ABSTRACT
Damage-associated molecular patterns (DAMPs), such as extracellular ATP, act as danger signals in response to biotic and abiotic stresses. Extracellular ATP is perceived by a plant purinoceptor, P2 receptor kinase 1 (P2K1), inducing downstream signaling for defense responses. How ATP induces these defense responses has not been well studied. A recent study by Tripathi et al. (Plant Physiology, 176: 511–523, 2018) revealed a synergistic interaction between extracellular ATP and jasmonate (JA) signaling during plant defense responses. This signaling crosstalk requires the formation of secondary messengers, i.e., cytosolic calcium, reactive oxygen species, and nitric oxide. This finding has given a new direction towards understanding the defense signals activated by DAMPs. In this addendum, we discuss possible insights into how extracellular ATP signaling interacts with the JA signaling pathway for plant defense responses.
KEYWORDS: Damage-associated molecular patterns, purinergic signaling, extracellular ATP, jasmonic acid, JAZ proteins, second messengers
All organisms use adenosine-5-triphosphate (ATP) as an essential energy source and as a basic substrate in various intracellular biochemical and signaling reactions.1 In plants, external stimuli, such as wounding or herbivore feeding, can cause the release of ATP from inside (the cytoplasm) to outside the cell (the extracellular matrix). The released ATP is recognized as a signal, a so-called damage-associated molecular pattern (DAMP), to activate plant defense responses.2 Although the role of extracellular ATP as a DAMP in plants has not been well understood, after the discovery of the first purinoceptor in plants, P2 receptor kinase 1 (P2K1), which was originally identified based on the isolation of an ATP-insensitive mutant, dorn1 (does not respond to nucleotide 1) by a forward genetic screening, it is now evident that extracellular ATP acts as an essential signal for plant life cycle activities.3,4 Unlike the purinoceptors in animals, P2K1 is a lectin-receptor kinase (also known as LecRK-I.9). As highlighted in several reviews, accumulating evidence has suggested a role for extracellular ATP in plant growth, development, and stress responses.2,4-7 Additionally, extracellular ATP is categorized as a classical DAMP, in contrast to secondary DAMPs or phytocytokines (e.g., elicitor peptides).8
There are several lines of evidence showing that extracellular ATP is involved in plant defense responses. For example, ATP induces the expression of defense-related genes, e.g., JA- and ethylene-induced genes.9,10 ATP treatment of wounded areas of lima bean leaves mimicked JA-dependent responses.11 Suppression of extracellular ATP-hydrolyzing enzymes, ectoapyrases, caused the accumulation of extracellular ATP, which consequently induced the expression of defense genes. Recent transcriptomic study revealed that approximately 60% of ATP-induced genes are also induced by wounding.3 In addition, gene Ontology enrichment analysis based on the ATP-induced transcriptome suggested that extracellular ATP is involved in a range of plant responses to abiotic and biotic stresses.12 All these studies directly indicate that extracellular ATP acts as a central signal in many plant defense responses.
In contrast, there are several reports indirectly suggesting involvement of extracellular ATP in plant defense responses. For example, some insects (e.g., herbivorous caterpillars and whitefly larvae) were reported to secrete saliva containing ATP-hydrolyzing enzymes.13,14 Application of the caterpillar apyrase blocked glandular trichome production (a plant defense reaction against herbivores) and the expression of defense-related genes at the wound site.14 Furthermore, enhanced apyrase catalytic activity in globose insect galls on Calliandra brevipes was detected.15 A clear explanation is that those apyrases dampen extracellular ATP, thus attenuating the plant defense response. Similar mechanisms for controlling extracellular ATP concentrations by parasitic worms or blood-sucking insects feeding on animals have been described16,17 (see the review by Guiguet et al.18 in detail). Interestingly, a reduction in extracellular ATP levels was observed following plant infection by a bacterial pathogen P. syringae, but not observed using its type III secretion system deficient hrcV mutant,19 suggesting that there are effector proteins for extracellular ATP depletion. Taken together, these observations suggest that pathogens and insects always seek to reduce extracellular ATP levels to elude plant defenses, thereby enhancing subsequent infestation of the plant host.
