. doi: 10.1017/S204017441600074X. [DOI] [PubMed] [Google Scholar]
- 54.van der Voorn B, Hollanders JJ, Ket JCF, Rotteveel J, Finken MJJ. Gender-specific differences in hypothalamus-pituitary-adrenal axis activity during childhood: a systematic review and meta-analysis. Biol Sex Differ. 2017;8:3. doi: 10.1186/s13293-016-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherman GD, Rice LK, Jin ES, Jones AC, Josephs RA. Sex differences in cortisol's regulation of affiliative behavior. Horm Behav. 2017;92:20–28. doi: 10.1016/j.yhbeh.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Rutherfurd-Markwick K, Starck C, Dulson DK, Ali A. Salivary diagnostic markers in males and females during rest and exercise. J Int Soc Sports Nutr. 2017;14:27. doi: 10.1186/s12970-017-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gifford RM, Reynolds RM. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans. Early Hum Dev. 2017 doi: 10.1016/j.earlhumdev.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Andiarena A, Balluerka N, Murcia M, Ibarluzea J, Glover V, Vegas O. Evening salivary cortisol and alpha-amylase at 14months and neurodevelopment at 4years: Sex differences. Horm Behav. 2017;94:135–144. doi: 10.1016/j.yhbeh.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Golfenshtein N, Srulovici E, Medoff-Cooper B. Investigating Parenting Stress across Pediatric Health Conditions - A Systematic Review. Issues Compr Pediatr Nurs. 2015:1–49. doi: 10.3109/01460862.2015.1078423. [DOI] [PubMed] [Google Scholar]
- 60.Franck LS, Wray J, Gay C, Dearmun AK, Lee K, Cooper BA. Predictors of parent post-traumatic stress symptoms after child hospitalization on general pediatric wards: a prospective cohort study. Int J Nurs Stud. 2015;52(1):10–21. doi: 10.1016/j.ijnurstu.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Goldberg AE, Smith JZ. Predictors of parenting stress in lesbian, gay, and heterosexual adoptive parents during early parenthood. J Fam Psychol. 2014;28(2):125–137. doi: 10.1037/a0036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menahem S, Poulakis Z, Prior M. Children subjected to cardiac surgery for congenital heart disease. Part 2 - parental emotional experiences. Interact Cardiovasc Thorac Surg. 2008;7(4):605–608. doi: 10.1510/icvts.2007.171066. [DOI] [PubMed] [Google Scholar]
- 63.Visconti KJ, Saudino KJ, Rappaport LA, Newburger JW, Bellinger DC. Influence of parental stress and social support on the behavioral adjustment of children with transposition of the great arteries. J Dev Behav Pediatr. 2002;23(5):314–321. doi: 10.1097/00004703-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Torowicz D, Irving S, Sumpter D, Medoff-Cooper B. Infant temperament and parental stress in 3 month old infant following surgery for complex congenital heart disease. Journal of Developmental & Behavioral Pediatrics. 2010;31(3):1–7. doi: 10.1097/DBP.0b013e3181d3deaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jordan B, Franich-Ray C, Albert N, et al. Early mother–infant relationships after cardiac surgery in infancy. Archives of disease in childhood. 2014;99(7):641–645. doi: 10.1136/archdischild-2012-303488. [DOI] [PubMed] [Google Scholar]
- 66.Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Health and well-being of children with congenital cardiac malformations, and their families, following open-heart surgery. Cardiol Young. 2006;16(2):157–164. doi: 10.1017/S1047951106000096. [DOI] [PubMed] [Google Scholar]
- 67.Uzark K, Jones K. Parenting stress and children with heart disease. Journal of Pediatric Healthcare. 2003;17(4):163–168. doi: 10.1067/mph.2003.22. [DOI] [PubMed] [Google Scholar]
- 68.Brosig CL, Whitstone BN, Frommelt MA, Frisbee SJ, Leuthner SR. Psychological distress in parents of children with severe congenital heart disease: the impact of prenatal versus postnatal diagnosis. J Perinatol. 2007;27(11):687–692. doi: 10.1038/sj.jp.7211807. [DOI] [PubMed] [Google Scholar]
- 69.Caris EC, Dempster N, Wernovsky G, et al. Anxiety Scores in Caregivers of Children with Hypoplastic Left Heart Syndrome. Congenital heart disease. 2016;11(6):727–732. doi: 10.1111/chd.12387. [DOI] [PubMed] [Google Scholar]
- 70.Kosta L, Harms L, Franich-Ray C, et al. Parental experiences of their infant's hospitalization for cardiac surgery. Child: care, health and development. 2015;41(6):1057–1065. doi: 10.1111/cch.12230. [DOI] [PubMed] [Google Scholar]
