Abstract
Background:
Single ventricle heart disease (SVHD) adolescents show cognitive impairments and anxiety and depressive symptoms, indicating the possibility of brain injury in regions that control these functions. However, brain tissue integrity in cognition, anxiety, and depression regulatory sites in SVHD remains unclear. We examined brain tissue changes in SVHD compared to controls using T2-relaxometry procedures, which measure free water content and show tissue injury.
Methods:
Proton-density and T2-weighted images, using a 3.0-Tesla MRI, as well as anxiety (Beck anxiety inventory [BAI]), depressive symptoms (patient health questionnaire-9 [PHQ-9]), and cognition (wide range assessment of memory and learning 2 [WRAML2] and Montreal cognitive assessment [MoCA]) data were collected from 20 SVHD (age: 15.8 ± 1.1 years, male/female: 11/ 9) and 36 controls (age: 16.0 ± 1.1 years, male/female: 19/17). Whole-brain T2-relaxation maps were calculated, normalized to a common space, smoothed, and compared between groups and sexes (analysis of covariance; covariates: age, sex; p < 0.001).
Results:
SVHD subjects showed significantly increased BAI and PHQ-9 and reduced MoCA and WRAML2 scores over controls. Several brain regions in SVHD showed increased T2-relaxation values (chronic injury), including the cingulate, and insula, hippocampus/para-hippocampal gyrus, thalamus, hypothalamus, amygdala, frontal white matter, corpus callosum, brainstem, and cerebellar areas. Decreased T2-relaxation values (acute injury) emerged in a few regions, including the prefrontal and cerebellar cortices in SVHD over controls. In addition, male SVHD showed more brain changes over female SVHD.
Conclusions:
Adolescents with SVHD showed significant brain injury with variable male-female differences in areas that control cognition, anxiety, and depression, which may contribute to functional deficits found in the condition.
Keywords: congenital heart disease, magnetic resonance imaging, T2-relaxometry, hippocampus, insula, cingulate
1 |. INTRODUCTION
Among the most challenging types of complex congenital heart disease (CHD) are conditions that lack two well-developed ventricles (single ventricle heart disease [SVHD]), which affect approximately 1 per 2,000 live births in the US (Khairy et al., 2008). The evolution of palliative and reconstructive cardiac operations has had an enormous impact on the increased life expectancy with the use of the Fontan procedure. However, SVHD patients are at greater risk for brain injury and neuro-cognitive deficits, both from innate or patient-related factors (Donofrio & Massaro, 2010; Marelli, Miller, Marino, Jefferson, & Newburger, 2016; Miller & McQuillen, 2007; Sethi et al., 2013) and multiple surgical procedures to treat the condition (Gaynor et al., 2015). Adolescents with palliated SVHD show autonomic dysfunction (Davos et al., 2003; Ohuchi et al., 2001), depression, anxiety (Bromberg, Beasley, D’Angelo, Landzberg, & DeMaso, 2003; Cohen, Mansoor, Langut, & Lorber, 2007; Moon et al., 2009), and cognitive (memory and executive function skills) abnormalities (Gerstle, Beebe, Drotar, Cassedy, & Marino, 2016; Pike, Poulsen, & Woo, 2016; Wernovsky et al., 2000), indicating the probability of brain injury in regions that control these functions.
Despite multiple reports of cognitive deficits, anxiety, and depression in complex SVHD, the underlying causes for these adverse symptoms remain unclear (Cohen et al., 2007; Moon et al., 2009; Pike et al., 2016). Preoperative brain injury has been identified in over 60% of newborns with SVHD, which may be explained in part by structural brain immaturity (Donofrio & Massaro, 2010; Marelli et al., 2016; Miller & McQuillen, 2007; Sethi et al., 2013). Reduced global brain volumes have been identified in infants with complex CHD prior to surgical intervention shedding light on potential innate contributing factors related to fetal brain development (von Rhein et al., 2015). Furthermore, scattered cerebral lesions have been recognized by conventional brain magnetic resonance imaging (MRI) as ischemic infarcts in gray and white matter and intraventricular hemorrhages (Donofrio & Massaro, 2010; Mahle et al., 2002; Sethi et al., 2013). Additional brain changes induced by hypoxemia and hypotension develop in over 3040% SVHD. These changes are described as either focal or scattered lesions in multiple brain areas (McQuillen et al., 2007), are not identified by routine cranial ultrasound scans (Abernethy, Klafkowski, Foulder-Hughes, & Cooke, 2003; Block et al., 2010; Latal et al., 2015; McQuillen et al., 2007), and are mostly clinically silent in the neonatal period (Clancy et al., 2005). Moreover, the functional manifestations of these brain deficits may not be apparent until later in development and have not been consistently reported in brain regions that control cognition, anxiety, and depression in SVHD. However, one study on adolescents with dextro-transposition of the great arteries (d-TGA) showed worse cognitive function mediated by global differences in white matter topology, suggesting that this disruption could drive neurocognitive dysfunction in this population (Panigrahy et al., 2015).
Among non-invasive techniques that can assess both white and gray matter injury, magnetic resonance imaging based T2-relaxometry is useful. The procedure indicates the relative proportion of free and bound water protons in tissue (Abernethy et al., 2003), such as macromolecules, myelin, and cell membranes. Increased T2-relaxation times could result from cerebral edema after hypoxia-ischemia (Rumpel et al., 1995) as well as chronic pathologic conditions, including long-lasting ischemia (Kato et al., 1986), gliosis (Kumar et al., 2002), and demyelination (Abernethy et al., 2003). The procedure has been used for evaluation of tissue changes in several brain conditions, including multiple sclerosis (Papanikolaou et al., 2004), Alzheimer’s disease (Kirsch, Jacobs, Butcher, & Beatty, 1992), and epilepsy (Kumar et al., 2002; Townsend, Bernasconi, N., Pike, & Bernasconi, A., 2004), even in conditions in which visible pathology was not apparent on routine brain MRI (Kumar et al., 2003). For these reasons, T2-relaxometry may be useful to evaluate the extent of structural brain injury in SVHD.
This study aimed to examine regional brain injury in SVHD subjects who have undergone Fontan completion over control subjects and sex differences in SVHD subjects, using T2-relaxometry procedures. Increased T2-relaxation indicates higher free water content and signature of chronic tissue condition. Decreased T2-relaxation shows free water shift from extra-axonal/cellular space to intra-axonal/cellular space due to acute pathology condition. We hypothesize that brain injury would predominantly show increased T2-relaxation values, indicating chronic tissue changes in SVHD in brain areas that control cognitive functions, anxiety, and depression deficiencies in the condition. In addition, male SVHD subjects will show more brain changes over female SVHD subjects, since mortality and morbidity are more prominent in male SVHD subjects (Engelfriet & Mulder, 2009).
2 |. MATERIALS AND METHODS
2.1 |. Subjects
This is a cross-sectional, comparative study of 56 adolescents (20 SVHD [male/female: 11/9] and 36 controls [male/female: 19/17]) who were recruited via flyers, provider referrals from the University of California Los Angeles (UCLA) Children’s Hospital Los Angeles (CHLA) pediatric cardiology clinics, and private practice cardiology groups in Southern California. We included adolescents with SVHD between the ages of 14–18 years who have undergone surgical palliation with Fontan completion.
Healthy controls were recruited from local high schools and the surrounding community. All controls were without history of chronic medical or psychiatric conditions or previous head injuries (e.g., concussions, trauma). Exclusion criteria for SVHD and controls were claustrophobia, non-removable metal (such as braces, pacemakers), severe developmental delay precluding active study participation or ability for self-report (e.g., cerebral palsy or severe hypoxic-injury), diagnosis of depression, premature birth (< 37 weeks gestation), history of extracorporeal membrane oxygenation (ECMO) use, and cardiac arrest. If eligible, control subjects were matched to SVHD participants for age (± 1 year), sex, and ethnicity. Clinical and demographic data were collected from participants and their medical records.
Parental permission and assent were obtained for all participants under the age of 18, and written informed consent was obtained from participants over 18 years of age before data collection. The Institutional Review Boards at the University of California at Los Angeles and Children’s Hospital Los Angeles approved the study protocol.
