Abstract
Objectives: Chronic low-back pain (CLBP) is burdensome and costly, and a common condition for which adults use integrative therapies. The effectiveness of multidisciplinary integrative approaches has not been well studied. The purpose of this observational study was to compare characteristics and outcomes of CLBP patients treated at the Osher Clinical Center (OCC) versus other clinics at Brigham and Women's Hospital.
Design: Observational comparative effectiveness study.
Setting: Tertiary care hospital.
Subjects: Patients ≥21 years with 3+ months of CLBP or 6+ months of intermittent low-back pain.
Intervention: All patients were observed for 12 months. OCC patients received care at the integrative clinic (7.3 visits on average over 13 weeks); non-OCC patients received usual care at other clinics of the same hospital.
Outcome measures: Primary outcomes: change from baseline to 6 months in functional status (Roland Disability Questionnaire [RDQ]) and bothersomeness of pain (BOP). Secondary outcomes: change in RDQ and BOP at 3 and 12 months, percentages of patients with clinically meaningful (≥30%) improvements.
Results: One hundred fifty-six OCC and 153 non-OCC participants were enrolled; follow-up was 90.4 and 98.0%, respectively, at 12 months. There were substantial differences in baseline characteristics between groups. For RDQ, the adjusted mean group difference was nonsignificant at 6 months; for BOP, the differences were significant, but clinically small. At 12 months, the observed benefit on RDQ was significant and clinically meaningful; for BOP, there were significant, but clinically small differences. Percentages of patients with ≥30% improvements in RDQ were significantly greater in the OCC group only at 12 months, and both 6 and 12 months for BOP.
Conclusions: Baseline characteristics can differ between those who select different sources of healthcare for CLBP. While benefits seen in the OCC versus non-OCC clinics were not large, further evaluation through randomized trials might be warranted to provide a more definitive evaluation.
Keywords: : chronic low-back pain, integrative medicine, pragmatic study, disability, interdisciplinary care, pain bothersomeness
Introduction
Despite widespread and growing popularity of complementary and integrative medical therapies in the United States, there currently exist significant gaps in our knowledge regarding complementary therapy use, its clinical effectiveness and safety, and its cost-effectiveness. Chronic low-back pain (CLBP) is the most common medical condition for which adults use integrative medical therapies.1–6 CLBP is a prevalent, costly health problem.7–16 Despite considerable investment over the past few decades, basic and clinical research have not translated into a decreased prevalence of CLBP nor in the development of unimodal therapies that result in consistently and markedly improved efficacy.17,18 CLBP is increasingly viewed as a complex biopsychosocial phenomenon in which anatomical injury interplays with psychosocial factors, including previous pain experiences, beliefs and fears about CLBP, and general and psychosocial health.19–22 This framework has catalyzed a shift in the focus for treating CLBP to a multimodal and multidisciplinary model, integrating pain management with physical, psychosocial, and behavioral strategies that address a patient's welfare in a holistic context.23–25
Limitations in conventional care for CLBP have resulted in patients seeking a combination of both conventional and complementary or alternative therapies.1,2,26 Moreover, patients who seek complementary therapies frequently use more than one complementary therapy for CLBP.27 Results from controlled clinical trials have supported the benefit of individual complementary and integrative therapies in treating CLBP symptoms, including chiropractic,28 acupuncture,29 massage,30 and mindfulness-based stress reduction (MBSR).31 However, studies of users of combined conventional and multiple complementary therapies have been varied.24,32–37 None has evaluated the comparative effectiveness of various methods of delivery for complementary and integrative approaches for CLBP in an academic medical center, in which complementary therapies are combined with conventional care delivered by a trained multidisciplinary team, and integration is made transparent through shared medical records and interprofessional referrals.
In 2007, the Brigham and Women's Hospital (BWH) and Harvard Medical School launched the Osher Clinical Center (OCC) for Complementary and Integrative Medical Therapies. The clinic is situated within the ambulatory center of the hospital and can be utilized by any BWH patient without referral, with insurance coverage as provided by their specific policy. The OCC is fully integrated with respect to shared use of electronic medical records and physical plant. OCC clinical services include chiropractic, acupuncture, psychiatry, physician-administered integrative medicine consultations, and multiple mind-body and movement-based therapies. A unique feature of the clinic is its extensive training of an integrated multidisciplinary team of clinicians to enhance interprofessional communication and understanding, and to optimize shared care.38,39 A primary focus of the clinic is the treatment of musculoskeletal pain.
The opening of the OCC clinic provided a unique opportunity to compare the characteristics of CLBP patients attending an integrative care clinic with CLBP patients using other sources of care in the same hospital, and provide preliminary evidence on the effectiveness of an integrative model of care versus usual care on treatment for CLBP. As the first study in this clinical setting and population, we utilized an observational cohort design to compare outcomes of self-selected patients seeking care for CLPB at the OCC, with outcomes of CLBP patients obtaining their treatment at other clinics at BWH. While definitive evaluation of the efficacy of an OCC model of care for treatment of CLBP would require a randomized comparative effectiveness trial, data from this prospective observational study can provide important information regarding characteristics and outcomes of CLBP patients in different treatment settings, which can inform the interpretation of existing studies and the design of any future CLBP trial.
Materials and Methods
Study design
This observational prospective cohort study was designed to examine the characteristics of patients and effectiveness of CLBP care provided by the OCC team compared with CLBP care provided in other settings in the same hospital (non-OCC). All CLBP patients at BWH could choose to receive care at the OCC, either by their personal choice or as a referral from a BWH provider. Both the OCC (n = 156) and the non-OCC (n = 153) patients were treated as per usual care of the treating clinicians. No treatments were provided by the investigators themselves. Patients were observed over a 12-month period. Primary outcomes were CLBP-related disability and bothersomeness of pain (BOP) assessed at 6 months postenrollment. Secondary outcomes included the two primary outcomes also assessed at both 3 months (to assess early effects) and 12 months (to assess maintenance of effects); postenrollment percentages of patients with clinically meaningful (≥30%)31,40 improvements at 3, 6, and 12 months; and additional measures of pain, worrisomeness of pain, mood, quality of life, medication use, and satisfaction with care, assessed at all outcome points.
