Abstract
Social anxiety disorder (SAD) is a prevalent and disabling mental disorder, associated with significant psychiatric co-morbidity. Previous research on structural brain alterations associated with SAD has yielded inconsistent results concerning the direction of the changes in gray matter (GM) in various brain regions, as well as on the relationship between brain structure and SAD-symptomatology. These heterogeneous findings are possibly due to limited sample sizes. Multi-site imaging offers new opportunities to investigate SAD-related alterations in brain structure in larger samples.
An international multi-center mega-analysis on the largest database of SAD structural T1-weighted 3T MRI scans to date was performed to compare GM volume of SAD-patients (n = 174) and healthy control (HC)-participants (n = 213) using voxel-based morphometry. A hypothesis-driven region of interest (ROI) approach was used, focusing on the basal ganglia, the amygdala-hippocampal complex, the prefrontal cortex, and the parietal cortex. SAD-patients had larger GM volume in the dorsal striatum when compared to HC-participants. This increase correlated positively with the severity of self-reported social anxiety symptoms. No SAD-related differences in GM volume were present in the other ROIs. Thereby, the results of this mega-analysis suggest a role for the dorsal striatum in SAD, but previously reported SAD-related changes in GM in the amygdala, hippocampus, precuneus, prefrontal cortex and parietal regions were not replicated. Our findings emphasize the importance of large sample imaging studies and the need for meta-analyses like those performed by the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) Consortium.
Keywords: Social anxiety disorder, Structural MRI, Voxel-based morphometry, Gray matter, Mega-analysis, Striatum
Graphical abstract
Gray matter (GM) alterations in social anxiety disorder (SAD) - an international voxel-based morphometry multi-center mega-analysis
Highlights
-
•
Multi-center mega-analysis on gray matter (GM) in social anxiety disorder (SAD)
-
•
Largest sample available for analysis to date: 174 SAD-patients vs 213 controls
-
•
Larger GM volume in the right putamen in SAD-patients
-
•
No SAD-related alterations in amygdala-hippocampal, prefrontal or parietal regions
-
•
Results stress need for larger samples and meta-analyses - cf. ENIGMA Consortium
1. Introduction
Social anxiety disorder (SAD) is one of the most common anxiety disorders (Stein and Stein, 2008), with an estimated lifetime prevalence between 6 and 13 percent (Kessler et al., 2012, Stein et al., 2010). Patients with SAD are characterized by intense fear of, distress in, and avoidance of situations in which they may be scrutinized (American Psychiatric Association, 2013). The disorder is highly disabling, as impairments in social life and work situations are frequently reported (Mack et al., 2015). In addition, the disorder is associated with significant psychiatric co-morbidity, such as depressive disorders and substance abuse (Stein and Stein, 2008). These findings stress the need for improvements in the treatment of SAD. Understanding the neurobiological mechanisms that underlie this disorder has the potential to advance treatment.
Previous magnetic resonance imaging (MRI) studies on brain anatomy differences in SAD have reported heterogeneous findings, implicating regions such as the frontal cortex, the parietal cortex, occipital cortex, temporal regions and subcortical limbic areas, as reviewed by Brühl et al. (2014a); see also Goodkind et al. (2015) reporting on a transdiagnostic meta-analysis of structural neuroimaging studies. Several of these changes were correlated with clinical characteristics, such as the severity of social anxiety symptoms (Brühl et al., 2014b, Frick et al., 2014a, Irle et al., 2014, Irle et al., 2010, Liao et al., 2011, Syal et al., 2012, Talati et al., 2013, Tükel et al., 2015) or disease duration (Meng et al., 2013). In addition, recent treatment studies in SAD-patients have identified structural changes in bilateral caudate and putamen, right thalamus and cerebellum after 8-weeks of paroxetine treatment (Talati et al., 2015) and alterations in parieto-occipital and prefrontal GM volumes after cognitive behavioral group therapy (Steiger et al., 2016), while a classification study using multi-voxel pattern analysis was able to discriminate SAD-patients from healthy control participants based on the pattern of regional gray matter (GM) volume over the whole brain (Frick et al., 2014b). Together, these studies provide evidence for the idea that certain brain regions are clinically associated with SAD.
Functional MRI (fMRI) studies have also identified important candidate brain regions that may be related to structural changes associated with SAD-related psychopathology. These fMRI studies, typically examining brain activity in response to emotional stimuli or in response to cognitive tasks (Brühl et al., 2014a), most consistently point towards an increase of brain activation in SAD in the bilateral amygdala and hippocampus, prefrontal brain regions, bilateral insula, bilateral parietal cortex and bilateral precuneus, while findings on the direction of changes in the basal ganglia are mixed (Brühl et al., 2014a, Cremers and Roelofs, 2016). In addition, studies on functional connectivity, during rest as well as during cognitive tasks (Brühl et al., 2014a), revealed changes in connectivity of, among others, the putamen (Cremers et al., 2015) and the amygdala (Hahn et al., 2011, Pannekoek et al., 2013, Sladky et al., 2015), while recent positron emission tomography (PET) studies showed decreased serotonin receptor binding (Lanzenberger et al., 2007) and increased serotonin synthesis and transporter availability in the hippocampus, amygdala, anterior cingulate cortex (ACC) and striatal regions like the putamen and globus pallidus (Frick et al., 2015, Furmark et al., 2016). These results, together with the findings of a treatment study revealing a relationship between changes in amygdala structure and amygdala function in SAD (Månsson et al., 2016), suggest that the brain regions showing functional changes in SAD overlap to a large extent with the regions that have showed differences in brain structure.
However, the available evidence with respect to structural brain alterations in SAD is inconclusive, as both increases as well as decreases in GM volumes in various brain regions have been reported (Brühl et al., 2014a). Furthermore, findings concerning the relationship between brain structure and SAD-symptoms are inconsistent (Brühl et al., 2014b, Frick et al., 2014a, Irle et al., 2014, Tükel et al., 2015). These heterogeneous results are possibly due to differences in the employed methods, as well as the relatively small sample sizes employed in studies on SAD-related changes in brain structure (ranging from 12 to 67 SAD-patients), and variability in clinical parameters between the samples. Recent advances in multi-site imaging offer new opportunities to investigate the structural brain alterations associated with SAD.
In this international multi-center mega-analysis, which is part of the European and South African Research Network in Anxiety Disorders (EURSANAD) program initiated by the Anxiety Disorders Research Network (Baldwin and Stein, 2012), we investigated GM volume in a-priori defined regions of interest (ROIs) in a sample of 174 SAD-patients and 213 healthy control participants, using an optimized voxel-based morphometry (VBM) protocol (Ashburner and Friston, 2000, Lerch et al., 2017). VBM analyses have the advantage of using unbiased, standardized methods to investigate brain structure, and have been extensively used to investigate alterations in brain morphology across numerous major psychiatric conditions (Ashburner and Friston, 2000, Goodkind et al., 2015). The large sample of the present work provides the best statistical power to date to investigate GM alterations associated with SAD. Data were collected in multiple scan centers located in five countries (Germany, South Africa, Sweden, the Netherlands and the United States of America). Based on the available evidence reviewed above, our analysis focused on changes in GM volume in four a-priori defined ROIs that seem to be most prominently involved in SAD: the basal ganglia, the amygdala-hippocampal complex, the prefrontal cortex and the parietal cortex including the precuneus. Given the mixed findings on SAD-related increases versus decreases in GM in the previous structural MRI studies (Brühl et al., 2014a), we did not make specific predictions about the direction of the changes within these ROIs. Significant results within the ROIs were followed up by regression analyses to investigate the relationship between GM volumes and the severity of social anxiety symptoms within the patient group.
2. Material and methods
2.1. Participants
Structural T1-weighted 3T MRI scans were collected at research centers located in Europe, Africa and North-America, and brought together for quality control and initial analysis in Cape Town, South-Africa. Final analyses took place in Leiden, the Netherlands. The initial sample consisted of 251 SAD-patients and 230 healthy control (HC)-participants (Table 1), and results on these datasets have been published previously (Boehme et al., 2015, Boehme et al., 2014a, Boehme et al., 2014b, Cremers et al., 2014, Geiger et al., 2016, Howells et al., 2015, Klumpp et al., 2016, Månsson et al., 2015, Månsson et al., 2013, Pannekoek et al., 2013, Phan et al., 2013, Syal et al., 2012, van Tol et al., 2010) – see Inline Supplementary Document 1 for more details on the in- and exclusion criteria and recruitment of participants for each sample. At each site, the local ethical committee approved data-collection and all participants provided written informed consent after the procedure had been fully explained.
Table 1.
