ABSTRACT
Recent studies have shown that it is possible to engineer substantial increases in triacylglycerol (TAG) content in plant vegetative biomass, which offers a novel approach for increasing the energy density of food, feed, and bioenergy crops or for creating a sink for the accumulation of unusual, high-value fatty acids. However, whether or not these changes in lipid metabolism affect plant responses to biotic and/or abiotic stresses is an open question. Here we show that transgenic Arabidopsis thaliana plant lines engineered for elevated leaf oil content, as well as lines engineered for accumulation of unusual conjugated fatty acids in leaf oil, had similar short-term responses to heat stress (e.g., 3 days at 37°C) as wild-type plants, including a reduction in polyunsaturated fatty acid (PUFA)-containing polar lipids and an increase in PUFA-containing neutral lipids. At extended time periods (e.g., 14 days at 37°C), however, plant lines containing accumulated conjugated fatty acids displayed earlier senescence and plant death. Further, no-choice feeding studies demonstrated that plants with the highest leaf oil content generated cabbage looper (Trichoplusia ni) insects with significantly heavier body weights. Taken together, these results suggest that biotic and abiotic responses will be important considerations when developing and deploying high-oil-biomass crops in the field.
KEYWORDS: Biofuel, cabbage looper, conjugated fatty acid, heat stress, oil in leaves
Plant oils are valuable agricultural commodities that are typically used for nutritional purposes, but given the increased demand for biofuels and bioproducts, there is significant interest in developing novel strategies for producing high amounts of oil in non-food crop plants.1 One promising approach is to increase the neutral lipid content in the leaves and stems of plants, which dominate the harvestable biomass of most crops. Although these organs typically lack appreciable amounts of oil, recent studies have shown that plants are remarkably amenable to metabolic engineering strategies that make them “fat”, with increases in leaf triacylglycerol (TAG) content up to 30% oil (dry weight) in tobacco leaves.2-4 Further, leaf oil has been recently shown to serve as an efficient sink for the accumulation of unusual, high-value fatty acids, which expands the number and types of oils that can be produced in the vegetative biomass of plants.5,6
While these results show great promise for developing high-oil, high-biomass crops, there is little information available on how these plants might respond to adverse field conditions. Given that TAG metabolism is involved in multiple aspects of plant growth, development, and stress responses,7,8 and also influences the caloric value of food,9 we were interested in understanding how high-leaf-oil transgenic plant lines respond to elevated temperature and insect predation. Toward this end, we employed three plant lines from our previous study,6 including i) wild-type Arabidopsis thaliana, ecotype Columbia (WT); ii) the same line transformed with constitutively-expressed tung (Vernicia fordii) tree diacylglycerol acyltransferase 2 (WT/DGAT2), which catalyzes the final, committed step in TAG biosynthesis10,11 and iii) the WT/DGAT2 line transformed with a constitutively-expressed tung fatty acid conjugase (WT/DGAT2/FADX), which synthesizes eleostearic acid (ESA), an unusual, conjugated fatty acid that has a variety of both industrial and nutritional end-uses.12,13
As shown in Fig. 1, expression of tung DGAT2 in control (i.e., non-heat stressed), 35-day-old Arabidopsis leaves elevated neutral lipid content 8-fold above WT, while co-expression of DGAT2 and FADX resulted in a 36-fold increase in neutral lipids (compare 0 hour values in Fig. 1). These results are consistent with data reported by Yurchenko et al.,6 who showed that co-expression of tung FADX and DGAT2 resulted in a synergistic increase in oil content and accumulation of ESA in Arabidopsis leaves. As shown also in Fig. 1, exposure of plants to high temperature (37°C) over a 24-hour period resulted in additional increases in neutral lipid content in all three plant lines. However, despite the 8- and 36-fold differences in starting neutral lipid contents, each plant line accumulated approximately similar amounts of neutral lipid by the end of the heat stress, i.e., an additional 2–3 µg neutral lipid per mg dry weight (Fig. 1). This increase in neutral lipid content was paralleled by decreases in polar lipid content in all three plant lines (Fig. 2A). Further investigation of fatty acid composition showed that the neutral lipids accumulating in response to elevated temperature were predominantly enriched in polyunsaturated fatty acids (PUFAs), e.g., 18:2 and 18:3 fatty acids, while the polar lipids were generally reduced in these same fatty acids (Fig. 2B). These trends are similar to other studies of heat responses in plants and are consistent with membrane remodeling processes, whereby membrane destabilizing lipids such as PUFA-containing monogalactosyl diacylglycerols are converted to diacylglycerols (DAGs), which are subsequently converted to TAG and stored in cytoplasmic lipid droplets.14-17 These alterations in polar lipid composition help to maintain the overall fluidity and integrity of cellular membranes, a process referred to as homeoviscous adaptation.14-17 Thus, the engineered plant lines appear to fully retain the capacity to adapt at the biochemical level to elevated temperature, despite their large, pre-existing differences in neutral lipid content. Furthermore, the increase in neutral lipid content in response to heat stress also increased the accumulation of ESA (Fig. 2B, bottom left panel), suggesting that the DAG utilized for TAG synthesis can also serve as a substrate for incorporation of ESA.