The study was approved by Partners HealthCare institutional review board and was registered at ClinicalTrials.gov (Identifier NCT01355237).
Participants
Eligibility criteria
To reflect clinical care of CLBP in a real-world setting, eligibility criteria were purposefully broad. Patients were included if they had a diagnosis of non-specific CLBP, or had herniated nucleus pulposus or stenosis. CLBP was defined, as in prior studies, as 3 months or more of chronic low-back pain or 6 months or more of intermittent low-back pain.41 Participants with CLBP related to cancer, fracture, or infection were excluded. Participants were ≥21 years of age and English speaking, and had to agree to three follow-up assessments by phone over a period of 12 months.
Identification, screening, and flow of participants
Participants were enrolled between February 2012 and November 2014. All patients coming to the OCC with a first-time complaint of CLBP completed a clinic intake questionnaire, which provided information on history of back pain, current back pain, medical care received, current activities, and general health, lifestyle, and demographics. On the basis of the clinic baseline questionnaire, clinicians completed an eligibility screen; those eligible were invited to talk with an onsite research assistant to learn more about the study, and if willing, to sign a study consent form.
Potential non-OCC participants were identified using programmed queries of electronic medical records at the hospital. Non-OCC patients were required to have two or more visits to BWH for their CLBP within the past 12 months, with one of these visits within the past 3 months, to increase comparability between the non-OCC and OCC groups with respect to currency of seeking care for CLBP. Permission was obtained from the BWH physician of each initially eligible non-OCC patient to send them a letter of invitation and detailed study information. If interested, a screening interview was administered by phone, and if willing and eligible, based on the same criteria used in the OCC group, a consent form and baseline questionnaire were completed. Participants received $50 for each study assessment.
CLBP treatment
Patients in both the OCC and non-OCC groups received the chosen medical care of their treatment sites: none of the patients in either the OCC or non-OCC group was provided supplemental treatments supported by the study, nor given any advice from the study investigators on treatment strategies to be selected by the OCC or hospital medical teams. In the OCC, the integrative treatments available to be combined with conventional care as delivered by a trained integrative multidisciplinary team included acupuncture, chiropractic, craniosacral therapy, dietary and nutritional consultation, integrative medicine consultation and health coaching, massage and movement therapies, and psychiatric counseling. We recorded self-reported information for both the OCC and non-OCC groups on CLBP treatments received both within and outside BWH. On each follow-up questionnaire (3, 6, and 12 months), both groups were asked whether, in the last 3 months, they had an office visit with a list of types of healthcare providers for treatment of their back pain (primary care physicians, specialists, physical therapists, chiropractors, acupuncturists, massage therapists, and mind-body therapists). We also asked if they had medical procedures (surgeries and injections) or hospitalizations for their back pain during this time, and requested information on current medication use. In addition, for the OCC group, we recorded, from the clinic medical record, the treatment modalities that had been used during this time for their CLBP care in the OCC.
Outcomes
Overview
Follow-up outcome measures were assessed for both groups by telephone at 3, 6, and 12 months postbaseline assessment. The assessment strategy is aligned with guidelines recommended by the “NIH Research Task Force (RTF) on Standards for Chronic Low-Back Pain”41 and parallels batteries of outcomes assessed in recent large-scale clinical trials evaluating complementary and alternative medicine therapies.30,31,42
Primary outcomes
The two co-primary clinical outcomes of this study were change from baseline to 6 months in back-pain related functional status and BOP.31 Back pain-related functional limitation was assessed by the Roland Disability Questionnaire (RDQ),43 modified to 23 (vs. 24 original) items asking about the past week.31 Higher scores (range 0–23) indicate greater functional limitation, and changes on the order of 2.0–3.0 points are generally considered to be clinically meaningful.43,44 The RDQ has demonstrated reliability, validity, and sensitivity to change.45 BOP in the past week was measured on a 0–10 scale (0 indicating “not at all bothersome” and 10 indicating “extremely bothersome”).31 While clinically meaningful differences in BOP have not yet been defined, a difference on the order of 1.5 has been commonly used in previous trials based on clinically meaningful differences in analogous pain scores.46–48 The primary analyses of this study examined adjusted mean change from baseline to 6 months between groups on each of these two measures.
Secondary outcomes
Secondary outcomes included changes in RDQ and BOP at 3 months (to assess early effects) and 12 months (to assess maintenance of effects), as well as the percentage of participants with ≥30% improvement in RDQ and BOP from baseline to 3, 6, and 12 months. This responder analyses threshold has been widely used as an alternative metric of clinically meaningful improvements for both outcomes.30,31,40,47
Analyses also evaluated pain and worrisomeness of back pain, assessed by self-reported severity of symptoms in the previous 7 days using a scale of 0–10, where higher scores reflect greater severity. Physical and mental health-related quality of life was assessed with the 12-item Short-Form Health Survey (SF-12 Physical and SF-12 Mental [0–100 scale; lower scores indicate poorer health status]).49 Participants were also asked about their overall and back-specific exercise during the previous week. Subjects' utilization of healthcare resources for back pain was assessed using self-reported information related to number of visits to conventional and nonconventional physicians and therapists, laboratory and radiologic examinations, procedures and hospitalizations, and use of prescription and over-the-counter medications. Patients' satisfaction with their back pain care was assessed by asking patients to rate how satisfied, overall, they have been with the care they have received from the hospital for back pain (five choices, ranging from 1 = very satisfied to 5 = very dissatisfied). Finally, expectation of improvement on the part of the patient was measured at baseline (0–10 point scale; 0 indicates expectation of “no improvement” and 10 indicates expectation of “complete recovery”).
Additional variables
We also recorded sociodemographic data, back pain history, comorbidities, current and past treatment-related information, and other factors that would help characterize similarities and differences between the OCC and non-OCC group, and could be considered potential confounding variables or effect modifiers in statistical analyses.