Sample composition.
|
Initial number of scans |
Excluded number of scans |
Included number of scans |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Research center | SAD | HC | Comorbiditya | Insufficient scan-qualityb | Brain pathology | Other reasonc | SAD | HC | Total |
| Germany (Boehme et al., 2015, Boehme et al., 2014a, Boehme et al., 2014b) | University of Jena; University of Münster | 53 | 22 | 31 | 3 | 0 | 0 | 19 | 22 | 41 |
| The Netherlands (Pannekoek et al., 2015, Pannekoek et al., 2013, Penninx et al., 2008, van Tol et al., 2010), (Cremers et al., 2014) | VU Medical Center Amsterdam - NESDA Study | 10 | 27 | 1 | 3 | 0 | 0 | 6 | 27 | 33 |
| University of Groningen - NESDA Study | 9 | 12 | 1 | 1 | 0 | 0 | 8 | 11 | 19 | |
| Leiden University Medical Center - NESDA Study | 9 | 26 | 1 | 0 | 0 | 0 | 8 | 26 | 34 | |
| Leiden University Medical Center - Social Anxiety Study | 20 | 20 | 0 | 0 | 0 | 0 | 20 | 20 | 40 | |
| South-Africa (Geiger et al., 2016, Howells et al., 2015, Syal et al., 2012) | University of Cape Town; Stellenbosch University | 18 | 17 | 2 | 10 | 0 | 0 | 12 | 11 | 23 |
| Sweden (Månsson et al., 2015, Månsson et al., 2013) | Umeå University | 26 | 26 | 0 | 3 | 0 | 0 | 26 | 23 | 49 |
| Uppsala University | 24 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | |
| United States of America (Klumpp et al., 2016, Phan et al., 2013) | University of Chicago | 27 | 25 | 3 | 4 | 0 | 0 | 24 | 21 | 45 |
| University of Illinois | 12 | 12 | 1 | 0 | 0 | 0 | 12 | 11 | 23 | |
| University of Michigan | 43 | 43 | 2 | 3 | 1 | 0 | 39 | 41 | 80 | |
| Total | 251 | 230 | 42 | 27 | 1 | 24 | 174 | 213 | 387 | |
SAD: Social Anxiety Disorder patients; HC: healthy control participants.
Other than depression or anxiety (SAD-patients only).
Insufficient scan-quality: scans with motion artefacts, scans being unsegmentable or scans for which brain extraction failed after multiple attempts.
No data from HC-participants to balance design.
Participants (18 years or older) were recruited through public announcements (online and within the community), consumer advocacy groups, general practitioners and clinical centers, and screened using structured clinical interviews in their native language: the Mini-International Neuropsychiatric Interview (Sheehan et al., 1997), the Composite Interview Diagnostic Instrument version (Kessler and Ustün, 2004) or the Structured Clinical Interview for DSM-IV disorders (First et al., 1998). SAD-patients had to meet criteria for a primary diagnosis of SAD, while HC-participants had to be free of any psychopathology. General MRI contraindications (ferromagnetic implants, claustrophobia, pregnancy) were a reason for exclusion in both groups.
In addition to the T1-weighted 3T MRI scans, demographic (age, gender, handedness) and clinical data were collected at each research center. Furthermore, information about education level, comorbidity, medication use and the scores on several questionnaires (Liebowitz Social Anxiety Scale (LSAS) (Heimberg et al., 1999), Beck Depression Inventory (BDI) (Beck et al., 1988) and State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1970)) were available for a subset of participants.
2.2. Data acquisition, quality checks and final sample
Parameters of the T1-weighted MRI scans are presented in Table 2. Scans from SAD-patients with comorbid psychopathology other than any other anxiety disorder or major depressive disorder (MDD) were excluded from the analysis (n = 42, see Table 1 and Inline Supplementary Table 1). Next, scans were extensively checked for pathology and quality, leading to the exclusion of an additional 28 scans (Table 1). Furthermore, all scans from the research center in Uppsala (n = 24 SAD-patients) were excluded due to the lack of scans from HC-participants from this center, necessary for our analytic approach. This resulted in a final sample of 174 SAD-patients and 213 HC-participants. Characteristics of the final sample are presented in Table 3. Statistical analyses on differences between groups were performed using IBM SPSS Statistics (Version 23), with a significance level of p < 0.05.
Table 2.
Characteristics of T1-weighted MRI scans.
| Country | Research site/sample | Scanner | Voxels | Dimensions |
|---|---|---|---|---|
| Germany | University of Jena; University of Münster | Siemens/TrioTim 3T | 192 × 256 × 256 | 1 × 1 × 1 mm |
| The Netherlands | VU Medical Center Amsterdam - NESDA study | Philips 3T | 170 × 256 × 256 | 1 × 1 × 1 mm |
| University of Groningen - NESDA study | Philips 3T | 170 × 256 × 256 | 1 × 1 × 1 mm | |
| Leiden University Medical Center - NESDA study | Philips 3T | 170 × 256 × 256 | 1 × 1 × 1 mm | |
| Leiden University Medical Center - Social Anxiety Study | Philips 3T | 256 × 256 × 140 | 0.875 × 0.875 × 1.2 mm | |
| South-Africa | University of Cape Town; Stellenbosch University | Siemens Magnetom Allegra 3T | 128 × 256 × 256 | 1.33 × 1 × 1 mm |
| Sweden | Umeå University | General Electric 3T | 512 × 512 × 176 | 0.48 × 0.48 × 1 mm |
| Uppsala University | Philips Achieva 3T | 480 × 480 × 170 | 0.5 × 0.5 × 1 mm | |
| United States of America | University of Chicago | GE Signa System 3T | 256 × 256 × 120 | 0.94 × 0.94 × 1.5 mm |
| University of Illinois | GE Signa System 3T | 256 × 256 × 182 | 0.86 × 0.86 × 1 mm | |
| University of Michigan | GE Signa System 3T | 256 × 256 × 124 | 1 × 1 × 1.2 mm |
2.3. Voxel-based morphometry analysis
Voxel-wise GM volumes were investigated using an optimized voxel-based morphometry (VBM) protocol, using the default pipeline as implemented in FSL (version 5.0.7) (Good et al., 2001, Smith et al., 2004). Structural T1-weighted images were first brain-extracted using FSL and Freesurfer software. Each brain was closely visually inspected and brain-extraction was repeated until all non-brain tissue was properly removed from the image. Subsequently, images were segmented into GM, white matter (WM) and cerebrospinal fluid (CSF) (Zhang et al., 2001). Next, a study-specific GM template was created, in order to avoid biases during registration that could favour either the SAD or HC-group (Good et al., 2001), by randomly selecting GM images from an equal number of SAD-patients and HC-participants from each research center (n = 166 SAD-patients and 166 HC-participants). These GM images were non-linearly registered to the Montreal Neurological Institute (MNI) T1-template brain, averaged and flipped along the x-axis to create a left-right symmetric study-specific GM template with a resolution of 2 × 2 × 2 mm. Subsequently, the original GM images from all participants were non-linearly registered to this template (Andersson et al., 2007), modulated to correct for local expansion or contraction and smoothed using a kernel with an isotropic Gaussian kernel (σ = 3 mm).
2.4. Region of interest (ROI) analysis: differences between groups
In order to maximize the statistical power to detect GM differences between SAD-patients and HC-participants, we used a region of interest (ROI) approach (Poldrack, 2007) focusing on brain areas in which functional and structural brain changes related to SAD have been reported previously (see Introduction). Four ROIs were created in standard space (resolution 2 × 2 × 2 mm) using the Harvard-Oxford Cortical Structural Atlas and Harvard-Oxford Subcortical Structural Atlas implemented in FSLView (version 3.2.0). The basal ganglia ROI consisted of voxels with a probability of at least 50% of belonging to the bilateral accumbens, caudate, pallidum or putamen (total size of ROI: 3224 voxels, 25,792 mm3). The second ROI, the amygdala-hippocampus ROI, consisted of voxels with a probability of at least 50% of belonging to the bilateral amygdala, hippocampus and the anterior and posterior parahippocampal gyrus (total size of ROI: 3066 voxels, 24,528 mm3). The prefrontal cortex ROI included voxels with a probability of at least 50% of belonging to the middle frontal gyrus, the subcallosal cortex, the anterior cingulate gyrus, paracingulate gyrus, frontal medial cortex and frontal orbital cortex (total size of ROI: 20,601 voxels, 164,808 mm3). Finally, the parietal ROI encompassed voxels with a probability of at least 50% of belonging to the superior parietal lobule, the precuneus cortex and the posterior cingulate gyrus (total size of ROI: 5478 voxels, 43,824 mm3).
Within these ROIs, we examined differences in GM volume between SAD-patients and HC-participants using a general linear model (GLM). In this model, scan center (coded by dummy variables) and gender were added as nuisance regressors and age and total GM volume were included as covariates. Before we analyzed this GLM, we tested the homogeneity of regression slopes assumption that applies to covariate analysis, by building a separate GLM that included a diagnosis-by-age and a diagnosis-by-total GM regressor in addition to the other regressors. No significant interactions at the whole-brain level were observed, thus justifying the use of the abovementioned GLM that investigated the effect of diagnosis while correcting for the covariates.
