Abstract
The pathogenesis of degenerative disc disease is a complex and multifactorial process in which genetics, mechanical trauma, altered loading and nutrition present significant etiological factors. Infection of the intervertebral disc with the anaerobic bacterium Propionibacterium acnes is now also emerging as a potentially new etiological factor. This human commensal bacterium is well known for its long association with the inflammatory skin condition acne vulgaris. A key component of inflammatory responses to P. acnes in acne appears to be interleukin (IL)-1β. Similarly, in degenerative disc disease (DDD) there is compelling evidence for the fundamental roles of IL-1β in its pathology. We therefore propose that P. acnes involvement in DDD is biologically very plausible, and that IL-1β is the key inflammatory mechanism driving the host response to P. acnes infection. Since there is a solid theoretical basis for this phenomenon, we further propose that the relationship between P. acnes infection and DDD is causal.
Keywords: Propionibacterium acnes, interleukin-1 beta, nerve growth factor (NGF), degenerative disc disease, acne vulgaris
The clinical problem and epidemiological evidence of Propionibacterium acnes in degenerative disc disease
Chronic low back pain (CLBP) is the leading cause of disability/worker's compensation claims, second leading cause for patient-doctor visits, the third most common cause of surgical procedures, and the fifth-ranking cause of admission to hospital (Hart et al., 1995). Although many conditions lead to CLBP, degenerative disc disease (DDD) is among the most common diagnoses. Because correlations among clinical symptoms, radiological signs of disc degeneration, and treatment outcomes are not satisfactory, the selection of treatment options is difficult and the options employed often yield less than satisfactory results. Clearly, a more detailed understanding of the causes and development of DDD is needed so patients can be stratified for tailored or personalized treatments where possible.
The identification of Propionibacterium acnes biofilms in intervertebral disc tissue obtained from patients who have undergone microdiscectomy, combined with previous studies demonstrating the success of antibiotic treatment for patients with CLBP (Albert et al., 2013), has led to the suggestion that P. acnes infection of the intervertebral disc may be another important causative factor for the development of DDD. The mechanisms by which P. acnes infection may lead to DDD have, however, yet to be elucidated. Here, we propose a model of P. acnes pathogenicity in DDD in which patients may benefit from biomarker-directed anti-inflammatory interventions, such as already available biologic drugs or small molecule inhibitors that could be re-purposed for a more precision-based approach to treatment, in combination with antibiotic or other antimicrobial treatments.
The seminal, potentially paradigm-shifting study that first provided evidence to link P. acnes infection of the degenerated disc to CLBP was published by Stirling et al. (2001). Since then, the role of this bacterium in CLBP has received considerable attention, as well as controversy, due to the possibility of novel treatment options for this debilitating condition (Birkenmaier, 2013). Subsequent independent research into this exciting area has been collectively examined in two independent meta-analyses published in 2015. These studies reported that the pooled prevalence of bacterial infection was 34% (Urquhart et al., 2015) or 36.2% (Ganko et al., 2015), and confirmed P. acnes as the major bacterial species present in retrieved disc samples. Very recently, P. acnes was further identified as the only significant bacterial organism present in degenerated disc tissue obtained from 32% of 368 patients undergoing microdiscectomy (Capoor et al., 2017). Importantly, this study also provided the first microscopic evidence of P. acnes biofilm within degenerated disc tissue as shown in Figures 1, 2 (Capoor et al., 2017). The presence of biofilm, which is consistent with colonization/ infection rather than perioperative contamination, which is a key concern when dealing with an organism which also forms part of the endogenous microbiota, provided compelling evidence that at least some of the culture-positive degenerated disc tissue had truly become infected with P. acnes (Capoor et al., 2017). Both rabbit and rat models have also shown that P. acnes infection of disc tissue can induce disc degeneration, inflammatory response in the endplate region and MC1-like changes in the adjacent bone marrow (Dudli et al., 2016; Shan et al., 2017).
