Abstract
Pancreatic cystic lesions are being detected with increasing frequency because of increased use and improved quality of cross-sectional imaging techniques. Pancreatic cystic lesions encompass non-neoplastic lesions (such as pancreatitis-related collections) and neoplastic tumors. Common cystic pancreatic neoplasms include serous cystadenomas, mucinous cystic neoplasms, intraductal papillary mucinous neoplasms, and solid pseudopapillary tumors. These cystic pancreatic neoplasms may have typical morphology, but at times show overlapping imaging features on cross-sectional examinations. This article reviews the classical and atypical imaging features of commonly encountered cystic pancreatic neoplasms and presents the limitations of current cross-sectional imaging techniques in accurately classifying pancreatic cystic lesions.
Keywords: Cystic pancreatic neoplasm, Pseudocyst, Serous cystadenoma, Mucinous cystic neoplasm, Solid pseudopapillary neoplasm, Intraductal papillary mucinous neoplasm
Introduction
In general, a pancreatic cystic lesion refers to any pancreatic (neoplastic and non-neoplastic) lesion consisting primarily of fluid. Among all tumors of the pancreas, cystic pancreatic neoplasms (CPNs) are relatively rare, representing around 10% of all pancreatic neoplasms [1]. This article will review the characteristic and atypical cross-sectional imaging features of commonly encountered CPNs. Also, limitations of cross-sectional imaging techniques in accurately classifying CPNs are addressed.
Cross-Sectional Imaging of Pancreatic Cystic Neoplasms
Most CPNs are characterized by the primary imaging modalities of multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP), each of which has its strengths and weaknesses. Over the last decades, the spatial resolution of computed tomography (CT) has improved significantly. Nowadays, thin-section MDCT of the pancreas allows for reliable detection of CPNs of a few millimeters, while the ability to perform high-quality data reformations allows for simultaneous multiplanar assessment of the pancreatic ductal system [1, 2]. The MDCT protocol for assessment of pancreatic pathology includes a bi- or triphasic protocol. At the very least, the CT protocol should include a pancreatic (40–50 s after intravenous (IV) administration of iodinated contrast medium) and portal venous phase (70–90 s after IV contrast) [2, 3, 4]. Advantages of CT are the wide availability, high spatial resolution, fast acquisition limiting motion artifacts, and the ease of interpretation for radiologists as well as for clinicians. Compared with MRI, MDCT is better in detecting (tiny) calcifications [2]. To increase specificity, MRI is generally used as a problem solving tool to better discriminate between CPNs that can be managed conservatively and those that require a more aggressive treatment [2, 5]. MRCP is mainly based on the acquisition of heavily T2-weighted images, with variants of fast spin echo sequences. An MRI protocol also includes typical sequences such as in-phase and out-of-phase T1-weighted images and multiphasic contrast-enhanced series for a complete evaluation of pancreatic pathology [4, 5, 6]. The role of diffusion-weighted imaging (DWI) in the characterization of CPNs is still controversial and appears rather limited. Particularly, it remains difficult to differentiate neoplastic from non-neoplastic cysts at DWI because of the freedom of water diffusion encountered in both serous and mucinous fluid-filled cystic lesions [7, 8]. MRI has superior sensitivity for detecting CPNs compared with MDCT and comes along with a reasonable accuracy in its characterization [5, 9]. MRI/MRCP has advantages over MDCT by better delineating the cyst fluid content due to the superior soft-tissue contrast resolution, thereby facilitating the recognition of internal septations and mural nodules, and is better in establishing the relationship between the CPN and the pancreatic duct [5, 6]. An additional advantage of MRI/MRCP over MDCT is in those patients who require repeated imaging for follow-up due to the lack of radiation exposure. Disadvantages of MRI include lower spatial resolution, low sensitivity for detecting calcifications, and motion-related artifacts.
Morphological Classification of Pancreatic Cystic Neoplasms
CPNs are generally assessed at cross-sectional imaging, primarily on CT and MRI. On CT, cysts are depicted as lesions with water density (Hounsfield unit between 0 and 15). On MRI, cystic lesions have low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. For non-radiologists, the fluid in the gallbladder and bladder may serve as a reference as uncomplicated CPNs show similar densities and intensities. Morphologically, cystic pancreatic lesions can be classified into 4 categories on CT and MRI based on specific imaging features [10, 11]:
(1) Unilocular cyst (one cyst without septa or solid component) - common lesions: pancreatitis-related collection, intraductal papillary mucinous neoplasm (IPMN), and mucinous cystadenoma; uncommon lesions: serous cystadenoma (oligocystic variant), non-neoplastic epithelial cysts, and cystic neuroendocrine tumor.
(2) Microcystic lesion (collection of microcysts) - serous cystadenoma (polycystic variant).
(3) Macrocystic lesion (multilocular cyst with fewer compartments, each >2 cm) - mucinous cystadenoma, IPMN, and lymphoepithelial cyst.
(4) Cyst with solid components - mucinous cystic neoplasm (MCN; mucinous cystadenoma and mucinous cystadenocarcinoma), IPMN, solid pseudopapillary neoplasm (SPN), and solid neoplasms showing cystic degeneration (adenocarcinoma and neuroendocrine tumors).
Morphologic features that need to be evaluated on cross-sectional imaging (CT and MRI) to arrive at a specific diagnosis or to narrow the differential diagnosis are related to the CPN and the mass effect it exerts on surrounding structures.
Items related to CPNs are the diameter of the lesion, location (head, neck, body, or tail), number (single or multiple), density and size of the cysts (microcystic or macrocystic), contour or shape of the cyst (round, oval, or lobulated), presence of (enhancing and non-enhancing) solid components or mural nodules in and outside of the cyst, internal septations (present or absent, uni- or multilocular), central scar (present or absent), wall thickness (thin: <2 mm, thick: >2 mm), margins (smooth or irregular), presence and location of calcifications (central, septal, peripheral), and visible communication with the main pancreatic duct (present or absent).
Items related to the mass effect of the CPN are the extent of main pancreatic duct dilatation (none, diffuse, upstream or downstream of the lesion), degree of main pancreatic duct dilatation (normal (1–3 mm), mild (4–5 mm), moderate (6–9 mm), or severe (10 mm or more)), relationship with vascular structures (encasement or abutment) and biliary structures (dilatation of the common bile duct), and upstream atrophy of the pancreas (present or absent).
A number of imaging features have been identified that are associated with a malignant or potentially malignant CPN. These imaging features include a thick or irregular wall, solid enhancing components, peripheral calcifications, and dilatation of the main pancreatic duct [9, 12, 13, 14]. None of these features alone, however, are specific by themselves, but when combined the likelihood of malignancy increases. Conversely, benign CPNs often have a lobulated shape, thin wall, and absence of solid components [15]. Some CPNs have characteristic imaging features that allow for a specific diagnosis, but many have equivocal appearances on imaging that do not permit a definitive diagnosis. The typical and atypical features of the most common CPNs are addressed in more detail below.