- 71.Schabert J, Browne JL, Mosely K, Speight J. Social stigma in diabetes : a framework to understand a growing problem for an increasing epidemic. Patient. 2013;6(1):1–10. doi: 10.1007/s40271-012-0001-0. [DOI] [PubMed] [Google Scholar]
- 72.Clair M, Daniel C, Lamont M. Destigmatization and health: Cultural constructions and the long-term reduction of stigma. Soc Sci Med. 2016;165:223–232. doi: 10.1016/j.socscimed.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17(5):941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 74.Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. American journal of public health. 2013;103(5):813–821. doi: 10.2105/AJPH.2012.301069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schomerus G, Schwahn C, Holzinger A, et al. Evolution of public attitudes about mental illness: a systematic review and meta-analysis. Acta Psychiatr Scand. 2012;125(6):440–452. doi: 10.1111/j.1600-0447.2012.01826.x. [DOI] [PubMed] [Google Scholar]
- 76.Whitley R, McKenzie K. Social capital and psychiatry: review of the literature. Harv Rev Psychiatry. 2005;13(2):71–84. doi: 10.1080/10673220590956474. [DOI] [PubMed] [Google Scholar]
- 77.Murayama H, Fujiwara Y, Kawachi I. Social Capital and Health: A Review of Prospective Multilevel Studies. Journal of Epidemiology. 2012;22(3):179–187. doi: 10.2188/jea.JE20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thuy NT, Berry HL. Social capital and mental health among mothers in Vietnam who have children with disabilities. Glob Health Action. 2013:6. doi: 10.3402/gha.v6i0.18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mussatto K. Adaptation of the child and family to life with a chronic illness. Cardiol Young. 2006;16(S3):110. doi: 10.1017/s104795110600103x. [DOI] [PubMed] [Google Scholar]
- 80.Thompson RJ. Coping with the stress of chronic childhood illness. In: O'Quinn AN, editor. Management of chronic disorders of childhood. Boston, MA: Hall; 1985. pp. 11–41. [Google Scholar]
- 81.McCubbin HI, Patterson J. The family stress process: the double ABCX model of adjustment and adaptation. Marriage and Family Review. 1983;6(7–37) [Google Scholar]
- 82.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 83.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and biobehavioral reviews. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 84.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 85.Ramey S, Schafer P, DeClerque J, et al. The Preconception Stress and Resiliency Pathways Model: A Multi-Level Framework on Maternal, Paternal, and Child Health Disparities Derived by Community-Based Participatory Research. Maternal & Child Health Journal. 2015;19(4):707–719. doi: 10.1007/s10995-014-1581-1. [DOI] [PubMed] [Google Scholar]
- 86.Dunkel Schetter C, Dolbier C. Resilience in the Context of Chronic Stress and Health in Adults. Soc Personal Psychol Compass. 2011;5(9):634–652. doi: 10.1111/j.1751-9004.2011.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep. 2015;17(2):5. doi: 10.1007/s11920-014-0546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gee GC, Payne–Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaugars A, Shields C, Brosig C. Stress and quality of life among parents of children with congenital heart disease referred for psychological services. Congenital heart disease. 2017 doi: 10.1111/chd.12547. [DOI] [PubMed] [Google Scholar]
- 90.Werner H, Latal B, Valsangiacomo Buechel E, Beck I, Landolt MA. The impact of an infant's severe congenital heart disease on the family: a prospective cohort study. Congenital heart disease. 2014;9(3):203–210. doi: 10.1111/chd.12123. [DOI] [PubMed] [Google Scholar]
- 91.Koehler A, Fagnano M, Montes G, Halterman J. Elevated Burden for Caregivers of Children with Persistent Asthma and a Developmental Disability. Maternal & Child Health Journal. 2014;18(9):2080–2088. doi: 10.1007/s10995-014-1455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solberg O, Dale MT, Holmstrom H, Eskedal LT, Landolt MA, Vollrath ME. Emotional reactivity in infants with congenital heart defects and maternal symptoms of postnatal depression. Arch Womens Ment Health. 2011;14(6):487–492. doi: 10.1007/s00737-011-0243-1. [DOI] [PubMed] [Google Scholar]