Voxelwise statistics were applied using permutation-based non-parametric testing (5000 permutations), correcting for multiple comparisons across space. FSL's default threshold-free cluster enhancement (TFCE) was used to detect significant clusters (Smith and Nichols, 2009) and we used a familywise error (FWE)-corrected threshold of p < 0.05 within each ROI. Given the fact that ROIs were a priori defined and are part of a network of brain areas involved in SAD (Brühl et al., 2014a), we report p-values uncorrected for the number of ROIs. Significant results within the ROIs were followed up by a multiple regression analysis using IBM SPSS Statistics, in order to examine the relationship between average individual GM volume in the extracted cluster and the severity of total social anxiety symptoms (measured with the LSAS), while controlling for scan center, gender, age and total GM volume. In line with previous work (Frick et al., 2014a, Irle et al., 2014, Meng et al., 2013, Syal et al., 2012), this analysis was performed in SAD-patients only.
For reasons of completeness, we also performed an exploratory whole-brain VBM analysis to examine a main effect of diagnosis and interactions with age and scan center outside the predefined ROIs using the same GLM. Again, we used TFCE-results based on an FWE-corrected threshold of p < 0.05.
3. Results
3.1. Sample characteristics
Characteristics of SAD-patients (n = 174) and HC-participants (n = 213) are presented in Table 3. SAD-patients did not differ from HC-participants in terms of age, gender, level of education, handedness and total GM volume, but they reported significantly more social anxiety symptoms (measured with the LSAS) and anxiety symptoms (measured with the STAI) in comparison to HC-participants. In addition, SAD-patients reported significantly more depressive symptoms than HC-participants as measured with the BDI. It should, however, be noted that the degree of reported depression symptoms in the SAD-patients indicates only minimal depression (mean ± standard deviation: 13.8 ± 8.8) (Beck et al., 1988), whereas the mean scores on the LSAS for the SAD-patients (mean ± standard deviation: 77.9 ± 17.9) are in line with a clinical diagnosis of SAD (Mennin et al., 2002).
Table 3.
Demographic and clinical characteristics of social anxiety disorder (SAD)-patients and healthy control (HC) participants
| SAD (n = 174) |
HC (n = 213) |
Statistical analysis |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | ||
| Age (years) | 30.6 | 10.0 | 32.4 | 10.5 | 0.13 | Independent Samples Mann-Whitney U test |
| Age of onset (years)a | 14.8 | 7.1 | ||||
| SAD (n = 174) |
HC (n = 213) |
Statistical analysis |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | p | ||
| Males | 72 | 41.4 | 97 | 45.5 | 0.41 | χ2 test |
| Education levelb | 0.10 | χ2 test | ||||
| Low | 1 | 0.7 | 6 | 3.2 | ||
| Intermediate | 56 | 36.8 | 54 | 29.0 | ||
| High | 95 | 62.5 | 126 | 67.7 | ||
| Right-handed | 172 | 98.9 | 206 | 96.7 | 0.17 | χ2 Test |
| Comorbidity | ||||||
| SAD only | 114 | 65.5 | ||||
| SAD + MDD | 8 | 4.6 | ||||
| SAD + MDD + PD | 2 | 1.1 | ||||
| SAD + GAD | 10 | 5.7 | ||||
| SAD + GAD + SP | 3 | 1.7 | ||||
| SAD + GAD + PD | 2 | 1.1 | ||||
| SAD + PD | 3 | 1.7 | ||||
| SAD + SP | 6 | 3.4 | ||||
| Unknown | 26 | 14.9 | ||||
| Medication use at time of scanc | 24 | 14.2 | ||||
| SSRI | 17 | |||||
| Betablocker | 2 | |||||
| Antidepressivum NOS | 4 | |||||
| Unknown medication | 1 | |||||
| SAD (n = 174) |
HC (n = 213) |
Statistical analysis |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | ||
| Liebowitz Social Anxiety Scale (LSAS)d | 77.9 | 17.9 | 14.3 | 12.6 | <0.001 | Independent Samples Mann-Whitney U test |
| Beck Depression Inventory (BDI)e | 13.8 | 8.8 | 2.3 | 3.2 | <0.001 | Independent Samples Mann-Whitney U test |
| State-Trait Anxiety Inventory - State scoref | 43.2 | 10.1 | 20.9 | 11.0 | <0.001 | Independent Samples T-Test |
| State-Trait Anxiety Inventory - Trait scoref | 50.1 | 10.2 | 22.6 | 11.5 | <0.001 | Independent Samples T-Test |
| Total Gray Matter Volume (mL) | 519.3 | 49.9 | 522.3 | 58.7 | 0.47 | Independent Samples Mann-Whitney U test |
GAD: generalized anxiety disorder; MDD: Major Depressive Disorder; NOS: not otherwise specified; PD: panic disorder; SP: specific phobia; SSRI: selective serotonin reuptake inhibitor
Data from 65 SAD-patients.
Data from 152 SAD-patients and 186 HC-participants.
Data from 169 SAD-patients.
Data from 148 SAD-patients and 140 HC-participants.
Data from 113 SAD-patients and 111 HC-participants.
Data from 75 SAD-patients and 73 HC-participants.
3.2. ROI analyses: differences between SAD-patients and HC-participants
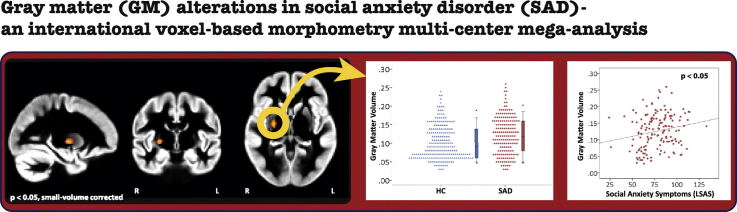
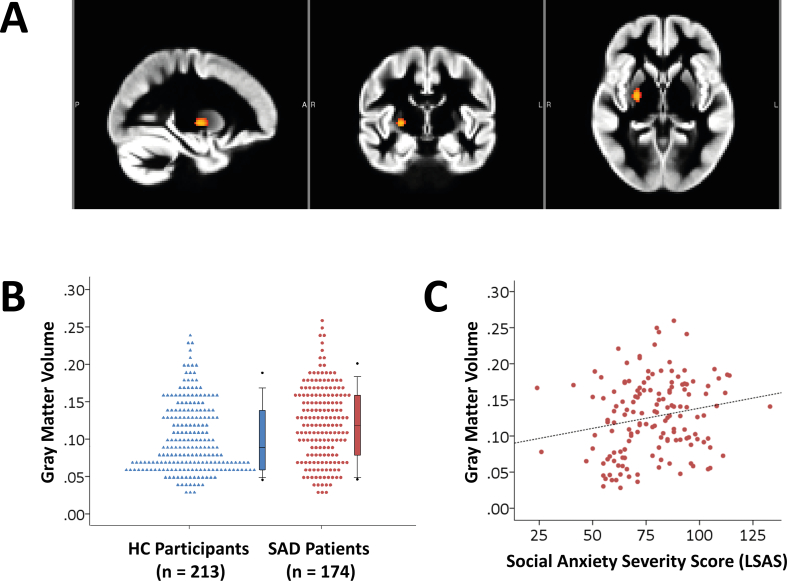
There was an effect of diagnosis in the basal ganglia ROI: SAD-patients had larger GM volume in the right putamen, extending into the pallidum (Fig. 1A and B; extent = 78 voxels, peak coordinate in MNI space: X = 26, Y = − 8, Z = 0; p = 0.022, small-volume corrected; result did not survive correction when all ROIs were taken together), with a small effect size (β = 0.14, Cohen's d = 0.20). A subsequent analysis, that regressed social anxiety symptoms within the SAD-patients on individual extracted GM volume in this region, revealed a significant positive correlation with a small effect size (zero-order correlation: Spearman's rho = 0.21, p = 0.010; multiple regression analysis while controlling for scan center, gender, age and total GM volume: β = 0.13, p = 0.048; see also Fig. 1C).
Fig. 1.
(A) Larger GM volume in social anxiety disorder (SAD)-patients (n = 174) relative to healthy control (HC)-participants (n = 213) in the right dorsal striatum (p < 0.05, small-volume corrected). (B) Dot density plot illustrating the group difference in GM volume in the dorsal striatum. (C) Scatterplot illustrating the relationship between social anxiety symptoms in a subset of SAD-patients (n = 148; measured with the Liebowitz Social Anxiety Scale, LSAS) and GM volume in the dorsal striatum (Spearman's rho = 0.21, p < 0.05).
Given the fact that SAD often co-occurs with major depressive disorder (MDD) (Stein and Stein, 2008), we investigated whether the GM difference in the putamen was influenced by comorbid depression, by performing three subsequent analyses. Firstly, we excluded SAD-patients with a diagnosis of comorbid MDD (excluded: n = 10 SAD-patients; Table 3) and performed a multiple regression analysis with individual GM volume of the right putamen cluster as dependent variable, and diagnosis as independent variable while controlling for scan center, age, gender and total GM volume (remaining sample: n = 164 SAD-patients and 213 HC-participants). This analysis still showed a significant effect of diagnosis (β = 0.14, p = 0.002). Secondly, we excluded participants with a BDI score ≥ 30, indicating severe depression (Beck et al., 1988), (excluded: n = 7 SAD-patients; remaining sample: n = 106 SAD-patients and 111 HC-participants). Again, the effect of diagnosis was significant (β = 0.14, p = 0.017). In the third analysis, we examined the relationship between BDI-score and GM volume in the SAD-group (n = 113 SAD-patients; regression analysis, controlling for scan center, age, gender, and total GM volume). This analysis revealed a significant effect of BDI-score on GM volume (β = 0.17, p = 0.034). Importantly, when LSAS-score and BDI-score were both entered in the regression model, the effect of BDI was not significant anymore (β = 0.13, p = 0.13), while LSAS-score was still a significant predictor of GM volume (β = 0.16, p = 0.049). These results indicate that variation in BDI-scores in the SAD-sample did not significantly account for GM variance in the putamen-pallidum over and above effects of LSAS.