Common Pancreatic Cystic Neoplasms
Serous Cystadenoma
Serous cystadenomas are benign cystic neoplasms of the pancreas that occur more commonly in older women who typically are in the 5th to 7th decade [16, 17]. Although it is stated that they are often located in the pancreatic head, they do occur in the pancreatic body and tail in up to 30% of cases. Serous cystadenomas displace surrounding organs instead of invading adjacent structures and may grow over time on serial follow-up imaging studies [13]. Especially lesions that are >4 cm at initial presentation exhibit a faster growth rate of almost 2 cm/year compared with their smaller counterparts (0.12 cm/year) [18, 19]. Serous cystadenomas can have a varied appearance on cross-sectional imaging with characteristic and atypical imaging features. Overall, the imaging appearance of a serous cystadenoma depends on the number of fibrous septa and the degree of enhancement. Lesions with a few fibrous septa show fluid density/intensity on CT and MRI, respectively [13, 20]. The thin septations are highly vascular (sometimes causing internal hemorrhage) and, therefore, enhance on post-contrast-enhanced imaging. The presence of numerous tiny cysts and septa may produce a solid appearance with increased contrast enhancement on CT, whereas MRI is better able to depict these small cysts [20]. On MRI, the signal intensity of serous cystadenoma may vary slightly depending on the degree of protein content. Atypical and rarely observed features of serous cystadenoma include communication with the main pancreatic duct, thick wall, intralesional hemorrhage, and portal hypertension secondary to splenic vein obstruction [21, 22]. When any of these atypical features are present, this may lead to difficulty in differentiating those from other CPNs. Traditionally, serous cystadenoma displays as one of the following three morphologic patterns: microcystic pattern in 70%, honeycomb pattern in 20%, and oligocystic pattern in 10% of cases [20, 23, 24].
The microcystic pattern (also referred to as multi- of polycystic pattern) has two salient morphologic features: external lobulation and a central fibrous scar with or without calcifications in a sunburst pattern (fig. 1) [25]. This pattern is composed of a conglomerate of small cysts ranging from a few millimeters up to 2 cm. The outer margin is regular with a typical lobulated contour and a thin almost imperceptible wall. The serous fluid-filled cysts are lined by glycogen-rich epithelial cells and separated by thin, fibrous septa. As the lesion grows, the retraction of fibrous tissue yields a central scar showing coarse calcifications in a stellate pattern in about 20–30% of cases, which is considered as a characteristic feature [23, 25, 26]. Generally, calcifications are seen in lesions larger than 5 cm [23, 25]. The fibrous portion enhances early after contrast administration. This is another distinguishing feature as serous cystadenoma is the only hypervascular lesion among the CPNs [27, 28]. The central scar is better depicted as areas of persistent enhancement within the cystic lesion on delayed imaging. Differential considerations are branch-duct IPMN and MCN as these may also have a polycystic appearance. Imaging features favoring branch-duct IPMN are communication with the pancreatic duct, pancreatic duct dilatation, and a pleomorphic cystic shape. Imaging features suggestive for a MCN are a smooth surface without lobulation, a relatively thick enhancing wall, and peripheral calcifications.
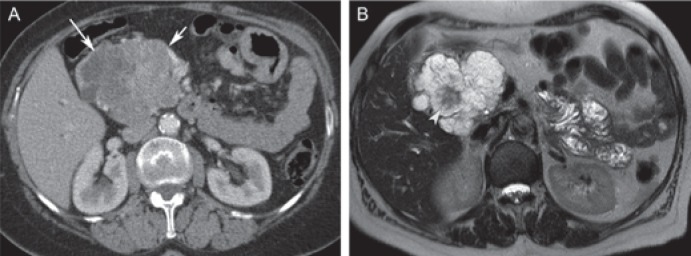
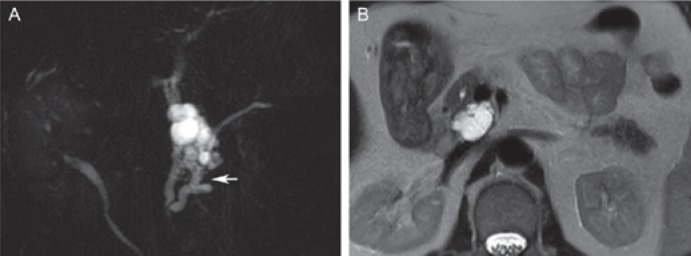
Fig. 1.
Typical serous cystadenoma in a 72-year-old female. A Axial post-contrast computed tomography shows a large lobulated lesion in the pancreatic head with hypodense cystic components (long arrow), but partly solid appearing (short arrow) due to the enhancement of numerous septations. B Axial T2-weighted magnetic resonance image clearly demonstrates the microcystic appearance of the lesion, with a T2 hypointense fibrous central scar (arrowhead), representing characteristic imaging features of serous cystic neoplasm.
The honeycomb pattern is characterized by numerous tiny cysts (sponge-like), which are at times too small to discern as individual cysts. At CT, this may then display as a solid mass lesion [20, 21]. MRI is superior and diagnostic in this regard to show the true cystic nature as a grapelike cluster of small fluid-containing cysts (considered as characteristic for serous cystadenoma). Cysts are depicted as high signal intensity on T2-weighted images, while septa show low signal intensity. Typically, the lesion is well-marginated and shows a slightly lobulated contour. Differential considerations are neuroendocrine tumor (MRI is useful in depicting the small cysts) and SPN (i.e. the small variant of SPN which is typically less hypervascular than serous cystadenoma).
The oligocystic pattern is the least typical pattern as the cystic lesion may consist of one or a few cysts with varying diameters (often larger than 2 cm) and has a thin (almost imperceptible) wall [23, 24]. The cyst may be round to oval or slightly lobulated. This pattern is almost indistinguishable from other CPNs, such as unilocular or oligocystic MCN, small branch-duct IPMN, and inflammatory cystic lesions.
Classical appearances of serous cystadenoma are the microcystic pattern with central scar and stellate pattern of calcifications and the honeycomb pattern with or without central calcification (grapelike appearance on MRI). Atypical manifestations of serous cystadenoma include the oligocystic pattern (especially if cysts are greater than 2 cm), giant serous cystadenoma (>10 cm) that may exhibit compression of adjacent structures, ductal dilatation and other findings of obstructive chronic pancreatitis, and solid serous cystadenomas (composed of microscopic serous cysts) (figs. 2, 3) [20, 21, 29, 30]. Even at MR imaging, solid serous cystadenoma may appear as a solid, well-circumscribed, well-vascularized pancreatic mass as the microscopic cysts are too small to be reliably depicted on MRI. This type of serous cystadenoma may be indistinguishable from pancreatic neuroendocrine tumors and hypervascular pancreatic metastases.
Fig. 2.
Atypical serous cystadenoma in a 48-year-old female. Coronal post-contrast computed tomography shows a hypervascular solid lesion in the pancreatic body (arrow) which was suspicious for a neuroendocrine tumor both on imaging and subsequent fine needle aspiration. The lesion proved to be a serous cystadenoma on resection.
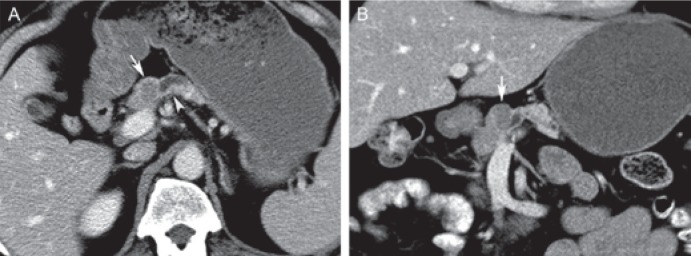
Fig. 3.