Sample size
The RDQ is the gold standard used for determining sample size in chronic back pain studies. Assuming the standard deviation for change in the RDQ to be ∼6.5 points, a sample of 150 per group was estimated to provide more than 80% power for detecting a difference of 2.5 on the RDQ40 for the unadjusted comparison of groups, testing at a nominal level of significance of 0.025 (Bonferroni adjustment for multiple comparisons).
Statistical analysis
We compared baseline characteristics of the OCC and non-OCC groups using Wilcoxon rank-sum tests for ordinal and continuous variables and chi-square tests or exact tests for categorical variables. Study outcomes were measured at three time points (3, 6, and 12 months); the primary outcomes are RDQ and BOP at 6 months. We fit longitudinal regression models using generalized estimating equations with an autoregressive correlation structure to account for within-patient correlations. Linear regression models were used for the primary outcome measures (RDQ and BOP) and other continuous measures. For binary outcomes (including responder analyses of clinically meaningful improvements), we fit modified Poisson regression models with a log link function and a robust sandwich variance estimator. In all models, we included independent variables for group (OCC versus non-OCC), indicators for time point, and group-by-time point interactions.
We fit a sequence of models for the primary outcomes incorporating additional covariates:
Model 1: Age, sex, and outcome baseline value.
Model 2: Model 1 + education and marital status.
Model 3: Model 2 + time since back pain treatment began, number of days with back pain in the last 180 days, and ever received injection for back pain.
Model 4: Model 3 + expectation for improvement.
Model 5: Model 4 + smoking status, body mass index, and exercise.
While race, employment, and litigation also differed significantly between the two groups as seen in Table 1, their inclusion in Model 5 did not change the results and were not included in that model to maximize statistical power.
Table 1.
Baseline Characteristics of Participants in the Osher Clinical Center and the Non-Osher Clinical Center Group
| No. (%)a | |||
|---|---|---|---|
| OCC (n = 156) | Non-OCC (n = 153) | p | |
| Sociodemographic characteristics | |||
| Age, mean (SD), year | 50.2 (16.5) | 52.2 (15.6) | 0.27 |
| Women | 105 (67.3) | 114 (74.5) | 0.16 |
| Education | <0.0001 | ||
| ≤High school | 14 (9.0) | 50 (32.9) | |
| Some college or vocational school | 11 (7.1) | 37 (24.3) | |
| College graduate | 131 (84.0) | 65 (42.8) | |
| Race | <0.0001 | ||
| White | 132 (86.8) | 81 (61.4) | |
| Asian | 4 (2.6) | 2 (1.5) | |
| African American | 12 (7.9) | 48 (36.4) | |
| Other | 4 (2.6) | 1 (0.8) | |
| Hispanic ethnicity | 10 (6.5) | 22 (14.4) | 0.025 |
| Married or living with partner | 97 (63.4) | 47 (36.2) | <0.0001 |
| Employed currently | 108 (70.1) | 57 (38.0) | <0.0001 |
| Back pain history and expectations | |||
| Years since first saw doctor for back pain, mean (SD) | 11.3 (12.1) | 10.7 (11.1) | 0.96 |
| Ever had injection for LBP | 71 (46.1) | 69 (45.1) | 0.86 |
| Back pain in the last 180 days, median (Q1–Q3), days | 179.5 (115–180) | 180 (137.5–180) | 0.12 |
| Improvement expected in next 6 weeks, mean (SD)b | 6.0 (2.7) | 3.0 (3.1) | <0.0001 |
| Office visit with healthcare provider for LBPc | 122 (78.2) | 108 (71.1) | 0.15 |
| Participated in group or guided activities for LBPc | 46 (29.7) | 20 (13.1) | 0.0004 |
| Baseline measures of primary outcome scores | |||
| RDQ (modified), mean (SD)d,e | 12.0 (5.8) | 15.9 (5.8) | <0.0001 |
| BOP, mean (SD)d,f | 6.3 (2.3) | 7.0 (2.1) | 0.016 |
| Baseline measures of secondary outcome scores | |||
| Pain, mean (SD)d,f | 5.8 (2.1) | 6.8 (2.1) | 0.0002 |
| Worried about pain, mean (SD)d,f | 5.9 (2.8) | 6.3 (3.0) | 0.16 |
| SF-12, Physical, mean (SD)g | 41.6 (10.8) | 36.9 (9.2) | <0.0001 |
| SF-12, Mental, mean (SD)g | 47.4 (10) | 43.3 (11.2) | 0.0024 |
| Baseline measures of other secondary outcomes | |||
| Medications, supplements, and topicals for LBPc | 147 (94.2) | 137 (90.1) | 0.18 |
| Opioid use for LBPc | 42 (26.9) | 53 (34.6) | 0.14 |
| Back-specific exercised | 103 (66.5) | 90 (59.2) | 0.19 |
| General exercised | 99 (63.5) | 102 (66.7) | 0.55 |
| Risk factors | |||
| Smoke currently | 10 (6.5) | 30 (19.9) | 0.0005 |
| BMI (kg/m2), mean (SD) | 25.9 (5.3) | 30.6 (8.0) | <0.0001 |
| Involved in litigation | 16 (10.5) | 34 (22.5) | 0.005 |
The numbers of subjects and their within-group percentage are reported for a given variable unless otherwise noted. The prevalence of missing responses for all variables ranged from 0 to <3%, except for race (8.1% missing).
Improvement-expected scores range from 0 to 10 with 0 being no improvement and 10 being complete recovery.
Time range was the previous 3 months.
Time range was the previous 7 days.
Modified RDQ range is from 0 to 23. Higher scores indicate worse function.
Pain bothersomeness, Pain, and Worried about pain used a range of 0–10. Higher values indicate poorer condition.
SF-12 physical and mental scores range from 0 to 100. Lower scores indicate poorer health status and higher ones indicate higher function.
BMI, body mass index; BOP, bothersomeness of pain; LBP, low-back pain; OCC, Osher Clinical Center; RDQ, Roland Disability Questionnaire; SD, standard deviation; SF-12, 12-item Short-Form Health Survey.