However, when we performed two additional sensitivity analyses to investigate the effect of 1st general comorbidity and 2nd medication use on the GM difference in the putamen, using multiple regression analyses with individual GM volume of the right putamen cluster as dependent variable, and diagnosis as independent variable while controlling for scan center, age, gender and total GM volume, the effect of diagnosis lacked significance (sensitivity analysis 1, including only SAD-patients without comorbidity: remaining sample: n = 114 SAD-patients and 213 HC-participants; β = 0.06, p = 0.28; sensitivity analysis 2, including only SAD-patients without present medication use: remaining sample: n = 59 SAD-patients and 117 HC-participants; β = 0.13, p = 0.13).
There were no clusters in the basal ganglia ROI where HC-participants had larger GM volume relative to SAD-patients. In addition, we did not find significant group-differences in the other ROIs using the VBM approach. To explore these null-findings, we extracted the individual GM volumes from the regions within each of the larger ROIs tested and examined the presence of between-group differences using multiple regression analyses controlled for scan center, age, gender and total GM volume. Because of the exploratory nature of these analyses, we corrected for the number of tests using Bonferroni-correction (13 regions, p ≤ 0.004). There were no regions in which the effect of diagnosis was significant at this Bonferroni-corrected significance level (Inline Supplementary Table 2), although two effects were significant at the uncorrected level. Furthermore, we explored the possibility that these null-findings were present due to gender differences between patients, by investigating gender x diagnosis interactions. Again, no significant interactions were found at the Bonferroni-corrected significance level (p ≤ 0.004) (see Inline Supplementary Table 2).
3.3. Whole-brain analysis: no group-differences
The exploratory whole-brain VBM analysis did not reveal a significant main effect of diagnosis. Significant diagnosis-by-age or diagnosis-by-scan center interactions were also not observed at whole-brain level.
4. Discussion
In this study we investigated differences in GM volume between SAD-patients and HC-participants, in the largest sample of 3T structural MRI scans available for analysis to date (n = 174 SAD-patients and 213 HC-participants). We used a hypothesis-driven ROI approach and focused on differences in GM volume in the amygdala-hippocampal complex, the basal ganglia, the prefrontal cortex and parietal areas. The results showed larger GM volume in the right putamen in SAD-patients in comparison to HC-participants (Fig. 1A and B), and this increase in GM was positively correlated with the total score on the Liebowitz Social Anxiety Scale (LSAS) within the patient group (Fig. 1C). This effect remained significant when we performed several sensitivity analyses examining the effect of comorbid depression; however, the effect did not survive in two other sensitivity analyses in which patients with any type of comorbidity and medication use were excluded, possibly due to the fact that the remaining sample size was relatively small.
We did, however, not find diagnosis-related alterations in GM volumes in the amygdala-hippocampal, prefrontal or parietal ROIs. Furthermore, there were no group differences in an exploratory whole-brain analysis. To examine these results, we performed post-hoc analyses to examine group differences in individual structures of these ROIs, but again, no SAD-related GM differences were present (Inline Supplementary Table 2). Furthermore, we checked whether GM differences between male and female SAD-patients might have confounded the results, but we did not find significant gender x diagnosis interactions (Inline Supplementary Table 2).
4.1. No SAD-related changes in amygdala-hippocampal, prefrontal and parietal ROIs
The null-findings in the amygdala-hippocampal, prefrontal and parietal ROIs were unexpected, because previous studies have reported SAD-related changes in GM in, among others, the amygdala, hippocampus, precuneus, prefrontal cortex and parietal regions (Brühl et al., 2014b, Irle et al., 2014, Irle et al., 2010, Liao et al., 2011, Machado-de-Sousa et al., 2014, Meng et al., 2013, Syal et al., 2012, Talati et al., 2013, Tükel et al., 2015). Although applying the usual caveats when interpreting null effects, our results based on the largest SAD-patient sample to date suggest that GM volume in regions outside the basal ganglia is likely not systematically related to SAD and thus might not underlie the alterations in brain functioning consistently reported and replicated in these regions (Brühl et al., 2014a). This idea is in line with the findings of a recent voxel-wise machine learning study, which suggested that SAD is easier to detect using multivariate analyses that take into account the global relationships between gray matter volume alterations in different regions than by applying analyses that only focus on local changes in specific brain regions (Frick et al., 2014b).
With respect to the previous studies reporting SAD-related GM differences, it should be noted that the findings of these studies were often inconsistent, with increases as well as decreases in the same regions having been reported (e.g. for the amygdala, see Irle et al., 2010, Machado-de-Sousa et al., 2014, Meng et al., 2013); see also Brühl et al. (2014b) and Syal et al. (2012) reporting no volumetric differences between SAD-patients and HC-participants, and Shang et al. (2014), who did not observe changes in amygdalar GM volumes in a meta-analysis on structural neuroimaging findings across several anxiety disorders. These inconsistencies are most likely due to small sample sizes, which may have increased the probability of obtaining false-positive findings (Blackford, 2017, Button et al., 2013) – see also Cremers and Roelofs (2016) for a critical overview of neuroimaging research findings in SAD. Furthermore, the inconsistencies are likely due to differences in methodology, for example the use of manual vs. automatic segmentation, the choice and size of ROIs, and to differences in clinical characteristics. Thus, the results of this study stress the need for studies with sufficient sample sizes and meta-analyses such as those performed by the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) Consortium and its working groups (Bearden and Thompson, 2017, Thompson et al., 2014).
4.2. Larger GM volume in right putamen
We did find GM differences in the right putamen, which, together with the caudate, forms the dorsal striatum (Marchand, 2010). The striatum is the major input structure of the basal ganglia, receiving information from the cortex, amygdala and hippocampus. The dorsal striatum is part of a network that is important for learning actions based on their predicted outcomes (i.e. reward-related behaviour), as well as for regulating cognitive and emotional behaviour (Marchand, 2010, Shohamy, 2011, Stathis et al., 2007); for a recent review on the role of the striatum in anxiety we refer to Lago et al., (2017). Interestingly, our findings converge with earlier research on the structural and functional basis of inhibited temperament, a characteristic that refers to the innate tendency to be shy, quiet and extremely cautious in novel social and non-social situations (Miskovic and Schmidt, 2012). Inhibited temperament substantially increases the risk for developing SAD (Clauss and Blackford, 2012, Fox and Kalin, 2014) and is correlated with larger volumes of both the amygdala and the caudate in young adults and hyperactivation in, among other areas, putamen, globus pallidus and caudate (Clauss et al., 2015, Clauss et al., 2014) – see Inline Supplementary Table 3 for coordinates of these and other findings discussed in this section. Moreover, Clauss and colleagues showed that the GM increase in the caudate was positively related to the level of activation in this area in response to neutral faces (Clauss et al., 2014). Because larger GM volume of the caudate was also associated with increased functional connectivity to regions that respond to social stimuli, the authors have proposed that larger caudate volume might facilitate the saliency of social and novel stimuli for individuals with an inhibited temperament, which could predispose them for developing SAD (Clauss et al., 2014). Combined with our observation that SAD is associated with larger GM volume in the putamen, it may be hypothesized that structural changes in the dorsal striatum, as an integral part of limbic circuitry (Stathis et al., 2007), might underlie the biased processing of stimuli typically observed in SAD (Miskovic and Schmidt, 2012).
Evidence consistent with this idea comes from recent fMRI studies on SAD-related threat processing (Cremers et al., 2015, Heitmann et al., 2016). Anticipation of social punishment versus reward was associated with increased local activity in the putamen in SAD-patients compared to healthy controls. In addition, SAD-patients showed increased negative connectivity between the putamen and the ACC during social punishment and reward compared to HC-participants (Cremers et al., 2015). Another study indicated that viewing ecologically valid, disorder-related complex visual scenes evoked increased activation in SAD-patients in, among others, the putamen and globus pallidus. Here, hyperactivation in the dorsal striatum was accompanied by increased connectivity with the amygdala, medial prefrontal cortex and ACC, regions playing an important role in emotion processing (Heitmann et al., 2016). These findings are supported by another resting-state study indicating hyperconnectivity of the putamen and the globus pallidus in SAD (Arnold Anteraper et al., 2014) and two meta-analyses on task-related activity in SAD, reporting increased activation of the globus pallidus (Gentili et al., 2016, Hattingh et al., 2013).