Small serous cystadenoma in a 52-year-old male. A Axial and B coronal post-contrast computed tomography displays a small, hypodense, well marginated lesion in the pancreatic neck (arrows). The lesion obstructs the pancreatic duct (arrowhead in A). Less typical features are the small size, ductal obstruction, and non-cystic appearance on computed tomography. Initial imaging diagnosis was a solid hypovascular tumor such as a ductal adenocarcinoma.
Mucinous Cystic Neoplasm (Mucinous Cystadenoma/Cystadenocarcinoma)
MCNs account for about 10% of CPNs [31]. The defining and characteristic histopathologic feature of MCNs is the presence of ovarian-type stroma akin to that observed in biliary cystadenomas [32]. Hence, MCNs almost exclusively occur in females, typically in their middle age [33]. The ovarian stromal elements differentiate MCNs from IPMNs, which have stromal elements of pancreatic ductal origin. They usually present as solitary, unilocular, well-circumscribed round or lobular cysts (about 80%) that can range from small to large dimensions (1–36 cm), mostly in the pancreatic body and tail (up to 75%) [33, 34]. Internal septa may be seen which can create a multilocular appearance. The cyst walls are usually thickened but may be smooth and thin. Peripheral curvilinear or eggshell calcifications may be seen in the wall or internal septa in up to 25% of cases and are better depicted on CT [2, 26, 30]. MCNs may grow slowly over time, at an average rate of 4 mm per year [33, 34].
At imaging, an MCN commonly displays as a unilocular or septated cystic lesion with a thickened wall with clear margins [30]. The thick wall corresponds to fibrotic changes observed at histopathology. In typical cases, the cyst or cysts vary in size but usually are larger than those observed in serous cystadenoma with less septa. The cystic lesion is typically filled with mucin. The most common MRI features are those of simple fluid, with homogeneous low T1 signal intensity and homogeneous high T2 signal intensity (fig. 4). On T1-weighted MR images, however, the signal intensity may vary depending on the proteinaceous content of the mucin [5, 30]. The typical T1 and T2 signal intensity may also be altered in case of intralesional hemorrhage (hyperintense on T1, mixed on T2), which is infrequently observed. The wall and internal septa or areas of nodules will show enhancement after gadolinium administration. Especially visualization of enhancing mural nodules (better displayed on MRI) is important as this may signify potential malignant degeneration [30]. Differential considerations are inflammatory cystic lesions (discriminators: clinical history of pancreatitis, in the absence of a proper clinical history; however, these lesions may be radiologically indistinguishable) and oligocystic serous cystadenoma. In contrast to serous cystadenoma, the septa in MCNs are located peripherally and in a disorganized fashion which may give a ‘pseudonodular’ appearance [33].
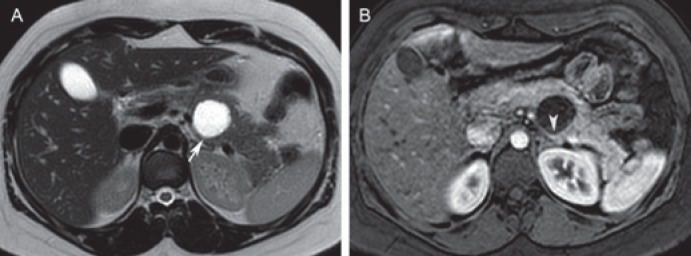
Fig. 4.
Mucinous cystic neoplasm in a 30-year-old female. A Axial T2-weighted image depicts a unilocular cystic lesion in the pancreatic body (arrow). The lesion is round and well circumscribed, and contains simple fluid. B Axial post-contrast T1-weighted image shows enhancement of a slightly thickened wall (arrowhead). Differential diagnosis includes an inflammatory cystic lesion. The patient did not have a history of pancreatitis, and the lesion was resected. On pathologic examination, this lesion proved to be a mucinous cystic neoplasm without dysplasia.
Atypical imaging features of MCNs are communication with the main pancreatic duct (due to malignant pancreatic fistula), internal hemorrhage, and upstream chronic obstructive pancreatitis changes (dilated pancreatic duct, parenchymal atrophy, coarse calculi, and areas of decreased enhancement and, at MRI, decreased signal intensity on fat-saturated unenhanced T1-weighted images) [11, 30, 35].
Besides the obvious features of malignancy (such as evidence of invasion of adjacent structures, nodal and distant metastases), other findings suggestive for possible malignant transformation of MCNs are larger size (cysts <3 cm are typically benign), irregular margins, enhancement of soft tissue components or mural nodules, peripheral calcifications, and a thick irregular wall (fig. 5) [30, 31, 35].
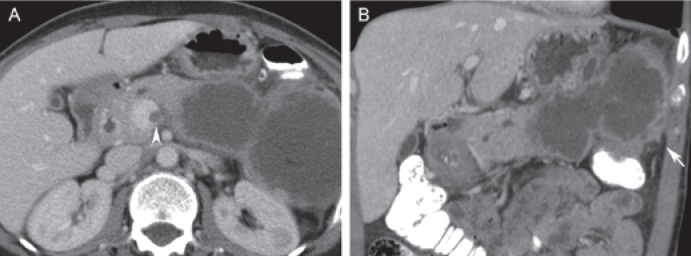
Fig. 5.
Mucinous cystadenocarcinoma in a 58-year-old female. A Axial and B coronal post-contrast computed tomography depicts a large cystic lesion arising from the pancreatic body and tail. The cystic lesion has a thick enhancing wall and irregular outer margins (arrow), both indicative of malignant transformation. The extension into the superior mesenteric vein (arrowhead) is another sign of malignancy.
Intraductal Papillary Mucinous Neoplasm
IPMNs account for up to 7% of all pancreatic neoplasms and represent the most common CPN (20%) [36, 37, 38]. IPMN is a mucin-producing tumor arising from pancreatic ductal epithelium and is clinically and histopathologically distinctly different from MCN. Opposed to other common CPNs, IPMNs occur more commonly in elderly males (mean age 60–70 years) while its prevalence increases with aging [36, 37]. The mucinous transformation of pancreatic ductal epithelium causes excessive viscous mucin production leading to obstructive dilation of the main duct or its side branches. Accordingly, IPMNs are classified according to the duct of origin: branch-duct IPMN, main-duct IPMN (focal or diffuse), or a combination of both [37, 38, 39]. IPMNs may be solitary or multiple and arise most commonly in the uncinate process (about 70%), but may occur in any part of the pancreas [37, 38]. The presence of multiple pancreatic cysts supports the diagnosis of IPMN as this is rarely observed in other CPNs (fig. 6). In 5–10% of cases, IPMNs involve the entire pancreas [6, 39]. Importantly, on imaging, the tumor itself is hardly visible, but the diagnosis of IPMN can be ascertained by the sequelae of excessive mucin production.
Fig. 6.