Figure 1.
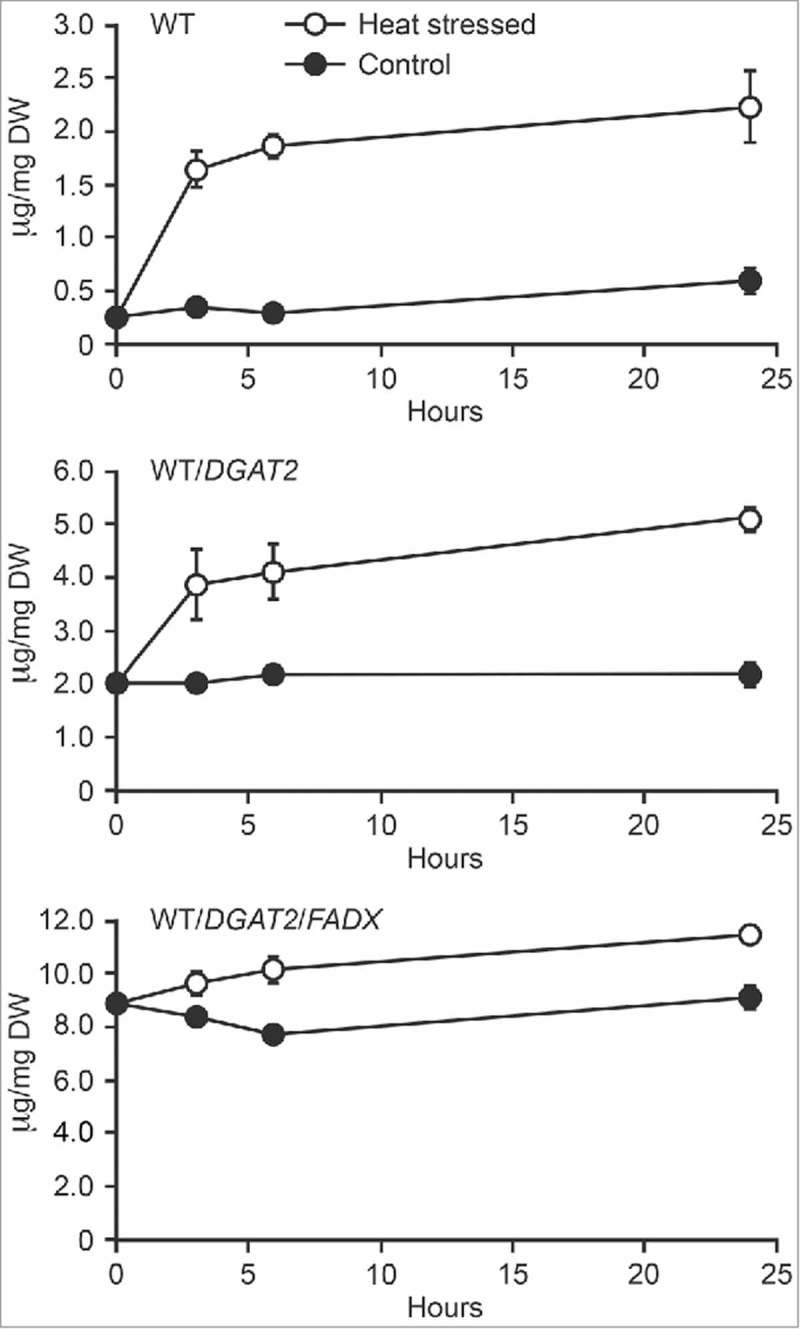
Changes in total neutral lipid content in response to heat stress. Arabidopsis plants of the indicated lines were grown in a growth chamber with a 16 h/8 h day-night cycle at 22°C/20°C, respectively. Then, after 35 days, a portion of the plants from each line were transferred to a second chamber with the same light cycle, but at a 37°C/33°C temperature regime. Fully-expanded leaves from each line were harvested and lipids extracted at 3, 6 and 24 hours, as indicated, then fractionated into neutral lipid and polar lipid classes and analyzed by gas chromatography with flame ionization detection. Values represent the means and standard error of the means based on a pooled estimate of the variance among replicates (n = 2).