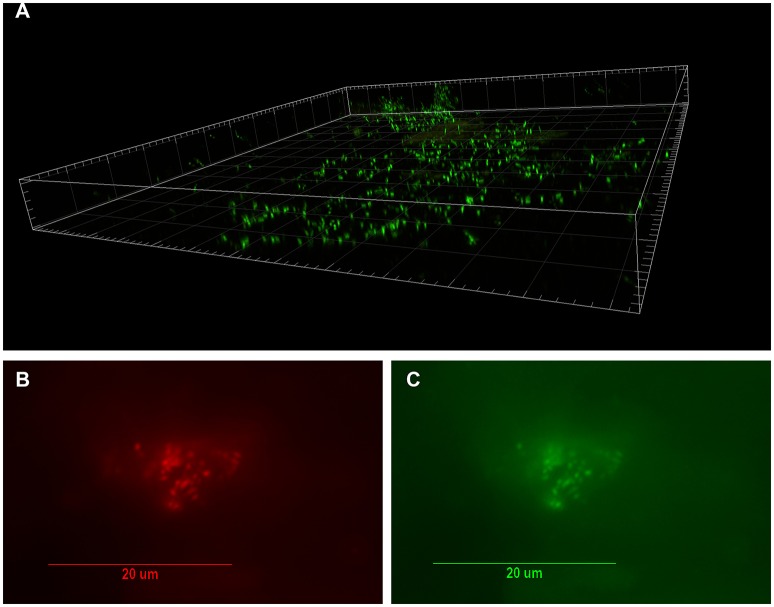
Figure 1.
Visualization of bacterial biofilm in the disc tissue by confocal laser scanning microscopy (CSLM) and confirmation of P. acnes by fluorescence in-situ hybridization (FISH) (reprinted from Capoor et al., 2017). (A) Three dimensional reconstructed CSLM image of biofilm bacteria stained with a DNA stain (SYTO9, green) in a disc tissue sample. (B,C) The presence of P. acnes biofilms in this sample verified using FISH. Epifluorescence micrographs of a biofilm cluster showing red fluorescence from the CY5-labeled EUB338 general eubacterial probe (B) and green fluorescence from the CY3-labled P. acnes-specific probe (C) Co-localization of the red and green fluorescence indicates that all of the bacteria in this biofilm were P. acnes.
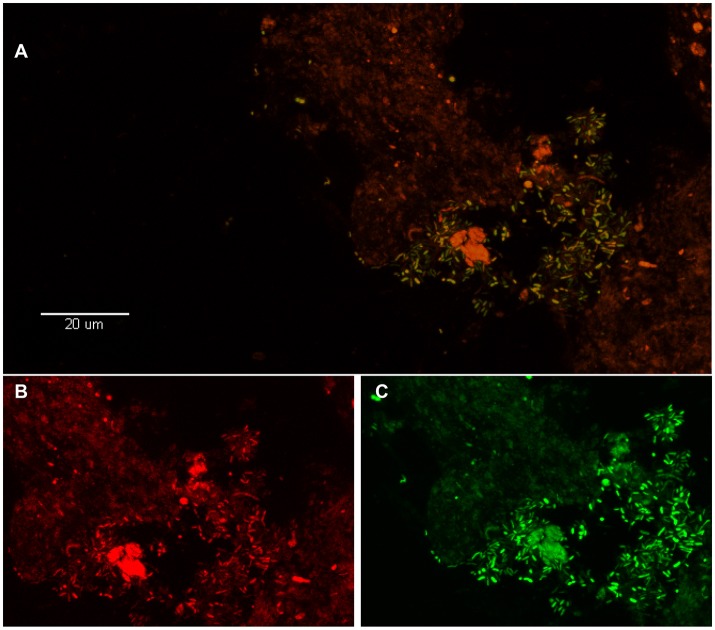
Figure 2.
Visualization of P. acnes biofilm in the disc tissue by use of FISH (reprinted from Capoor et al., 2017). (A) This color-combined image shows the “pocket” of green fluorescent P. acnes cells (biofilm) near the center right of the image. The presence of P. acnes biofilms in this sample was verified using FISH. (B,C) Red fluorescence is the general eubacterial probe (B) and green is the P. acnes probe (C). The (B,C) image is a zoom of (A) showing fluorescence from the red and green channels separately. Almost all of the cells in (A) are emitting both red and green fluorescence, indicating that they are P. acnes.