Additional support for our hypothesis comes from a within-subject longitudinal study on the neuro-anatomical effects of paroxetine in a small sample of fourteen patients with SAD, showing treatment-related decreases in symptom severity and concomitant reductions in GM in bilateral caudate and putamen (Talati et al., 2015). Furthermore, a 1H-magnetic resonance spectroscopy study demonstrated a relationship between social anxiety symptoms and the concentration of choline metabolites in the left caudate and right putamen (Howells et al., 2015), while single-photon emission computed tomography (SPECT) studies reported on alterations in the striatal dopaminergic system in patients with SAD (Schneier et al., 2000, Tiihonen et al., 1997, van der Wee et al., 2008), which are possibly related to striatal dysfunction (Sareen et al., 2007). In addition, two recent PET studies indicated enhanced serotonin synthesis capacity in the striatum (Frick et al., 2015, Furmark et al., 2016). Given the role of serotonin in neuroplasticity and brain circuit development (Lesch and Waider, 2012), concomitant brain structure alterations may be expected in this region. Combined with these previous findings, our results support the idea stated before (Brühl et al., 2014a, Gentili et al., 2016), that SAD-related changes in brain function and structure may be found outside the traditional fear circuitry, consisting of the amygdala, insula, prefrontal cortex and anterior cingulate cortex (Etkin and Wager, 2007).
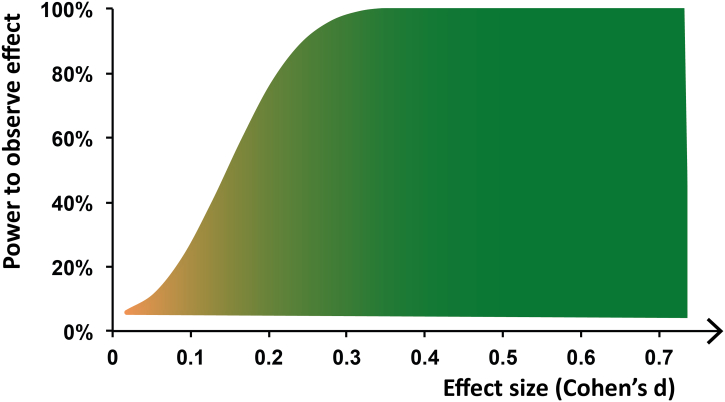
Notwithstanding the results of the present study, it should be noted that, despite the use of the largest database of structural MRI scans of SAD-patients available to date, the effect sizes obtained in our study were small (see Fig. 2 for an illustration of the relationship between effect size and the power to detect an effect, given the sample size of our study). However, small effect sizes are not uncommon for studies on structural brain abnormalities in mental disorders (Ioannidis, 2011); we refer the reader to the recent viewpoint articles by Blackford (2017) and Reddan et al. (2017) for important insights on improving the validity and reproducibility of neuroimaging studies in psychiatry. Furthermore, because of the hypothesis-driven ROI approach, we did not correct the p-value for the number of ROIs tested. In addition, it should be mentioned that the GM increase was present in a region with a low GM density (mean GM volume ± SD in significant cluster: SAD-patients: 0.12 ± 0.05; HC-participants 0.10 ± 0.05; see also Fig. 1B). Together with the fact that it is hard to link neuroimaging results showing changes in brain structure directly to underlying cellular and molecular mechanisms like synaptogenesis, neurogenesis and changes in neuronal morphology (Lerch et al., 2017, Zatorre et al., 2012), this finding underscores that more research is needed to understand how the macroscopic SAD-related GM increase relates to effects at the microscopic level. It is also unclear, given the correlational nature of this study, whether and how structural differences in the dorsal striatum might play a causal or compensatory role in the pathogenesis of SAD. This underscores the need for future longitudinal studies on SAD, as well as for experiments that incorporate the dorsal striatum in animal models of social anxiety (compare Fox and Kalin (2014)).
Fig. 2.
Illustration of relation between effect size and power to detect effect, given the current sample size (n = 174 SAD-patients and n = 213 HC-participants), calculated using https://www.ai-therapy.com/psychology-statistics/power-calculator.
4.3. Study limitations and future studies
The present study has several limitations. First, data on medication use and comorbidity were not available for all participants (Table 3). Furthermore, only the current use of medication and present comorbidity were known, so we could not exclude heterogeneity within the sample due to past medication-use or past comorbidity. Another possible source of heterogeneity within the sample arises from the fact that we pooled data from multiple research centers located in various countries, which could add confounding effects of, for example, ethnicity and differences in scanner settings. However, we do not believe that these potential confounds have substantially influenced our results, as we corrected for scan center within our statistical model and since we did not find any diagnosis-by-scan center effects.
In the present study, we have exclusively investigated SAD-related differences in GM volumes. Future studies on structural brain alterations should examine changes in other parameters of brain anatomy, like cortical thickness, white matter integrity, and the shape of brain structures. The latter is especially interesting, given the recent insight that the shape of the putamen exhibits moderate-to-high heritability (Ge et al., 2016, Roshchupkin et al., 2016). This, together with the understanding that SAD is familial and moderately heritable (Isomura et al., 2015, Middeldorp et al., 2005, Scaini et al., 2014, Torvik et al., 2016), raises the question whether putamen shape could be considered a candidate endophenotype of SAD (compare Bas-Hoogendam et al. (2016)) and it will be interesting to investigate this in future studies. In addition, it would be worthwhile to perform multivariate pattern analyses (MVPA) (Adluru et al., 2013, Pereira et al., 2009) to examine whether it is possible to discriminate SAD-patients from HC-participants based on GM volumes – see for example Frick et al. (2014b). Together with ongoing work on the functional brain alterations, as well as with the results of PET studies on brain metabolism in SAD, these findings may aid in unraveling the neurobiological basis of this serious and disabling disorder.
5. Conclusions
In summary, the results of the present mega-analysis of the largest database of SAD brain scans to date showed larger GM volume in the dorsal striatum in SAD, which correlated positively with the severity of self-reported social anxiety symptoms. Combined with previous work on inhibited temperament and imaging studies on SAD, our results suggest that the dorsal striatum may play a role in the biased processing of social stimuli that is characteristic of SAD psychopathology. Importantly, we could not replicate GM alterations in the amygdala, hippocampus, prefrontal cortex and precuneus, regions previously implicated in SAD in imaging studies with smaller sample sizes. We take these null-findings as an indication that large sample sizes and investigations such as the meta-analyses performed by the ENIGMA Consortium are necessary for the reliable detection of neuro-anatomical changes in SAD.
The following are the supplementary data related to this article.
Details on in- and exclusion criteria for each site.
Scans excluded based on comorbidity other than anxiety and/or MDD.
Overview results multiple regression analyses individual regions.
Coordinates of findings summarized in the Discussion.
Acknowledgments
Acknowledgements
We thank Tanja Kreuk (research intern, Leiden University) for her contribution to the visual inspection of the data, and the Anxiety Disorders Research Network of the European College of Psychopharmacology for its scientific and administrative support.
Funding sources
Funding: Janna Marie Bas-Hoogendam is funded by the Leiden University Research Profile ‘Health, Prevention and the Human Life Cycle’. Henk van Steenbergen was supported by a grant from the Netherlands Organization for Scientific Research (NWO) to Bernhard Hommel. Henk van Steenbergen, J. Nienke Pannekoek and Jean-Paul Fouche were partially supported by the EU 7th Frame Work Marie Curie Actions International Staff Exchange Scheme grant ‘European and South African Research Network in Anxiety Disorders’ (EUSARNAD). Jean-Paul Fouche is funded by the South African Medical Research Council National Health Scholarship. Münster (Jena) collaborators were partially supported by the Collaborative Research Center “Fear, Anxiety, and Anxiety disorders” in Münster, funded by the German Research Society (SFB/TRR-58, project C07 awarded to Thomas Straube) and by the Research Group “Person Perception” in Jena, funded by the German Research Society (grant number STR 987/6-1 to Thomas Straube). The infrastructure for the Netherlands Study of Depression and Anxiety (NESDA) was funded through the Geestkracht programme of the Netherlands Organization for Health Research and Development (ZonMw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, IQ Healthcare, Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos Institute)). Studies in Umea and Uppsala were supported by the Swedish Research Council and the Swedish Research Council for Health, Working Life and Welfare.
The funding sources had no involvement in writing this paper nor in the decision to submit this work for publication.
The data of this manuscript were presented previously at the International Congress of the World Psychiatric Association, Cape Town, South Africa (2016).
References
- Adluru N., Hanlon B.M., Lutz A., Lainhart J.E., Alexander A.L., Davidson R.J. Penalized likelihood phenotyping: unifying Voxelwise analyses and multi-voxel pattern analyses in neuroimaging. Neuroinformatics. 2013;11:227–247. doi: 10.1007/s12021-012-9175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Fifth Edition. DSM-5. 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Andersson J.L., Jenkinson M., Smith S. Non-linear registration aka Spatial normalisation [WWW Document] 2007. www.fmrib.ox.ac.uk/analysis/techrep FMRIB Tech. Rep. TR07JA2 from.