2.2 |. Experimental design
2.2.1 |. Assessment of depression and anxiety symptoms
Two self-reported questionnaires, the Beck Anxiety Inventory (BAI) and the Patient Health Questionnaire-9 (PHQ-9; Allgaier, Pietsch, Fruhe, Sigl-Glockner, & Schulte-Korne, 2012; Beck, Epstein, Brown, & Steer, 1988; Kroenke et al., 2001; Steer, Kumar, Ranieri, & Beck, 1995), were used for assessment of anxiety and depressive symptoms, respectively, in all subjects. The BAI inventory had 21 multiple-choice questions (each question score ranged 0–3), with score ranging from 0–63 based on symptom severity. A subject with a score > 9 was considered to have anxiety symptoms (Beck et al., 1988; Steer et al., 1995). The PHQ-9 is a 9-item depression module with scores ranging from 0–27 (each question score ranged 0–3). A score from 5–9, 10–14, 15–19, and > 20 were considered minimal, moderate, moderately severe, and severe depressive symptoms, respectively (Allgaier et al., 2012; Kroenke et al., 2001).
2.2.2 |. Cognition assessment
The Montreal Cognitive Assessment (MoCA) test was used to screen all subjects for various cognitive domains, including attention and concentration, executive functions, language, memory, visuo-constructional skills, conceptual thinking, calculations, and orientation (Nasreddine et al., 2005). The maximum score on the MoCA can be 30, and a score < 26 is considered abnormal. The MoCA has been validated for use in adolescents with CHD (Pike et al., 2016).
In addition, the Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML2) was administered in all subjects for assessment of memory and learning functions. The WRAML2 measures various domains of memory, including verbal and visual memory, attention/concentration, working memory, visual recognition, and verbal recognition. The core battery consists of six subtests that are combined to yield a general memory index (GMI) score: story memory, verbal learning, design memory, picture memory, finger window (short-term memory of a visual sequential pattern), and numbers/letters (digit-span format using both numbers and letters). Also, general memory recognition index (GRI) scores are comprised of working memory, visual, and verbal recognition. The GMI measures immediate recall, and GRI shows delayed recall. A score of < 85 in either measures is considered impaired (Sheslow & Adams, 2003).
2.2.3 |. Socioeconomic status
Socioeconomic status of each subject was derived from the American Community Survey data available on Population Studies Center, Institute for Social Research, based on residential postal codes. The annual household income of each subject was evaluated through his or her zip code.
2.2.4 |. Magnetic resonance imaging
While participants lay supine, brain images were acquired using 3.0-Tesla MRI scanners (Magnetom Tim-Trio [6 SVHD and 8 controls] & Prisma [14 SVHD and 28 controls]). Foam pads on either side of the head were used to minimize head movement. Proton density (PD) and T2-weighted images (repetition time [TR] = 10,000 ms; echo-time [TE1, TE2] = 12, 123/124 ms; flip angle [FA] = 130°) were collected simultaneously using a dual-echo turbo spin-echo pulse sequence in the axial plane, with a 256 × 256 matrix size, 230 × 230 mm field of view (FOV), 3.5 mm slice thickness, and no interslice gap. High resolution T1-weighted images were also collected using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence (TR = 2200 ms; TE = 2.4 ms; inversion time = 900 ms; FA = 9 °; matrix size = 320 × 320; FOV = 230 × 230 mm; slice thickness = 0.9 mm; number of slices = 192) to aid in anatomical identification and evaluation for any anatomical defects.
2.2.5 |. Data processing
We used the statistical parametric mapping (SPM12) package (Wellcome Department of Cognitive Neurology, UK; http://www.fil.ion.ucl.ac.uk/spm/), and MATLAB-based (The MathWorks Inc) custom software to process images. All brain images of individual subjects, including T1-weighted, PD-weighted, and T2-weighted images were evaluated for any major anatomical defects, including cystic lesions, infarcts, or other types of brain lesions before T2-relaxation calculation. Proton-density and T2-weighted images were also examined for motion artifacts to ensure that images were acceptable for subsequent processing.
2.2.6 |. Calculation of T2-relaxation maps, normalization, and smoothing
Using PD and T2-weighted images, pixel-by-pixel T2-relaxation values were calculated as described elsewhere (Duncan, Bartlett, & Barker, 1996; Kumar et al., 2005). T2 maps were constructed using T2-relaxation times calculated for each voxel, in which voxel intensity corresponded to the T2-relaxation value (Kumar et al., 2005). We normalized T2 maps to the standard Montreal Neurological Institute (MNI) space template. T2-weighted images of each patient were normalized to the MNI template, using a priori-defined distributions of tissue types, and the resulting parameters were applied to the corresponding T2 maps. The normalized T2 maps were smoothed using a Gaussian filter (full-width-at-half-maximum = 8 mm).
2.2.7 |. Validation for combining data
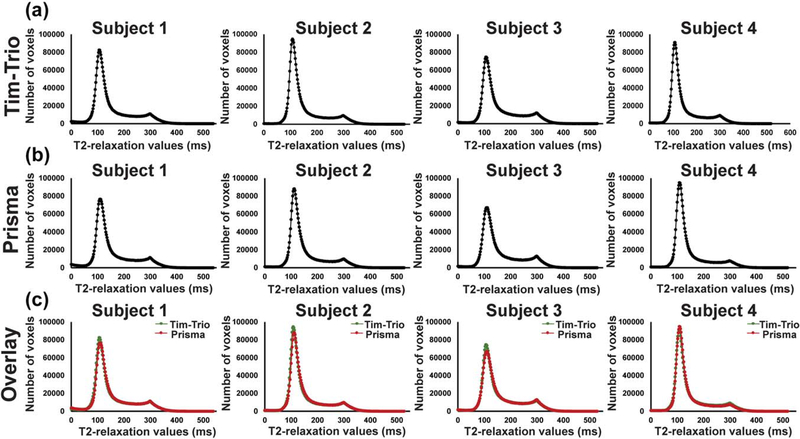
Four control subjects were scanned in the two different scanners (3.0-Tesla, Tim-Trio, and 3.0-Tesla, Prisma) for validation of combining data from two scanners. T2-relaxation maps of each subject were normalized and descalped to remove noise signal from non-brain regions. We plotted histograms for each normalized and descalped T2-relaxation maps to look for differences in distribution of T2-relaxation values from two different scanners (Figure 1a,b). Both histograms appeared identical (Figure 1c), indicating no differences between scanners, which suggested the ability to combine data together.
FIGURE 1.
T2-relaxation histograms of four control subjects scanned on two different scanners (3.0-Tesla, magnetom, Tim-Trio; 3.0-Tesla, magnetom, Prisma) for validation of combining data. (a) T2-relaxation histograms were plotted using normalized and descalped T2-relaxation maps obtained from Tim-Trio and (b) Prisma scanners. (c) T2-relaxation histograms of two different scanners for each subject are superimposed
2.2.8 |. Background image
High-resolution T1-weighted images of all SVHD and controls were normalized to the MNI space template. The normalized images were averaged to create a mean anatomical image, which was used as a background image for anatomical identification.
2.2.9 |. Region-of-interest analyses
Region-of-interest (ROI) analyses were performed to calculate regional T2-relaxation values to determine magnitude differences between groups. Regional brain masks were created based on significant whole-brain voxel-by-voxel differences between the groups in those regions. The values were extracted using these regional brain masks and smoothed T2-relaxation maps of SVHD and controls.
2.3 |. Statistical analyses
We used the statistical package for social sciences (SPSS v22) for assessment of demographic and clinical data. The numerical demographic data and characteristics of SVHD and controls were compared with independent samples t-tests (two sample t-test [SVHD, Control], n = 56), and categorical characteristics were compared using the Chi-square test (χ2 [SVHD, Control], n = 56). Wilcoxon rank-sum test (w [SVHD, Control], n = 56) was performed to check the consistency of the histograms across scanners. Statistical threshold values of p < 0.05 were considered a significant difference for all tests.
For identification of brain regions with significant T2-relaxation value differences between SVHD and controls, we used voxel-based-relaxometry procedures, which allowed for a multi-participant whole brain analysis and the ability to compare regional T2-relaxation values between groups. The normalized and smoothed T2-relaxation maps of SVHD and controls were compared at each voxel using analysis of covariance (ANCOVA; covariates, age, and sex; SPM12; SVHD vs. controls, n = 56, p < 0.001, uncorrected; minimum extended cluster size, 10 voxels). The extended cluster size 10 was chosen to avoid brain sites with small voxels having less than 10 (Kumar et al., 2012a; Kumar et al., 2011b; Tummala et al., 2016; Woo et al., 2015). The statistical parametric maps showing regions of significant T2-relaxation value differences were superimposed onto the mean anatomical image for anatomical identification.
Regional brain T2-relaxation values, calculated from ROI analyses, were examined for significant differences between SVHD and controls using analysis of covariance (ANCOVA; n = 56; covariates, age, and sex; p < 0.05). The normality of the distribution was evaluated for control and SVHD data using Shapiro-Wilk test.