A key role for IL-1β in P. acnes driven acne vulgaris
The involvement of P. acnes in acne was first suggested as early as 1896 when Unna isolated the bacterium from an acne lesion (Unna, 1895). Even since then, the exact role the organism plays in the initiation and progression of this chronic inflammatory skin condition remains unclear, especially as some inflamed lesions show no evidence of viable bacterial colonization and no correlation exists between bacterial counts and severity of inflammation (Shaheen and Gonzalez, 2011). Acne samples do, however, demonstrate a higher prevalence of follicular P. acnes colonization, both in terms of follicles containing P. acnes and the greater numbers of bacteria in macrocolonies/biofilms than in control samples (Jahns et al., 2012). The strong inflammatory potential of P. acnes is well known, and the bacterium can upregulate a wide range of inflammatory cytokines (e.g., TNFα, IL-6, IL-8, IL-1 family) and activate keratinocytes and sebocytes via CD1, CD14, and Toll-like receptors (TLRs) (Moradi Tuchayi et al., 2015). The bacterium can also induce the expression of IL-17 from peripheral blood mononuclear cells and promote Th17 and Th1 response pathways (Agak et al., 2014); activation of the Th17 axis and Th17 cytokines (IL-17, IL-1β, IL-6, and TGF-β) appears to play a pivotal role in acne (Sardana and Verma, 2017). Furthermore, the organism produces lipases which facilitate breakdown of the sebum (carbon source) into free short chain fatty acids which are pro-inflammatory (Sanford et al., 2016). The critical role of the IL-1 family of cytokines in the pathogenesis of acne vulgaris, especially IL-1β which is abundant in acneic lesions, has now been well described (Kistowska et al., 2014). This strongly pro-inflammatory cytokine has been associated with IL-17 production highlighting the strong link between IL-1β and IL-17 immunity (Lasigliè et al., 2011).
The induction of IL-1β is believed to be a key driver of inflammatory responses in acne and is mediated via inflammasome activation (Kistowska et al., 2014). We can easily envisage, therefore, how P. acnes pathogen-associated molecular patterns (PAMPs) will bind to TLR2 on sebocytes (Li et al., 2014) and myeloid cells (Kistowska et al., 2014), activating NF-κB signaling and consequently transcription of the IL-1β gene, thus leading to the production of pro-IL-1β within the pilosebaceous unit. This initial interaction between P. acnes and myeloid cells can occur either by migration of the latter into the follicular space or by leakage of P. acnes into the peri-follicular space. Myeloid cells and sebocytes constitutively express inflammasome proteins. P. acnes PAMPs trigger NLRP3 inflammasome activation, resulting in increased caspase 1 activity, facilitated cleavage of pro-IL-1β, and excessive release of mature IL-1β. Furthermore, IL-1β promotes dermal matrix destruction by inducing proteolytic matrix metalloproteases (MMPs). Progression of the inflammation results in P. acnes overgrowth and follicular obstruction, which provokes follicular rupture. P. acnes can then leak out of the pilosebaceous unit and get into close contact with the peri-follicular myeloid cells, finally leading to excessive release of IL-1β and neutrophil-rich peri-follicular inflammation.
A key role for IL-1β in degenerative disc disease
Disc degeneration is also known to be mediated by the abnormal secretion of pro-inflammatory cytokines by the inner nucleus pulposus (NP) and outer annulus fibrosus (AF) cells of the intervertebral disc, as well as by immune cells attracted to the site of disc degeneration. These cytokines, including IL-1α, IL-1β, IL-6, IL-8, IL-17, TNF-α and IFN-γ, serve to promote a range of pathogenic responses by disc cells including matrix breakdown and immune cell recruitment (Risbud and Shapiro, 2014). As with acne vulgaris, IL-1β appears to play a key role in disc degeneration, including matrix destruction, angiogenesis and cellular apoptosis and senescence (Yang et al., 2015). Levels of the cytokine in degenerated discs also appear to correlate with the degree of degeneration (Le Maitre et al., 2005). Also, IL-17 expression is increased in degenerated discs as well as intervertebral disc cells exposed to IL-1β (Le Maitre et al., 2005; Yang et al., 2015). As in acne, we see an infiltration of CD68+ macrophages, neutrophils and T cells (CD4+; CD8+) in herniated discs (Risbud and Shapiro, 2014).