For secondary outcomes, we fit only the full Model 5. Based on these longitudinal models, we obtained estimates of the effect of group at each time point and the associated p-value. For continuous outcomes, the effect was the estimated difference in outcome between the integrative clinic and usual care along with a 95% confidence interval for the difference. For binary outcomes, the effect was the estimated relative risk between groups with its 95% confidence interval.
Results
Flow of patients
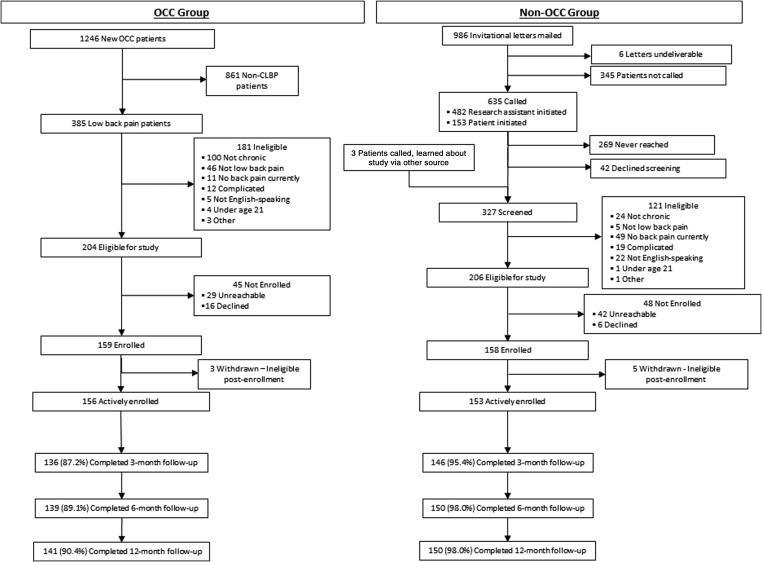
Details of the flow of participants through the study are summarized in Figure 1. A total of 156 OCC patients and 153 non-OCC patients were actively enrolled. Follow-up rates in the OCC and non-OCC groups, respectively, were 87.2% and 95.4% at 3 months, 89.1% and 98.0% at 6 months, and 90.4% and 98.0% at 12 months.
FIG. 1.
Flow of participants through an observational study comparing CLBP treated in a multidisciplinary integrative care team (OCC) with conventional back pain care in the same hospital (non-OCC). CLBP, chronic low-back pain; OCC, Osher Clinical Center.
Baseline characteristics
The baseline characteristics of study participants are summarized in Table 1. The OCC and non-OCC groups were comparable with regard to age, sex, and multiple back pain-related characteristics, including mean time since the subject first saw a doctor for back pain, ever use of injections, number of past 180 days with back pain, worried about back pain, office visit with healthcare provider for back pain, use of medications for back pain, and back-specific and general exercise in the last 7 days. However, baseline scores of the primary outcomes were significantly higher in the non-OCC group for both RDQ (15.9 vs. 12.0) and BOP (7.0 vs. 6.3). The non-OCC group was also racially and ethnically more diverse, and reported significantly higher levels of smoking, body mass index, and involvement in litigation, and lower levels of marriage or living with partner, current employment, education, physical and mental quality of life, and expectations for improvement.
Medical utilization in the OCC and non-OCC groups
As shown in Table 2, over the 12 months of the study, both groups utilized conventional as well as integrative providers. Total provider visits overall were significantly higher in the OCC group, driven primarily by higher primary care provider visits, but the two groups were similar with respect to visits to specialists or physical therapists, or use of medical procedures or hospitalizations. As expected, the OCC group had significantly greater numbers of visits to integrative providers due to their primary treatment at the integrative medicine clinic. Among the OCC group, the average number of visits to the OCC during the study based on medical records was 7.3 (range: 1–32) over the course of 13 weeks (range: 1–59) of active intervention. During the study period, 39% of the OCC group used only integrative medicine, while 61% used both integrative and conventional therapies. Of the non-OCC group, ∼67% used only conventional therapies, 13% used both, 16% used neither, and 3% used only integrative therapies. Among the OCC group, the most commonly used therapies were chiropractic (78%) and acupuncture (26%). Of the 36 (23%) patients who used more than one therapy, the most commonly used combinations were chiropractic plus acupuncture (28%) and chiropractic plus movement therapy (25%).
Table 2.
Mean Number of Self-Reported Provider Visits/Medical Treatments per Subject During 12 Months of Study Participation
| Mean per subjecta,b(SD) | |||
|---|---|---|---|
| OCC (n = 156) | Non-OCC (n = 153) | pc | |
| Conventional | |||
| Total provider visits | 9.08 (16.80) | 8.42 (13.29) | 0.0327 |
| PCP visits | 0.76 (1.77) | 1.20 (1.93) | 0.0002 |
| Specialist visits | 1.37 (2.72) | 1.96 (3.90) | 0.0686 |
| Physical therapy visits | 6.95 (14.58) | 5.26 (11.92) | 0.8124 |
| Medical procedures | 0.88 (2.45) | 1.91 (5.71) | 0.4534 |
| Hospitalizations | 0.11 (0.42) | 0.05 (0.21) | 0.2391 |
| Integrative | |||
| Total provider visits | 9.95 (11.40) | 2.08 (7.85) | <0.0001 |
| Chiropractic | 5.55 (7.81) | 0.97 (5.34) | <0.0001 |
| Acupuncture | 1.95 (4.85) | 0.22 (1.66) | <0.0001 |
| Massage | 2.35 (5.85) | 0.88 (4.69) | <0.0001 |
| Mind body | 0.09 (0.65) | 0.00 (0.00) | |
Means are calculated as the sum of the visits reported by each subject at 3-, 6-, and 12-month follow-ups divided by the total number of subjects.
All means include visits within and outside Brigham and Women's Hospital for both groups.
Groups are compared using a Wilcoxon Rank-Sum (nonparametric) Test.
OCC, Osher Clinical Center; PCP, primary care provider; SD, standard deviation.