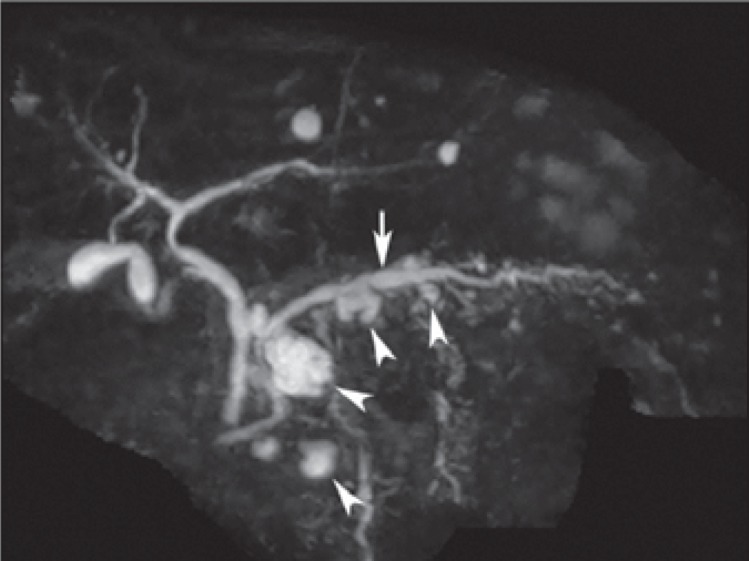
Multiple branch-duct intraductal papillary mucinous neoplasm (IPMN) in a 72-year-old male. Coronal magnetic resonance cholangiopancreatography shows multiple cystic lesions in the entire pancreas (arrowheads), which favors the diagnosis of (branch-duct) IPMN. There is possible involvement of a slightly dilated main pancreatic duct (arrow). Upon surgical resection, pathology showed a combined IPMN with low-grade dysplasia.
The imaging diagnosis of IPMNs is highly variable (grape-like, multicystic, unilocular, or finger-like) and depends on determining the communication of a CPN with the pancreatic duct, especially in the case of branch-duct IPMN. Establishing this connection is an important feature in the diagnosis of IPMN because this is rare in other neoplastic cystic lesions (MCN and serous cystadenoma) [11, 39]. MRI and particularly using heavily T2-weighted sequences or MRCP is superior compared with CT in showing the connection of a cyst to the pancreatic duct non-invasively and in characterizing the IPMN type. The degree of cystic dilatation (in branch-duct IPMN) and main pancreatic duct dilatation (in main-duct and combined IPMN) varies with the amount of mucin production. Calcification may be seen with any type of IPMN (in about 20% of cases). The less commonly observed coarse calcifications are correlated with malignant degeneration (mostly with concurrent malignant features), whereas the more common punctate calcifications are not [26, 40]. Invasive IPMNs may be solid appearing if the cystic component is replaced by tumor.
Branch-duct IPMN are most frequently seen in the uncinate process (fig. 7) [39]. The morphology of branch-duct IPMN varies with the number of affected side branches that become dilated by the production of large amounts of mucin. A single cyst in proximity of the main duct is depicted as round or oval lesions, and its diagnosis hinges on the communication with the non-dilated main pancreatic duct. The main differential diagnoses are the oligocystic variant of serous cystadenoma (which rarely shows communication with the pancreatic duct) and a pseudocyst which may also communicate with the pancreatic duct. A true pseudocyst tends to be round or oval as well, but often there is a clinical history of pancreatitis. Clubbed finger-like cysts are seen when one or two cysts are involved, and the morphology becomes more pleomorphic when there are three or more cysts [24]. Typically, a lobulated contour with tiny septa is seen. The communication with the main duct is best visualized on CT using coronal or curved reformatted images and on MRCP [39]. Contrast-enhanced images are used to depict nodular components and degree of wall or septal thickening. A pleomorphic cystic IPMN may mimic an MCN. Ductal communication, however, favors the diagnosis of branch-duct IPMN.
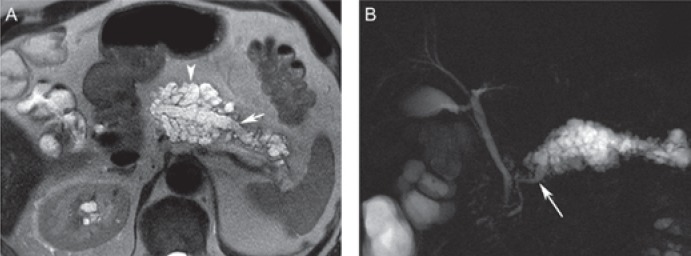
Fig. 7.
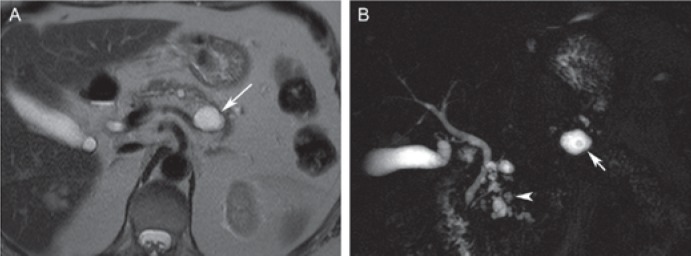
Branch-duct intraductal papillary mucinous neoplasm (IPMN) in a 70-year-old male. A Coronal magnetic resonance cholangiopancreatography (MRCP) and B axial T2-weighted MR image displays a lobulated cystic lesion with tiny septa arising from the pancreatic head/uncinate process. MRCP clearly demonstrates the communication with the main pancreatic duct (arrow) consistent with a branch-duct IPMN. The lesion was resected based on the worrisome feature of size >3 cm and showed low-grade dysplasia on pathological examination.
Main-duct IPMN is characterized by dilatation of the main pancreatic duct (i.e. 5 mm or greater by international consensus guidelines) in a diffuse pattern or a segmental portion without a discernable obstructive lesion or stenosis [11, 37]. The diffuse form of IPMN may cause bulging of the duodenal papilla into the lumen of the duodenum and is considered virtually diagnostic of IPMN [37, 38]. A focally dilated main duct may resemble a cyst and can be difficult to distinguish from other CPNs. Again, delineating the relationship with the main pancreatic duct will usually provide the diagnosis. Parenchymal atrophy may be present depending on the severity of main-duct IPMNs. Main-duct IPMNs may thus resemble or even coincide with chronic pancreatitis [11, 41]. In advanced cases of chronic pancreatitis, a focally or diffusely dilated main pancreatic duct is almost always associated with pancreatic parenchymal changes, such as parenchymal atrophy, loss of lobulated parenchymal contour, and, on MRI, by loss of inherent hyperintensity on T1 fat-suppressed images and delayed enhancement after administration of contrast material; both latter findings are suggestive of fibrosis [11]. Also, obstructive ductal calculi and irregularity of the main duct with strictures or stenosis favors the diagnosis of chronic pancreatitis [41]. Conversely, features suggestive of IPMN are communication with the main pancreatic duct, location in the pancreatic head or uncinate process, and bulging of the duodenal papilla.
Combined IPMNs show features of both branch-duct and main-duct IPMNs and may also closely resemble chronic pancreatitis (fig. 8) [41]. The same characteristic features in favor of IPMN and chronic pancreatitis as aforementioned apply here. Importantly, an imaging diagnosis of combined IPMNs may be difficult in cases where microscopic involvement of the main pancreatic duct is observed or in cases where the excessive mucin production by branch-duct IPMN protrudes into the main duct, causing dilatation of the pancreatic duct in absence of a tumor component [38, 42].
Fig. 8.