Irrespective of the initiating factor in DDD, it is thought to trigger NF-κB signaling through TLRs, which stimulates the production of pro-IL-1β (Risbud and Shapiro, 2014). Increased levels of NLRP3 and caspase-1 have also been described in degenerated disc tissue, indicating inflammasome activation and facilitated maturation of pro-IL-1β (Chen et al., 2015). Inflammatory actions are further amplified because IL-1β is not only an NF-κB target gene, but also an NF-κB activator, forming a positive feedback loop (Chen et al., 2015). This IL-1β/IL-1 receptor (IL-1R) signaling promotes extracellular matrix degradation through the induction of proteolytic enzymes, including MMPs 1, 2, 3, 9, and 13 and aggrecanases of “a disintegrin and metalloproteinase with thrombospondin motifs' (ADAMTS) families 4/5 (Le Maitre et al., 2007). Specific damage-associated molecular patterns (DAMPs), which include fragmented collagen, aggrecan, or hyaluronic acid, bind to TLRs on NP and AF cells and activate NF-κB, forming another positive feedback loop (Quero et al., 2013). IL-1β also promotes the production of chemotactic CC-chemokines, mainly CCL3 and CCL4, leading to the recruitment and activation of infiltrating immune cells that further amplify the inflammatory cascade (Wang et al., 2013). These pathways form the core of a model in which P. acnes infection causes IL-1β release by NP, AF and immune cells, which leads to extracellular matrix degradation within the intervertebral disc (Nazipi et al., 2017).
The ingrowth of nociceptive nerve fibers into the degenerated disc, usually accompanied by the presence of annular fissures, is considered a main source of nociception related to CLBP (Freemont et al., 1997). IL-1β has been shown to induce the expression of neurotrophin-like nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in both disc and immune cells, supporting nerve ingrowth into the degenerated disc (Gruber et al., 2012). IL-1β-stimulated NGF and BDNF production further induces expression of pain-associated cation channels in the dorsal root ganglion (Risbud and Shapiro, 2014), the depolarization of which is likely to promote low back and radicular pain. Finally, NGF has a direct activating or sensitizing effect on nociceptors, and its upregulation in CLBP has been demonstrated (Denk et al., 2017).
Hypothesis: the central role of IL-1β in DDD is evidence of a P. acnes-driven etiology
Based on the (i) compelling epidemiological evidence (Stirling et al., 2001; Urquhart et al., 2015; Capoor et al., 2016, 2017; Coscia et al., 2016), (ii) the uniqueness of P. acnes in the degenerated disc tissue (Capoor et al., 2017), (iii) direct microscopic evidence of P. acnes biofilm in degenerated disc tissue, as shown in Figures 1, 2 (Capoor et al., 2017), (iv) experimental data showing P. acnes induces disc degeneration in animal models (Dudli et al., 2016; Shan et al., 2017), and (v) the shared central role of IL-1β within acne vulgaris and DDD (Le Maitre et al., 2005; Wuertz and Haglund, 2013), we hypothesize, that a role for P. acnes in the etiology of DDD is biologically plausible, with IL-1β expression representing the driving mechanism behind its pathogenesis.
As shown in Figure 3, P. acnes can affect the initiation and/or progression of DDD by stimulating IL-1β synthesis through several potential routes: (i) P. acnes infection can promote IL-1β production by AF and NP cells through a direct interaction between P. acnes-specific PAMPs and TLR2 on the surfaces of NP and AF cells, which activates NF-κB signaling and leads to the production of pro-IL-1β; (ii) hyaluronidase released by P. acnes cleaves hyaluronic acid within the disc matrix to form short fragments with ability to activate NF-κB signaling leading to production of pro-IL-1β; (iii) P. acnes-specific PAMPs can also activate NLRP3-inflammasome and caspase-1, which can convert pro-IL-1β to mature IL-1β; (iv) the IL-1β produced through the action of P. acnes can amplify inflammatory signaling through interaction with IL-1R, which creates a positive feedback loop; and (v) the IL-1β produced can further unleash signaling pathways that lead to the production of MMPs and ADAMTS, which degrade the extracellular matrix, producing P. acnes-related DAMPs that can interact with AF, NP, and myeloid cells to further activate NF-κB signaling and produce a second positive feedback loop.
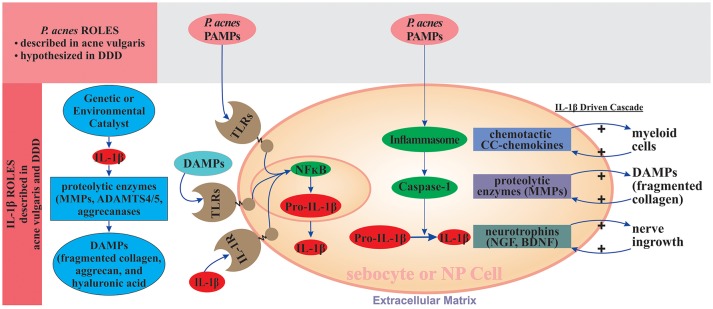
Figure 3.
