Abstract
Chemotherapeutic agents used to treat acute lymphoblastic leukemia (ALL), the most common cancer affecting young children, have been associated with long-term cognitive impairments that reduce quality of life. Executive dysfunction is one of the most consistently observed deficits and can have substantial and pervasive effects on academic success, occupational achievement, psychosocial function, and psychiatric status. We examined the neural mechanisms of executive dysfunction by measuring structural and functional connectomes in 161 long-term survivors of pediatric ALL, age 8–21 years, who were treated on a single contemporary chemotherapy-only protocol for standard/high- or low-risk disease. Lower global efficiency, a measure of information exchange and network integration, of both structural and functional connectomes was found in survivors with executive dysfunction compared with those without dysfunction (p < 0.046). Patients with standard/high- versus low-risk disease and those who received greater number of intrathecal treatments containing methotrexate had the lowest network efficiencies. Patients with executive dysfunction also showed hyperconnectivity in sensorimotor, visual, and auditory-processing regions (p = 0.037) and poor separation between sensorimotor, executive/attention, salience, and default mode networks (p < 0.0001). Connectome disruption was consistent with a pattern of delayed neurodevelopment that may be associated with reduced resilience, adaptability, and flexibility of the brain network. These findings highlight the need for interventions that will prevent or manage cognitive impairment in survivors of pediatric acute lymphoblastic leukemia.
Keywords: : chemotherapy, connectome, DTI, executive function, leukemia, resting state fMRI
Introduction
We previously showed that survivors of pediatric acute lymphoblastic leukemia (ALL) treated only with chemotherapy have significant impairments in memory, processing speed, and executive function (Cheung et al., 2016; Kesler et al., 2014; Krull et al., 2016). These deficits persist decades beyond treatment cessation and negatively impact educational and occupational functioning as well as health-related behaviors (Krull et al., 2011, 2013; Schuitema et al., 2013).
Widespread injury to brain structure and function has been observed following treatment for pediatric ALL (Edelmann et al., 2014; ElAlfy et al., 2014; Kesler et al., 2014; Tamnes et al., 2015; Zeller et al., 2013). Many of these studies show correlations between neuroimaging metrics and cognitive outcomes, but few if any have examined differences in neurobiological status between cognitively impaired versus nonimpaired patients.
Additionally, few studies have examined interactions between brain regions within affected neural networks that subserve cognitive functions. Connectomics is a method for evaluating brain networks based on graph theory, the study of objects, and their connections. Connectomes are frequently constructed from neuroimaging data, including diffusion tensor imaging (DTI) for structural connectivity and resting-state functional magnetic resonance imaging (fMRI) for functional connectivity. We previously demonstrated alterations in connectome organization in small samples of young survivors of ALL compared with typically developing controls (Hosseini et al., 2012; Kesler et al., 2016).
In the present study, we compared connectome organization between ALL survivors with or without executive dysfunction at long-term follow-up. Deficits in executive function are among the most common impairments in these patients (Cheung et al., 2016; Kesler et al., 2014; Krull et al., 2016). Executive function refers to a set of cognitive skills critical for goal-oriented behaviors, adaptive function, and self-regulation. Executive function is associated with health behavior trajectories and longevity among noncancer populations (Hall and Fong, 2013; Williams and Thayer, 2009). We hypothesized that patients with executive dysfunction would demonstrate lower functional and structural connectome organization compared with those without executive dysfunction. We also examined the effects of age at diagnosis, sex, and chemotherapy exposure on connectome organization.
Materials and Methods
Participants
From 2000 to 2010, 408 children with ALL were treated at St. Jude Children's Research Hospital (SJCRH) on the Total Therapy XV protocol (ClinicalTrials.gov, NCT00137111). Survivors who were receiving pediatric follow-up care at SJCRH, were at least 5 years from diagnosis, and were at least 8 years of age were eligible to participate. Survivors were excluded for the following reasons: death before long-term follow-up (n = 35); treatment with cranial radiation for central nervous system (CNS) relapse or bone marrow transplantation (n = 30); neurodevelopmental condition, genetic disorder, or brain injury unrelated to cancer, but associated with cognitive impairment (n = 22); lack of proficiency in English (n = 1); and not eligible for follow-up (e.g., discharged from pediatric follow-up care [n = 13], under foster care [n = 4], or in prison [n = 1]). Of the 302 eligible survivors, 218 participated in neurocognitive testing beginning on January 1, 2010.
Thirty-eight survivors refused brain imaging studies, 12 had imaging contraindications (e.g., orthodontia), and seven produced nonevaluable data (i.e., excessive movement and technical complications), resulting in 161 survivors with neurocognitive and imaging studies. This study was approved by the institutional review board and conducted at St. Jude Children's Research Hospital. Informed consent/assent was obtained from parents and/or participants, as appropriate.
Chemotherapy exposure
We recorded the number of intrathecal treatments containing methotrexate, hydrocortisone, and cytarabine that each patient received as well as exposure to high-dose intravenous methotrexate. Blood samples were drawn before methotrexate treatment and at 6, 23, and 42 h following the start of each course. Exposure was quantified as area under the curve (AUC).