Sex differences on T2-relaxation value were evaluated using voxel-based-relaxometry procedures, comparing regional T2-relaxation values between male SVHD (n = 11) and male controls (19), female SVHD (n = 9) and female controls (n = 17), and male SVHD (n = 11) and female SVHD (n = 9) subjects. The smoothed T2-relaxation maps were used for each comparison with analysis of covariance (ANCOVA; covariate, age; SPM12; p < 0.001, uncorrected; minimum extended cluster size, 10 voxels).
3 |. RESULTS
3.1 |. Demographic and clinical characteristics
Demographic, anxiety and depressive symptoms, cognitive, and clinical variables of SVHD and controls are summarized in Table 1. No significant differences in age (p = 0.43), sex (p = 0.87), body mass index (p = 0.16), ethnicity (p = 0.42), socioeconomic status (p = 0.20), or handedness (p = 0.64) appeared between groups. The PHQ-9 and BAI scores were significantly higher in SVHD over controls (PHQ-9, p = 0.004; BAI,p = 0.006). The total MoCA scores and majority of sub-scales were significantly lower in SVHD as compared to controls (p < 0.001). The GMI and GRI scores were significantly reduced along with all other sub-divisions, including verbal memory, visual memory, attention, working memory, and verbal and visual recognition in SVHD compared to controls (p < 0.001).
Table 1.
Demographic, anxiety, mood, cognitive, and clinical characteristics of SVHD and control subjects
| Variables | SVHD n = 20 (Mean ± SD) | Controls n = 36 (Mean ± SD) | P values |
|---|---|---|---|
| Age (years) | 15.8 ± 1.1 | 16.0 ± 1.1 | 0.427 |
| Sex [male] (%) | 11 (55%) | 19 (53%) | 0.873 |
| Ethnicity (%) | White, 10 (50%); Hispanic, 7 (35%); Other, 3 (15%) | White, 18 (50%); Hispanic, 16 (44%); Other, 2 (6%) | 0.458 |
| BMI (kg/m2) | 20.7 ± 3.0 | 22.5 ± 5.3 (n=35) | 0.158 |
| Handedness [right] (%) | 19 (95%) | 33 (92%) | 0.643 |
| Ventricle type [right] (%) | 10 (50%) | N/A | N/A |
| Extracardiac Fontan (%) | 18 (90%) | N/A | N/A |
| Fenestration (%) | 3 (15%) | N/A | N/A |
| Oxygen saturation < 93% | 5 (25%) | N/A | N/A |
| Socioeconomic status (Annual household income) | $83294.7 ± 40072.9 (n = 19) | $98699.6 ± 41724.9 (n = 35) | 0.195 |
| PHQ-9 | 7.6 ± 5.5 | 3.4 ± 2.6 | 0.004 |
| BAI | 18.2 ± 12.0 | 9.3 ± 7.4 | 0.006 |
| Total MoCA scores | 22.4 ± 3.5 | 28.8 ± 1.5 | <0.001 |
| MoCA: Visuospatial | 3.6 ± 1.1 | 4.9 ± 0.3 | <0.001 |
| MoCA: Naming | 2.9 ± 0.4 | 3.0 ± 0.0 | 0.083 |
| MoCA: Attention | 4.3 ± 1.2 | 5.7 ± 0.7 | <0.001 |
| MoCA: Language | 1.6 ± 0.8 | 2.5 ± 0.6 | <0.001 |
| MoCA: Abstraction | 1.3 ± 0.7 | 1.9 ± 0.4 | 0.002 |
| MoCA: Delayed recall | 1.9 ± 1.4 | 4.2 ± 0.6 | <0.001 |
| MoCA: Orientation | 5.9 ± 0.3 | 5.9 ± 0.3 | 0.838 |
| WRAML2 GMI | 83.5 ± 13.2 | 111.0 ± 7.9 | <0.001 |
| Verbal memory | 88.4 ± 10.1 | 107.1 ± 9.4 | <0.001 |
| Visual memory | 96.1 ± 10.5 | 107.3 ± 9.4 | <0.001 |
| Attention | 85.9 ± 10.5 | 110.8 ± 8.7 | <0.001 |
| WRAML2 GMRI | 93.3 ± 11.4 | 112.2 ± 10.1 (n = 35) | <0.001 |
| Working memory | 87.0 ± 10.3 | 112.6 ± 12.9 | <0.001 |
| Verbal recognition | 93.4 ± 11.1 | 106.1 ± 12.2 (n = 35) | <0.001 |
| Visual recognition | 95.6 ± 14.1 | 109.3 ± 10.9 | <0.001 |
Abbreviations: SD = standard deviation; N/A = not applicable; BMI = body mass index; PHQ-9 = Patient Health Questionnaire-9; BAI= Beck Anxiety Inventory; MoCA= Montreal Cognitive Assessment; WRAML2 = Wide Range Assessment of Memory and Learning, 2nd Edition; GMI = General Memory Index; GMRI = General Memory Recognition Index.
3.1.1 |. Validation for combining data
No significant differences were observed in histograms obtained from two different scanners (Subject 1, p = 0.79; Subject 2, p = 0.69; Subject 3, p = 0.91; Subject 4, p = 0.35). Since no significant differences appeared between data collected from two different scanners (Figure 1), data collected from both scanners (14 subjects [Magnetom, TimTrio] and 42 subjects [Magnetom, Prisma]) were combined to obtain sufficient statistical power. Each variable had 54 degrees of freedom.
3.1.2 |. Visual examination of MRI data
None of the healthy controls showed any major anatomical brain lesions on visual examination. However, six SVHD subjects showed subtle brain tissue changes, including old infarcts, benign cystic focus, periventricular white matter changes, and chronic tissue loss.
3.2 |. Brain regions with significant T2-relaxation value differences
Several brain areas in SVHD participants showed significantly higher T2-relaxation values, indicating tissue injury, compared to controls (Figures 2 and 3). Few brain sites showed significantly lower T2-relaxation values in SVHD compared to controls. Regions with significantly prolonged T2-relaxation values in SVHD participants appeared in the frontal cortices, hippocampus, amygdala, insular cortices, anterior, mid and posterior cingulate cortices, thalamus, hypothalamus, mammillary bodies, and lingual and para-hippocampal gyrus (Figures 2 and 3; p < 0.001). Other sites, including white matter areas, were also detected with increased T2-relaxation values in SVHD over controls in regions that link important gray matter regions associated with cognition, anxiety, and depression, including frontal white matter, corpus callosum, pons, midbrain, and cerebellar peduncles (p < 0.001). Brain regions showing decreased T2-relaxation values in SVHD emerged in the caudate nuclei, cerebellar cortex, and prefrontal cortices.
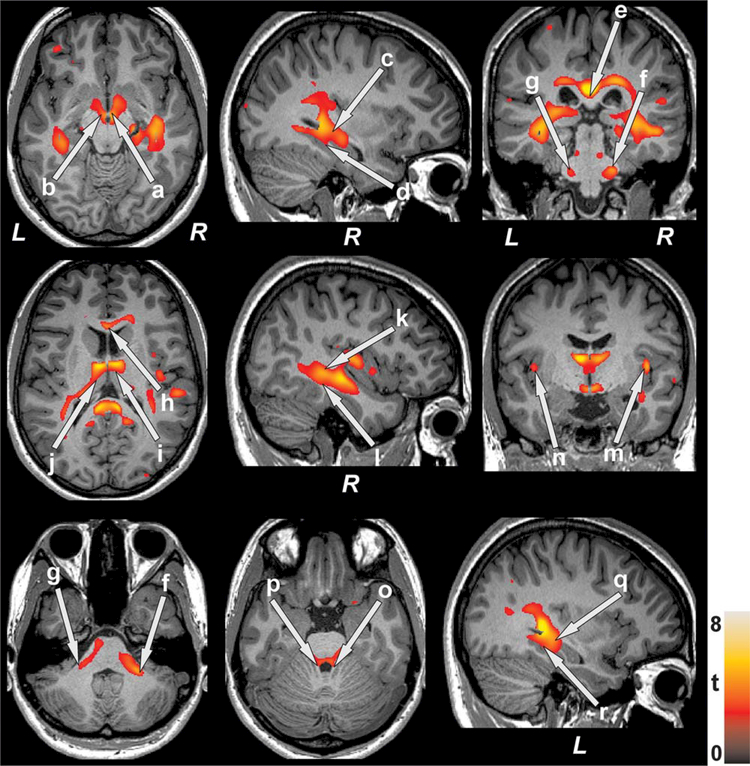
FIGURE 2.