Primary outcomes
Adjusted mean differences between groups for the primary outcomes of RDQ and BOP at 6 months are shown in Table 3 with the five models accounting for increasing numbers of covariates. Higher scores on both RDQ and BOP indicate worse clinical condition. A negative adjusted mean difference between OCC and non-OCC groups indicates a greater improvement in the OCC group. For RDQ, the adjusted mean differences in change scores showed statistically significant benefits in Models 1–4, which were attenuated and no longer significant in the full Model 5, which included the full set of potential confounding variables (−0.95, −2.17 to 0.27; p = 0.13). Except for Model 1, none of the benefits observed in RDQ was of the magnitude considered to be clinically meaningful (change in RDQ of 2–3). For BOP, the adjusted mean differences in change scores reflected statistically significant benefits in all models (Model 5: −1.08, −1.68 to 0.47; p = 0.0005), but were in the range considered to be clinically meaningful (change in BOP of 1.5) only in Models 1–3. Across these five models, for both outcomes, there were reductions in effect size as additional variables were controlled, illustrating the importance of adjusting for the substantial differences in baseline characteristics between these groups.
Table 3.
Adjusted Mean Differences Between the Osher Clinical Center and Non-Osher Clinical Center Groups for Primary Outcomes at 6 Months and 95% Confidence Intervals
| Modela | Adjusted mean differenceb(95% CI) | p | |
|---|---|---|---|
| Roland disability | 1 | −2.50 (−3.67 to −1.33) | <0.0001 |
| 2 | −1.78 (−2.95 to −0.62) | 0.003 | |
| 3 | −1.86 (−3.01 to −0.71) | 0.002 | |
| 4 | −1.36 (−2.58 to −0.14) | 0.03 | |
| 5 | −0.95 (−2.17 to 0.27) | 0.13 | |
| BOP | 1 | −2.01 (−2.58 to −1.44) | <0.0001 |
| 2 | −1.60 (−2.16 to −1.04) | <0.0001 | |
| 3 | −1.62 (−2.17 to −1.06) | <0.0001 | |
| 4 | −1.28 (−1.86 to −0.70) | <0.0001 | |
| 5 | −1.08 (−1.68 to −0.47) | 0.0005 |
Models are adjusted with the following variables:
Model 1: age, sex, and outcome baseline value.
Model 2: Model 1 + education and marital status.
Model 3: Model 2 + time since treatment began, number of days of back pain in the last 180 days, and ever received injection for back pain.
Model 4: Model 3 + expectation for improvement.
Model 5: Model 4 + current smoking, BMI, and exercise.
Higher scores on Roland Disability and BOP indicate a worse clinical condition. A negative adjusted mean difference between OCC and non-OCC groups indicates a greater improvement in the OCC group.
BMI, body mass index; BOP, bothersomeness of pain; CI, confidence intervals; OCC, Osher Clinical Center.
Secondary outcomes
Table 4 provides the full Model 5 adjusted mean differences between groups for all secondary outcomes, including RDQ and BOP at 3 months (reflecting short term) and 12 months (reflecting long term) of observation. For RDQ, there was no significant benefit seen at 3 or 6 months, but at 12 months, the benefit was statistically significant and of an effect size considered to be clinically meaningful. For BOP, the adjusted mean differences were statistically significantly increased at all time periods, although not of a magnitude considered clinically meaningful.
Table 4.
Adjusted Mean Differences Between the Osher Clinical Center and Non-Osher Clinical Center Groups for the Primary and Secondary Outcomes at 3, 6, and 12 Months and 95% Confidence Intervals
| Measure | Time | Adjustedamean differenceband 95% CI | p |
|---|---|---|---|
| Primary outcomes | |||
| RDQ | 3 month | −0.30 (−1.47 to 0.87) | 0.61 |
| 6 month | −0.95 (−2.17 to 0.27) | 0.13 | |
| 12 month | −2.08 (−3.36 to −0.81) | 0.001 | |
| BOP | 3 month | −0.73 (−1.35 to −0.12) | 0.02 |
| 6 month | −1.08 (−1.68 to −0.47) | 0.0005 | |
| 12 month | −1.06 (−1.69 to −0.43) | 0.001 | |
| Secondary outcomes | |||
| Pain | 3 month | −0.74 (−1.27 to −0.20) | 0.007 |
| 6 month | −1.06 (−1.62 to −0.50) | 0.0002 | |
| 12 month | −1.18 (−1.75 to −0.61) | <0.0001 | |
| Worry | 3 month | −1.02 (−1.79 to −0.26) | 0.009 |
| 6 month | −1.14 (−1.86 to −0.42) | 0.002 | |
| 12 month | −0.81 (−1.56 to −0.05) | 0.04 | |
| Satisfactionc | 3 month | −0.18 (−0.47 to 0.12) | 0.25 |
| 6 month | −0.14 (−0.43 to 0.15) | 0.36 | |
| 12 month | −0.07 (−0.35 to 0.21) | 0.63 | |
| SF-12 physical scale | 3 month | −1.97 (−3.90 to −0.04) | 0.05 |
| 6 month | 0.23 (−1.72 to 2.18) | 0.82 | |
| 12 month | 0.13 (−1.76 to 2.01) | 0.89 | |
| SF-12 mental scale | 3 month | 3.52 (1.21 to 5.82) | 0.003 |
| 6 month | 2.18 (−0.16 to 4.51) | 0.07 | |
| 12 month | 2.96 (0.67 to 5.25) | 0.01 | |
| Exercised in past week | 3 month | 0.98 (0.84 to 1.13) | 0.74 |
| 6 month | 1.03 (0.89 to 1.19 | 0.73 | |
| 12 month | 1.03 (0.94 to 1.14) | 0.50 | |
| Exercise days | 3 month | 0.49 (−0.11 to 1.09) | 0.11 |
| 6 month | 0.72 (0.12 to 1.31) | 0.02 | |
| 12 month | 0.57 (0.06 to 1.08) | 0.03 | |
| Any Meds (binary) | 3 month | 1.00 (0.89 to 1.12) | 0.98 |
| 6 month | 1.01 (0.90 to 1.14) | 0.82 | |
| 12 month | 0.93 (0.82 to 1.06) | 0.28 | |
| NSAID (binary) | 3 month | 1.25 (0.98 to 1.59) | 0.08 |
| 6 month | 1.06 (0.83 to 1.36) | 0.64 | |
| 12 month | 1.00 (0.77 to 1.30) | 0.99 | |
| Opioids (binary) | 3 month | 0.97 (0.65 to 1.46) | 0.90 |
| 6 month | 1.02 (0.67 to 1.54) | 0.92 | |
| 12 month | 0.81 (0.52 to 1.26) | 0.35 | |
Model 5 is adjusted for age, gender, baseline outcome value, education, married or living with partner, time since first saw doctor for back pain, number of days of back pain in the last 180 days, injections, expectations for improvement, smoke currently, BMI, and exercise.