Neurocognitive function
All participants completed neurocognitive testing with certified examiners under the supervision of a board-certified clinical neuropsychologist. Measures of executive function, processing speed, intelligence, attention, and memory span were examined (Supplementary Table S1; Supplementary Data are available online at www.libertpub.com/brain). These included selected subtests from the Delis–Kaplan Executive Function System (Delis et al., 2008), Wechsler intelligence and memory scales (Wechsler, 1997, 2003, 2008), and Rey Complex Figure Test (Meyers and Meyers, 1996). Impairment was defined as an age-adjusted score falling below the 10th percentile of national normative data.
Structural and functional connectivity
fMRI was obtained during 5 min of eyes-open rest on a 3T scanner (Siemens Trio or Skyra MR; Siemens, Malvern, PA) using a single-shot T2*-weighted echo-planar imaging (EPI) pulse sequence (TR = 2.06 sec, TE = 30 msec, FOV = 192 mm, matrix = 64 × 64, slice thickness = 5 mm). DTI was obtained on a 1.5T scanner (Siemens Avanto; Siemens) using a double spin-echo EPI pulse sequence (TR/TE = 10,000/100 msec, b = 1000, 3.0 × 1.8 × 1.8 mm, acquisition time ∼1.5 min each) with 4 acquisitions and 12 gradient directions.
Visual artifact inspection resulted in exclusion of five DTIs. There were no significant differences in demographic, treatment, or neurocognitive variables between excluded and included participants. DTIs were preprocessed using the fMRIB Software Library (FSL), v5.0, as previously described (Kesler et al., 2015, 2016), including eddy current correction, tensor reconstruction, and probabilistic tractography. fMRIs were preprocessed using Statistical Parametric Mapping, v8 (SPM8), as previously described (Bruno et al., 2012; Kesler et al., 2013, 2014; Kesler and Blayney, 2016), including realignment, normalization, and smoothing (8 mm full width half maximum).
Functional volumes were further denoised to reduce motion- and signal-related artifacts using a wavelet despiking method (Patel et al., 2014). In the nonimpaired group, 97 participants had usable DTI, 65 had usable fMRI, and 62 had both. In the impaired group, these numbers were 57, 36, and 32, respectively.
Functional connectivity matrices were obtained using CONN Toolbox, as previously described (Kesler et al., 2013, 2014; Kesler and Blayney, 2016), including filtering data to the <0.1 Hz range of spontaneous activity (Whitfield-Gabrieli and Ford, 2012) and correction of motion and physiologic/non-neuronal artifacts (Behzadi et al., 2007). Correlation coefficients were calculated between fMRI time courses for each pair of 90 regions of interest (ROIs) (Tzourio-Mazoyer et al., 2002) in standard space. The resulting z-score connectivity matrices were thresholded to minimum connection density and then submitted to graph theoretical analysis (Rubinov and Sporns, 2010).
We determined the number of DTI tractography streamlines connecting each pair of ROIs in native space (Kesler et al., 2015, 2016). Regions were considered connected if one streamline end-point terminated within one region and the other end-point terminated within the other region. A threshold of three streamlines was applied to minimize false positive connections (Kesler et al., 2015, 2016). We weighted each valid edge using the product of the streamline number and fractional anisotropy divided by average ROI volume. These weighted connectivity matrices were submitted to graph theoretical analysis.
We focused on connectome efficiency of information processing. The connectome is organized in such a manner that most regions are connected to their neighbors and can be reached by every other region through a small number of steps or paths (Latora and Marchiori, 2001). Efficient information processing is assumed to follow the shortest paths between regions (Supplementary Fig. S1) (Latora and Marchiori, 2001). We previously demonstrated impaired connectome efficiency associated with adult-onset cancer (Kesler et al., 2015, 2017b).
Statistical analyses
Global and local connectome efficiencies were compared between executive function groups (impaired or unimpaired) using analysis of covariance (ANCOVA) with sex, age at diagnosis, maternal education, and treatment (low vs. standard/high risk) as covariates. Age at evaluation also differed between functional groups, but was highly collinear with age at diagnosis (r = 0.93, p < 0.001), so only age at diagnosis was included in our models.
To further evaluate the effect of age at diagnosis and sex on connectome efficiency, we tested differences in global efficiency between four age at diagnosis groups defined arbitrarily using quantiles and in a data-driven manner using K-means clustering. Age groups were evaluated across the entire sample and within females and males separately using omnibus ANCOVA, followed by pairwise t-tests, controlled for multiple comparisons with the false discovery rate (FDR) (Benjamini and Yekutieli, 2001). These analyses were conducted using the R statistical package, v3.3.2 (R Foundation). Hypothesis tests were two-sided and considered significant when p-values were <0.05.
We used a network-based statistic approach to determine the specific regional profile of structural and functional connectivity differences between the executive function groups. This method identifies connected substructures, or components, within the larger network. Permutation testing with 5000 permutations was then used to determine group differences in components, controlling for multiple comparisons using family-wise error (FWE) (Zalesky et al., 2010). Covariates as described above were included in these models. We also examined network modularity, which involves decomposing the brain into nonoverlapping groups of regions (modules) that have maximal within-group connections and minimal between-group connections (Sporns and Betzel, 2016). Modularity was compared between groups using permutation testing with 1000 permutations (Pereira et al., 2016; Zalesky et al., 2010).