IL-1β driven degenerative state in acne vulgaris and degenerative disc disease. Following the initiating event, sebocytes and NP and AF cells promote production of IL-1β. IL-1β signaling facilitates extracellular matrix degradation through induction of proteolytic enzymes, including MMPs and ADAMTS4/5. P. acnes PAMPs and extracellular matrix degradation products DAMPs (e.g., fragmented collagen, aggrecan, and hyaluronic acid) bind to TLRs, which activate NF-κB, leading to the synthesis of pro-IL-1β; P. acnes PAMPs also activate inflammasomes (NLRP3) and caspase-1, which convert pro-IL-1β to IL-1β. IL-1β stimulates production of CC-chemokines that recruit myeloid cells and proteolytic matrix metalloproteases (MMPs) that degrade the ECM. Positive feedback loops arise when IL-1β binds to IL-1R and when extracellular matrix degradation products (DAMPs, e.g., fragmented collagen) activate TLRs. Both binding events stimulate IL-1β production. Inflammatory signaling is amplified since IL-1β is not only NF-κB target gene, but also its activator through binding to IL-R1. The nerve ingrowth into the degenerated disc, usually accompanied by presence of the annular fissures eventually herniation, is considered a main source of nociception and thus low back and/or radicular pain. IL-1β induces the expression of neurotrophins like NGF and BDNF supporting the nerve. ADAMTS-4/5, a disintegrin and metalloproteinase with thrombospondin motifs 4/5; AF, annulus fibrosus; BDNF, brain-derived neurotrophic factor; DAMPs, damage-associated molecular patterns; PAMPs, pathogen-associated molecular patterns; DDD, degenerative disc disease; ECM, extracellular matrix; IL-1R, interleukin-1 receptor; MMPs, matrix metalloproteinases; NF-κB, nuclear factor κB; NGF, nerve growth factor; NP, nucleus pulposus; TLRs, Toll-like receptors.
Since there are several independent routes by which P. acnes can promote the synthesis of IL-1β, and there is a positive feedback loop in the regulation of IL-1β levels, we propose that P. acnes is a strong contributor to IL-1β-based pathogenetic mechanisms that lead to DDD. We further hypothesize that if there are other etiological factors contributing to DDD through the promotion of IL-1β, P. acnes significantly amplifies the significance of this pre-existing IL-1β action and facilitates the pathogenesis in synergy with other etiological factors of DDD.
Furthermore, we propose that in some patients where no P. acnes is detected directly by culture- or non-culture methods within disc material, the central role of IL-1β may still be a “smoking gun” for an infection that may no longer be present but has created a state of chronic inflammation and bone destruction that still persists in some form.
Finally, complex inherited alterations in the inflammasome/ IL-1β system may fine-tune its functional capacity and cause an imbalance of pro- and anti-inflammatory responses that determine the individual risk, time of onset, and severity of P. acnes-associated DDD.
Testing the hypothesis and implications for health care provision
Since there is strong experimental evidence of IL-1β involvement in the pathogenesis of DDD (Wuertz and Haglund, 2013; Risbud and Shapiro, 2014), our aim here is to propose experimental approaches to assess the ability and mechanism by which P. acnes promotes IL-1β synthesis and function, and not to prove its effects on DDD pathogenesis per se. NP and AF cells have been shown to express TLR2 (Le Maitre et al., 2005), and there is evidence of P. acnes interaction with TLR2 (Kistowska et al., 2014; Li et al., 2014). Therefore, the ability of P. acnes to induce IL-1β should be demonstrated by monitoring IL-1β levels in NP and AF cell cultures and in vivo models of DDD that have been infected with P. acnes strains isolated from degenerated human discs, and covering different phylogenetic groups that are known to vary in their inflammatory nature as well as other pathogenic characteristics (McDowell et al., 2013). Since P. acnes has been shown to form biofilm in DDD, its ability to promote IL-1β production should be compared with heavy biofilm formers and isolates that fail to actively form biofilms (Holmberg et al., 2009). Finally, clinical samples of degenerated disc tissue shown to be infected with P. acnes should be associated with IL-1β levels higher than those seen with non-infected degenerated discs.