- Arnold Anteraper S., Triantafyllou C., Sawyer A.T., Hofmann S.G., Gabrieli J.D., Whitfield-Gabrieli S. Hyper-connectivity of subcortical resting state networks in social anxiety disorder. Brain Connect. 2014;4:81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baldwin D.S., Stein D.J. A joint European and South African research network in anxiety disorders. Hum. Psychopharmacol. 2012;27:4–5. doi: 10.1002/hup.1267. [DOI] [PubMed] [Google Scholar]
- Bas-Hoogendam J.M., Blackford J.U., Brühl A.B., Blair K.S., van der Wee N.J.A., Westenberg P.M. Neurobiological candidate endophenotypes of social anxiety disorder. Neurosci. Biobehav. Rev. 2016;71:362–378. doi: 10.1016/j.neubiorev.2016.08.040. [DOI] [PubMed] [Google Scholar]
- Bearden C.E., Thompson P.M. Emerging global initiatives in neurogenetics: the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) consortium. Neuron. 2017;94:232–236. doi: 10.1016/j.neuron.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Carbin M.G. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8:77–100. [Google Scholar]
- Blackford J.U. Leveraging statistical methods to improve validity and reproducibility of research findings. JAMA Psychiat. 2017;74:119–120. doi: 10.1001/jamapsychiatry.2016.3730. [DOI] [PubMed] [Google Scholar]
- Boehme S., Mohr A., Becker M.P., Miltner W.H., Straube T. Area-dependent time courses of brain activation during video-induced symptom provocation in social anxiety disorder. Biol. Mood Anxiety Disord. 2014;4:6. doi: 10.1186/2045-5380-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S., Ritter V., Tefikow S., Stangier U., Strauss B., Miltner W.H.R., Straube T. Brain activation during anticipatory anxiety in social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2014;9:1413–1418. doi: 10.1093/scan/nst129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S., Ritter V., Tefikow S., Stangier U., Strauss B., Miltner W.H.R., Straube T. Neural correlates of emotional interference in social anxiety disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in social anxiety disorder – a meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Brühl A.B., Hänggi J., Baur V., Rufer M., Delsignore A., Weidt S., Jäncke L., Herwig U. Increased cortical thickness in a frontoparietal network in social anxiety disorder. Hum. Brain Mapp. 2014;35:2966–2977. doi: 10.1002/hbm.22378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Clauss J.A., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J.A., Seay A.L., Vanderklok R.M., Avery S., Cao A., Cowan R.L., Benningfield M.M., Blackford J.U. Structural and functional bases of inhibited temperament. Soc. Cogn. Affect. Neurosci. 2014;9:2049–2058. doi: 10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss J.A., Avery S.N., Blackford J.U. The nature of individual differences in inhibited temperament and risk for psychiatric disease: a review and meta-analysis. Prog. Neurobiol. 2015;127–128:23–45. doi: 10.1016/j.pneurobio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers H.R., Roelofs K. Social anxiety disorder: a critical overview of neurocognitive research. Wiley Interdiscip. Rev. Cogn. Sci. 2016;7:218–232. doi: 10.1002/wcs.1390. [DOI] [PubMed] [Google Scholar]
- Cremers H.R., Veer I.M., Spinhoven P., Rombouts S.A.R.B., Yarkoni T., Wager T.D., Roelofs K. Altered cortical-amygdala coupling in social anxiety disorder during the anticipation of giving a public speech. Psychol. Med. 2014;1–9 doi: 10.1017/S0033291714002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers H.R., Veer I.M., Spinhoven P., Rombouts S.A.R.B., Roelofs K. Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Front. Behav. Neurosci. 2015;8 doi: 10.3389/fnbeh.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J., Benjamin L. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. [Google Scholar]
- Fox A.S., Kalin N.H. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am. J. Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A., Engman J., Alaie I., Björkstrand J., Faria V., Gingnell M., Wallenquist U., Agren T., Wahlstedt K., Larsson E.-M., Morell A., Fredrikson M., Furmark T. Enlargement of visual processing regions in social anxiety disorder is related to symptom severity. Neurosci. Lett. 2014;583:114–119. doi: 10.1016/j.neulet.2014.09.033. [DOI] [PubMed] [Google Scholar]
- Frick A., Gingnell M., Marquand A.F., Howner K., Fischer H., Kristiansson M., Williams S.C.R., Fredrikson M., Furmark T. Classifying social anxiety disorder using multivoxel pattern analyses of brain function and structure. Behav. Brain Res. 2014;259:330–335. doi: 10.1016/j.bbr.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A., Åhs F., Engman J., Jonasson M., Alaie I., Björkstrand J., Frans Ö., Faria V., Linnman C., Appel L., Wahlstedt K., Lubberink M., Fredrikson M., Furmark T. Serotonin synthesis and reuptake in social anxiety disorder: a positron emission tomography study. JAMA Psychiatry. 2015;72:794–802. doi: 10.1001/jamapsychiatry.2015.0125. [DOI] [PubMed] [Google Scholar]
- Furmark T., Marteinsdottir I., Frick A., Heurling K., Tillfors M., Appel L., Antoni G., Hartvig P., Fischer H., Långström B., Eriksson E., Fredrikson M. Serotonin synthesis rate and the tryptophan hydroxylase-2: G-703T polymorphism in social anxiety disorder. J. Psychopharmacol. 2016;30:1028–1035. doi: 10.1177/0269881116648317. [DOI] [PubMed] [Google Scholar]
- Ge T., Reuter M., Winkler A.M., Holmes A.J., Lee P.H., Tirrell L.S., Roffman J.L., Buckner R.L., Smoller J.W., Sabuncu M.R. Multidimensional heritability analysis of neuroanatomical shape. Nat. Commun. 2016;7:13291. doi: 10.1038/ncomms13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger M.J., Domschke K., Ipser J., Hattingh C., Baldwin D.S., Lochner C., Stein D.J. Altered executive control network resting-state connectivity in social anxiety disorder. World J. Biol. Psychiatry. 2016;17:47–57. doi: 10.3109/15622975.2015.1083613. [DOI] [PubMed] [Google Scholar]
- Gentili C., Cristea I.A., Angstadt M., Klumpp H., Tozzi L., Phan K.L., Pietrini P. Beyond emotions: a meta-analysis of neural response within face processing system in social anxiety. Exp. Biol. Med. 2016;241:225–237. doi: 10.1177/1535370215603514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodkind M., Eickhoff S.B., Oathes D.J., Jiang Y., Chang A., Jones-Hagata L.B., Ortega B.N., Zaiko Y.V., Roach E.L., Korgaonkar M.S., Grieve S.M., Galatzer-Levy I., Fox P.T., Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A., Stein P., Windischberger C., Weissenbacher A., Spindelegger C., Moser E., Kasper S., Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hattingh C.J., Ipser J., Tromp S.A., Syal S., Lochner C., Brooks S.J., Stein D.J. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front. Hum. Neurosci. 2013;6:347. doi: 10.3389/fnhum.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg R.G., Horner K.J., Juster H.R., Safren S.A., Brown E.J., Schneier F.R., Liebowitz M.R. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol. Med. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- Heitmann C.Y., Feldker K., Neumeister P., Zepp B.M., Peterburs J., Zwitserlood P., Straube T. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder. Hum. Brain Mapp. 2016;37:1559–1572. doi: 10.1002/hbm.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells F.M., Hattingh C.J., Syal S., Breet E., Stein D.J., Lochner C. (1)H-magnetic resonance spectroscopy in social anxiety disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;58:97–104. doi: 10.1016/j.pnpbp.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Ioannidis J.P.A. Excess significance bias in the literature on brain volume abnormalities. Arch. Gen. Psychiatry. 2011;68:773. doi: 10.1001/archgenpsychiatry.2011.28. [DOI] [PubMed] [Google Scholar]