We computed two-tailed partial correlations controlling for sex, age at diagnosis, treatment, and maternal education between connectome efficiencies and executive function test scores. Efficiencies included global and local efficiencies as well as regional efficiency (i.e., mean efficiency of significant network components). Correlations were FDR corrected and only those that survived correction for global/local efficiencies were examined for regional efficiency. We also calculated partial correlations between connectome efficiencies and chemotherapy exposure variables.
We predicted functional connectivity from structural connectivity by generating simulated functional connectivity matrices based on network communication measures (path transitivity, search information, and shortest path length) derived from structural connectivity and then computing the correlation between the simulated and observed matrices for each participant (Goñi et al., 2014; Honey et al., 2009). The between-group difference in these correlations was measured using ANCOVA, as described above.
Results
Participants
We included 161 participants, of whom 61 (37.9%) were impaired on measures of executive function. On average, participants in both groups were 14 years old at the time of data collection and between 6 and 7 years old at the time of their ALL diagnosis. Sixty-four percent of the impaired group was male compared with 41% of the nonimpaired group. Demographic and medical data for the nonimpaired and impaired groups are displayed in Table 1.
Table 1.
Demographic and Medical Data Shown as Mean (Standard Deviation) Unless Otherwise Noted
| Nonimpaired (n = 100) | Impaired (n = 61) | t/chi-square | p | |
|---|---|---|---|---|
| Age at evaluation (years) | 14.87 (4.9) | 14.23 (4.4) | 0.857 | 0.393 |
| Age at diagnosis (years) | 7.08 (4.6) | 6.47 (4.1) | 0.875 | 0.383 |
| Maternal education (years) | 14.04 (2.5) | 12.81 (2.3) | 3.10 | 0.002 |
| Gender (male) | 40% | 64% | 8.68 | 0.003 |
| Treatment intensity (standard/high risk) | 41% | 44% | 0.165 | 0.684 |
| Intrathecal methotrexate | 14.43 (4.1) | 14.77 (4.0) | 0.519 | 0.605 |
| Methotrexate area under the curve | 32.20 (11.3) | 33.72 (12.15) | 0.779 | 0.438 |
Functional connectome
All participants demonstrated expected small-world connectome organization as indicated by the small-world index greater than one (Humphries and Gurney, 2008). Global efficiency was significantly lower (F = 4.09, p = 0.046), while local efficiency was moderately higher (F = 3.42, p = 0.068), in survivors with executive dysfunction (Table 2). Sex was a significant covariate in both models (p < 0.04) as more males had executive dysfunction. Treatment group was also a significant covariate in both models (p < 0.05), indicating that standard/high-risk patients had lower global efficiency and higher local efficiency than low-risk patients. Age at diagnosis did not contribute to the models (p > 0.25) and age group analyses were not significant (p > 0.46).
Table 2.
Connectome Efficiency Data Shown as Mean (Standard Deviation)
| n | Nonimpaired | n | Impaired | F | p | |
|---|---|---|---|---|---|---|
| Functional connectome | ||||||
| Global efficiency | 65 | 0.580 (0.005) | 36 | 0.578 (0.007) | 4.09 | 0.046 |
| Local efficiency | 65 | 0.717 (0.012) | 36 | 0.721 (0.014) | 3.42 | 0.068 |
| Modularity* | 65 | 0.348 | 36 | 0.3001 | — | <0.0001 |
| Structural connectome | ||||||
| Global efficiency | 97 | 0.162e-2 (0.12e-3) | 57 | 0.158e-2 (0.13e-3) | 5.02 | 0.027 |
| Local efficiency | 97 | 0.146e-2 (0.94e-4) | 57 | 0.142e-2 (0.11e-3) | 5.31 | 0.023 |
| Modularity* | 97 | 0.4999 | 57 | 0.4990 | — | 0.458 |
| Relationship between structural and functional connectivity | ||||||
| Pearson R | 62 | 0.280 (0.049) | 32 | 0.283 (0.054) | 0.025 | 0.876 |
p values for modularity are obtained using permutation testing of the mean difference, so there is no associated F statistic or standard deviation. The mean difference for functional modularity was 0.0479 with a 95% confidence interval from −0.019 to 0.016. The mean difference for structural modularity was 0.0009 with a 95% confidence interval from −0.014 to 0.014.
Lower global efficiency was associated with higher number of intrathecal methotrexate treatments (r = −0.21, p = 0.036), but was not significantly correlated with methotrexate AUC (r = 0.09, p = 0.416). Local efficiency was not correlated with either treatment variable (p > 0.45). The effect of treatment group on connectome efficiencies was likely driven, in part, by the number of intrathecal treatments, which was significantly higher in standard/high compared with low-risk patients (t = 10.70, p < 0.0001).