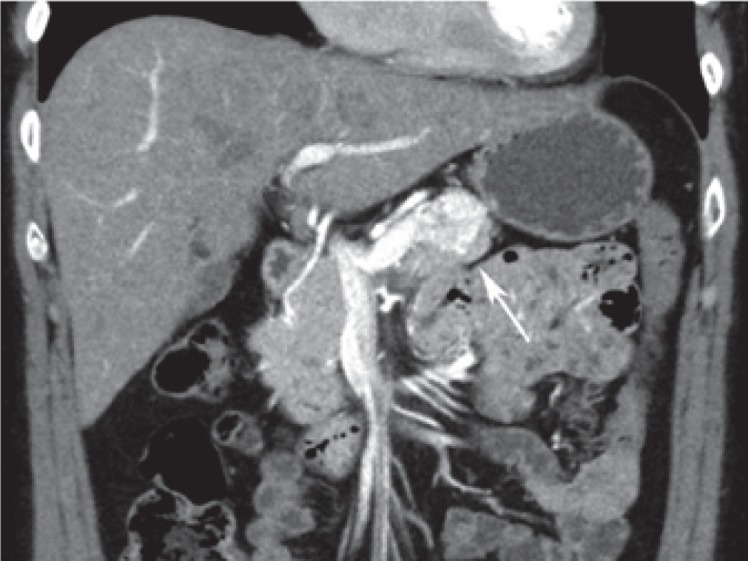
Combined intraductal papillary mucinous neoplasm (IPMN) in a 64-year-old male. A Axial T2-weighted magnetic resonance image shows extensive cystic change of the pancreatic body and tail, consisting of both a dilated main pancreatic duct (short arrow) and numerous dilated side branches (arrowhead). B Coronal magnetic resonance cholangiopancreatography again demonstrates the combined IPMN in body and tail. The involvement of the pancreas is segmental with an abrupt change to a normal main duct in the pancreatic head (arrow). Upon surgical resection, this proved to be a combined IPMN with low-grade dysplasia.
Uncommon presentations of IPMNs include the dilatation of the entire ductal system (pan-ductal-ectasia), invasion or fistulation of adjacent structures such as bowel loops or common bile duct, and rarely intra-abdominal perforation resulting in pseudomyxoma peritonei [43].
The 2012 international consensus guidelines of the International Association of Pancreatology (with a minor update in 2017) on the management of mucinous lesions discriminates between ‘high-risk stigmata’ (indication for surgical resection) and ‘worrisome features’ (assessed during surveillance and prompting endoscopic ultrasound evaluation), all of which are correlated with a higher likelihood of malignancy [37, 38]. High-risk stigmata include main duct diameter ≥ 10 mm in main-duct and combined-type IPMNs, presence of solid enhancing mural nodules ≥ 5 mm within the cyst in branch-duct IPMNs, or obstructive jaundice in the presence of a cystic lesion of the pancreatic head (fig. 9). During follow-up of asymptomatic branch-duct IPMNs, assessment of the following worrisome features includes cyst size ≥ 3 cm, main duct dilatation between 5 and 9 mm, thickened or irregular (enhancing) cyst wall (>2 mm), enhancing mural nodules < 5 mm, lymphadenopathy, abrupt change in caliber of pancreatic duct with upstream pancreatic atrophy without discernable obstructive lesion, and rapid cyst growth at a rate of >5 mm/2 years [38]. Of these features, structural change in morphology of the cystic lesion (development of solid nodules and thick septations) seems to be more associated with malignant degeneration than lesion growth alone [38].
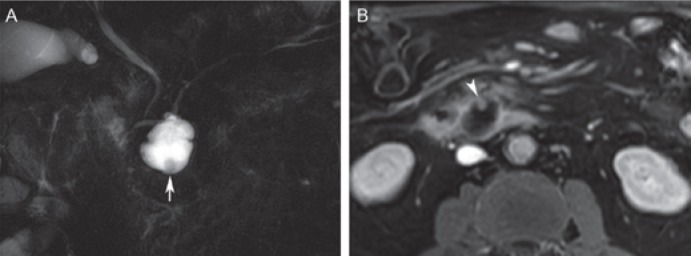
Fig. 9.
Branch-duct intraductal papillary mucinous neoplasm (IPMN) with ‘high-risk stigmata’ in a 73-year-old male. A Coronal magnetic resonance cholangiopancreatography with a branch-duct IPMN in the uncinate process (communication with the pancreatic duct not shown on this image) displays a mural nodule (arrow). B Post-contrast T1-weighted magnetic resonance image shows enhancement of the nodule (arrowhead), which typifies a high-risk stigma for malignancy.
Solid Pseudopapillary Neoplasm
SPN is a rare and slow-growing pancreatic neoplasm and predominantly occurs in young women (mean age 30 years), who are most often asymptomatic at presentation [44, 45]. Mostly, the tumor arises in the pancreatic head and tail and has a tendency to displace structures rather than invading them. These tumors exhibit a soft consistency which rarely causes obstruction of the common bile duct or main pancreatic duct, even if located in the pancreatic head [46].
Histopathological analysis of SPNs varies with tumor size. Small SPNs show predominantly solid sheets of cells with ample cytoplasm and degenerative changes. Larger lesions often have a combination of solid, cystic, and pseudopapillary tissue patterns as well as intratumoral hemorrhage [44, 45, 46]. Indeed, it is hypothesized that SPNs begin as solid tumors when small, and as they enlarge the tumor growth is not supported by an adequate vascular network, resulting in cystic and hemorrhagic degeneration. The cystic components are not true cysts as epithelial lining is lacking but rather denote a degenerative or necrotic process comprised of blood and debris. The imaging features of SPNs parallel those of histopathology and, thus, vary depending on tumor size with typical imaging features for larger lesions (>3 cm) and more atypical imaging features for lesions smaller than 3 cm.
Classical imaging features of large SPNs consist of a well-circumscribed heterogeneous lesion with a thick (sometimes discontinuous) pseudocapsule (representing fibrosis and compressed pancreatic tissue), typically depicted as low density and low signal intensity (on both T1- and T2-weighted images) on CT and MRI, respectively [45, 46, 47]. Another characteristic imaging hallmark of SPNs is the combination of a central area of internal hemorrhage and cystic degeneration, and peripheral rim of solid components (fig. 10) [45, 46, 47]. Peripheral calcification is present in up to 60% of cases, and central dystrophic calcifications may occur in areas of hemorrhage [40, 48]. Hemorrhage is best delineated at MRI due to its superior contrast resolution, and its signal intensity varies with age and with the amount of blood products. In the subacute phase, hemorrhage is depicted as T1 hyperintensity and variable signal intensity on T2-weighted images, whereas chronic hemorrhage is depicted as hypointense signal on both T1- and T2-weighted images. A fluid-fluid level (in cystic areas) or fluid-debris level (in areas of hemorrhagic degeneration) is present in about 10–20% of cases due to sedimentation [46, 49]. After contrast administration, the solid (peripheral) components of SPNs show heterogeneous enhancement during the arterial phase and progressive enhancement in the portal and delayed phase, albeit lower than that of normal pancreas [46, 48]. The main differential considerations are mucinous cystadenocarcinoma and cystic neuroendocrine tumors as both types of CPN are well-defined, may show cystic degeneration, and have enhancing solid components as well. Mucinous cystadenocarcinoma occur mostly in older females and rarely show hemorrhage. Also, there are several clinical and imaging features that distinguish SPNs from cystic neuroendocrine tumors. Neuroendocrine tumors are rarely seen younger than 30 years of age, have low signal intensity on T1-weighted images (no hemorrhage), and show hypervascularity depicted as early arterial enhancement in either a diffuse or ring-like enhancement pattern. Furthermore, on CT, large SPNs with minimal cystic component and absence of hemorrhage may mimic the honeycomb pattern of serous cystadenoma. In such cases, MRI can be helpful in showing the true cystic nature of serous cystadenoma.