Using knockout mutants and a P2K1 overexpression line, Balagué et al. showed that P2K1 was involved in increased resistance to a bacterial pathogen, P. syringae, through JA signaling.20 Extracellular ATP is also reported to enhance stomatal immunity against bacterial pathogens,21 in which P2K1 directly controls the activity of an NADPH oxidase, RBOHD, that catalyzes the production of superoxide, a type of reactive oxygen species (ROS). Ectopic expression of P2K1 in Arabidopsis, tobacco, and potato showed enhanced resistance to the oomycete pathogen Phytophthora infestans or P. brassicae.22,23 In a recent study, Tripathi et al. showed the ATP induced resistance against a necrotrophic fungus, Botrytis cinerea, in a P2K1-dependent manner.24 In this case, ATP and JA act synergistically to maximize the plant defense response. All these abovementioned data strongly suggest the essential role of ATP in mediating plant immunity against pathogens via P2K1. However, a dearth of information about the function of P2K1 has restricted our further understanding of ATP signaling.
Although a mechanism for extracellular ATP in plant immunity has not been explored in great detail, Tripathi et al. dissected the downstream signaling pathway of extracellular ATP, which interplays with other signaling pathways of plant defense hormones.24 Data mining using publicly available software identified the P2K1-associated coexpression network containing a set of defense-related genes, some of which encode jasmonate ZIM-domain (JAZ) proteins, key regulators of JA signaling. A reporter-based approach measuring protein stability revealed that the stability of the JAZ1 protein is directly regulated by ATP addition in a proteasome-dependent manner. This protein stability change required the interaction of JAZ1 with a JA receptor, coronatine-insensitive 1 (COI1), which is indispensable for the induction of JA-specific primary transcription factors.25 Interestingly, ATP-induced JAZ1 degradation was not attenuated upon the addition of JA biosynthesis inhibitors or in a JA biosynthesis mutant. These results were corroborated by the finding that ATP does not change the basal levels of JA content, showing the direct interaction of extracellular ATP signaling with JA signaling pathways. In addition, ATP-induced JAZ1 degradation requires the formation of secondary messengers. A synergistic effect of ATP and JA on plant defense is attributed to direct activation of JA signaling by ATP-induced changes in the signatures of secondary messengers. These results demonstrate that ATP activates stress hormone signaling, which eventually activates additional defense systems.
In summary, the proposed model (Fig. 1) indicates crosstalk between extracellular ATP and JA signaling for plant defense responses, where intracellular ATP molecules are released into the extracellular matrix upon damage caused by pathogen attack. The released ATP acts as a danger signal, which binds to its receptor P2K1, followed by the formation of secondary messenger signatures: Ca2+, NO and ROS. This extracellular ATP signaling via P2K1 enhances the COI1-JAZ protein interaction and ultimately activates JA-signaling-mediated gene expression for plant defense responses. This event primes the JA-dependent defense responses prior to JA accumulation upon pathogen attack.
Figure 1.