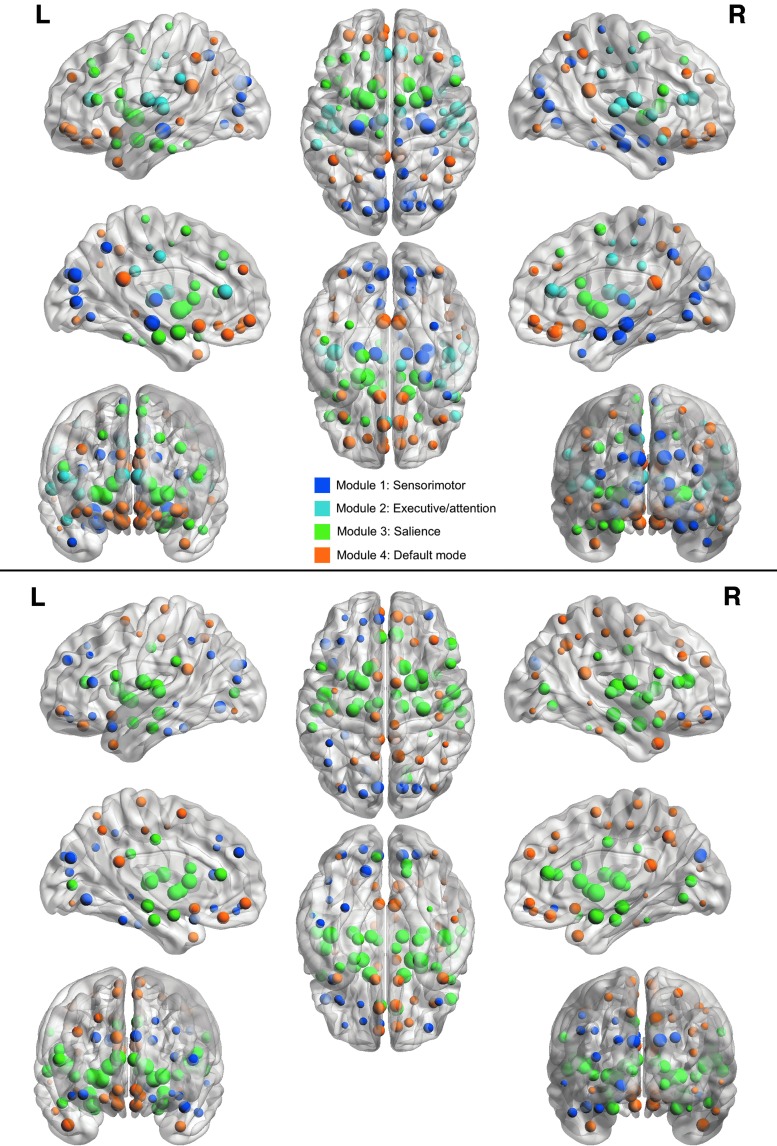
Regional analysis indicated hyperconnectivity of a medial–lateral–posterior network in survivors with executive dysfunction (p = 0.037, FWE corrected, Fig. 1). Modularity was lower in the impaired group (p < 0.0001). Nonimpaired participants showed a typical profile of modules consistent with distinct salience, sensorimotor, default mode, and attention/executive networks. The impaired group demonstrated poor separation between sensorimotor and attention/executive, default mode and attention/executive, and salience and sensorimotor networks (Fig. 2).
FIG. 1.
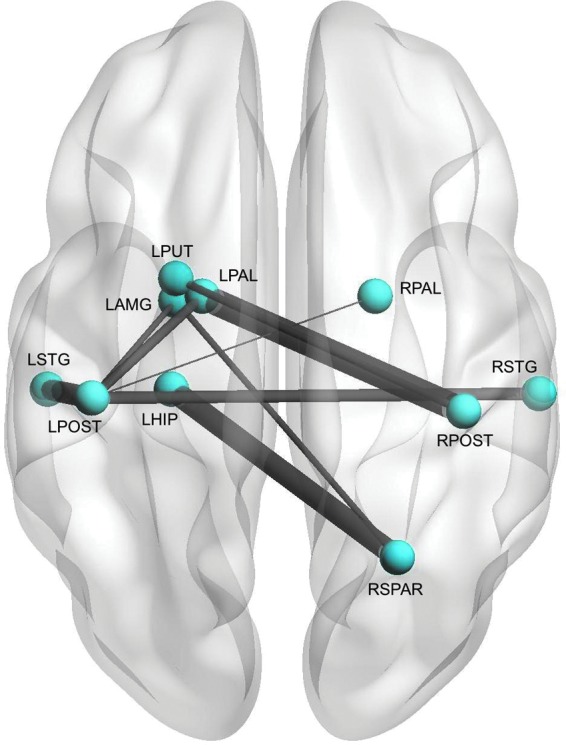
Functional Regional Connectivity. Patients with executive dysfunction demonstrated hyperconnectivity among several regions compared with patients without executive dysfunction (p = 0.037, corrected). Regions are shown as spheres and their connections as lines, which are weighted by the test statistic for that connection (i.e., thicker line = greater hyperconnectivity). LAMG, left amygdala; LHIP, left hippocampus; LPAL, left palladium; LPOST, left postcentral gyrus; LSTG, left superior temporal gyrus; LPUT, left putamen; RPAL, right palladium; RPOST, right postcentral gyrus; RSPAR, right superior parietal; RSTG, right superior temporal gyrus.
FIG. 2.
Functional Network Modules. Patients with executive dysfunction demonstrated lower modularity (p < 0.001) compared with patients without executive dysfunction, indicating lower separation among networks, consistent with hyperconnectivity. Nonimpaired patients (top) showed distinct salience, sensorimotor, default mode, and executive/attention networks, while the impaired group (bottom) demonstrated overlap between sensorimotor and attention/executive, default mode and attention/executive, and salience and sensorimotor networks. Regions are shown as spheres colored by module membership.
Partial correlations demonstrated direct relationships between global efficiency and executive function test scores (p < 0.006) and negative correlations between local/regional efficiencies (p < 0.005) and executive function test scores (Supplementary Table S2).