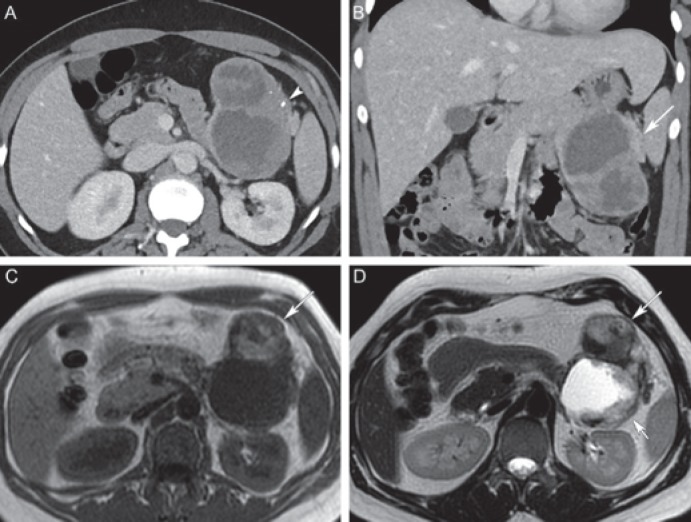
Fig. 10.
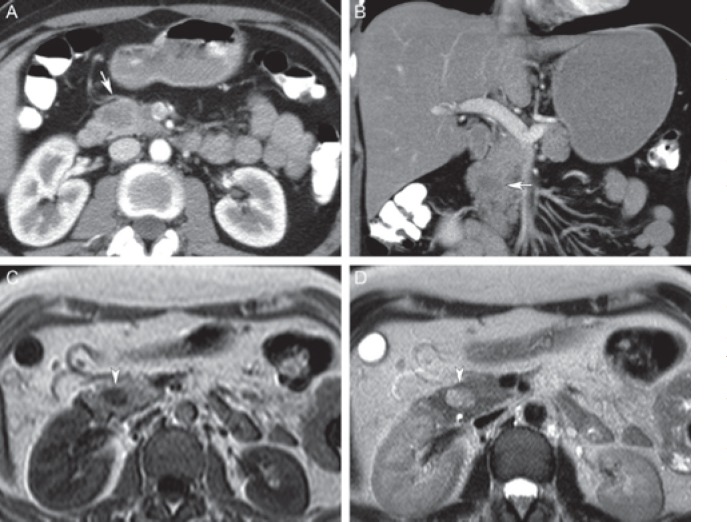
Solid pseudopapillary neoplasm (SPN) in a 45-year-old female. A Axial and B coronal post-contrast computed tomography shows a large well circumscribed mixed solid and cystic lesion in the pancreatic tail (long arrow), with tiny peripheral calcifications (arrowhead). C Axial T1-weighted magnetic resonance image shows hyperintensity in the anterior part of the lesion corresponding to internal hemorrhage (long arrow). D Axial T2-weighted magnetic resonance image shows corresponding T2 hypointense hemorrhage in the anterior part (long arrow) and extensive cystic degeneration of the posterior part (short arrow). Differential diagnosis includes mucinous cystadenocarcinoma, but the presence of hemorrhage in a well circumscribed lesion favors SPN.
Imaging features of small SPNs consists of an ill- or well-defined homogeneous, solid mass with less frequent calcification (about 25%) (fig. 11) [47, 50, 51]. Peripheral capsule, cystic degeneration, and internal hemorrhage is usually not seen. Signal intensity on MRI of these small SPNs are non-specific, i.e. low on T1- and high on T2-weighted images. These tumors show an early, heterogeneous, and gradually increasing enhancement after administration of contrast media [46, 52]. The appearance of small SPNs may mimic that of pancreatic adenocarcinoma (discriminators: dilatation of pancreatic duct, upstream pancreatic atrophy, and infiltrative growth pattern), neuroendocrine tumors (discriminator: hypervascular neoplasms with early enhancement in the arterial or pancreatic phase), solid variant of serous cystadenoma, and pancreatic metastases (discriminator: history of malignancy).
Fig. 11.
Small solid pseudopapillary neoplasm (SPN) in a 37-year-old female. A Axial and B coronal post-contrast computed tomography shows a hypodense mass in the pancreatic head (arrows) without biliary or pancreatic duct obstruction (i.e. no signs of malignancy). C, D Axial T1- and T2-weighted magnetic resonance imaging shows a T1 hypointense (arrowhead in C) and slightly T2 hyperintense (arrowhead in D) lesion without cystic components. Patient underwent surgical resection, and pathological examination revealed an SPN.
Although the majority of SPNs are indolent tumors with low-grade malignant potential, about 5–15% are more aggressive with metastases and poor prognosis [53, 54]. Several imaging features can be helpful in differentiating benign from malignant SPNs. Besides the obvious findings of nodal and distant metastases, features suggestive of malignant SPN include local invasion of adjacent organs or vessels, extracapsular extension (lobulated margins and focal discontinuity of the pseudocapsule), and ductal dilatation [55, 56].
Limitations of Imaging Diagnosis of Pancreatic Cystic Neoplasms
With the improvement of technical quality of the primary cross-sectional imaging modalities MDCT and MRI, the detection of a CPN by the radiologist has become relatively easy. The differentiation between the various CPNs by imaging alone, however, is still problematic. Interestingly, there is a difference between the rate of diagnostic accuracy reported in radiology and non-radiology journals. Reported radiology series mention accuracies of cross-sectional imaging of around 75–85% [9, 12, 57], whereas clinical series report radiology misdiagnosis in up to 50% of cases [58, 59, 60, 61, 62].
There are a number of reasons why the diagnostic accuracy lags behind the technical advancements of cross-sectional imaging modalities. First, as has become clear based on the abovementioned review of imaging features of the various CPNs, many CPNs present with overlapping and non-specific imaging characteristics at a macroscopic level. This has become even more pronounced with the decreasing size of the detected CPNs. Second, at a microscopic level, the lining of the various CPNs differs considerably (with varying prognosis), but imaging modalities have limited accuracy in distinguishing between normal and absent epithelial lining as well as between normal and dysplastic epithelium (low-/high-grade dysplasia, in situ and invasive carcinomas) as these disease states often lack distinct imaging features. Indeed, there is a poor correlation between the complexity of the composition of the various CPNs observed at histopathology and the rather simple morphologic characteristics used at radiology. The radiology-pathology correlation is unlikely to improve unless imaging techniques are able to visualize the epithelial lining of cystic cavities more accurately. Third, reader familiarity with the different aspects of the various CPNs at both CT and MRI undoubtedly plays a role in diagnosing and characterizing CPNs [63]. This could be one explanation of the varying reported diagnostic accuracies between radiology and non-radiology series. Still, a substantial rate of misdiagnosis occurs (with both CT and MRI) even when review certainty by expert abdominal radiologists is high [14, 64, 65]. Indeed, interpretation of cross-sectional imaging is often confounded by morphologic overlap between the different CPNs, and this explains the moderate sensitivity in differentiating inflammatory cysts from neoplastic CPNs, benign from malignant cysts, and mucinous from non-mucinous neoplasms, even by expert abdominal radiologists (figs. 12, 13). Notably, the classical appearances of the various CPNs occur in about 50–70% of cases, and this number fairly equals the diagnostic accuracy of radiologists [13, 65]. The remainder of CPNs presents with atypical imaging features. Therefore, the differential diagnosis of a CPN based on cross-sectional imaging alone must include a variety of neoplasms, particularly in the absence of a clinical history of pancreatitis.