Brain regions with higher T2-relaxation values in SVHD over controls. These sites with increased T2-relaxation values, suggesting brain changes, included the bilateral hypothalamus (a, b), bilateral hippocampus (c, q), bilateral parahippocampal gyrus (d, r), splenium (e), bilateral middle (f, g) and superior (o, p) cerebellar peduncles, genu (h), bilateral thalamus (i, j), middle (l) and superior (k) temporal white matter, and bilateral insular cortices (m, n). All images are in neurological convention (L = Left; R = Right). Color bar indicates t-statistic values
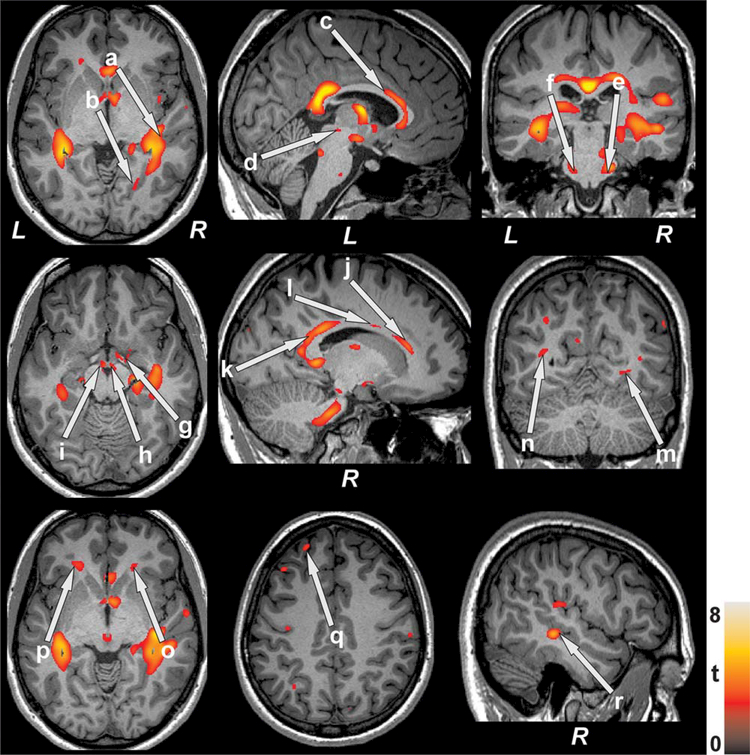
FIGURE 3.
Brain regions with prolonged T2-relaxation values in SVHD over controls. Elevated T2 values, indicating brain injury, appeared in the right superior (a) and middle (r) temporal gray matter, right lingual gyrus (b), body of corpus callosum (c), midbrain (d), bilateral pons (e, f), right amygdala (g), bilateral mammillary bodies (h, i), anterior (j), mid (l), posterior (k) cingulate, right inferior (m) and left middle (n) occipital white matter, bilateral frontal white matter (o, p), and left frontal gyrus (q). Figure conventions are same as in fig. 2
3.3 |. Regional brain T2-relaxation values: ROI analyses
The voxel-wise analyses indicated 52 brain sites with altered T2-relaxation values between SVHD and control subjects. The ROI analyses were performed in these 52 brain sites and average T2-relaxation values were computed. Of 52 brain regions, T2-relaxation values of nine sites in SVHD were not normally distributed, but remaining 43 brain areas were normally distributed. Of 52 brain regions in controls, T2-relaxation values of 46 regions were normally distributed. Since majority of brain regions had normally distributed T2-relaxation values, ANCOVA analyses were performed to examine differences between SVHD and control subjects. Regional brain T2-relaxation values of SVHD and control subjects are tabulated in Tables 2 and 3. Mean T2-relaxation values were significantly altered in SVHD compared to controls with large to very-large effect sizes that varied from 0.96–1.73 between groups for each different individual brain regions (Faul, Erdfelder, Buchner, & Lang, 2009; Faul, Erdfelder, Buchner, & Lang, 2013). Between groups, degree of freedom was one and within group, degree of freedom was 52 for all brain regions.
Table 2.
Regional brain T2-relaxation values (mean ± SD) of SVHD and control subjects (corrected for age and sex) and effect sizes
| Brain regions | SVHD n = 20 (unit, ms) | Controls n = 36 (unit, ms) | P values | Effect Sizes |
|---|---|---|---|---|
| L Anterior cingulate | 127.97 ± 11.8 | 116.15 ± 11.7 | 0.001 | 1.01 |
| R Anterior cingulate | 129.64 ± 8.5 | 119.19 ± 8.5 | <0.001 | 1.23 |
| L Middle cingulate | 125.77 ± 10.4 | 114.71 ± 10.3 | <0.001 | 1.07 |
| R Middle cingulate | 130.54 ± 9.8 | 119.88 ± 9.7 | <0.001 | 1.09 |
| L Posterior cingulate | 128.43 ± 5.5 | 119.59 ± 5.5 | <0.001 | 1.61 |
| R Posterior cingulate | 125.61 ± 5.8 | 116.52 ± 5.8 | <0.001 | 1.57 |
| L Genu | 131.22 ± 7.7 | 121.00 ± 7.7 | <0.001 | 1.33 |
| R Genu | 129.37 ± 6.4 | 120.24 ± 6.4 | <0.001 | 1.43 |
| L Splenium | 141.33 ± 10.1 | 124.76 ± 10.1 | <0.001 | 1.64 |
| R Splenium | 137.58 ± 8.3 | 123.18 ± 8.3 | <0.001 | 1.73 |
| L Body corpus callosum | 150.54 ± 8.8 | 139.33 ± 8.7 | <0.001 | 1.28 |
| R Body corpus callosum | 151.01 ± 8.3 | 140.25 ± 8.3 | <0.001 | 1.30 |
| R Amygdala | 162.89 ± 10.8 | 152.12 ± 10.8 | 0.001 | 1.00 |
| L Hippocampus | 153.27 ± 11.5 | 136.16 ± 11.5 | <0.001 | 1.49 |
| R Hippocampus | 144.56 ± 8.7 | 131.79 ± 8.7 | <0.001 | 1.47 |
| L Parahippocampus | 142.51 ± 8.7 | 132.26 ± 8.7 | <0.001 | 1.18 |
| R Parahippocampus | 139.27 ± 5.8 | 132.16 ± 5.8 | <0.001 | 1.23 |
| L Thalamus | 131.53 ± 6.8 | 121.21 ± 6.7 | <0.001 | 1.53 |
| R Thalamus | 124.78 ± 5.3 | 115.70 ± 5.3 | <0.001 | 1.71 |
| L Hypothalamus | 148.91 ± 7.7 | 139.50 ± 7.7 | <0.001 | 1.22 |
| R Hypothalamus | 143.21 ± 7.6 | 133.42 ± 7.6 | <0.001 | 1.29 |
| L Insula | 164.69 ± 8.0 | 155.94 ± 8.0 | <0.001 | 1.09 |
| R Insula | 155.33 ± 6.4 | 145.61 ± 6.4 | <0.001 | 1.52 |
| L Mammillary body | 185.27 ± 12.1 | 172.34 ± 12.1 | <0.001 | 1.07 |
| R Mammillary body | 186.28 ± 13.2 | 172.58 ± 13.2 | 0.001 | 1.04 |
| L Midbrain | 136.00 ± 7.1 | 128.68 ± 7.1 | 0.001 | 1.03 |
| R Midbrain | 139.65 ± 7.7 | 131.84 ± 7.7 | 0.001 | 1.01 |
| L Frontal gyrus | 203.19 ± 13.4 | 188.20 ± 13.4 | <0.001 | 1.12 |
| R Lingual gyrus | 129.40 ± 6.4 | 121.64 ± 6.4 | <0.001 | 1.21 |
| L Inferior cerebellar peduncle | 152.26 ± 8.4 | 142.58 ± 8.4 | <0.001 | 1.15 |
| R Inferior cerebellar peduncle | 147.64 ± 6.4 | 138.57 ± 6.4 | <0.001 | 1.42 |
| L Middle cerebellar peduncle | 151.65 ± 8.0 | 142.40 ± 8.0 | <0.001 | 1.16 |
| R Middle cerebellar peduncle | 149.91 ± 7.0 | 140.01 ± 6.9 | <0.001 | 1.42 |
| L Superior cerebellar peduncle | 165.10 ± 7.1 | 156.30 ± 7.1 | <0.001 | 1.24 |
| R Superior cerebellar peduncle | 157.71 ± 5.8 | 149.06 ± 5.8 | <0.001 | 1.49 |
| L Frontal WM | 114.81 ± 5.3 | 108.91 ± 5.3 | <0.001 | 1.11 |
| R Frontal WM | 110.25 ± 5.1 | 104.88 ± 5.1 | <0.001 | 1.05 |
| L Middle temporal WM | 128.66 ± 7.1 | 118.01 ± 7.1 | <0.001 | 1.50 |
| R Middle temporal WM | 121.78 ± 6.1 | 112.91 ± 6.0 | <0.001 | 1.47 |
| R Middle temporal GM | 121.26 ± 4.9 | 115.02 ± 4.9 | <0.001 | 1.27 |
| R Superior temporal GM | 117.36 ± 4.2 | 112.22 ± 4.2 | <0.001 | 1.22 |
| L Superior temporal WM | 125.38 ± 6.6 | 116.07 ± 6.6 | <0.001 | 1.41 |
| R Superior temporal WM | 121.58 ± 5.6 | 113.49 ± 5.6 | <0.001 | 1.44 |
| R Inferior occipital WM | 121.17 ± 7.4 | 114.10 ± 7.4 | <0.001 | 0.96 |
| L Middle occipital WM | 123.37 ± 7.6 | 115.81 ± 7.5 | 0.001 | 1.00 |
| R Superior occipital GM | 140.55 ± 11.9 | 128.75 ± 11.9 | 0.001 | 0.99 |
| L Pons | 158.33 ± 10.3 | 146.67 ± 10.3 | <0.001 | 1.13 |
| R Pons | 153.53 ± 8.8 | 143.07 ± 8.8 | <0.001 | 1.19 |
Abbreviations: SD = standard deviation; SVHD = Single ventricle heart disease; ms = millisecond; L = left; R = right; WM = white matter; GM = gray matter.