The ability of P. acnes to activate TLR2 signaling in myeloid cells, especially macrophages, has been already demonstrated in acne vulgaris confirming another route of P. acnes-associated IL-1β production. Finally, short fragments of hyaluronic acid have been shown to interact with TLR2 present on NP cells (Quero et al., 2013), providing evidence for the P. acnes-associated IL-1β production, through hyaluronidase secretion. To provide a definitive test of the hypothesis that P. acnes is a strong contributor to IL-1β-based DDD pathogenesis, in vitro and in vivo experiments will be performed to quantitatively compare the ability of P. acnes to induce IL-1β with that of other known activators of IL-1β commonly used in DDD.
To identify the concrete mechanisms affecting the propensity of P. acnes to activate the various routes of IL-1β induction, the following experimental approaches could be used in vitro and in animal models: (i) a series of knock-in and knock-down experiments of the critically important genes linked to various mechanisms of IL-1β induction and functioning (e.g., TLR2, NLR3, MyD88 or caspase-1), (ii) experiments with a panel of inhibitors specifically affecting signaling pathways associated with the complex cascade of IL-1β processing and function, and finally (iii) experiments with models for which the signaling ligands are naturally or experimentally altered.
Evidence that P. acnes infection may incite a chronic inflammatory response that persists after the organism is no longer present could be investigated via serological studies and analysis of samples for bacterial products and signature metabolites that could still be perpetuating inflammation (Magnitsky et al., 2017), or via a series of model infections in which animals will be treated for P. acnes with antibiotics over a wide range of time-points post-infection and the grade of disc degeneration compared between the groups. A prior history of moderate-to-severe acne vulgaris may also be important in this context as has been shown in relation to the potential role of P. acnes in prostate cancer (Ugge et al., 2018).
Even if the hypothesis put forward here turns out to be incorrect or only partially correct, it will at least have served to stimulate more research on the potential role of P. acnes in DDD, which is vitally important because of the potential benefits for patients and wider society.
The main clinical presentation of DDD is chronic low back pain (CLBP). CLBP is one of the world's most debilitating conditions, presenting with substantial socio-economic and health-care consequences (Vos et al., 2012). Based on our theoretical concept, the causal therapy for patients with DDD associated with P. acnes infection is appropriate antibiotic treatment. However, even if P. acnes infection is successfully eradicated by antibiotic treatment, ongoing inflammatory processes with IL-1β playing the central role, along with other etiological factors, can cause treatment failure.
Since anti-IL-1β drugs have already been approved for the treatment of inflammatory and autoinflammatory diseases (Dinarello et al., 2012), therapeutic approaches targeting IL-1β in combination with appropriate antibiotic treatment, concomitantly or in sequence, should be considered as a novel treatment approach. Inhibition of IL-1β signaling can be via direct targeting of the molecule with biologics or via pro-drug inhibitors of the inflammasome that target caspase-1 or NLRP3 (Risbud and Shapiro, 2014). As an exemplar of this approach, treatment of patients with acne using the humanized anti-IL-1β antibody Gevokizumab demonstrated significant clinical response with reductions in inflammatory lesions (Fenini et al., 2017). While acknowledging the complicated nature of DDD, and the potential for different drivers of inflammation, there is already promising experimental evidence of the ability of IL-1β-targeted approaches to affect pathogenic mechanisms of the condition both in vitro and in vivo, promoting ECM repair and inhibiting disc degeneration (Gorth et al., 2012).
The prerequisite for successful CLBP treatment is an accurate diagnosis. From this perspective, diagnosis of P. acnes infection and direct evidence of IL-1β over-expression present a strategy to rationalize a more tailored treatment and management strategy for patients with DDD, which is essential in a growing era of precision/ stratified medicine.
Author contributions
OS and MC conceptualized the hypothesis and drafted the initial manuscript. AM, HB, AR, SD-D, TF, and PL all contributed to writing and editing of the subsequent drafts and final version of the manuscript.