Fig. 12.
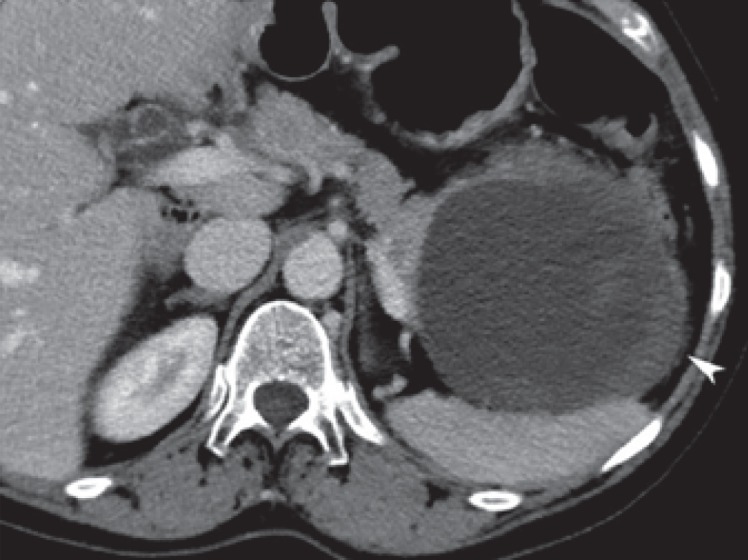
Inflammatory cystic lesion (pseudocyst) in a 66-year-old female. Large cystic lesion with a partial thickened wall or debris (arrowhead) in the pancreatic tail. Preoperative diagnosis was a mucinous cystadenoma/cystadenocarcinoma, but pathologic specimen showed a large pseudocyst.
Fig. 13.
Branch-duct intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) in a 68-year-old female. A Axial T2-weighted magnetic resonance image shows a round unilocular cystic lesion in the pancreatic body (long arrow). B Coronal magnetic resonance cholangiopancreatography shows multiple small cystic lesions in the pancreatic head consistent with branch-duct IPMN (arrowhead) and a nodule in the larger cystic lesion in the body of the pancreas. Based on the multiplicity, the larger cystic lesion was thought to represent an IPMN as well, but this proved to be an MCN with low-grade dysplasia upon surgical resection.
Conclusion
CPNs constitute a diverse category including inflammatory lesions as well as neoplasms that range from benign lesions, low-grade indolent neoplasia, to frankly malignant tumors. Awareness of the strengths and weaknesses of the primary cross-sectional imaging modalities (MDCT and MRI/MRCP) and the varying spectrum of imaging findings of commonly encountered CPNs is important for making an accurate diagnosis or for narrowing the differential diagnosis. Some CPNs present with characteristic imaging findings combined with gender- and age-related features. The integration of such clinical and imaging data facilitates accurate characterization of CPNs and often prevents unnecessary invasive procedures in non-mucinous CPNs or expedites immediate surgical resection in mucinous CPNs. A substantial percentage of CPNs, however, shows non-specific and overlapping imaging findings so that ancillary testing (cytologic evaluation, tumor markers, and molecular analysis) is necessary to reach a definitive diagnosis. Ideally, all benign CPNs are treated conservatively and (pre-)malignant CPNs are timely detected before the stage of invasive carcinoma. To this end, the individual patient with a CPN is best served by a team of specialists including an abdominal radiologist, pathologist, gastroenterologist, and gastrointestinal surgeon specialized in hepatopancreaticobiliary surgery, preferably during a multidisciplinary team meeting.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–848. doi: 10.1053/j.gastro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Sahani DV, Kambadakone A, Macari M, Takahashi N, Chari S, Fernandez-del Castillo C. Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol. 2013;200:343–354. doi: 10.2214/AJR.12.8862. [DOI] [PubMed] [Google Scholar]
- 3.Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31:993–1015. doi: 10.1148/rg.314105731. [DOI] [PubMed] [Google Scholar]
- 4.Vernuccio F, Borhani AA, Dioguardi Burgio M, Midiri M, Furlan A, Brancatelli G. Common and uncommon pitfalls in pancreatic imaging: it is not always cancer. Abdom Radiol (NY) 2016;41:283–294. doi: 10.1007/s00261-015-0557-y. [DOI] [PubMed] [Google Scholar]
- 5.Barral M, Soyer P, Dohan A, Laurent V, Hoeffel C, Fishman EK, Boudiaf M. Magnetic resonance imaging of cystic pancreatic lesions in adults: an update in current diagnostic features and management. Abdom Imaging. 2014;39:48–65. doi: 10.1007/s00261-013-0048-y. [DOI] [PubMed] [Google Scholar]
- 6.Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics. 2009;29:1749–1765. doi: 10.1148/rg.296095506. [DOI] [PubMed] [Google Scholar]
- 7.Sandrasegaran K, Akisik FM, Patel AA, Rydberg M, Cramer HM, Agaram NP, Schmidt CM. Diffusion-weighted imaging in characterization of cystic pancreatic lesions. Clin Radiol. 2011;66:808–814. doi: 10.1016/j.crad.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Pozzessere C, Castaños Gutiérrez SL, Corona-Villalobos CP, Righi L, Xu C, Lennon AM, Wolfgang CL, Hruban RH, Goggins M, Canto MI, Kamel IR. Diffusion-weighted magnetic resonance imaging in distinguishing between mucin-producing and serous pancreatic cysts. J Comput Assist Tomogr. 2016;40:505–512. doi: 10.1097/RCT.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sainani NI, Saokar A, Deshpande V, Fernández-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193:722–731. doi: 10.2214/AJR.08.1253. [DOI] [PubMed] [Google Scholar]
- 10.Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–1484. doi: 10.1148/rg.256045161. [DOI] [PubMed] [Google Scholar]
- 11.Khan A, Khosa F, Eisenberg RL. Cystic lesions of the pancreas. AJR Am J Roentgenol. 2011;196:W668–677. doi: 10.2214/AJR.10.4378. [DOI] [PubMed] [Google Scholar]
- 12.Sahani DV, Sainani NI, Blake MA, Crippa S, Mino-Kenudson M, Fernandez-del Castillo C. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR Am J Roentgenol. 2011;197:W53–61. doi: 10.2214/AJR.10.5866. [DOI] [PubMed] [Google Scholar]
- 13.Freeny PC, Saunders MD. Moving beyond morphology: new insights into the characterization and management of cystic pancreatic lesions. Radiology. 2014;272:345–363. doi: 10.1148/radiol.14131126. [DOI] [PubMed] [Google Scholar]
- 14.Do RK, Katz SS, Gollub MJ, Li J, LaFemina J, Zabor EC, Moskowitz CS, Klimstra DS, Allen PJ. Interobserver agreement for detection of malignant features of intraductal papillary mucinous neoplasms of the pancreas on MDCT. AJR Am J Roentgenol. 2014;203:973–979. doi: 10.2214/AJR.13.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Lim JH, Lee WJ, Lim HK. Macrocystic pancreatic lesions: differentiation of benign from premalignant and malignant cysts by CT. Eur J Radiol. 2009;71:122–128. doi: 10.1016/j.ejrad.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas) Gut. 2016;65:305–312. doi: 10.1136/gutjnl-2015-309638. [DOI] [PubMed] [Google Scholar]
- 17.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited. Part I: serous cystic neoplasms. Surg Oncol. 2011;20:e84–92. doi: 10.1016/j.suronc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–419. doi: 10.1097/01.sla.0000179651.21193.2c. discussion 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menard A, Tomlinson G, Cleary S, Wei A, Gallinger S, Haider MA. Serous cystadenomas of the pancreas: long-term follow-up measurement of growth rate. Can Assoc Radiol J. 2011;62:190–196. doi: 10.1016/j.carj.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Chu LC, Singhi AD, Haroun RR, Hruban RH, Fishman EK. The many faces of pancreatic serous cystadenoma: radiologic and pathologic correlation. Diagn Interv Imaging. 2017;98:191–202. doi: 10.1016/j.diii.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Choi JY, Kim MJ, Lee JY, Lim JS, Chung JJ, Kim KW, Yoo HS. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2009;193:136–142. doi: 10.2214/AJR.08.1309. [DOI] [PubMed] [Google Scholar]