Table 3.
Brain areas with decreased T2-relaxation values (mean ± SD) in SVHD over control subjects (corrected for age and sex) and effect sizes
| Brain regions | SVHD n = 20 (unit, ms) | Controls n = 36 (unit, ms) | P values | Effect sizes |
|---|---|---|---|---|
| L Caudate | 117.50 ± 10.4 | 129.64 ± 10.4 | <0.001 | 1.17 |
| L Cerebellar cortex | 123.23 ± 9.9 | 138.85 ± 9.9 | <0.001 | 1.58 |
| L Prefrontal cortex | 164.04 ± 11.9 | 181.74 ± 11.9 | <0.001 | 1.49 |
| R Prefrontal cortex | 166.85 ± 12.2 | 184.18 ± 12.2 | <0.001 | 1.42 |
Abbreviations: SD = standard deviation; SVHD = single ventricle heart disease; ms = millisecond; L = left; R = right.
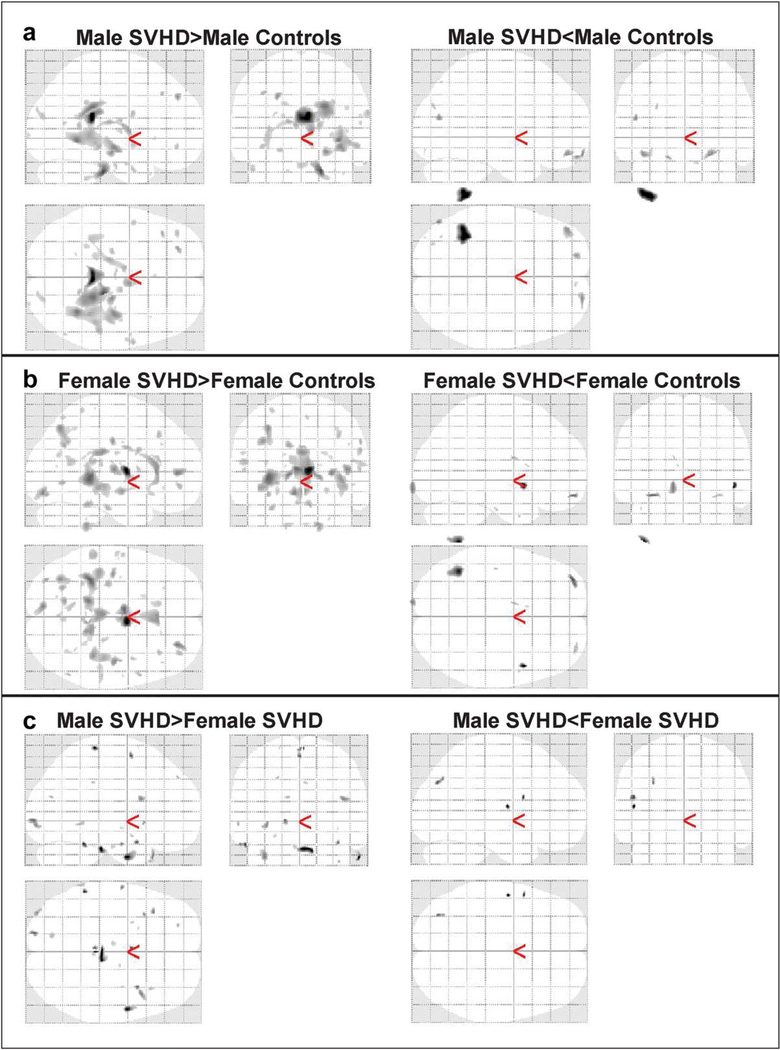
3.4 |. Sex differences on brain injury
Male SVHD showed significantly increased T2-relaxation values in several brain regions compared to male controls, with very few brain sites showing lower T2-relaxation values (Figure 5a). Significantly higher and wide spread T2-relaxation values were observed in female SVHD over female controls, with limited brain areas showing decreased T2-relaxation values (Figure 5b). Male-female comparison of SVHD subjects showed more brain areas with injury in male SVHD compared to female SVHD subjects (Figure 5c).
FIGURE 5.
Extent of brain injury differed in male as compare to female. (a) Widespread brain regions showed increased T2-relaxation values in male SVHD over male healthy controls, and limited brain regions appeared with lowered T2-relaxation values. (b) Female SVHD subjects predominantly illustrated elevated T2-relaxation values over female healthy subjects, with few brain regions emerged with diminished T2-relaxation values. (c) Male SVHD subjects showed predominantly more brain injury as compare to female SVHD patients
4 |. DISCUSSION
Single ventricle heart disease patients showed brain injury in multiple sites, including the cingulate, caudate, prefrontal, frontal, temporal, occipital, cerebellar and insular cortices, lingual gyrus, pons, mammillary bodies, mid-brain, thalamus, extending to hypothalamus, hippocampus, amygdala, parahippocampal gyrus, and other neighboring white matter areas, such as genu, splenium, corpus callosum, and cerebellar peduncle. Such changes in specific brain areas may contribute to cognitive and behavioral deficits (e.g., executive function, visual-spatial, and memory, language, and attention disorders), as well as anxiety and depression functions accompanying the condition (Woo, Kumar, Macey, Fonarow, & Harper, 2009). Previous neuroimaging studies in adolescents with SVHD have qualitatively examined either gray and white matter infarcts visually, or gross estimation of total gray matter or brain volumes (Cordina et al., 2014; Miller & McQuillen, 2007). The current study utilizes a quantitative T2-relaxometry approach to localize gray and white matter injury as opposed to visual examination of tissue infarcts or global gray/white matter volumes. Also, this study utilized a 3.0-Tesla compared to a 1.5-Tesla MRI machine, which produces higher-resolution images with greater anatomic details. Lateralization of brain tissue abnormalities was noteworthy, however lateralized brain injury in autonomic, anxiety, depression, and cognition regulatory sites are often observed in other conditions (Kumar et al., 2014; Roy et al., 2017; Tummala et al., 2016). The aberrations here might result from low cardiac output, endothelial dysfunction, ischemic changes, and underlying perfusion deficits in the condition.
Injury in the prefrontal cortex has adverse implications in planning complex cognitive behavior, personality expression, decision-making, and moderating social behavior. The site receives input and has reciprocal connections to limbic structures, and thus, affects working memory, attention, and executive functions (Frith & Dolan, 1996). Procedural and associative learning are implicated with caudate nuclei (Bauer, Toepper, Gebhardt, Gallhofer, & Sammer, 2015; Manelis & Reder, 2012; Williams & Eskandar, 2006), which are an integral part of cortico-basal ganglia-thalamic loop and are well recognized for their contributions in cognition. Frontal cortex is functionally connected with specific areas in basal ganglia structures, where dorsolateral prefrontal cortex, coordinating for strategic planning and working memory, has projections terminating in rostral and dorsal caudate and consequently any injury in caudate nuclei can disconnect the dorsolateral prefrontal neural circuitry. Spatial working memory (Levy, Friedman, Davachi, & Goldman-Rakic, 1997) and executive function have been linked with caudate through various fMRI studies and play fundamental roles in the decision-making process (Haruno et al., 2004; Seger & Cincotta, 2005). Visuospatial processing and episodic memory are disrupted by damage to parahippocampal gyrus, an area that is part of a large network with connections to temporal, parietal, and frontal cortices and inputs signals to the entorhinal cortex, which feeds direct information to the hippocampus (Aminoff, Kveraga, & Bar, 2013). Frontal white matter is highly connected to parietal lobe with the parahippocampal gyrus, temporal, and occipital areas. Also, frontal white matter forms a network and ensures rapid neuronal conduction to pass information among those sites, therefore contributing to the efficiency of information processing for cognition. The corpus callosum is a major commissural fiber system connecting the left and right cerebral hemispheres and coordinates perception, memory, and cognition from bilateral hemispheres by communicating and transferring information between corresponding sites. Thus, altered T2-relaxation values observed in prefrontal cortices, hippocampus, frontal white matter, corpus callosum, caudate nuclei, and para-hippocampal gyrus might have contributed to cognitive deficits in SVHD.