Figure 2.
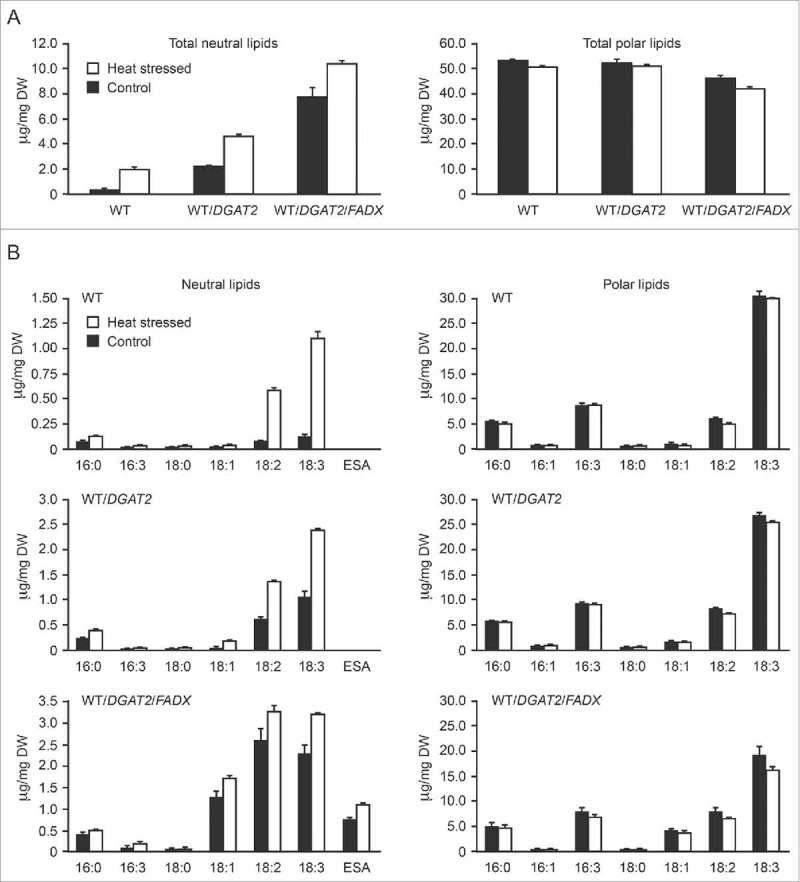
Comparison of changes in total neutral and polar lipid content (A) or fatty acid composition (B) after six hours of control or heat treatment. Arabidopsis plants from each of the three lines (as indicated) were cultivated and analyzed as described in the legend for Fig. 1. Values represent the means and standard error of the means based on a pooled estimate of the variance among replicates (n = 2).
As shown in Fig. 3, both engineered plant lines, i.e., WT/DGAT2 and WT/DGAT2/FADX, displayed similar overall morphologies in comparison to WT plants after three days of prolonged heat stress. After 14 days, however, WT/DGAT2/FADX plants showed more pronounced yellowing, necrosis and plant death in comparison to WT or WT/DGAT2 plants (Fig. 3).