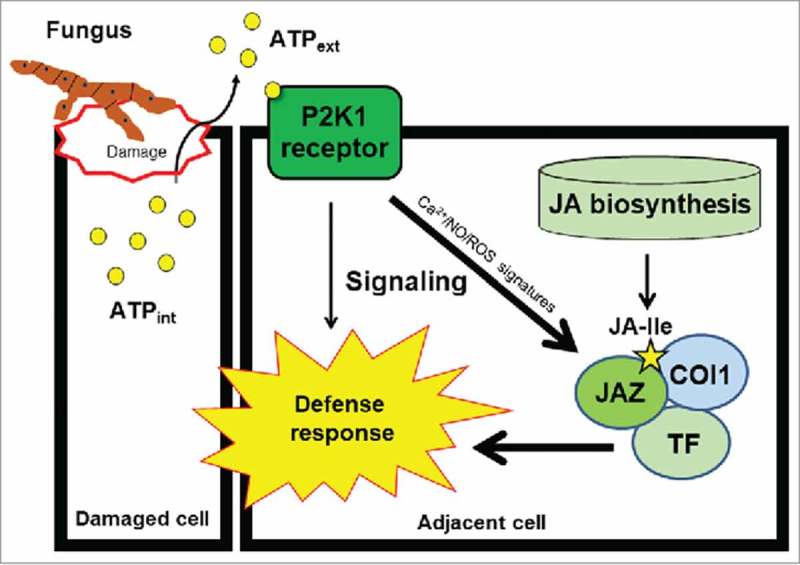
A proposed model of the interaction between extracellular ATP signaling and JA signaling in plants based on Tripathi et al. (2018).24 Extracellular ATP, released after cellular damage by pathogen attack, binds to the P2K1 receptor on the plasma membrane. The activated P2K1 receptor turns on extracellular ATP signaling, which directly enhances JA signaling by changing the interaction of the COI1-JAZ1 complex. Eventually, P2K1-mediated ATP signaling induces the defense response against pathogens by crosstalk with the JA-mediated pathway in addition to the ATP-specific pathway. This ATP-activated JA signaling requires the formation of second messengers (Ca2+, ROS, and NO) and is probably a bypass event that occurs before bona fide JA synthesis to prime plant defense responses. TF: transcription factor.
Further studies, e.g., identifying the modifications of COI1 and/or JAZ1 proteins upon ATP addition, can help determine how the COI1-JAZ1 complex forms upon activation of extracellular ATP signaling, which depends on the formation of secondary messenger signatures.
Additionally, Tripathi et al. showed that salicylic acid (SA)-dependent genes were induced by extracellular ATP.24 Given that ATP treatment does not alter SA levels,19,24 there is the possibility of direct activation of SA signaling by extracellular ATP signaling via secondary messengers, as seen in the case of the crosstalk between extracellular ATP and JA signaling, which needs to be further explored in the future.
Funding Statement
National Science Foundation, IOS-1557813.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This project was supported by the National Science Foundation (grant no. IOS-1557813 to K.T.). PPNS No. 0757, Department of Plant Pathology, College of Agriculture, Human and Natural Resource Sciences, Agricultural Research Center, Hatch Project No. WNP00833, Washington State University, Pullman, WA, 99164–6430, USA.
References
- 1.Khakh BS & Burnstock G. The double life of ATP. Sci Am. 2009; 301:84–90, 92. doi: 10.1038/scientificamerican1209-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka K, Choi J, Cao Y & Stacey G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 2014;5:446 doi: 10.3389/fpls.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J et al.. Identification of a plant receptor for extracellular ATP. Science. 2014;343:290–294. doi: 10.1126/science.343.6168.290. [DOI] [PubMed] [Google Scholar]
- 4.Choi J, Tanaka K, Liang Y, Cao Y, Lee SY, Stacey G. Extracellular ATP, a danger signal, is recognized by DORN1 in Arabidopsis. Biochem J. 2014;463:429–437. doi: 10.1042/BJ20140666. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K, Gilroy S, Jones AM & Stacey G. Extracellular ATP signaling in plants. Trends Cell Biol. 2010;20:601–608. doi: 10.1016/j.tcb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chivasa S & Slabas AR. Plant extracellular ATP signalling: new insight from proteomics. Mol Biosyst. 2012;8:445. doi: 10.1039/C1MB05278K. [DOI] [PubMed] [Google Scholar]
- 7.Clark GB, Morgan RO, Fernandez M-P, Salmi ML & Roux SJ. Breakthroughs spotlighting roles for extracellular nucleotides and apyrases in stress responses and growth and development. Plant Sci. 2014;225:107–116. doi: 10.1016/j.plantsci.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Gust AA, Pruitt R, Nürnberger T. Sensing Danger: Key to Activating Plant Immunity. Trends Plant Sci. 2017;22:779–791. doi: 10.1016/j.tplants.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ. Evidence of a Novel Cell Signaling Role for Extracellular Adenosine Triphosphates and Diphosphates in Arabidopsis. Plant Cell. 2004;16:2652–2664. doi: 10.1105/tpc.104.