- 93.Solberg O, Gronning Dale MT, Holmstrom H, Eskedal LT, Landolt MA, Vollrath ME. Trajectories of maternal mental health: a prospective study of mothers of infants with congenital heart defects from pregnancy to 36 months postpartum. J Pediatr Psychol. 2012;37(6):687–696. doi: 10.1093/jpepsy/jss044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lawoko S, Soares JJF. Social Support among Parents of Children with Congenital Heart Disease, Parents of Children with Other Diseases and Parents of Healthy Children. Scand J Occup Ther. 2003;10:177–187. [Google Scholar]
- 95.Turan T, Basbakkal Z, Ozbek S. Effect of nursing interventions on stressors of parents of premature infants in neonatal intensive care unit. Journal of clinical nursing. 2008;17(21):2856–2866. doi: 10.1111/j.1365-2702.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 96.Vander Haegen M, Luminet O. Stress, Psychosocial Mediators, and Cognitive Mediators in Parents of Child Cancer Patients and Cancer Survivors: Attention and Working Memory Pathway Perspectives. J Psychosoc Oncol. 2015;33(5):504–550. doi: 10.1080/07347332.2015.1067279. [DOI] [PubMed] [Google Scholar]
- 97.Re J, Dean S, Menahem S. Infant cardiac surgery: mothers tell their story: a therapeutic experience. World journal for pediatric & congenital heart surgery. 2013;4(3):278–285. doi: 10.1177/2150135113481480. [DOI] [PubMed] [Google Scholar]
- 98.Lawoko S, Soares JJF. Distress and hopelessness among parents of children with congenital heart disease, parents of children with other diseases, and parents of healthy children. Journal of Psychosomatic Research. 2002;52(4):193–208. doi: 10.1016/s0022-3999(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 99.Ahn JA, Lee S, Choi JY. Comparison of coping strategy and disease knowledge in dyads of parents and their adolescent with congenital heart disease. The Journal of cardiovascular nursing. 2014;29(6):508–516. doi: 10.1097/JCN.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 100.Lee S, Yoo JS, Yoo IY. Parenting stress in mothers of children with congenital heart disease. Asian nursing research. 2007;1(2):116–124. doi: 10.1016/S1976-1317(08)60014-6. [DOI] [PubMed] [Google Scholar]
- 101.Anderson LS, Riesch SK, Pridham KA, Lutz KF, Becker PT. Furthering the understanding of parent-child relationships: a nursing scholarship review series. Part 4: parent–child relationships at risk. Journal for specialists in pediatric nursing : JSPN. 2010;15(2):111–134. doi: 10.1111/j.1744-6155.2009.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stremler R, Haddad S, Pullenayegum E, Parshuram C. Psychological Outcomes in Parents of Critically Ill Hospitalized Children. J Pediatr Nurs. 2017;34:36–43. doi: 10.1016/j.pedn.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Bruce E, Lilja C, Sundin K. Mothers' lived experiences of support when living with young children with congenital heart defects. Journal for specialists in pediatric nursing : JSPN. 2014;19(1):54–67. doi: 10.1111/jspn.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harper FWK, Peterson AM, Uphold H, et al. Longitudinal study of parent caregiving self-efficacy and parent stress reactions with pediatric cancer treatment procedures. Psycho-Oncology. 2013;22(7):1658–1664. doi: 10.1002/pon.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Streisand R, Mackey E, Herge W. Associations of parent coping, stress, and well-being in mothers of children with diabetes: examination of data from a national sample. Maternal & Child Health Journal. 2010;14(4):612–617. doi: 10.1007/s10995-009-0497-7. [DOI] [PubMed] [Google Scholar]
- 106.Aftyka A, Rozalska-Walaszek I, Rosa W, Rybojad B, Karakula-Juchnowicz H. Post-traumatic growth in parents after infants' neonatal intensive care unit hospitalisation. Journal of clinical nursing. 2017;26(5–6):727–734. doi: 10.1111/jocn.13518. [DOI] [PubMed] [Google Scholar]
- 107.Dawson G, Ashman SB, Carver LJ. The role of early experience in shaping behavioral and brain development and its implications for social policy. Dev Psychopathol. 2000;12(4):695–712. doi: 10.1017/s0954579400004089. [DOI] [PubMed] [Google Scholar]