4.1 |. Injury in cognitive control areas
Cognitive deficits, including memory and executive functions are well documented in over 60% SVHD adolescents (Cassidy, White, DeMaso, Newburger, & Bellinger, 2015; Gerstle et al., 2016; Pike et al., 2016). Those with hypoplastic left heart syndrome, one of the subgroup of SVHD, are at higher risk for cognitive deficits. Previous brain MRI studies of adolescents with SVHD following Fontan completion have identified focal and multifocal abnormalities, brain mineralization or iron deposit, and evidence of stroke (Bellinger et al., 2015). However, none of the previous studies examined consistent regional brain changes in SVHD patients. We found altered T2-relaxation values, indicating tissue abnormality in the prefrontal cortices, hippocampus, frontal white matter, corpus callosum, lingual gyrus, caudate nuclei, and
4.2 |. Anxiety and depression regulation and brain injury
Depression and anxiety symptoms are commonly seen in over 50% of adolescents and adults with SVHD (Bromberg et al., 2003; Cohen et al., 2007). Unfortunately, most SVHD patients go underdiagnosed and undertreated (Pauliks 2013). In our study, multiple brain areas, including the cingulate, insula, cerebellum, and para-hippocampal gyrus showed tissue injury. Most of these brain sites have been associated with injury in patients with isolated depression (Fitzgerald, Laird, Maller, & Daska-lakis, 2008); however, these areas also showed altered T2-relaxation values in SVHD participants. Cingulate gyrus, insula, cerebellum, and para-hippocampal gyus have been implicated in the modulation of emotional behavior by regulating depressive and anxious states as indicated by numerous neuroimaging studies (Avery et al., 2014; Ebert & Ebmeier, 1996; Gorka et al., 2014; Liu et al., 2012; Schutter, Koolschijn, Peper, & Crone, 2012; Shang et al., 2014; Talati, Pantazatos, Schneier, Weissman, & Hirsch, 2013; Zamoscik et al., 2014). The cingulate is involved in four parallel functional circuits through the basal ganglia and limbic loop that begins its association in cortical areas, where ventral striatum is the principal input nucleus, with output through the ventral pallidum and the thalamic dorsomedial nuclei. The anterior cingulate, together with the medial orbital gyri, parahippocampal gyrus, and temporal pole, forms an integral part of limbic cortical association. Three major neocortical association areas converge on limbic cortex in the cingulate gyrus and parahippocampal gyrus and are connected with the hippocampus, amygdala, and hypothalamus (Ebert & Ebmeier, 1996). Dorsal anterior cingulate cortex, superior temporal lobe, and insula form a part of the salience network involved in the coordination of behavioral responses. The anterior insula displays altered functional connectivity within the salience network and with other brain networks in major depressive disorders (Wiebking et al., 2015). The functioning mechanism of the cerebellum suggests that the information flows through mossy fibers, parallel fibers, Purkinje cells, and cerebellar and vestibular nuclear neurons. A climbing fiber codes an error signal reflecting the motor performance failure and the signal conveyed by a climbing fiber works to depress the synaptic transmission between parallel fibers and a Purkinje cell. This coupled activation of parallel fibers and a climbing fiber hampers the information flow from the parallel fibers to a Purkinje cell, plausibly causing depressive symptoms (Hirano 2013). Thus, the increased T2-relaxation values in cingulate, insula, and para hippocampal gyrus and cerebellar sites might have steered toward depression and anxiety dysregulation in the SVHD.
4.3 |. Potential pathological processes
Various pathological processes may contribute to brain tissue injury in SVHD. SVHD patients often have delayed brain development due to underlying compromised cardiac anatomy (Sethi et al., 2013). Prior to Fontan completion, the single ventricular chamber may not be able to increase cardiac output enough to compensate for the cerebral hypoxemia caused by intracardiac mixing, which may lead to abnormal brain development, despite intact cerebral autoregulation (Donofrio & Mas-saro, 2010). Surgical procedures undertaken by these patients involve cardiopulmonary bypass and, in some cases, deep hypothermia circulatory arrest, which may result in brain injury due to embolism, inflammation, and ischemia (Miller & McQuillen, 2007). Low cardiac output in the early postoperative period, and alteration of cerebral blood flow may also lead to deficient cerebral auto-regulatory process, and thus, brain injury in the condition.
4.4 |. T2-relaxation values and tissue injury
T2-relaxation values show non-invasively free water content (water molecules not associated with cell membrane and macromolecules) in the tissue (Abernethy et al., 2003). T2-relaxation values rise with increase in free water content, which is often associated with injury to or loss of axons, myelin or cells seen in chronic conditions or the normal aging processes (Kumar, Delshad, Woo, Macey, & Harper, 2012b; Kumar et al., 2002). However, during adolescence, the brain continues to develop more densely packed axonal structures (Kumar, Delshad, Macey, Woo, & Harper, 2011a; Reiss, Abrams, Singer, Ross, & Denckla, 1996) and myelination increases (Barkovich 2000), which restricts free water, resulting in reduced T2-relaxation values. Despite the adolescent lacking complete maturation, T2-relaxation values were found to be higher in our study in the SVHD group compared to controls. Although T2-relaxation values do not discern the specific cause of injury, we can speculate the increase of T2-relaxation values is due to likely sources, including developmental or innate processes, open heart surgery procedures, hypoxemia, and low cardiac output induced hypoxia/ischemia processes contributing to tissue changes. The decreased T2-relaxation values as observed in our study might be due to acute tissue changes, including axonal and neuronal inflammation, shifting free water from extra-axonal/cellular space to intra-axonal/cellular space, contributing to the reduction of T2-relaxation values.
4.5 |. Study limitations
One noticeable limitation to this study was the moderate sample size. However, despite the sample size, significant differences emerged between groups, which was indicative of (and as shown) very large effect sizes on brain injury in SVHD (Tables 2–3). Another limitation of this study was the sample characteristics (age 14–18, gestation > 37 weeks, and lack of psychological or clinical morbidities), reflecting better health within their chronic condition, and thus, may not be generalizable to all SVHD participants. Nonetheless, significant brain injury was still identified. The SVHD subjects had their last heart surgery over a decade ago and may not have benefited from modern surgical techniques. Furthermore, this study was also a cross-sectional design or snapshot in time of brain structures in SVHD adolescents and is more reflective of cumulative injury in the condition. The altered T2-relaxation values were observed in brain sites that control anxiety, depression, and cognition, and might result from underlying compromised cardiac anatomy, surgical procedures undergone, or low cardiac output in postoperative periods. However, the correlations between these causal factors and T2-relaxation values and functional deficits were not studied due to limited number of SVHD subjects. This is one of the other limitations. Recent literature supports different brain fiber wiring between men and women (Macey et al., 2016), hence it is imperative to look for sex differences affecting T2 relaxation changes in SVHD as compared to control. However, although we examined, due to availability of limited subjects in both groups and underpowered study, male-female differences should be examined with a large number of subjects.
5 |. CONCLUSIONS
Adolescents with palliated SVHD showed significant brain structural changes, as evidenced by altered T2-relaxation values that were indicative of tissue injury in brain regions that control cognitive functions, anxiety, and depression. These sites included the prefrontal, cingulate, caudate nuclei, insular cortices, cerebellum, hippocampus, amygdala, thalamus, hypothalamus, midbrain, pons, mammillary bodies, and parahippocampal gyrus; and neighboring white matter areas including bilateral frontal white matter, anterior corpus callosum, and middle cerebellar peduncles. Several of these regions may play significant roles in both anxiety and depression (cingulate, insula, cerebellum, parahippocampal gyrus) and cognitive (prefrontal cortices, caudate nuclei, para-hippocampal gyrus, frontal white matter, and corpus callosum) capabilities that are frequently identified as abnormal in SVHD patients. The pathological mechanisms that contribute to brain changes may include altered brain maturation and hypoxemia induced processes accompanying the underlying SVHD condition.