Figure 3.

Changes in plant morphology in response to prolonged heat stress. Arabidopsis plants from each line (as indicated) were cultivated and heat stressed as described in the legend of Fig. 1. Pictures were taken at daily intervals to monitor susceptibility to heat stress, and images shown for 3 and 14 days are representative of four independent replicates. Note that two independent transgenic lines were used to evaluate the WT/DGAT2/FADX genotype/transgene combination.6
Taken together, the results from biochemical and physiological studies (Fig. 1–3) suggest that the engineered plant lines have similar short-term responses to elevated temperature as WT plants, and this abiotic stress treatment can even increase the yields of oil and ESA in vegetative biomass. At extended time periods, however, the engineered lines containing ESA showed greater susceptibility to heat stress.
To determine whether the high-leaf-oil plant lines might also be more or less susceptible to biotic stress, we conducted no-choice feeding studies whereby newly-hatched cabbage looper (Tricholplusia ni) caterpillars, a generalist lepidopteran herbivore, were fed a diet of WT or engineered plants and then weighed after 8 days.18 As shown in Fig. 4, insects cultivated on WT/DGAT2/FADX plants, the highest oil and only ESA-containing line (Fig. 1 and 2), were significantly heavier than insects fed on WT or WT/DGAT2 plants. This indicates that the increased oil content and perhaps the resulting increased energy density may have caused the insects to perform better; however, multiple factors, such as changes in stress signaling molecules such as jasmonate (which are oxylipins derived from fatty acids), or specialized metabolites with anti-herbivory properties (e.g., glucosinolates), could have contributed to the final outcome19,20 and, thus the exact reason needs to be further clarified. Still, these and other studies9 suggest that elevating leaf oil content might alter plant-pest interactions and be an important consideration when developing and testing these kinds of crops in the field. One possibility for protecting high-oil crops from animal predation might be to engineer higher percentages of unusual fatty acids in leaf oil, which might render the oil less digestible or cause other physiological disturbances.21 For instance, our WT/DGAT2/FADX line contains approximately 12% ESA in the neutral lipid fraction of leaves, while seed oil of the tung tree contains about 80% ESA.22 Notably, many plant species are known to produce seed oils containing up to 80% of a single unusual fatty acid22 and some of these fatty acids, such as the hydroxy fatty acids in castor bean seed oil, are known to act as laxatives and can induce labor in pregnant females.21 Whether such alterations in leaf oil composition might discourage animal predation remains to be determined and this would, of course, be applicable only in those cases where high-oil crops are engineered for production of industrial feedstocks or biofuels, not for food or feed.
