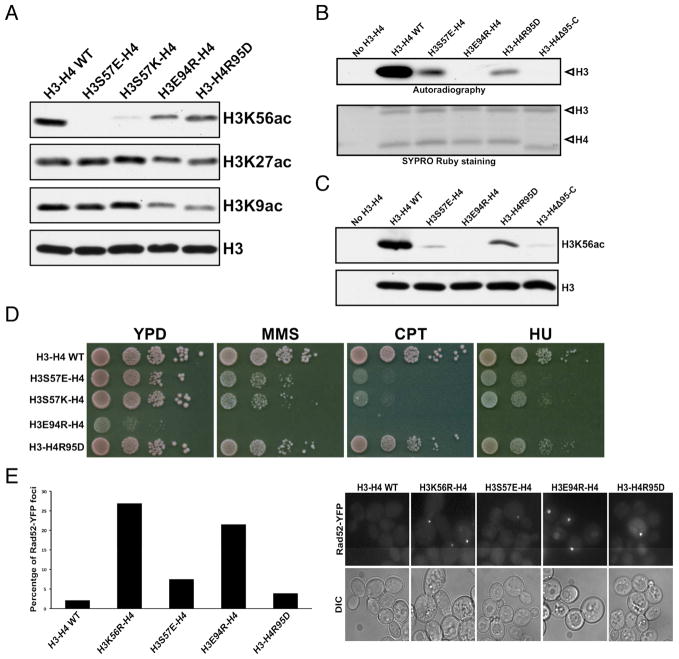
Figure 6. Histone residues important for H3K56 acetylation.
(A) Western blot analyses of impacts on H3K56, H3K27 and H3K9 acetylation by indicated histone mutations in budding yeast cells. (B) Autoradiography detection of H3 acetylation in an in vitro HAT assay using purified wild-type AfRtt109, and the indicated mutant histone-AfAsf1 complexes. The bottom panel: SYPRO Ruby staining of the histones used. (C) H3K56 acetylation of wild-type or histone mutants by AfRtt109 in vitro in the presence of AfAsf1 detected by Western blot. (D) Cell growth analysis of budding yeast cells expressing the indicated histone mutants in the absence and presence of the denoted DNA-damaging agents. (E) Spontaneous chromosome breaks in budding yeast cells expressing indicated histone mutants determined by the Rad52-YFP foci. Left panel: percentage of cells with Rad52-YFP foci from two independent experiments. Right panel: representative fields of live cell images. See also Figure S6; Tables S2, S3.