- 108.McCusker CG, Doherty NN, Molloy B, et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Archives of disease in childhood. 2007;92(2):137–141. doi: 10.1136/adc.2005.092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hynan MT, Steinberg Z, Baker L, et al. Recommendations for mental health professionals in the NICU. J Perinatol. 2015;35(Suppl 1):S14–18. doi: 10.1038/jp.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simeone S, Pucciarelli G, Perrone M, et al. Comparative Analysis: Implementation of a Pre-operative Educational Intervention to Decrease Anxiety Among Parents of Children With Congenital Heart Disease. J Pediatr Nurs. 2017;35:144–148. doi: 10.1016/j.pedn.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 111.McCusker CG, Doherty NN, Molloy B, et al. A controlled trial of early interventions to promote maternal adjustment and development in infants born with severe congenital heart disease. Child: care, health and development. 2010;36(1):110–117. doi: 10.1111/j.1365-2214.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 112.Golfenshtein N, Deatrick JA, Lisanti AJ, Medoff-Cooper B. Coping with the Stress in the Cardiac Intensive Care Unit: Can Mindfulness Be the Answer? J Pediatr Nurs. 2017;37:117–126. doi: 10.1016/j.pedn.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 113.Lion KC, Raphael JL. Partnering health disparities research with quality improvement science in pediatrics. Pediatrics. 2015;135(2):354–361. doi: 10.1542/peds.2014-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ylmaz HB, Kavlak O, Isler A, Liman T, Van Sell SL. A Study of Maternal Attachment Among Mothers of Infants With Congenital Anomalies in Turkey. Infants & Young Children. 2011;24(3):259–266. [Google Scholar]
- 115.Sikora K, Janusz B. Maternal bond with cardiosurgically treated infant. Qualitative analysis of mothers' narratives. Dev Period Med. 2014;18(4):439–446. [PubMed] [Google Scholar]
- 116.Boztepe H, Ay A, Kerimoglu Yildiz G, Cinar S. Does the visibility of a congenital anomaly affect maternal-infant attachment levels? Journal for specialists in pediatric nursing : JSPN. 2016;21(4):200–211. doi: 10.1111/jspn.12157. [DOI] [PubMed] [Google Scholar]
- 117.Goldberg S, Simmons RJ, Newman J, Campbell K, Fowler RS. Congenital heart disease, parental stress, and infant-mother relationships. J Pediatr. 1991;119(4):661–666. doi: 10.1016/s0022-3476(05)82425-4. [DOI] [PubMed] [Google Scholar]
- 118.Fuchs A, Mohler E, Reck C, Resch F, Kaess M. The Early Mother-to-Child Bond and Its Unique Prospective Contribution to Child Behavior Evaluated by Mothers and Teachers. Psychopathology. 2016;49(4):211–216. doi: 10.1159/000445439. [DOI] [PubMed] [Google Scholar]
- 119.Treyvaud K, Anderson VA, Howard K, et al. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics. 2009;123(2):555–561. doi: 10.1542/peds.2008-0477. [DOI] [PubMed] [Google Scholar]
- 120.Berant E, Mikulincer M, Shaver PR. Mothers' attachment style, their mental health, and their children's emotional vulnerabilities: a 7-year study of children with congenital heart disease. J Pers. 2008;76(1):31–65. doi: 10.1111/j.1467-6494.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- 121.Enlow E, Faherty LJ, Wallace-Keeshen S. Perspectives of Low Socioeconomic Status Mothers of Premature Infants. Pediatrics. 2017;139(3):1–8. doi: 10.1542/peds.2016-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taylor JY, Washington OG, Artinian NT, Lichtenberg P. Parental stress among African American parents and grandparents. Issues in Mental Health Nursing. 2007;28(4):373–387. doi: 10.1080/01612840701244466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shah R, Sobotka SA, Yi-Fan C, Msall ME. Positive Parenting Practices, Health Disparities, and Developmental Progress. Pediatrics. 2015;136(2):318–326. doi: 10.1542/peds.2014-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jacobs R, Boyd L, Brennan K, Sinha CK, Giuliani S. The importance of social media for patients and families affected by congenital anomalies: A Facebook cross-sectional analysis and user survey. J Pediatr Surg. 2016;51(11):1766–1771. doi: 10.1016/j.jpedsurg.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 125.Agrawal H, Wright OK, Carberry KE, Sexson Tejtel SK, Mery CM, Molossi S. Family perception of unmet support needs following a diagnosis of congenital coronary anomaly in children: Results of a survey. Congenital heart disease. 2017;12(6):721–725. doi: 10.1111/chd.12473. [DOI] [PubMed] [Google Scholar]
- 126.Davis MA, Anthony DL, Pauls SD. Seeking and receiving social support on Facebook for surgery. Soc Sci Med. 2015;131:40–47. doi: 10.1016/j.socscimed.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]