Figure 4.
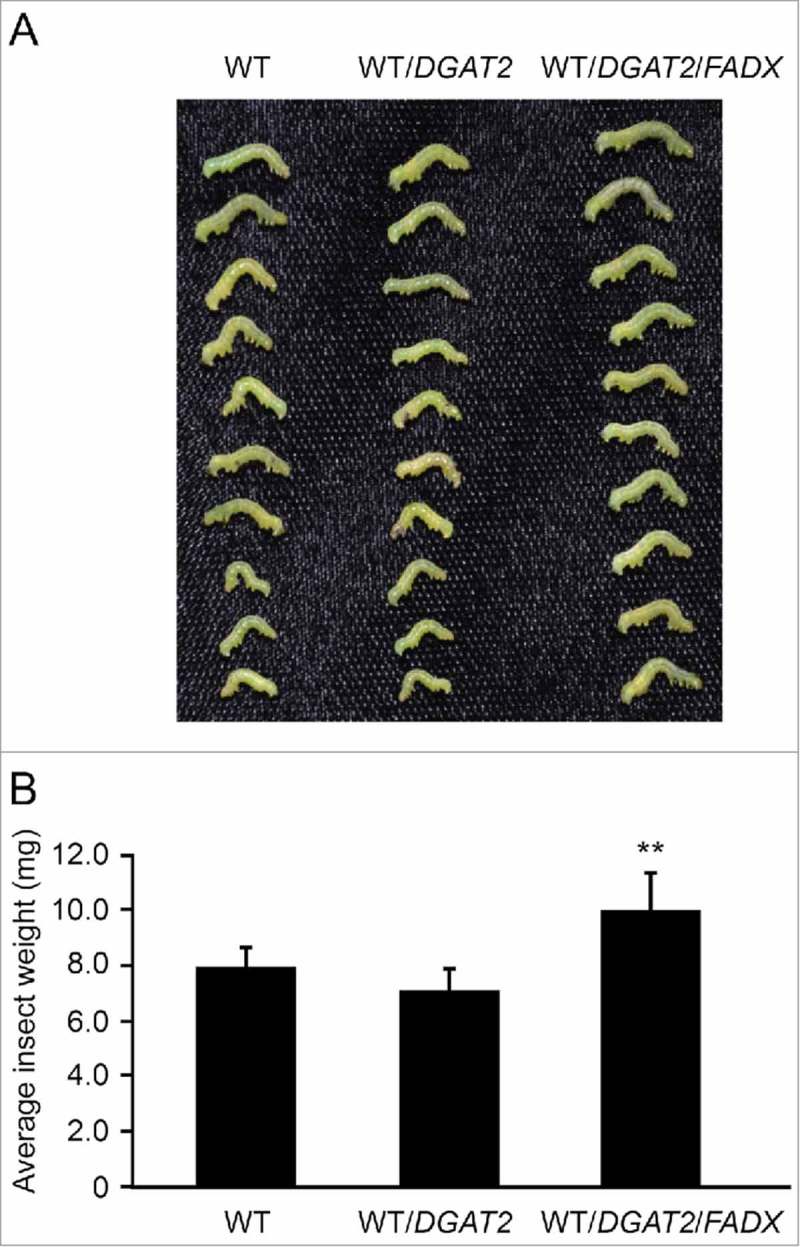
Comparison of cabbage looper caterpillar weights after cultivation on WT, WT/DGAT2 or WT/DGAT2/FADX plant lines. Newly-hatched cabbage looper (Trichoplusia ni) caterpillars were allowed to feed on individual 3-4-week-old Arabidopsis WT, WT/DGAT2, or WT/DGAT2/FADX plant lines for 8 days after which the insects were recovered and individually weighed. Shown are representative images of insects cultivated on the different plant lines (A) and quantification of average insect weights (B). (Mean ± standard deviation, n>50; asterisks denote significant difference in comparison to WT [(P ≤ 0.01]).
Finally, there is increasing evidence that TAGs and lipid droplets in leaves play important roles during both biotic and abiotic stress responses, providing substrates for lipid signaling pathways, a cellular platform for remodeling membrane lipids, or serving as centers for organizing proteins involved in stress responses.7,8,23,24 As such, it is likely that new lipid droplet proteins will be identified in the future that, when co-expressed in high-leaf-oil lines, might render the plants more resistant to biotic and/or abiotic stresses.
Materials and methods
Heat treatment and analysis of lipids
Arabidopsis thaliana plant lines included ecotype Columbia (WT), the same line transformed with tung DGAT2 (WT/DGAT2), or the WT/DGAT2 line transformed with tung FADX (WT/DGAT2/FADX).6 Plants were grown in pots in soil and randomized in a growth chamber, with a 16 h/8 h day-night cycle at 22°C/20°C. After 35 days, a portion of the plants from each line were transferred to a second chamber with the same light conditions, but a 37°C/33°C temperature regime. Six to eight fully expanded leaves, corresponding to one biological replicate (approximately 500 mg fresh weight), were harvested from each plant line at 3, 6 and 24 hours, then samples were snap-frozen in liquid nitrogen and stored at −80°C until used. Two biological replicates were used for the experiments. Internal standards including 17:0 TAG (Sigma-Aldrich, St. Louis, MO) and 15:0 phosphatidylcholine (Avanti, Alabaster, AL) were added, then total lipids were extracted using a hot isopropanol method,7 which suppresses phospholipase activity.25 Total lipids were separated into polar and neutral lipid fractions using solid-phase extraction cartridges, then fatty acid methyl esters were prepared using sodium methoxide.6 Fatty acids were identified and quantified by gas chromatography with flame ionization detection, using the appropriate internal standard as a reference. Data are reported as means and standard error of the means based on a pooled estimate of the variance among replicates (n = 2).