For RDQ and BOP, higher scores indicate a worse clinical condition, and a negative adjusted mean difference between OCC and non-OCC indicates a greater improvement in the OCC group. For binary outcomes, the values represent risk ratios.
Note: outcome for satisfaction is actual level, not change. There is no adjustment for baseline level.
BMI, body mass index; BOP, bothersomeness of pain; CI, confidence intervals; OCC, Osher Clinical Center; RDQ, Roland Disability Questionnaire; SF-12, 12-item Short-Form Health Survey.
For self-reported severity of back pain and worry related to back pain during the prior week, the adjusted mean differences showed statistically significant, but not clinically meaningful improvement in the OCC versus non-OCC group for all time periods. For SF-12 Mental, there were statistically significant as well as clinically meaningful improvements over all time periods; there were no differences seen for the SF-12 Physical. The OCC group showed statistically significant increases at 12 months in the number of days they engaged in exercise. There were no group differences in other outcomes, including satisfaction with care or reported use of back pain-related medications.
Responder analyses
A common alternative metric of a clinically meaningful result is an improvement of greater or equal to 30% (Table 5).31,40 For RDQ, there was a statistically significantly greater proportion of responders at 12 months in the OCC versus the non-OCC group (27.2% vs. 15.4%) (RR, 1.77 [95% CI, 1.09–2.86]), but not at 3 or 6 months. For BOP, there were statistically significantly greater proportions of responders in the OCC group for all three time periods.
Table 5.
Adjusted Proportions (%) and Relative Risks of Participants in the Osher Clinical Center and Non-Osher Clinical Center Group with Clinically Meaningful Improvements (≥30%) for Primary Outcomes
| Adjusted proportions (95% CI) with clinically meaningful improvements | |||||
|---|---|---|---|---|---|
| Outcome | Time | OCC | Non-OCC | Relative risk (95% CI) | p |
| Roland disability | |||||
| 3 month | 20.0 (13.4–29.9) | 16.3 (10.9–24.3) | 1.23 (0.75–2.02) | 0.41 | |
| 6 month | 23.4 (16.1–34.0) | 20.0 (14.0–28.4) | 1.17 (0.77–1.79) | 0.46 | |
| 12 month | 27.2 (18.8–39.4) | 15.4 (10.4–22.8) | 1.77 (1.09–2.86) | 0.02 | |
| Pain bothersomeness | |||||
| 3 month | 36.1 (27.7–47.1) | 19.9 (14.0–28.2) | 1.82 (1.22–2.71) | 0.003 | |
| 6 month | 37.6 (28.9–48.9) | 19.4 (13.7–27.5) | 1.94 (1.29–2.90) | 0.001 | |
| 12 month | 42.7 (33.3–54.7) | 23.2 (17.1–31.3) | 1.84 (1.29–2.64) | 0.0008 | |
CI, confidence intervals; OCC, Osher Clinical Center.
Discussion
There were substantial differences observed between the CLBP patients seen at the OCC and those seen in other clinics of the same hospital (non-OCC). The OCC and non-OCC groups were comparable with regard to age, sex, and multiple back pain-related characteristics. However, the non-OCC group had a number of other characteristics that put them at a higher risk of poor outcomes, including higher baseline scores for the primary outcomes of RDQ and BOP; racially and ethnically more diverse; lower levels of marriage, employment, education, physical and mental quality of life, and expectations for improvement; and higher levels of smoking, body mass index, and involvement in litigation. These findings clearly illustrate that different groups of people use different sources of healthcare, even for the same clinical condition. This supports that these and other potential confounders are important to consider in the interpretation of findings from any observational study or trial of small sample size, and supports the need for randomized trials of adequate size to definitively evaluate the effectiveness of integrative medical care for the treatment of CLBP.
After including these known baseline differences in the fullest model (Model 5), the adjusted mean improvements in RDQ at 6 months between the OCC and non-OCC groups were not statistically significant nor of a magnitude considered to be clinically meaningful. Similarly, for BOP, while the adjusted mean differences in change scores did reflect statistically significant benefits, they were not in the range considered to be clinically meaningful. For both outcomes, there were reductions in effect size as additional variables were controlled, underscoring the importance of adjusting for the substantial differences in baseline characteristics between these groups in any observational analyses.
With regard to the secondary endpoints of RDQ and BOP at 3 and 12 months, there were no significant improvements on the RDQ seen at 3 months, but the observed benefit at 12 months was statistically significant and of an effect size considered to be clinically meaningful. For BOP, the adjusted mean differences were statistically significantly increased at 3 and 12 months, although not of a magnitude considered clinically meaningful. For the remaining secondary outcomes, self-reported severity of back pain and worry related to back pain during the prior week, there were statistically significant, but not clinically meaningful improvements in the OCC versus non-OCC group for 3, 6, and 12 months; for SF-12 Mental, statistically significant as well as clinically meaningful improvements at all time periods; and no differences for the SF-12 Physical, satisfaction with care, or reported use of back pain-related medications at any time period. Finally, with regard to the secondary endpoint of the proportions of those with clinically meaningful improvements in the two groups (≥30%), for RDQ, there was a statistically significantly greater proportion of responders at 12 months, but not 3 or 6 months, in the OCC versus the non-OCC group; for BOP, there were statistically significantly greater proportions of responders in the OCC group for all three time periods.