Furthermore, the functional consequences of brain injury are affecting various domains, especially sites that rely on widespread neural networks, such as higher cognitive functions, anxiety and depression regulation. The clinical implication of this study was to demonstrate the widespread tissue abnormality and chronic, as well as acute, changes across brain regions responsible for appropriate functioning of anxiety, depression, and cognition. These functional deficits have the potential to impact the ability for self-care, educational achievement, employability, and quality of life.
FIGURE 4.
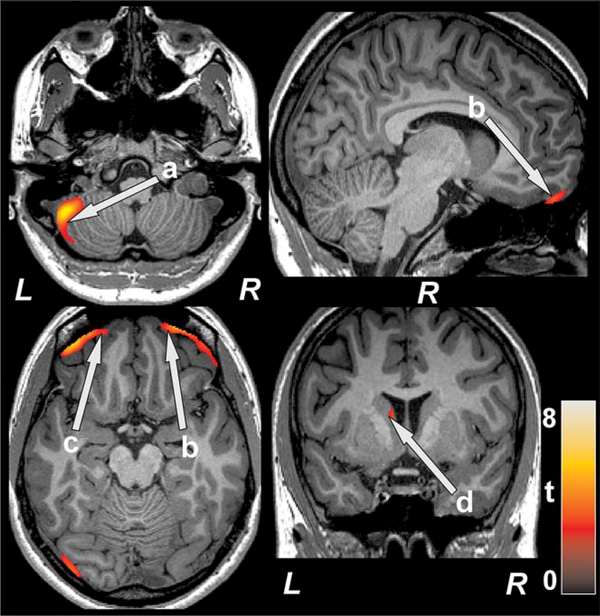
Brain regions with decreased T2-relaxation values in SVHD over controls. Decreased T2-relaxation values, indicated altered tissue integrity, were observed in left cerebellar cortex (a), bilateral prefrontal cortices (b, c), and left caudate (d). Figure conventions are same as in fig. 2
Significance.
Adolescents with single ventricle heart disease (SVHD), a rare disorder with an unknown cause, often show cognitive dysfunction and anxiety and depressive symptoms that suggest the possibility of brain injury. This study is the first to evaluate regional brain tissue changes in SVHD using T2-relaxometry procedures. It also describes how these changes affect the brain’ anxiety, depression, and cognitive control sites. Our findings indicate that SVHD subjects have a brain structural basis for these functional deficits in the condition.
ACKNOWLEDGEMENTS
Authors would like to thank Ruchi Vig, Luke Ehlert, Ariana Dideban, and Patty Chung for assistance with data collection.
Funding information: This research was supported by the National Institutes of Health, grant numbers: R01-NR013930 and R01-NR016463.
Footnotes
CONFLICT OF INTEREST
All authors have no conflicts of interest to declare.
REFERENCES
- Abernethy LJ, Klafkowski G, Foulder-Hughes L, & Cooke RW (2003). Magnetic resonance imaging and T2 relaxometry of cerebral white matter and hippocampus in children born preterm. Pediatric Research, 54(6), 868–874. [DOI] [PubMed] [Google Scholar]
- Allgaier AK, Pietsch K, Fruhe B, Sigl-Glockner J, & Schulte-Korne G (2012). Screening for depression in adolescents: validity of the patient health questionnaire in pediatric care. Depression and Anxiety, 29(10), 906–913. [DOI] [PubMed] [Google Scholar]
- Aminoff EM, Kveraga K, & Bar M (2013). The role of the parahippocampal cortex in cognition. Trends in Cognative Science, 17(8), 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, & Simmons WK (2014). Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biological Psychiatry, 76(3), 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ (2000). Concepts of myelin and myelination in neuroradiology. American Journal of Neuroradiology, 21(6), 1099–1109. [PMC free article] [PubMed] [Google Scholar]
- Bauer E, Toepper M, Gebhardt H, Gallhofer B, & Sammer G (2015). The significance of caudate volume for age-related associative memory decline. Brain Research, 1622, 137–148. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Watson CG, Rivkin MJ, Robertson RL, Roberts AE, Stopp C, ... Newburger JW (2015). Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. Journal of the American Heart Association, 4(12). dor.orghttps://10.1161/JAHA.115.002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AJ, McQuillen PS, Chau V, Glass H, Poskitt KJ, Barkovich AJ, ... Miller SP (2010). Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery, 140(3), 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JI, Beasley PJ, D’Angelo EJ, Landzberg M, & DeMaso DR 2003. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart & Lung, 32(2), 105–110. [DOI] [PubMed] [Google Scholar]
- Cassidy AR, White MT, DeMaso DR, Newburger JW, & Bellinger DC (2015). Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. Journal of the International Neuropsychological Society, 21(1), 34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy RR, Sharif U, Ichord R, Spray TL, Nicolson S, Tabbutt S, ... Gaynor JW (2005). Electrographic neonatal seizures after infant heart surgery. Epilepsia, 46(1), 84–90. [DOI] [PubMed] [Google Scholar]
- Cohen M, Mansoor D, Langut H, & Lorber A (2007). Quality of life, depressed mood, and self-esteem in adolescents with heart disease. Psychosomatic Medicine, 69(4), 313–318. [DOI] [PubMed] [Google Scholar]
- Cordina R, Grieve S, Barnett M, Lagopoulos J, Malitz N, & Cele-rmajer DS (2014). Brain volumetric, regional cortical thickness and radiographic findings in adults with cyanotic congenital heart disease. NeuroImage: Clinical, 4, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davos CH, Francis DP, Leenarts MF, Yap SC, Li W, Davlouros PA, ... Gatzoulis MA (2003). Global impairment of cardiac autonomic nervous activity late after the Fontan operation. Circulation, 108(1), II180–185. [DOI] [PubMed] [Google Scholar]
- Donofrio MT, & Massaro AN (2010). Impact of congenital heart disease on brain development and neurodevelopmental outcome. International Journal of Pediatrics, 2010. doi.orghttps://10.1155/2010/359390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Bartlett P, & Barker GJ (1996). Technique for measuring hippocampal T2 relaxation time. American Journal of Neuroradiology, 17(10), 1805–1810. [PMC free article] [PubMed] [Google Scholar]
- Ebert D, & Ebmeier KP (1996). The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biological Psychiatry, 39(12), 1044–1050. [DOI] [PubMed] [Google Scholar]
- Engelfriet P, & Mulder BJ (2009). Gender differences in adult congenital heart disease. Netherlands Heart Journal, 17(11), 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang AG (2009). Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang AG (2013). G*Power Version 3.1.7 [computer software]. Uiversität Kiel, Germany: http://www.gpower.hhu.de/en.html. Accessed on 23 May 2016. [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, & Daskalakis ZJ (2008). A meta-analytic study of changes in brain activation in depression. Human Brain Mapping, 29(6), 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C, & Dolan R (1996). The role of the prefrontal cortex in higher cognitive functions. Brain Research, Cognative Brain Research, 5(1–2), 175–181. [DOI] [PubMed] [Google Scholar]
- Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, ... International Cardiac Collaborative on Neurodevelopment I. (2015). Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics, 135(5), 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstle M, Beebe DW, Drotar D, Cassedy A, & Marino BS (2016). Executive functioning and school performance among pediatric survivors of complex congenital heart disease. Journal of Pediatrics, 173, 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Phan KL, & Shankman SA (2014). Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression. Biology of Mood & Anxiety Disorders, 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, ... Kawato M (2004). A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. Journal of Neuroscience, 24(7), 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T (2013). Long-term depression and other synaptic plasticity in the cerebellum. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 89(5), 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Kogure K, Ohtomo H, Izumiyama M, Tobita M, Matsui S, ... Watanabe T (1986). Characterization of experimental ischemic brain edema utilizing proton nuclear magnetic resonance imaging. Journal of Cerebral Blood Flow & Metabolism, 6(2), 212–221. [DOI] [PubMed] [Google Scholar]
- Khairy P, Fernandes SM, Mayer JE Jr., Triedman JK, Walsh EP, Lock JE, & Landzberg MJ (2008). Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation, 117(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Kirsch SJ, Jacobs RW, Butcher LL, & Beatty J (1992). Prolongation of magnetic resonance T2 time in hippocampus of human patients marks the presence and severity of Alzheimer’s disease. Neuroscience Letters, 134(2), 187–190. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, & Harper RM (2012a). Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. Journal of Neuroscience Research, 90(10), 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Delshad S, Macey PM, Woo MA, & Harper RM (2011a). Development of T2-relaxation values in regional brain sites during adolescence. Magnetic Resonance Imaging, 29(2), 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Delshad S, Woo MA, Macey PM, & Harper RM (2012b). Age-related regional brain T2-relaxation changes in healthy adults. Journal of Magnetic Resonance Imaging, 35(2), 300–308. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gupta RK, Rao SB, Chawla S, Husain M, & Rathore RK (2003). Magnetization transfer and T2 quantitation in normal appearing cortical gray matter and white matter adjacent to focal abnormality in patients with traumatic brain injury. Magnetic Resonance Imaging, 21(8), 893–899. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gupta RK, Rathore RK, Rao SB, Chawla S, & Pradhan S (2002). Multiparametric quantitation of the perilesional region in patients with healed or healing solitary cysticercus granuloma. Neuroimage, 15(4), 1015–1020. [DOI] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Keens TG, & Harper RM (2005). Neuroanatomic deficits in congenital central hypoventilation syndrome. Journal Comprehensive Neurology, 487(4), 361–371 [DOI] [PubMed] [Google Scholar]