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ. Extracellular ATP Induces the Accumulation of Superoxide via NADPH Oxidases in Arabidopsis. Plant Physiol. 2006;140:1222–1232. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heil M, Ibarra-Laclette E, Adame-Álvarez RM, Martínez O, Ramirez-Chávez E, Molina-Torres J, Herrera-Estrella L. How Plants Sense Wounds: Damaged-Self Recognition Is Based on Plant-Derived Elicitors and Induces Octadecanoid Signaling. PLoS ONE. 2012;7:e30537. doi: 10.1371/journal.pone.0030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Tanaka K, Nguyen CT, Stacey G. Extracellular ATP is a central signaling molecule in plant stress responses. Curr Opin Plant Biol. 2014;20:82–87. doi: 10.1016/j.pbi.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Su Y-L, Li JM, Li M, Luan JB, Ye XD, Wang XW, Liu SS. Transcriptomic Analysis of the Salivary Glands of an Invasive Whitefly. PLoS ONE. 2012;7:e39303. doi: 10.1371/journal.pone.0039303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Peiffer M, Luthe DS, Felton GW. ATP Hydrolyzing Salivary Enzymes of Caterpillars Suppress Plant Defenses. PLoS ONE. 2012;7:e41947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detoni M, de L, et al.. Galls from Calliandra brevipes BENTH (Fabaceae: Mimosoidae): evidence of apyrase activity contribution in a plant - insect interaction. Aust J Bot. 2012;60:559. doi: 10.1071/BT12096. [DOI] [Google Scholar]
- 16.Valenzuela JG, Belkaid Y, Rowton E, Ribeiro JM. The salivary apyrase of the blood-sucking sand fly Phlebotomus papatasi belongs to the novel Cimex family of apyrases. J Exp Biol. 2001;204:229–237. [DOI] [PubMed] [Google Scholar]
- 17.Da'dara AA, Bhardwaj R, Skelly PJ. Schistosome apyrase SmATPDase1, but not SmATPDase2, hydrolyses exogenous ATP and ADP. Purinergic Signal. 2014;10:573–580. doi: 10.1007/s11302-014-9416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiguet A, Dubreuil G, Harris MO, Appel HM, Schultz JC, Pereira MH, Giron D. Shared weapons of blood- and plant-feeding insects: Surprising commonalities for manipulating hosts. J Insect Physiol. 2016;84:4–21. doi: 10.1016/j.jinsphys.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Chivasa S, Murphy AM, Hamilton JM, Lindsey K, Carr JP, Slabas AR. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009;60:436–448. doi: 10.1111/j.1365-313X.2009.03968.x. [DOI] [PubMed] [Google Scholar]
- 20.Balagué C, Gouget A, Bouchez O, Souriac C, Haget N, Boutet-Mercey S, Govers F, Roby D, Canut H. The Arabidopsis thaliana lectin receptor kinase LecRK-I.9 is required for full resistance to Pseudomonas syringae and affects jasmonate signalling. Mol Plant Pathol. 2017;18(7):937–948. doi: 10.1111/mpp.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Cao Y, Li H, Kim D, Ahsan N, Thelen J, Stacey G. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat Commun. 2017;8:2265. doi: 10.1038/s41467-017-02340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouwmeester K, de Sain M, Weide R, Gouget A, Klamer S, Canut H, Govers F. The Lectin Receptor Kinase LecRK-I.9 Is a Novel Phytophthora Resistance Component and a Potential Host Target for a RXLR Effector. PLoS Pathog. 2011;7:e1001327. doi: 10.1371/journal.ppat.1001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouwmeester K, Han M, Blanco-Portales R, Song W, Weide R, Guo LY, van der Vossen EA, Govers F. The Arabidopsis lectin receptor kinase LecRK-I.9 enhances resistance to Phytophthora infestans in Solanaceous plants. Plant Biotechnol J. 2014;12:10–16. doi: 10.1111/pbi.12111. [DOI] [PubMed] [Google Scholar]
- 24.Tripathi D, Zhang T, Koo AJ, Stacey G, Tanaka K. Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiol. 2018;176:511–523. doi: 10.1104/pp.17.01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al.. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]