- Irle E., Ruhleder M., Lange C., Seidler-Brandler U., Salzer S., Dechent P., Weniger G., Leibing E., Leichsenring F. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J. Psychiatry Neurosci. 2010;35:126–131. doi: 10.1503/jpn.090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irle E., Barke A., Lange C., Ruhleder M. Parietal abnormalities are related to avoidance in social anxiety disorder: a study using voxel-based morphometry and manual volumetry. Psychiatry Res. 2014;224:175–183. doi: 10.1016/j.pscychresns.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Isomura K., Boman M., Rück C., Serlachius E., Larsson H., Lichtenstein P., Mataix-Cols D. Population-based, multi-generational family clustering study of social anxiety disorder and avoidant personality disorder. Psychol. Med. 2015;45:1581–1589. doi: 10.1017/S0033291714002116. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Ustün T.B. The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int. J. Methods Psychiatr. Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Petukhova M., Sampson N.A., Zaslavsky A.M., Wittchen H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Fitzgerald D.A., Piejko K., Roberts J., Kennedy A.E., Phan K.L. Prefrontal control and predictors of cognitive behavioral therapy response in social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2016;11:630–640. doi: 10.1093/scan/nsv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago T., Davis A., Grillon C., Ernst M. Striatum on the anxiety map: small detours into adolescence. Brain Res. 2017;1654:177–184. doi: 10.1016/j.brainres.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzenberger R.R., Mitterhauser M., Spindelegger C., Wadsak W., Klein N., Mien L.-K., Holik A., Attarbaschi T., Mossaheb N., Sacher J., Geiss-Granadia T., Kletter K., Kasper S., Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol. Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Lerch J.P., van der Kouwe A.J.W., Raznahan A., Paus T., Johansen-Berg H., Miller K.L., Smith S.M., Fischl B., Sotiropoulos S.N. Studying neuroanatomy using MRI. Nat. Neurosci. 2017;20:314–326. doi: 10.1038/nn.4501. [DOI] [PubMed] [Google Scholar]
- Lesch K.-P., Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron. 2012;76:175–191. doi: 10.1016/j.neuron.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Liao W., Xu Q., Mantini D., Ding J., Machado-de-Sousa J.P., Hallak J.E.C., Trzesniak C., Qiu C., Zeng L., Zhang W., Crippa J.A.S., Gong Q., Chen H. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Res. 2011;1388:167–177. doi: 10.1016/j.brainres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Machado-de-Sousa J.P., de L. Osório F., Jackowski A.P., Bressan R.A., Chagas M.H.N., Torro-Alves N., Depaula A.L.D., Crippa J.A.S., Hallak J.E.C. Increased amygdalar and hippocampal volumes in young adults with social anxiety. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack S., Jacobi F., Beesdo-Baum K., Gerschler A., Strehle J., Höfler M., Busch M.A., Maske U., Hapke U., Gaebel W., Zielasek J., Maier W., Wittchen H.-U. Functional disability and quality of life decrements in mental disorders: results from the mental health module of the German health interview and examination survey for adults (DEGS1-MH) Eur. Psychiatry. 2015;30:793–800. doi: 10.1016/j.eurpsy.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Månsson K.N.T., Carlbring P., Frick A., Engman J., Olsson C.-J., Bodlund O., Furmark T., Andersson G. Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Res. 2013;214:229–237. doi: 10.1016/j.pscychresns.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Månsson K.N.T., Frick A., Boraxbekk C.-J., Marquand A.F., Williams S.C.R., Carlbring P., Andersson G., Furmark T. Predicting long-term outcome of internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Månsson K.N.T., Salami A., Frick A., Carlbring P., Andersson G., Furmark T., Boraxbekk C.-J. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand W.R. Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct. Funct. 2010;215:73–96. doi: 10.1007/s00429-010-0280-y. [DOI] [PubMed] [Google Scholar]
- Meng Y., Lui S., Qiu C., Qiu L., Lama S., Huang X., Feng Y., Zhu C., Gong Q., Zhang W. Neuroanatomical deficits in drug-naïve adult patients with generalized social anxiety disorder: a voxel-based morphometry study. Psychiatry Res. 2013;214:9–15. doi: 10.1016/j.pscychresns.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Mennin D.S., Fresco D.M., Heimberg R.G., Schneier F.R., Davies S.O., Liebowitz M.R. Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. J. Anxiety Disord. 2002;16:661–673. doi: 10.1016/s0887-6185(02)00134-2. [DOI] [PubMed] [Google Scholar]
- Middeldorp C.M., Birley A.J., Cath D.C., Gillespie N.A., Willemsen G., Statham D.J., de Geus E.J.C., Andrews J.G., van Dyck R., Beem A.L., Sullivan P.F., Martin N.G., Boomsma D.I. Familial clustering of major depression and anxiety disorders in Australian and Dutch twins and siblings. Twin Res. Hum. Genet. 2005;8:609–615. doi: 10.1375/183242705774860123. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Schmidt L.A. Social fearfulness in the human brain. Neurosci. Biobehav. Rev. 2012;36:459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Pannekoek J.N., Veer I.M., van Tol M.-J., van der Werff S.J.A., Demenescu L.R., Aleman A., Veltman D.J., Zitman F.G., Rombouts S.A.R.B., van der Wee N.J.A. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur. Neuropsychopharmacol. 2013;23:186–195. doi: 10.1016/j.euroneuro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Pannekoek J.N., van der Werff S.J.A., van Tol M.J., Veltman D.J., Aleman A., Zitman F.G., Rombouts S.A.R.B., van der Wee N.J.A. Investigating distinct and common abnormalities of resting-state functional connectivity in depression, anxiety, and their comorbid states. Eur. Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Penninx B.W.J.H., Beekman A.T.F., Smit J.H., Zitman F.G., Nolen W.A., Spinhoven P., Cuijpers P., De Jong P.J., Van Marwijk H.W.J., Assendelft W.J.J., Van Der Meer K., Verhaak P., Wensing M., De Graaf R., Hoogendijk W.J., Ormel J., Van Dyck R. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int. J. Methods Psychiatr. Res. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F., Mitchell T., Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. NeuroImage. 2009;45:S199–S209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Coccaro E.F., Angstadt M., Kreger K.J., Mayberg H.S., Liberzon I., Stein M.B. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biol. Psychiatry. 2013;73:329–336. doi: 10.1016/j.biopsych.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddan M.C., Lindquist M.A., Wager T.D. Effect size estimation in neuroimaging. JAMA Psychiatry. 2017;74:207–208. doi: 10.1001/jamapsychiatry.2016.3356. [DOI] [PubMed] [Google Scholar]
- Roshchupkin G.V., Gutman B.A., Vernooij M.W., Jahanshad N., Martin N.G., Hofman A., McMahon K.L., van der Lee S.J., van Duijn C.M., de Zubicaray G.I., Uitterlinden A.G., Wright M.J., Niessen W.J., Thompson P.M., Ikram M.A., Adams H.H.H., Bressler S.L., Menon V., Doyon J., Benali H., Andreasen N.C., Tekin S., Cummings J.L., Verstraete E., Veldink J.H., den Berg L.H., den Heuvel M.P., Blokland G.A.M., de Zubicaray G.I., McMahon K.L., Wright M.J., Peper J.S., den Braber A., Reuter M., Wolter F.-E., Peinecke N., Wang Y., Yonggang S., Cole J.H., McKeown M.J., Bron E.E., Bis J.C., Stein J.L., Hibar D.P., Chen C.-H., Chen C.-H., Chen C.-H., Whelan C.D., Eyler L.T., Adams H.H.H., Ge T., Loh P.-R., Roshchupkin G.V., Hofman A., Ikram M.A., Zubicaray D.G., Chiang M.C., McMahon K.L., Howie B., Fuchsberger C., Stephens M., Marchini J., Abecasis G.R., Fischl B., Gutman B.A., Verhaaren B.F.J., Almasy L., Blangero J., Benjamini Y., Hochberg Y. Heritability of the shape of subcortical brain structures in the general population. Nat. Commun. 2016;7:13738. doi: 10.1038/ncomms13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen J., Campbell D.W., Leslie W.D., Malisza K.L., Stein M.B., Paulus M.P., Kravetsky L.B., Kjernisted K.D., Walker J.R., Reiss J.P. Striatal function in generalized social phobia: a functional magnetic resonance imaging study. Biol. Psychiatry. 2007;61:396–404. doi: 10.1016/j.biopsych.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Scaini S., Belotti R., Ogliari A. Genetic and environmental contributions to social anxiety across different ages: a meta-analytic approach to twin data. J. Anxiety Disord. 2014;28:650–656. doi: 10.1016/j.janxdis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Liebowitz M.R., Abi-Dargham A., Zea-Ponce Y., Lin S.H., Laruelle M. Low dopamine D2 receptor binding potential in social phobia. Am. J. Psychiatry. 2000;157:457–459. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- Shang J., Fu Y., Ren Z., Zhang T., Du M., Gong Q., Lui S., Zhang W. The common traits of the ACC and PFC in anxiety disorders in the DSM-5: meta-analysis of voxel-based morphometry studies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D., Lecrubier Y., Harnett Sheehan K., Janavs J., Weiller E., Keskiner A., Schinka J., Knapp E., Sheehan M., Dunbar G. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur. Psychiatry. 1997;12:232–241. [Google Scholar]