A number of trials have evaluated the impact of individual integrative health therapies on functional limitations using the RDQ. The magnitude of our nonsignificant between-group mean difference in RDQ at our primary endpoint of 6 months (−0.95) was smaller than that observed at the primary endpoints of Cherkin's trials of acupuncture (−2.47),29 structural massage (−2.5),30 and MBSR (−1.37).31 In the MBSR study, the relative risk for experiencing a 30% reduction in RDQ was 1.37,31 higher than the relative risk of 1.17 we observed at 6 months. However, we observed an increase in relative risk of 30% improvement in RDQ (1.77) by 12 months. These long-term improvements in functional status contrast with findings in acupuncture, massage, and MBSR, which were stable or had modest declines.29–31 The long-term persistence of effects may be attributable to the explicit emphasis on self-care, which was a primary focus of the team-based training at the OCC.38,39
With regard to BOP, the magnitude of our observed between-group mean difference of integrative therapy at our primary endpoint of 6 months (−1.08) was relatively small, and not clinically significant. However, it is similar to that observed in recent large-scale randomized trials of individual therapies, including acupuncture (−1.05),29 structural massage (−1.4),30 and MBSR (−0.64)31 when compared to usual care. Cherkin et al.' MBSR study also provides comparable data on the proportion of individuals experiencing a 30% reduction in pain bothersomeness.31 At 6 months, they reported a relative risk of reduction in BOP between the MBSR and usual care group of 1.64, which is similar to the relative risk of 1.94 that we observed at the same time point. Of note, in the trials above, the magnitudes of the between-group differences tended to become smaller over time, with the exception of MBSR between-group differences.31 This contrasts with our findings, which observed improvements in BOP which were sustained at 12 months.
Multidisciplinary integrative therapies for CLBP have been evaluated in a limited number of studies. Two observational cohort studies suggested multidisciplinary rehabilitation34 showed promise on quality of life, and a multidisciplinary occupational training program35 on short-term return to work. One trial did not observe benefit for a multidisciplinary rehabilitation program for female CLBP patients,36 but a 2015 meta-analysis24 showed that multidisciplinary biopsychosocial rehabilitation interventions were more effective than usual care in decreasing pain and disability. An economic analysis showed no difference in total costs in a trial evaluating intensive group training versus usual care physiotherapy.37
Limitations
While our analyses controlled for known baseline differences between the OCC- and non-OCC-treated groups, it is possible that other residual confounders, unknown or unmeasurable, could have contributed to our observed group differences in this observational study. We were unable to obtain information about referral patterns to the OCC and other BWH clinics, therefore we cannot rule out the possibility of selection bias. With this limitation in mind, however, our findings suggesting modest improvements in functional status and BOP, along with observed improvements in clinically relevant secondary outcomes of pain severity and pain worrisomeness, support the potential value of evaluating multimodal integrative models for the treatment of CLBP in randomized controlled trials.
Another limitation is that for BOP, one of our two primary outcomes, there are no clear guidelines as to what magnitude of differences in scores would suggest a clinically meaningful outcome. As such, we have chosen to report the observed magnitudes, so they can be compared to other investigations and be judged by clinicians as to their importance. In addition, this study focused on the effectiveness of care delivered by a multimodal integrative care clinic in a single academic center hospital. Larger scale multisite randomized trials, including the range/diversity of clinical care models currently being delivered in academic centers, would be needed to make broader generalizations regarding the effectiveness of multimodal integrative approaches to CLBP.
Finally, our study was not designed to inform optimal treatment strategies or mechanisms underlying observed improvements. It would be informative to systematically evaluate the effectiveness of the most common combinations of therapies used by patients. In our study, the most common therapies among the OCC patients were chiropractic plus acupuncture and chiropractic plus movement therapy.
Conclusions
These findings clearly indicate that baseline characteristics can differ between those who select different sources of healthcare for CLBP. While the benefits on CLBP function and BOP seen in the OCC versus the non-OCC clinics were not large in magnitude, further evaluation might be warranted to test whether a multidisciplinary integrative care model for CLBP that includes cross-disciplinary training and is delivered within an academic hospital may be more effective than usual care. Consideration of its cost-effectiveness, as well as further research evaluating patterns of integrative treatments of CLBP that are most beneficial might also be warranted. To answer such questions, only randomized trials of adequate sample size could provide a more definitive evaluation because of their unique ability to control for potential confounding.