Insect feeding studies
Cabbage looper caterpillar feeding studies were carried out essentially as described.18 Briefly, newly-hatched cabbage looper (Trichoplusia ni) caterpillars (n>50) were allowed to feed on individual 3-4-week-old Arabidopsis WT, WT/DGAT2, or WT/DGAT2/FADX plants lines for 8 days in a growth chamber maintained at 21°C with a 10 h/14 h day-night cycle. Thereafter, insects were recovered and individually weighed. Student's t-tests were used to determine significant difference of weights of insects grown on WT/DGAT2 or WT/DGAT2/FADX plants in comparison to WT plants (P ≤ 0.01).
Funding Statement
This work was supported by grants from the US Department of Energy (DOE) Office of Science, BES-Physical Biosciences program (DE-SC0016536), the National Science Foundation (IOS-1557439), and the Natural Sciences and Engineering Research Council of Canada (217291-2013). Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). The USDA is an equal opportunity provider and employer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ms. Brenda Singleton for excellent technical assistance, Dr. Bruce Mackey for help with statistical analysis, and Dr. Satinder Gidda for critical reading of the manuscript.
References
- 1.Horn PJ, Benning C. The plant lipidome in human and environmental health. Science. 2016;353:1228–32. doi: 10.1126/science.aaf6206. PMID:27634522. [DOI] [PubMed] [Google Scholar]
- 2.Chapman KD, Dyer JM, Mullen RT. Commentary: why don't plant leaves get fat? Plant Sci. 2013;207:128–34. doi: 10.1016/j.plantsci.2013.03.003. PMID:23602107. [DOI] [PubMed] [Google Scholar]
- 3.Xu C, Shanklin J. Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu Rev Plant Biol. 2016; 67:179–206. doi: 10.1146/annurev-arplant-043015-111641. PMID:26845499. [DOI] [PubMed] [Google Scholar]
- 4.Vanhercke T, Divi UK, El Tahchy A, Liu Q, Mitchell M, Taylor MC, Eastmond PJ, Bryant F, Mechanicos A, Blundell C, Zhi Y, Belide S, Shrestha P, Zhou XR, Ral JP, White RG, Green A, Singh SP, Petrie JR. Step changes in leaf oil accumulation via iterative metabolic engineering. Metab Eng. 2017;39:237–46. doi: 10.1016/j.ymben.2016.12.007. PMID:27993560. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds KB, Taylor MC, Cullerne DP, Blanchard CL, Wood CC, Singh SP, Petrie JR. A reconfigured Kennedy pathway which promotes efficient accumulation of medium-chain fatty acids in leaf oils. Plant Biotechnol J. 2017;15:1397–408. doi: 10.1111/pbi.12724. PMID:28301719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yurchenko O, Shockey JM, Gidda SK, Silver MI, Chapman KD, Mullen RT, Dyer JM. Engineering the production of conjugated fatty acids in Arabidopsis thaliana leaves. Plant Biotech J 2017; 15:1010–23. doi: 10.1111/pbi.12695. PMID:28083898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gidda SK, Park S, Pyc M, Yurchenko O, Cai Y, Wu P, Andrews DW, Chapman KD, Dyer JM, Mullen RT. Lipid droplet-associated proteins (LDAPs) are required for the dynamic regulation of neutral lipid compartmentation in plant cells. Plant Physiol. 2016;170:2052–71. doi: 10.1104/pp.15.01977. PMID:26896396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Benning C. Functions of triacylglycerols during plant development and stress. Curr Opin Biotechnol. 2017;49:191–8. doi: 10.1016/j.copbio.2017.09.003. PMID:28987914. [DOI] [PubMed] [Google Scholar]