Structural connectome
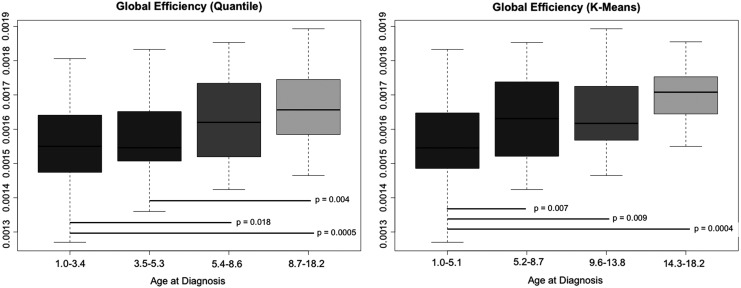
All participants demonstrated expected small-world connectome organization as indicated by the small-world index greater than one (Humphries and Gurney, 2008). Both global (F = 5.02, p = 0.027) and local (F = 5.31, p = 0.023) efficiencies were lower in the executive dysfunction group (Table 2). Age at diagnosis was the only significant covariate in these models (p < 0.004). The quantile-based age at diagnosis group analyses were significant (p < 0.0001) and indicated that children diagnosed at approximately age 5 or younger tend to be the most vulnerable to alterations of structural brain network efficiency. A similar finding was observed using K-means clustering (Fig. 3). There did not appear to be a sex interaction given that both younger females and younger males demonstrated lower efficiency compared with older children. There were no differences in efficiency between sexes within each age group and no differences in sex between the age groups or clusters (p > 0.507).
FIG. 3.
Structural Network Efficiency by Age at Diagnosis Groups. Dividing the sample by age at diagnosis into quantiles or K-means clusters suggested that children approximately age 5 or younger at diagnosis demonstrated the greatest vulnerability to disruption of brain network efficiency; p values are FDR corrected. FDR, false discovery rate.
There were no significant differences between the groups in terms of regional structural connectivity or modularity.
None of the partial correlations between efficiencies and executive function test scores survived correction (Supplementary Table S3). There were no significant correlations between efficiencies and chemotherapy exposure variables.
Relationship between structure and function
Structural connectivity significantly predicted functional connectivity in all participants (mean r = 0.28 [0.05], range = 0.18–0.41, p < 0.0001), but there was no between-group difference (p = 0.876).
Discussion
We examined neural mechanisms underlying cognitive impairment in adolescent and young adult survivors of pediatric ALL by measuring structural and functional connectome properties in survivors with and without executive dysfunction. Global efficiency of information exchange, a measure of brain network integration and capacity for parallel information processing, was significantly lower in survivors with executive function impairment compared with those without impairment. This finding was observed in both functional and structural connectomes.
Global efficiency was lowest for survivors who were younger at diagnosis, had a history of standard/high treatment with higher dose of dexamethasone, and had a higher number of intrathecal methotrexate injections. Additionally, we noted direct correlations between global efficiency and performance on several executive function tests, including those measuring abstract reasoning, verbal fluency, and shifting attention. Global efficiency deficits have been observed in several other pediatric conditions that are associated with cognitive impairment (Rudie et al., 2012; Thompson et al., 2016; Yuan et al., 2015).
Brain injury and associated cognitive deficits following pediatric ALL are believed to stem, in part, from direct and indirect effects of chemotherapy. Methotrexate is an antimetabolite agent that targets the folate pathway and is administered throughout the entire treatment of ALL, generally 2 or more years in duration (Cooper and Brown, 2015). Methotrexate is also administered intrathecally to treat and/or prevent CNS disease. Methotrexate has been associated with several neuropathological effects, including glial progenitor cell death, suppression of neurogenesis, microvascular damage, and leukoencephalopathy, among others (Monje and Dietrich, 2012; Seigers et al., 2009, 2010; Shuper et al., 2002). Accordingly, we demonstrated that the higher number of intrathecal injections containing methotrexate was associated with lower functional global efficiency.
Patients with standard/high-risk disease also received 50% higher cumulative dosage of dexamethasone compared with low-risk patients. The cytotoxic action of dexamethasone and other glucocorticoids involves binding of glucocorticoid receptors (Inaba and Pui, 2010). These receptors have been shown to play important roles in cognition, particularly memory functions (Barsegyan et al., 2010; Meir Drexler and Wolf, 2017). Previous studies involving survivors of pediatric ALL have shown associations between dexamethasone treatment and brain function (Edelmann et al., 2013; Waber et al., 2013).
Previous studies investigating steroid-induced, long-term neurocognitive outcomes have not found significant differences between prednisone and dexamethasone (Warris et al., 2014). However, these previous studies have been confounded by the inclusion of survivors treated with cranial radiation therapy. For example, in one trial, investigators did not adjust for radiation dose or check for radiation/corticoid–steroid interactions, thus any differences between prednisone and dexamethasone may have been confounded by radiation therapy. Another trial did find that the dexamethasone group used more special education services and this intervention may have ameliorated some of the neurocognitive late effects. Finally, all three trials that have previously compared prednisone and dexamethasone outcomes have used intrathecal hydrocortisone, which when combined with intrathecal methotrexate, would produce the same proposed injury as dexamethasone and methotrexate (Warris et al., 2014).