Acknowledgments
This study was supported by grant no. AT005065 from the National Center for Complementary and Integrative Health (NCCIH)/National Institutes of Health (NIH). Dr. Wayne was also supported by an NCCIH-funded K24 (AT009282). The funding source had no role in the collection, management, analysis, or interpretation of data; or preparation, review, or approval of the article. The authors would like to acknowledge the contributions of the clinicians and staff at the Osher Clinic, and the support of the Bernard Osher Foundation, which enabled this investigation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA 1998;280:1569–1575 [DOI] [PubMed] [Google Scholar]
- 2.Wolsko PM, Eisenberg DM, Davis RB, et al. Patterns and perceptions of care for treatment of back and neck pain: Results of a national survey. Spine (Phila Pa 1976) 2003;28:292–297; discussion 298 [DOI] [PubMed] [Google Scholar]
- 3.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 2008;12:1–23 [PubMed] [Google Scholar]
- 4.Black LI, Clarke TC, Barnes PM, et al. Use of complementary health approaches among children aged 4–17 years in the United States: National Health Interview Survey, 2007–2012. Natl Health Stat Report 2015;78:1–19 [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke TC, Nahin RL, Barnes PM, Stussman BJ. Use of complementary health approaches for musculoskeletal pain disorders among adults: United States, 2012. Natl Health Stat Rep 2016;98:1–12 [PubMed] [Google Scholar]
- 6.Murthy V, Sibbritt DW, Adams J. An integrative review of complementary and alternative medicine use for back pain: A focus on prevalence, reasons for use, influential factors, self-perceived effectiveness, and communication. Spine 2015;15:1870–1883 [DOI] [PubMed] [Google Scholar]
- 7.Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003;290:2443–2454 [DOI] [PubMed] [Google Scholar]
- 8.Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain 2010;11:1230–1239 [DOI] [PubMed] [Google Scholar]
- 9.Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res Clin Rheumatol 2010;24:769–781 [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Tsui-Wu Y-J. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987;12:264–268 [DOI] [PubMed] [Google Scholar]
- 11.Buchbinder R, Blyth FM, March LM, et al. Placing the global burden of low back pain in context. Best Pract Res Clin Rheumatol 2013;27:575–589 [DOI] [PubMed] [Google Scholar]
- 12.Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 2004;29:79–86 [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976) 2006;31:2724–2727 [DOI] [PubMed] [Google Scholar]
- 14.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoy D, March L, Brooks P, et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:968–974 [DOI] [PubMed] [Google Scholar]
- 16.Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: Data from the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res 2016;68:1688–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morlion B. Chronic low back pain: Pharmacological, interventional and surgical strategies. Nat Rev Neurol 2013;9:462–473 [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ 2015;350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000;85:317–332 [DOI] [PubMed] [Google Scholar]
- 20.Waddell G. Biopsychosocial analysis of low back pain. Baillieres Clin Rheumatol 1992;6:523–557 [DOI] [PubMed] [Google Scholar]
- 21.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 2002;27:E109–E120 [DOI] [PubMed] [Google Scholar]
- 22.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147:478–491 [DOI] [PubMed] [Google Scholar]
- 23.Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;133:581–624 [DOI] [PubMed] [Google Scholar]
- 24.Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015;350:h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166:514–530 [DOI] [PubMed] [Google Scholar]
- 26.Ghildayal N, Johnson PJ, Evans RL, Kreitzer MJ. Complementary and alternative medicine use in the US adult low back pain population. Glob Adv Health Med 2016;5:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolsko PM, Eisenberg DM, Davis RB, et al. Insurance coverage, medical conditions, and visits to alternative medicine providers: Results of a national survey. Arch Intern Med 2002;162:281–287 [DOI] [PubMed] [Google Scholar]
- 28.Bronfort G, Hondras MA, Schulz CA, et al. Spinal manipulation and home exercise with advice for subacute and chronic back-related leg pain: A trial with adaptive allocation. Ann Intern Med 2014;161:381–391 [DOI] [PubMed] [Google Scholar]
- 29.Cherkin DC, Sherman KJ, Avins AL, et al. A randomized trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Arch Intern Med 2009;169:858–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherkin D, Sherman KJ, Kahn J, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: A randomized, controlled trial. Ann Intern Med 2011;155:1–9, W-1-W-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs. cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: A randomized clinical trial. JAMA 2016;315:1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundberg T, Petzold M, Wandell P, et al. Exploring integrative medicine for back and neck pain—A pragmatic randomised clinical pilot trial. BMC Complement Altern Med 2009;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha FJ, Bruning A, Barcelona C, et al. Integrative medicine for chronic pain: A cohort study using a process-outcome design in the context of a department for internal and integrative medicine. Medicine (Baltimore) 2016;95:e4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang E, Liebig K, Kastner S, et al. Multidisciplinary rehabilitation versus usual care for chronic low back pain in the community: Effects on quality of life. Spine J 2003;3:270–276 [DOI] [PubMed] [Google Scholar]
- 35.Koopman FS, Edelaar M, Slikker R, et al. Effectiveness of a multidisciplinary occupational training program for chronic low back pain: A prospective cohort study. Am J Phys Med Rehabil 2004;83:94–103 [DOI] [PubMed] [Google Scholar]
- 36.Kaapa EH, Frantsi K, Sarna S, Malmivaara A. Multidisciplinary group rehabilitation versus individual physiotherapy for chronic nonspecific low back pain: A randomized trial. Spine (Phila Pa 1976) 2006;31:371–376 [DOI] [PubMed] [Google Scholar]
- 37.van der Roer N, van Tulder M, van Mechelen W, de Vet H. Economic evaluation of an intensive group training protocol compared with usual care physiotherapy in patients with chronic low back pain. Spine (Phila Pa 1976) 2008;33:445–451 [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg DM, Kaptchuk TJ, Post DE, et al. Establishing an integrative medicine program within an academic health center: Essential considerations. Acad Med 2016;91:1223–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor BLDB, Eisenberg DM. Case Study: Osher Clinical Center for Complementary and Integrative Medical Therapies (Chapter 5). Beyond The Checklist: What Else Healthcare Can Learn From Aviation Safety and Teamwork. Ithaca, NY: Cornell University Press, 2013 [Google Scholar]
- 40.Jordan K, Dunn KM, Lewis M, Croft P. A minimal clinically important difference was derived for the Roland Disability Questionnaire for low back pain. J Clin Epidemiol 2006;59:45–52 [DOI] [PubMed] [Google Scholar]
- 41.Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on research standards for chronic low back pain. J Pain 2014;15:569–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman K, Cherkin DC, Wellman RD, et al. A randomized trial comparing yoga, stretching, and a self-care book for chronic low back pain. ARch Intern Med 2011;171:2019–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrick D, Deyo RQ, Atlas SJ, et al. Assessing health-related quality of life in patients with sciatica. Spine 1995;20:1899–1909 [DOI] [PubMed] [Google Scholar]
- 44.Bombardier C, Hayden J, Beaton DE. Minimal clinically important difference. Low back pain: Outcome measures. J Rheumatol 2001;28:431–438 [PubMed] [Google Scholar]
- 45.Roland M, Fairbank J. The roland-morris disabilty Questionnaire and the Oswestery Disability Questionnaire. Spine 2000;25:3115–3124 [DOI] [PubMed] [Google Scholar]
- 46.Farrar JT, Young JP, Jr., LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–158 [DOI] [PubMed] [Google Scholar]
- 47.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90–94 [DOI] [PubMed] [Google Scholar]
- 48.Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: A randomized, controlled trial. Ann Intern Med 2005;143:849–856 [DOI] [PubMed] [Google Scholar]
- 49.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scale and preliminary tests of reliability and validity. Med Care 1996;34:220–223 [DOI] [PubMed] [Google Scholar]