- 9.Sanjaya Miller R, Durrett TP, Kosma DK, Lydic TA, Muthan B, Koo AJ, Bukhman YV, Reid GE, Howe GA, Ohlrogge J, Benning C. Altered lipid composition and enhanced nutritional value of Arabidopsis leaves following introduction of an algal diacylglycerol acyltransferase 2. Plant Cell. 2013;25:677–93. doi: 10.1105/tpc.112.104752. PMID:23417035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 2006;18:2294–313. doi: 10.1105/tpc.106.043695. PMID:16920778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Siloto RM, Lehner R, Stone SJ, Weselake RJ. Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog Lipid Res. 2012;51:350–77. doi: 10.1016/j.plipres.2012.06.001. PMID:22705711. [DOI] [PubMed] [Google Scholar]
- 12.Sonntag NOV. Composition and characteristics of individual fats and oils. In: Swern D, editor Bailey's industrial oil and fat products. New York: (USt: ): John Wiley & Sons; 1979. p. 289–477. [Google Scholar]
- 13.Yuan GF, Chen XE, Li D. Conjugated linolenic acids and their bioactivities: a review. Food Funct. 2014;5:1360–8. doi: 10.1039/c4fo00037d. PMID:24760201. [DOI] [PubMed] [Google Scholar]
- 14.Légeret B, Schulz-Raffelt M, Nguyen HM, Auroy P, Beisson F, Peltier G, Blanc G, Li-Beisson Y. Lipidomic and transcriptomic analyses of Chlamydomonas reinhardtii under heat stress unveil a direct route for the conversion of membrane lipids into storage lipids. Plant Cell Environ. 2016;39:834–47. doi: 10.1111/pce.12656. PMID:26477535. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Shen W, Zheng Q, Fowler DB, Zou J. Adjustments of lipid pathways in plant adaptation to temperature stress. Plant Signal Behav. 2016;11:e1058461. doi: 10.1080/15592324.2015.1058461. PMID:26734889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayanan S, Tamura PJ, Roth MR, Prasad PV, Welti R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016;39:787–803. doi: 10.1111/pce.12649. PMID:26436679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller SP, Unger M, Guender L, Fekete A, Mueller MJ. Phospholipid:diacylglycerol acyltransferase-mediated triacylglyerol synthesis augments basal thermotolerance. Plant Physiol. 2017;175:486–97. doi: 10.1104/pp.17.00861. PMID:28733391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herde M, Koo AK, Howe G. Elicitation of jasmonate-mediated defense responses by mechanical wounding and insect herbivory In: Goossens A, Pauwels L, editors. Jasmonate signaling, Vol 1011 New York: (US: ): Humana Press; 2013. p. 51–61. [DOI] [PubMed] [Google Scholar]
- 19.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. PMID:18031220; . [DOI] [PubMed] [Google Scholar]
- 20.Koo AJ. Metabolism of the plant hormone jasmonate: a sentinel for tissue damage and master regulator of stress response. Phytochem Rev. 2017;17:51–80. doi: 10.1007/s11101-017-9510-8 [DOI] [Google Scholar]
- 21.Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci U S A. 2012;109:9179–84. doi: 10.1073/pnas.1201627109. PMID:22615395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badami RC, Patil KB. Structure and occurrence of unusual fatty acids in minor seed oils. Prog Lipid Res. 1980;19:119–53. doi: 10.1016/0163-7827(80)90002-8. PMID:7033990. [DOI] [PubMed] [Google Scholar]
- 23.Kim EY, Park KY, Seo YS, Kim WT. Arabidopsis small rubber particle protein homolog SRPs play dual roles as positive factors for tissue growth and development and in drought stress responses. Plant Physiol. 2016;170:2494–510. doi: 10.1104/pp.16.00165. PMID:26903535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyc M, Cai Y, Greer MS, Yurchenko O, Chapman KD, Dyer JM, Mullen RT. Turning over a new leaf in lipid droplet biology. Trends Plant Sci. 2017;22:596–609. doi: 10.1016/j.tplants.2017.03.012. PMID:28454678. [DOI] [PubMed] [Google Scholar]
- 25.de la Roche IA, Andrews CJ. Changes in phospholipid composition of a winter wheat cultivar during germination at 2 C and 24 C. Plant Physiol. 1973;51:468–73. doi: 10.1104/pp.51.3.468. PMID:16658353. [DOI] [PMC free article] [PubMed] [Google Scholar]