- Kumar R, Pham TT, Macey PM, Woo MA, Yan-Go FL, & Harper RM (2014). Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea. Sleep, 37 (4), 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, & Harper RM (2011b). Brain axonal and myelin evaluation in heart failure. Journal of Neurological Science, 307(1–2), 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latal B, Kellenberger C, Dimitropoulos A, Hagmann C, Balmer C, Beck I, & Bernet V (2015). Can preoperative cranial ultrasound predict early neurodevelopmental outcome in infants with congenital heart disease? Dev Med Child Neurol. doi.orghttps://10.1111/dmcn.12701. [DOI] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, & Goldman-Rakic PS (1997). Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. Journal of Neuroscience, 17(10), 3870–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zeng LL, Li Y, Ma Q, Li B, Shen H, & Hu D (2012). Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PLoS One, 7(6), e39516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Rieken NS, Kumar R, Ogren JA, Middlekauff HR, Wu P, ... Harper RM (2016). Sex differences in insular cortex gyri responses to the valsalva maneuver. Frontiers in Neurology, 7, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, ... Kurth CD (2002). An MRI study of neurological injury before and after congenital heart surgery. Circulation, 106(12 Suppl 1), I109–114. [PubMed] [Google Scholar]
- Manelis A, & Reder LM (2012). Procedural learning and associative memory mechanisms contribute to contextual cueing: Evidence from fMRI and eye-tracking. Learning & Memory, 19(11), 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli A, Miller SP, Marino BS, Jefferson AL, & Newburger JW (2016). Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation, 133(20), 1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, ... Miller SP (2007). Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke, 38(2 Suppl), 736–741. [DOI] [PubMed] [Google Scholar]
- Miller SP, & McQuillen PS (2007). Neurology of congenital heart disease: insight from brain imaging. Archives of Disease in Childhood. Fetal and Neonatal Edition, 92(6), F435–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JR, Huh J, Kang IS, Park SW, Jun TG, & Lee HJ (2009). Factors influencing depression in adolescents with congenital heart disease. Heart & Lung, 38(5), 419–426. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, ... Chertkow H (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society, 53(4), 695–699. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hasegawa S, Yasuda K, Yamada O, Ono Y, & Echigo S (2001). Severely impaired cardiac autonomic nervous activity after the Fontan operation. Circulation, 104(13), 1513–1518. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Schmithorst VJ, Wisnowski JL, Watson CG, Bellinger DC, Newburger JW, & Rivkin MJ (2015). Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. NeuroImage: Clinical, 7, 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou N, Papadaki E, Karampekios S, Spilioti M, Maris T, Prassopoulos P, & Gourtsoyiannis N (2004). T2 relaxation time analysis in patients with multiple sclerosis: correlation with magnetization transfer ratio. European Radiology, 14(1), 115–122. [DOI] [PubMed] [Google Scholar]
- Pauliks LB (2013). Depression in adults with congenital heart disease-public health challenge in a rapidly expanding new patient population. World Journal of Cardiology, 5(6), 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike NA, Poulsen MK, & Woo MA (2016). Validity of the Montreal cognitive assessment screener in adolescents and young adults with and without congenital heart disease. Nursing Research, 66(3), 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike NA, Woo MA, Poulsen MK, Evangelista W, Faire D, Halnon NJ, ... Kumar R (2016). Predictors of memory deficits in adolescents and young adults with congenital heart disease compared to healthy controls. Frontiers in Pediatrics, 4, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, & Denckla MB (1996). Brain development, gender and IQ in children. A volumetric imaging study. Brain, 119(Pt 5), 1763–1774. [DOI] [PubMed] [Google Scholar]
- Roy B, Woo MA, Wang DJJ, Fonarow GC, Harper RM, & Kumar R (2017). Reduced regional cerebral blood flow in patients with heart failure. Eur J Heart Fail, 19(10), 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel H, Buchli R, Gehrmann J, Aguzzi A, Illi O, & Martin E (1995). Magnetic resonance imaging of brain edema in the neonatal rat: a comparison of short and long term hypoxia-ischemia. Pediatric Research, 38(1), 113–118. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, Koolschijn PC, Peper JS, & Crone EA (2012). The cerebellum link to neuroticism: a volumetric MRI association study in healthy volunteers. PLoS One, 7(5), e37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, & Cincotta CM (2005). The roles of the caudate nucleus in human classification learning. Journal of Neuroscience, 25(11), 2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi V, Tabbutt S, Dimitropoulos A, Harris KC, Chau V, Poskitt K, ... McQuillen PS (2013). Single-ventricle anatomy predicts delayed microstructural brain development. Pediatric Research, 73(5), 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Fu Y, Ren Z, Zhang T, Du M, Gong Q, ... Zhang W (2014). The common traits of the ACC and PFC in anxiety disorders in the DSM-5: meta-analysis of voxel-based morphometry studies. PLoS One, 9(3), e93432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheslow D, & Adams W (2003). Wide range assessment of memory and learning administration and technical manual (2nd ed.). Wilmington, DE: Wide Range Inc. [Google Scholar]
- Steer RA, Kumar G, Ranieri WF, & Beck AT (1995). Use of the Beck Anxiety Inventory with adolescent psychiatric outpatients. Psychological Reports, 76(2), 459–465. [DOI] [PubMed] [Google Scholar]
- Talati A, Pantazatos SP, Schneier FR, Weissman MM, & Hirsch J (2013). Gray matter abnormalities in social anxiety disorder: primary, replication, and specificity studies. Biological Psychiatry, 73(1), 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend TN, Bernasconi N, Pike GB, & Bernasconi A (2004). Quantitative analysis of temporal lobe white matter T2 relaxation time in temporal lobe epilepsy. Neuroimage, 23(1), 318–324. [DOI] [PubMed] [Google Scholar]
- Tummala S, Roy B, Park B, Kang DW, Woo MA, Harper RM, & Kumar R (2016). Associations between brain white matter integrity and disease severity in obstructive sleep apnea. Journal of Neuroscience Research, 94(10), 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rhein M, Buchmann A, Hagmann C, Dave H, Bernet V, Scheer I, ... Heart and Brain Research Group. (2015). Severe congenital heart defects are associated with global reduction of neonatal brain volumes. Journal of Pediatrics, 167(6), 1259–1263. [DOI] [PubMed] [Google Scholar]
- Wernovsky G, Stiles KM, Gauvreau K, Gentles TL, duPlessis AJ, Bellinger DC, ... Newburger JW (2000). Cognitive development after the Fontan operation. Circulation, 102(8), 883–889. [DOI] [PubMed] [Google Scholar]
- Wiebking C, de Greek M, Duncan NW, Tempelmann C, Bajbouj M, & Northoff G (2015). Interoception in insula subregions as a possible state marker for depression-an exploratory fMRI study investigating healthy, depressed and remitted participants. Frontiers in Behavioral Neuroscience, 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZM, & Eskandar EN (2006). Selective enhancement of associative learning by microstimulation of the anterior caudate. Nature Neuroscience, 9(4), 562–568. [DOI] [PubMed] [Google Scholar]
- Woo MA, Kumar R, Macey PM, Fonarow GC, & Harper RM (2009). Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail 15(3), 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Palomares JA, Macey PM, Fonarow GC, Harper RM, Kumar R. 2015. Global and regional brain mean diffusivity changes in patients with heart failure. Journal of Neuroscience, Res 93(4), 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamoscik V, Huffziger S, Ebner-Priemer U, Kuehner C, & Kirsch P (2014). Increased involvement of the parahippocampal gyri in a sad mood predicts future depressive symptoms. Social Cognitive and Affective Neuroscience, 9(12), 2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]