- Shohamy D. Learning and motivation in the human striatum. Curr. Opin. Neurobiol. 2011;21:408–414. doi: 10.1016/j.conb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Sladky R., Höflich A., Küblböck M., Kraus C., Baldinger P., Moser E., Lanzenberger R., Windischberger C. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for FMRI. Cereb. Cortex. 2015;25:895–903. doi: 10.1093/cercor/bht279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spielberger C., Gorsuch R., Lushene R. Consulting Psychologists Press; Palo Alto, CA: 1970. STAI Manual for the State-trait Anxiety Inventory. [Google Scholar]
- Stathis P., Panourias I.G., Themistocleous M.S., Sakas D.E. Connections of the basal ganglia with the limbic system: implications for neuromodulation therapies of anxiety and affective disorders. Acta Neurochir. Suppl. 2007;97:575–586. doi: 10.1007/978-3-211-33081-4_67. [DOI] [PubMed] [Google Scholar]
- Steiger V.R., Brühl A.B., Weidt S., Delsignore A., Rufer M., Jäncke L., Herwig U., Hänggi J. Pattern of structural brain changes in social anxiety disorder after cognitive behavioral group therapy: a longitudinal multimodal MRI study. Mol. Psychiatry. 2016;0:1–8. doi: 10.1038/mp.2016.217. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Stein D.J. Social anxiety disorder. Lancet. 2008;371:1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- Stein D.J., Ruscio A.M., Lee S., Petukhova M., Alonso J., Andrade L.H.S.G., Benjet C., Bromet E., Demyttenaere K., Florescu S., de Girolamo G., de Graaf R., Gureje O., He Y., Hinkov H., Hu C., Iwata N., Karam E.G., Lepine J.-P., Matschinger H., Oakley Browne M., Posada-Villa J., Sagar R., Williams D.R., Kessler R.C. Subtyping social anxiety disorder in developed and developing countries. Depress. Anxiety. 2010;27:390–403. doi: 10.1002/da.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal S., Hattingh C.J., Fouché J.-P., Spottiswoode B., Carey P.D., Lochner C., Stein D.J. Grey matter abnormalities in social anxiety disorder: a pilot study. Metab. Brain Dis. 2012;27:299–309. doi: 10.1007/s11011-012-9299-5. [DOI] [PubMed] [Google Scholar]
- Talati A., Pantazatos S.P., Schneier F.R., Weissman M.M., Hirsch J. Gray matter abnormalities in social anxiety disorder: primary, replication, and specificity studies. Biol. Psychiatry. 2013;73:75–84. doi: 10.1016/j.biopsych.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati A., Pantazatos S.P., Hirsch J., Schneier F. A pilot study of gray matter volume changes associated with paroxetine treatment and response in social anxiety disorder. Psychiatry Res. 2015;231:279–285. doi: 10.1016/j.pscychresns.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Stein J.L., Medland S.E., Hibar D.P., Vasquez A.A., Renteria M.E., Toro R., Jahanshad N., Schumann G., Franke B., Wright M.J., Martin N.G., Agartz I., Alda M., Alhusaini S., Almasy L., Almeida J., Alpert K., Andreasen N.C., Andreassen O.A., Apostolova L.G., Appel K., Armstrong N.J., Aribisala B., Bastin M.E., Bauer M., Bearden C.E., Bergmann O., Binder E.B., Blangero J., Bockholt H.J., Bøen E., Bois C., Boomsma D.I., Booth T., Bowman I.J., Bralten J., Brouwer R.M., Brunner H.G., Brohawn D.G., Buckner R.L., Buitelaar J., Bulayeva K., Bustillo J.R., Calhoun V.D., Cannon D.M., Cantor R.M., Carless M.A., Caseras X., Cavalleri G.L., Chakravarty M.M., Chang K.D., Ching C.R.K., Christoforou A., Cichon S., Clark V.P., Conrod P., Coppola G., Crespo-Facorro B., Curran J.E., Czisch M., Deary I.J., de Geus E.J.C., den Braber A., Delvecchio G., Depondt C., de Haan L., de Zubicaray G.I., Dima D., Dimitrova R., Djurovic S., Dong H., Donohoe G., Duggirala R., Dyer T.D., Ehrlich S., Ekman C.J., Elvsåshagen T., Emsell L., Erk S., Espeseth T., Fagerness J., Fears S., Fedko I., Fernández G., Fisher S.E., Foroud T., Fox P.T., Francks C., Frangou S., Frey E.M., Frodl T., Frouin V., Garavan H., Giddaluru S., Glahn D.C., Godlewska B., Goldstein R.Z., Gollub R.L., Grabe H.J., Grimm O., Gruber O., Guadalupe T., Gur R.E., Gur R.C., Göring H.H.H., Hagenaars S., Hajek T., Hall G.B., Hall J., Hardy J., Hartman C.A., Hass J., Hatton S.N., Haukvik U.K., Hegenscheid K., Heinz A., Hickie I.B., Ho B.-C., Hoehn D., Hoekstra P.J., Hollinshead M., Holmes A.J., Homuth G., Hoogman M., Hong L.E., Hosten N., Hottenga J.-J., Hulshoff Pol H.E., Hwang K.S., Jack C.R., Jenkinson M., Johnston C., Jönsson E.G., Kahn R.S., Kasperaviciute D., Kelly S., Kim S., Kochunov P., Koenders L., Krämer B., Kwok J.B.J., Lagopoulos J., Laje G., Landen M., Landman B.A., Lauriello J., Lawrie S.M., Lee P.H., Le Hellard S., Lemaître H., Leonardo C.D., Li C.-S., Liberg B., Liewald D.C., Liu X., Lopez L.M., Loth E., Lourdusamy A., Luciano M., Macciardi F., Machielsen M.W.J., Macqueen G.M., Malt U.F., Mandl R., Manoach D.S., Martinot J.-L., Matarin M., Mather K.A., Mattheisen M., Mattingsdal M., Meyer-Lindenberg A., McDonald C., McIntosh A.M., McMahon F.J., McMahon K.L., Meisenzahl E., Melle I., Milaneschi Y., Mohnke S., Montgomery G.W., Morris D.W., Moses E.K., Mueller B.A., Muñoz Maniega S., Mühleisen T.W., Müller-Myhsok B., Mwangi B., Nauck M., Nho K., Nichols T.E., Nilsson L.-G., Nugent A.C., Nyberg L., Olvera R.L., Oosterlaan J., Ophoff R.A., Pandolfo M., Papalampropoulou-Tsiridou M., Papmeyer M., Paus T., Pausova Z., Pearlson G.D., Penninx B.W., Peterson C.P., Pfennig A., Phillips M., Pike G.B., Poline J.-B., Potkin S.G., Pütz B., Ramasamy A., Rasmussen J., Rietschel M., Rijpkema M., Risacher S.L., Roffman J.L., Roiz-Santiañez R., Romanczuk-Seiferth N., Rose E.J., Royle N.A., Rujescu D., Ryten M., Sachdev P.S., Salami A., Satterthwaite T.D., Savitz J., Saykin A.J., Scanlon C., Schmaal L., Schnack H.G., Schork A.J., Schulz S.C., Schür R., Seidman L., Shen L., Shoemaker J.M., Simmons A., Sisodiya S.M., Smith C., Smoller J.W., Soares J.C., Sponheim S.R., Sprooten E., Starr J.M., Steen V.M., Strakowski S., Strike L., Sussmann J., Sämann P.G., Teumer A., Toga A.W., Tordesillas-Gutierrez D., Trabzuni D., Trost S., Turner J., Van den Heuvel M., van der Wee N.J., van Eijk K., van Erp T.G.M., van Haren N.E.M., van't Ent D., van Tol M.-J., Valdés Hernández M.C., Veltman D.J., Versace A., Völzke H., Walker R., Walter H., Wang L., Wardlaw J.M., Weale M.E., Weiner M.W., Wen W., Westlye L.T., Whalley H.C., Whelan C.D., White T., Winkler A.M., Wittfeld K., Woldehawariat G., Wolf C., Zilles D., Zwiers M.P., Thalamuthu A., Schofield P.R., Freimer N.B., Lawrence N.S., Drevets W. The ENIGMA consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J., Kuikka J., Bergström K., Lepola U., Koponen H., Leinonen E. Dopamine reuptake site densities in patients with social phobia. Am. J. Psychiatry. 1997;154:239–242. doi: 10.1176/ajp.154.2.239. [DOI] [PubMed] [Google Scholar]
- van Tol M.-J., van der Wee N.J.A., van den Heuvel O.A., Nielen M.M.A., Demenescu L.R., Aleman A., Renken R., van Buchem M.A., Zitman F.G., Veltman D.J. Regional brain volume in depression and anxiety disorders. Arch. Gen. Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Torvik F.A., Welander-Vatn A., Ystrom E., Knudsen G.P., Czajkowski N., Kendler K.S., Reichborn-Kjennerud T. Longitudinal associations between social anxiety disorder and avoidant personality disorder: a twin study. J. Abnorm. Psychol. 2016;125:114–124. doi: 10.1037/abn0000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tükel R., Aydın K., Yüksel Ç., Ertekin E., Koyuncu A., Taş C. Gray matter abnormalities in patients with social anxiety disorder: a voxel-based morphometry study. Psychiatry Res. 2015;234:106–112. doi: 10.1016/j.pscychresns.2015.09.003. [DOI] [PubMed] [Google Scholar]
- van der Wee N.J., van Veen J.F., Stevens H., van Vliet I.M., van Rijk P.P., Westenberg H.G. Increased serotonin and dopamine transporter binding in psychotropic medication-naive patients with generalized social anxiety disorder shown by 123I-beta-(4-iodophenyl)-tropane SPECT. J. Nucl. Med. 2008;49:757–763. doi: 10.2967/jnumed.107.045518. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Fields R.D., Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details on in- and exclusion criteria for each site.
Scans excluded based on comorbidity other than anxiety and/or MDD.
Overview results multiple regression analyses individual regions.
Coordinates of findings summarized in the Discussion.