Patients treated with standard/high-risk ALL regimens showed lower functional global efficiency compared with those treated with low-risk regimens. Since patients were treated with risk-adapted therapy, those with higher risk disease received more aggressive therapy regimens (Cooper and Brown, 2015). However, leukemia itself likely has an independent effect on brain structure and function. Emerging evidence suggests that before treatment, solid tumors originating outside the CNS are associated with altered structural and functional connectome organization as well as cognitive impairment (Kesler et al., 2017a; Patel et al., 2015).
Little is known regarding the direct impact of hematologic cancer cells on CNS integrity and long-term function. However, we have recently demonstrated that children diagnosed with ALL have evidence of brain white matter injury before initiation of chemotherapy (Cheung et al., 2017). The contribution of disease pathogenesis alone to brain injury and cognitive impairment in ALL has received limited attention and requires further study.
Younger age at diagnosis was another significant risk factor, which has been noted in several previous studies (Kahalley et al., 2013; Kesler et al., 2014; Reddick et al., 2014). Our findings further demonstrated that children diagnosed at approximately age five or younger tend to have the highest risk for impaired connectome efficiency. The number of white matter tracts is established by ∼4 years of age and structural connectomes become more integrated over time, that is, global efficiency increases with age as longer tracts continue to develop (Richmond et al., 2016).
Our regional findings also point to neurodevelopmental mechanisms. The impaired group demonstrated regional hyperconnectivity between postcentral gyri and other regions important for somatosensory, auditory, and visual functioning. These brain regions are among those that mature earliest and may therefore be preferentially vulnerable to early brain injury (Gogtay and Thompson, 2010). Our group and others have shown that temporal lobe regions are particularly sensitive to early brain injury (Kesler et al., 2006). In the context of lower global efficiency, our regional results suggest that local functional networks may be overly segregated and not well integrated with other processing centers. Accordingly, there were significant negative correlations between functional local efficiency and performance on executive function tests.
It is unclear why age was associated with structural, but not functional, connectome properties or why sex was associated with functional, but not structural, metrics. DTI and fMRI measure different neurobiological properties and therefore tend to have divergent associations with demographic and other data. It is possible that age has a greater effect on white matter development, while sex has a larger impact on functional dynamics. Few studies in this population (and others) include examination of multimodal connectomes and therefore these relationships require further examination.
Additionally, the impaired group demonstrated significantly fewer modules in the functional connectome compared with the nonimpaired group. Previous studies demonstrate that the brain network is decomposable into distinct modules or subnetworks representing known functional components (Chen et al., 2013; Grayson and Fair, 2017). In the impaired group, the executive function/attention network was overly connected to default mode and sensorimotor networks.
Modularity is present very early in brain development, although in a primitive form that is dominated by sensorimotor networks. Salience, executive/attention, and default mode networks tend to be refined and distinguished over time (Grayson and Fair, 2017). This is consistent with our observation that regions of functional hyperconnectivity largely involved sensory systems and again suggests that ALL and its treatments disrupt normal brain maturation. Lower modularity may result in a brain network that is less adaptable, flexible, and resilient (Sporns and Betzel, 2016).
Structural and functional connectomes showed global correspondence, but there were also inconsistencies, including local efficiency, regional differences, modularity, and correlations, with chemotherapy exposure variables and executive function performance. Our findings may simply reflect differences in neural properties, network densities, and imaging modalities (Bassett et al., 2011). Cunningham et al. (2017) suggested that differences in structural and functional connectivity may reflect the dynamic nature of functional networks and/or limitations of DTI. It is also probable that structure–function relationships are nonlinear, which were beyond the scope of this study and require further investigation.
This study had several limitations. Certain results are difficult to interpret without data from a typically developing comparison group. It is possible that our nonimpaired group would be impaired relative to healthy noncancer controls. However, comparing patients with and without executive dysfunction is a strength of the study and represents an important examination of cognitive deficit that is unique in this field. This comparison provides insight regarding potential neural mechanisms of deficit that are independent of disease and treatment, not just the divergence from typical development.
The cross-sectional design prevented evaluation of independent effects of chemotherapy, cancer pathogenesis, and other factors. Currently, there is no standard definition of cognitive impairment and while ours was consistent with methods used in other studies, alternate definitions of impairment may yield different results.
Our DTI acquisition had lower resolution than many similar studies, which may have contributed to the lack of overlap with functional connectome topology. This possibility seems unlikely given that other studies observing inconsistencies between structural and functional connectivity have employed high-resolution DTI (Cunningham et al., 2017; Kesler et al., 2017a; Rudie et al., 2012).
Our fMRI acquisition was relatively short (5 min) and obtained with a different field strength compared with DTI. It is well known that test-retest reliability of connectome properties increases with increased scan time (Andellini et al., 2015). However, studies have noted stable and accurate graph metrics using scans as short as 2 min (Whitlow and Maldjian, 2011). Our imaging sequences had to be completed on clinical scanners and were designed and implemented over 8 years ago and acquired over a 5-year period given the relative rarity of childhood leukemia. Efficiency of the protocol with respect to scheduling of clinical scanner time had to be balanced with study goals. Additionally, we demonstrated significant and robust correlations between functional connectome properties and neuropsychological test performance, suggesting that our fMRI graph metrics represent important biomarkers of cognitive function in this sample. Furthermore, structural connectivity was highly predictive of functional connectivity despite the difference in scanner field strength, which we have also demonstrated pre-clinically, showing that this correlation discriminates between genetic models (Kesler et al., 2018).