Conflict of interest statement
MC, OS, and AR have stock ownership or options in DiscitisDx, Inc. MC and OS have filed several patent applications, which have been assigned to DiscitisDx, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Agak G. W., Qin M., Nobe J., Kim M. H., Krutzik S. R., Tristan G. R., et al. (2014). Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J. Invest. Dermatol. 134, 366–373. 10.1038/jid.2013.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert H. B., Sorensen J. S., Christensen B. S., Manniche C. (2013). Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur. Spine J. 22, 697–707. 10.1007/s00586-013-2675-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmaier C. (2013). Should we start treating chronic low back pain with antibiotics rather than with pain medications? Korean J. Pain 26, 327–335. 10.3344/kjp.2013.26.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoor M. N., Ruzicka F., Machackova T., Jancalek R., Smrcka M., Schmitz J. E., et al. (2016). Prevalence of Propionibacterium acnes in intervertebral discs of patients undergoing lumbar microdiscectomy: a prospective cross-sectional study. PLoS ONE 11:e0161676. 10.1371/journal.pone.0161676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoor M. N., Ruzicka F., Schmitz J. E., James G. A., Machackova T., Jancalek R., et al. (2017). Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS ONE 12:e0174518. 10.1371/journal.pone.0174518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. H., Jin S. H., Wang M. Y., Jin X. L., Lv C., Deng Y. F., et al. (2015). Enhanced NLRP3, caspase-1, and IL- 1beta levels in degenerate human intervertebral disc and their association with the grades of disc degeneration. Anat. Rec. 298, 720–726. 10.1002/ar.23059 [DOI] [PubMed] [Google Scholar]
- Coscia M. F., Denys G. A., Wack M. F. (2016). Propionibacterium acnes, coagulase-negative Staphylococcus, and the “Biofilm-like” intervertebral disc. Spine 41, 1860–1865. 10.1097/BRS.0000000000001909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk F., Bennett D. L., McMahon S. B. (2017). Nerve growth factor and pain mechanisms. Annu. Rev. Neurosci. 40, 307–325. 10.1146/annurev-neuro-072116-031121 [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Simon A., van der Meer J. W. (2012). Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652. 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudli S., Liebenberg E., Magnitsky S., Miller S., Demir-Deviren S., Lotz J. C. (2016). Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J. Orthop. Res. 34, 1447–1455. 10.1002/jor.23265 [DOI] [PubMed] [Google Scholar]
- Fenini G., Contassot E., French L. E. (2017). Potential of IL-1, IL-18 and inflammasome inhibition for the treatment of inflammatory skin Diseases. Front. Pharmacol. 8:278. 10.3389/fphar.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J., Peacock T. E., Goupille P., Hoyland J. A., O'Brien J., Jayson M. I. (1997). Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 350, 178–181. [DOI] [PubMed] [Google Scholar]
- Ganko R., Rao P. J., Phan K., Mobbs R. J. (2015). Can bacterial infection by low virulent organisms be a plausible cause for symptomatic disc degeneration? A systematic review. Spine 40, E587–E592. 10.1097/BRS.0000000000000832 [DOI] [PubMed] [Google Scholar]
- Gorth D. J., Mauck R. L., Chiaro J. A., Mohanraj B., Hebela N. M., Dodge G. R., et al. (2012). IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1beta-mediated degradation of nucleus pulposus in vitro. Arthritis Res. Ther. 14:R179. 10.1186/ar3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber H. E., Hoelscher G. L., Bethea S., Hanley E. N., Jr. (2012). Interleukin 1-beta upregulates brain-derived neurotrophic factor, neurotrophin 3 and neuropilin 2 gene expression and NGF production in annulus cells. Biotech. Histochem. 87, 506–511. 10.3109/10520295.2012.703692 [DOI] [PubMed] [Google Scholar]
- Hart L. G., Deyo R. A., Cherkin D. C. (1995). Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine 20, 11–19. 10.1097/00007632-199501000-00003 [DOI] [PubMed] [Google Scholar]
- Holmberg A., Lood R., Mörgelin M., Söderquist B., Holst E., Collin M., et al. (2009). Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin. Microbiol. Infect. 15, 787–795. 10.1111/j.1469-0691.2009.02747.x [DOI] [PubMed] [Google Scholar]
- Jahns A. C., Lundskog B., Ganceviciene R., Palmer R. H., Golovleva I., Zouboulis C. C., et al. (2012). An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br. J. Dermatol. 167, 50–58. 10.1111/j.1365-2133.2012.10897.x [DOI] [PubMed] [Google Scholar]
- Kistowska M., Gehrke S., Jankovic D., Kerl K., Fettelschoss A., Feldmeyer L., et al. (2014). IL-1beta drives inflammatory responses to propionibacterium acnes in vitro and in vivo. J. Invest. Dermatol. 134, 677–685. 10.1038/jid.2013.438 [DOI] [PubMed] [Google Scholar]