Conclusion
Our findings contribute novel insights regarding the cognitive effects of ALL and its treatments as well as the neural mechanisms underlying these effects. The innovative aspects of this study include the comparison between functional groups, the combination of structural and functional imaging, the focus on connectome organization, and measurement of chemotherapy exposure. ALL and/or its treatments appear to disrupt normal brain maturational processes, including refinement of functional networks that support higher-order cognitive skills such as executive function, attention, and monitoring. Our findings highlight the need for interventional strategies that will help prevent and manage cognitive impairment and normal brain neural network development in patients with pediatric ALL and assist these patients with maintaining brain health across the life span.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health (MH085849 to K.R.K.; CA195547 to M.M.H. and L.L.R.; Cancer Center Support [CORE] grant CA21765; 1R01NR014195; and 1R01CA172145 to S.K.) and by ALSAC. The authors wish to thank Vikram Rao, MS, for assistance with neuroimaging preprocessing.
Author Disclosure Statement
No competing financial interests exist.
References
- Andellini M, Cannata V, Gazzellini S, Bernardi B, Napolitano A. 2015. Test-retest reliability of graph metrics of resting state MRI functional brain networks: a review. J Neurosci Methods 253:183–192 [DOI] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. 2010. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci U S A 107:16655–16660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Brown JA, Deshpande V, Carlson JM, Grafton ST. 2011. Conserved and variable architecture of human white matter connectivity. Neuroimage 54:1262–1279 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. 2001. The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188 [Google Scholar]
- Bruno J, Hosseini SM, Kesler S. 2012. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis 48:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Liu M, Gross DW, Beaulieu C. 2013. Graph theoretical analysis of developmental patterns of the white matter network. Front Hum Neurosci 7:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y, Khan R, Liu W, Brinkman T, Edelmann M, Reddick W, et al. 2017. Biomarkers of brain injury and neurologic outcomes in children treated with chemotherapy for acute lymphoblastic leukemia. J Clin Oncol 35(suppl):abstr 10521 [Google Scholar]
- Cheung YT, Sabin ND, Reddick WE, Bhojwani D, Liu W, Brinkman TM, et al. 2016. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol 3:e456–e466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SL, Brown PA. 2015. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am 62:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SI, Tomasi D, Volkow ND. 2017. Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum Brain Mapp 38:938–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. 2008. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corp [Google Scholar]
- Edelmann MN, Krull KR, Liu W, Glass JO, Ji Q, Ogg RJ, et al. 2014. Diffusion tensor imaging and neurocognition in survivors of childhood acute lymphoblastic leukaemia. Brain 137(Pt 11):2973–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann MN, Ogg RJ, Scoggins MA, Brinkman TM, Sabin ND, Pui CH, et al. 2013. Dexamethasone exposure and memory function in adult survivors of childhood acute lymphoblastic leukemia: a report from the SJLIFE cohort. Pediatr Blood Cancer 60:1778–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElAlfy M, Ragab I, Azab I, Amin S, Abdel-Maguid M. 2014. Neurocognitive outcome and white matter anisotropy in childhood acute lymphoblastic leukemia survivors treated with different protocols. Pediatr Hematol Oncol 31:194–204 [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. 2010. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn 72:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi J, van den Heuvel MP, Avena-Koenigsberger A, Velez de Mendizabal N, Betzel RF, Griffa A, et al. 2014. Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci U S A 111:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Fair DA. 2017. Development of large-scale functional networks from birth to adulthood: a guide to neuroimaging literature. Neuroimage 160:15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Fong GT. 2013. Conscientiousness versus executive function as predictors of health behaviors and health trajectories. Ann Behav Med 45:398–399 [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. 2009. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A 106:2035–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Hoeft F, Kesler SR. 2012. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One 7:e40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Gurney K. 2008. Network “small-world-ness”: a quantitative method for determining canonical network equivalence. PLoS One 3:e0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba H, Pui C-H. 2010. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol 11:1096–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahalley LS, Conklin HM, Tyc VL, Hudson MM, Wilson SJ, Wu S, et al. 2013. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology 22:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Acton P, Rao V, Ray W. 2018. Functional and structural connectome properties in the 5XFAD transgenic mouse model of Alzheimer's disease. Netw Neurosci 2:241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Adams M, Packer M, Rao V, Henneghan AM, Blayney DW, Palesh O. (2017a). Disrupted brain network functional dynamics and hyper-correlation of structural and functional connectome topology in patients with breast cancer prior to treatment. Brain Behav 7:e00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Blayney DW. 2016. Neurotoxic effects of anthracycline-vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol 2:185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Gugel M, Huston-Warren E, Watson C. 2016. Atypical structural connectome organization and cognitive impairment in young survivors of acute lymphoblastic leukemia. Brain Connect 6:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Gugel M, Pritchard-Berman M, Lee C, Kutner E, Hosseini SM, et al. 2014. Altered resting state functional connectivity in young survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer 61:1295–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Noll K, Cahill DP, Rao G, Wefel JS. (2017b). The effect of IDH1 mutation on the structural connectome in malignant astrocytoma. J Neurooncol 131:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR. 2006. Increased temporal lobe gyrification in preterm children. Neuropsychologia 44:445–453 [DOI] [PubMed] [Google Scholar]
- Kesler SR, Watson CL, Blayney DW. 2015. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging 36:2429–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F. 2013. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A 110:11600–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Annett RD, Pan Z, Ness KK, Nathan PC, Srivastava DK, et al. 2011. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer 47:1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, et al. 2013. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol 31:4407–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Cheung YT, Liu W, Fellah S, Reddick WE, Brinkman TM, et al. 2016. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 34:2644–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. 2001. Efficient behavior of small-world networks. Phys Rev Lett 87:198701. [DOI] [PubMed] [Google Scholar]
- Meir Drexler S, Wolf OT. 2017. The role of glucocorticoids in emotional memory reconsolidation. Neurobiol Learn Mem 142:126–134 [DOI] [PubMed] [Google Scholar]
- Meyers JE, Meyers KR. 1996. Rey Complex Figure and Recognition Trial. Odessa, FL: Psychological Assessment Resources [Google Scholar]
- Monje M, Dietrich J. 2012. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav Brain Res 227:376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vertes PE, Ersche KD, et al. 2014. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage 95:287–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Wong AL, Wong FL, Breen EC, Hurria A, Smith M, et al. 2015. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst 107:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Mijalkov M, Kakaei E, Mecocci P, Vellas B, Tsolaki M, et al. 2016. Disrupted network topology in patients with stable and progressive mild cognitive impairment and Alzheimer's disease. Cereb Cortex 26:3476–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick WE, Taghipour DJ, Glass JO, Ashford J, Xiong X, Wu S, et al. 2014. Prognostic factors that increase the risk for reduced white matter volumes and deficits in attention and learning for survivors of childhood cancers. Pediatr Blood Cancer 61:1074–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S, Johnson KA, Seal ML, Allen NB, Whittle S. 2016. Development of brain networks and relevance of environmental and genetic factors: a systematic review. Neurosci Biobehav Rev 71:215–239 [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069 [DOI] [PubMed] [Google Scholar]
- Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, et al. 2012. Altered functional and structural brain network organization in autism. Neuroimage Clin 2:79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitema I, Deprez S, Van Hecke W, Daams M, Uyttebroeck A, Sunaert S, et al. 2013. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol 31:3378–3388 [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Coppens CM, van der Most PJ, van Dam FS, Koolhaas JM, Buwalda B. 2009. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res 201:279–284 [DOI] [PubMed] [Google Scholar]
- Seigers R, Timmermans J, van der Horn HJ, de Vries EF, Dierckx RA, Visser L, et al. 2010. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res 207:265–272 [DOI] [PubMed] [Google Scholar]
- Shuper A, Stark B, Kornreich L, Cohen IJ, Avrahami G, Yaniv I. 2002. Methotrexate-related neurotoxicity in the treatment of childhood acute lymphoblastic leukemia. Isr Med Assoc J 4:1050–1053 [PubMed] [Google Scholar]
- Sporns O, Betzel RF. 2016. Modular brain networks. Annu Rev Psychol 67:613–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Zeller B, Amlien IK, Kanellopoulos A, Andersson S, Due-Tonnessen P, et al. 2015. Cortical surface area and thickness in adult survivors of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer 62:1027–1034 [DOI] [PubMed] [Google Scholar]
- Thompson DK, Chen J, Beare R, Adamson CL, Ellis R, Ahmadzai ZM, et al. 2016. Structural connectivity relates to perinatal factors and functional impairment at 7 years in children born very preterm. Neuroimage 134:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Waber DP, McCabe M, Sebree M, Forbes PW, Adams H, Alyman C, et al. 2013. Neuropsychological outcomes of a randomized trial of prednisone versus dexamethasone in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute All Consortium Protocol 00-01. Pediatr Blood Cancer 60:1785–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warris LT, van den Heuvel-Eibrink MM, den Hoed MA, Aarsen FK, Pieters R, van den Akker EL. 2014. Does dexamethasone induce more neuropsychological side effects than prednisone in pediatric acute lymphoblastic leukemia? A systematic review. Pediatr Blood Cancer 61:1313–1318 [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1997. Wechsler Memory Scale– 3rd ed. San Antonio, TX: The Psychological Corporation [Google Scholar]
- Wechsler D. 2003. Wechsler Intelligence Scale for Children, 4th ed. San Antonio: The Psychological Corporation [Google Scholar]
- Wechsler D. 2008. Wechsler Adult Intelligence Scale, 4th ed. San Antonio, TX: The Psychological Corporation [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. 2012. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76 [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Maldjian JA. 2011. Effect of resting-state functional MR imaging duration on stability of graph theory metrics of brain network connectivity. Radiology 259:516–524 [DOI] [PubMed] [Google Scholar]
- Williams PG, Thayer JF. 2009. Executive functioning and health: introduction to the special series. Ann Behav Med 37:101–105 [DOI] [PubMed] [Google Scholar]
- Yuan W, Wade SL, Babcock L. 2015. Structural connectivity abnormality in children with acute mild traumatic brain injury using graph theoretical analysis. Hum Brain Mapp 36:779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. 2010. Network-based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207 [DOI] [PubMed] [Google Scholar]
- Zeller B, Tamnes CK, Kanellopoulos A, Amlien IK, Andersson S, Due-Tonnessen P, et al. 2013. Reduced neuroanatomic volumes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 31:2078–2085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.