- Lasigliè D., Traggiai E., Federici S., Alessio M., Buoncompagni A., Accogli A., et al. (2011). Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS ONE 6:e20014. 10.1371/journal.pone.0020014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre C. L., Freemont A. J., Hoyland J. A. (2005). The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 7, R732–R745. 10.1186/ar1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre C. L., Hoyland J. A., Freemont A. J. (2007). Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res. Ther. 9:R83. 10.1186/ar2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. J., Choi D. K., Sohn K. C., Seo M. S., Lee H. E., Lee Y., et al. (2014). Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J. Invest. Dermatol. 134, 2747–2756. 10.1038/jid.2014.221 [DOI] [PubMed] [Google Scholar]
- Magnitsky S., Dudli S., Tang X., Kaur J., Diaz J., Miller S., et al. (2017). Quantification of propionic acid in the bovine spinal disk after infection of the tissue with P. Acnes bacteria. Spine 43, E634–E638. 10.1097/BRS.0000000000002448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell A., Nagy I., Magyari M., Barnard E., Patrick S. (2013). The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS ONE 8:e70897. 10.1371/journal.pone.0070897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi Tuchayi S., Makrantonaki E., Ganceviciene R., Dessinioti C., Feldman S. R., Zouboulis C. C. (2015). Acne vulgaris. Nat. Rev. Dis. Primers 1:15029. 10.1038/nrdp.2015.29 [DOI] [PubMed] [Google Scholar]
- Nazipi S., Stødkilde-Jørgensen K., Scavenius C., Bruggemann H. (2017). The Skin bacterium Propionibacterium acnes employs two variants of hyaluronate lyase with distinct properties. Microorganisms 5:57. 10.3390/microorganisms5030057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quero L., Klawitter M., Schmaus A., Rothley M., Sleeman J., Tiaden A. N., et al. (2013). Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways. Arthritis Res. Ther. 15:R94. 10.1186/ar4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud M. V., Shapiro I. M. (2014). Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 10, 44–56. 10.1038/nrrheum.2013.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford J. A., Zhang L. J., Williams M. R., Gangoiti J. A., Huang C. M., Gallo R. L. (2016). Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. 1:eaah4609. 10.1126/sciimmunol.aah4609 [DOI] [PubMed] [Google Scholar]
- Sardana K., Verma G. (2017). Propionibacterium acnes and the Th1/Th17 axis, implications in acne pathogenesis and treatment. Indian J. Dermatol. 62, 392–394. 10.4103/ijd.IJD_483_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen B., Gonzalez M. (2011). A microbial aetiology of acne: what is the evidence? Br. J. Dermatol. 165, 474–485. 10.1111/j.1365-2133.2011.10375.x [DOI] [PubMed] [Google Scholar]
- Shan Z., Zhang X., Li S., Yu T., Liu J., Zhao F. (2017). Propionibacterium acnes incubation in the discs can result in time-dependent modic changes: a long-term rabbit model. Spine 42, 1595–1603. 10.1097/BRS.0000000000002192 [DOI] [PubMed] [Google Scholar]
- Stirling A., Worthington T., Rafiq M., Lambert P. A., Elliott T. S. (2001). Association between sciatica and Propionibacterium acnes. Lancet 357, 2024–2025. 10.1016/S0140-6736(00)05109-6 [DOI] [PubMed] [Google Scholar]
- Ugge H., Udumyan R., Carlsson J., Andrén O., Montgomery S., Davidsson S., et al. (2018). Acne in late adolescence and risk of prostate cancer. Int. J. Cancer 142, 1580–1585. 10.1002/ijc.31192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unna P. G. (1895). The histological pathology of diseases of the skin. Br. J. Dermatol. 7, 83–85. 10.1111/j.1365-2133.1895.tb16839.x [DOI] [Google Scholar]
- Urquhart D. M., Zheng Y., Cheng A. C., Rosenfeld J. V., Chan P., Liew S., et al. (2015). Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med. 13:13. 10.1186/s12916-015-0267-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Flaxman A. D., Naghavi M., Lozano R., Michaud C., Ezzati M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tian Y., Phillips K. L., Chiverton N., Haddock G., Bunning R. A., et al. (2013). Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 65, 832–842. 10.1002/art.37819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuertz K., Haglund L. (2013). Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine J. 3, 175–184. 10.1055/s-0033-1347299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Yu X. H., Wang C., He W. S., Zhang S. J., Yan Y. G., et al. (2015). Interleukin-1beta in intervertebral disk degeneration. Clin. Chim. Acta 450, 262–272. 10.1016/j.cca.2015.08.029 [DOI] [PubMed] [Google Scholar]