- 22.Jung JH, Lee JK, Lee KT, Kim MH, Kim JH, Kim DH, Song BG, Paik SW, Yoo BC, Rhee JC. A case of serous cystadenoma of the pancreas communicating with the pancreatic duct. Korean J Gastroenterol. 2003;42:440–443. [PubMed] [Google Scholar]
- 23.Kim HJ, Lee DH, Ko YT, Lim JW, Kim HC, Kim KW. CT of serous cystadenoma of the pancreas and mimicking masses. AJR Am J Roentgenol. 2008;190:406–412. doi: 10.2214/AJR.07.2808. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, Han CJ, Han JK, Choi BI. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192–1198. doi: 10.2214/AJR.05.0337. [DOI] [PubMed] [Google Scholar]
- 25.Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, Fishman EK. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99–103. doi: 10.2214/ajr.175.1.1750099. [DOI] [PubMed] [Google Scholar]
- 26.Javadi S, Menias CO, Korivi BR, Shaaban AM, Patnana M, Alhalabi K, Elsayes KM. Pancreatic calcifications and calcified pancreatic masses: pattern recognition approach on CT. AJR Am J Roentgenol. 2017;209:77–87. doi: 10.2214/AJR.17.17862. [DOI] [PubMed] [Google Scholar]
- 27.Sidden CR, Mortele KJ. Cystic tumors of the pancreas: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28:339–356. doi: 10.1053/j.sult.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Shankar PR, Wasnik AP, Al-Hawary MM, Francis IR, Kaza RK. Hypervascular pancreatic ‘lesions’: a pattern-based approach to differentiation. Abdom Radiol (NY) 2018;43:1013–1028. doi: 10.1007/s00261-017-1363-5. [DOI] [PubMed] [Google Scholar]
- 29.Ishigami K, Nishie A, Asayama Y, Ushijima Y, Takayama Y, Fujita N, Takahata S, Ohtsuka T, Ito T, Igarashi H, Ikari S, Metz CM, Honda H. Imaging pitfalls of pancreatic serous cystic neoplasm and its potential mimickers. World J Radiol. 2014;6:36–47. doi: 10.4329/wjr.v6.i3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manfredi R, Ventriglia A, Mantovani W, Mehrabi S, Boninsegna E, Zamboni G, Salvia R, Pozzi Mucelli., R Mucinous cystic neoplasms and serous cystadenomas arising in the body-tail of the pancreas: MR imaging characterization. Eur Radiol. 2015;25:940–949. doi: 10.1007/s00330-014-3493-2. [DOI] [PubMed] [Google Scholar]
- 31.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: part II. Mucinous cystic neoplasms. Surg Oncol. 2011;20:e93–101. doi: 10.1016/j.suronc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, Farnell MB, Sarr MG, Chari ST. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026–1031. doi: 10.1016/s1542-3565(04)00450-1. [DOI] [PubMed] [Google Scholar]
- 33.Garces-Descovich A, Beker K, Castillo-Angeles M, Brook A, Resnick E, Shinagare S, Najarian RM, Mortele KJ. Mucinous cystic neoplasms of the pancreas: high-resolution cross-sectional imaging features with clinico-pathologic correlation. Abdom Radiol (NY) 2018;43:1413–1422. doi: 10.1007/s00261-017-1326-x. [DOI] [PubMed] [Google Scholar]
- 34.Naveed S, Qari H, Banday T, Altaf A, Para M. Mucinous cystic neoplasms of pancreas. Gastroenterology Res. 2014;7:44–50. doi: 10.14740/gr600e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morel A, Marteau V, Chambon E, Gayet B, Zins M. Pancreatic mucinous cystadenoma communicating with the main pancreatic duct on MRI. Br J Radiol. 2009;82:e243–245. doi: 10.1259/bjr/98185084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited. Part III. Intraductal papillary mucinous neoplasms. Surg Oncol. 2011;20:e109–118. doi: 10.1016/j.suronc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K;, International Association of Pancreatology International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Campbell NM, Katz SS, Escalon JG, Do RK. Imaging patterns of intraductal papillary mucinous neoplasms of the pancreas: an illustrated discussion of the International Consensus Guidelines for the Management of IPMN. Abdom Imaging. 2015;40:663–677. doi: 10.1007/s00261-014-0236-4. [DOI] [PubMed] [Google Scholar]
- 40.Verde F, Fishman EK. Calcified pancreatic and peripancreatic neoplasms: spectrum of pathologies. Abdom Radiol (NY) 2017;42:2686–2697. doi: 10.1007/s00261-017-1182-8. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Hong SS, Kim YJ, Kim JK, Eun HW. Intraductal papillary mucinous neoplasm of the pancreas: differentiate from chronic pancreatitis by MR imaging. Eur J Radiol. 2012;81:671–676. doi: 10.1016/j.ejrad.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 42.Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, Sandrasegaran K, Akisik F, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12:101–109. doi: 10.1007/s11605-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 43.D'Onofrio M, De Robertis R, Capelli P, Tinazzi Martini P, Crosara S, Gobbo S, Butturini G, Salvia R, Barbi E, Girelli R, Bassi C, Pederzoli P. Uncommon presentations of common pancreatic neoplasms: a pictorial essay. Abdom Imaging. 2015;40:1629–1644. doi: 10.1007/s00261-015-0388-x. [DOI] [PubMed] [Google Scholar]
- 44.Coleman KM, Doherty MC, Bigler SA. Solid-pseudopapillary tumor of the pancreas. Radiographics. 2003;23:1644–1648. doi: 10.1148/rg.236035006. [DOI] [PubMed] [Google Scholar]
- 45.Ventriglia A, Manfredi R, Mehrabi S, Boninsegna E, Negrelli R, Pedrinolla B, Pozzi Mucelli R. MRI features of solid pseudopapillary neoplasm of the pancreas. Abdom Imaging 201. 39:1213–1220. doi: 10.1007/s00261-014-0169-y. [DOI] [PubMed] [Google Scholar]
- 46.Ganeshan DM, Paulson E, Tamm EP, Taggart MW, Balachandran A, Bhosale P. Solid pseudo-papillary tumors of the pancreas: current update. Abdom Imaging. 2013;38:1373–1382. doi: 10.1007/s00261-013-0015-7. [DOI] [PubMed] [Google Scholar]
- 47.Anil G, Zhang J, Al Hamar NE, Nga ME. Solid pseudopapillary neoplasm of the pancreas: CT imaging features and radiologic-pathologic correlation. Diagn Interv Radiol. 2017;23:94–99. doi: 10.5152/dir.2016.16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantisani V, Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R, Ros PR, Silverman SG. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am J Roentgenol. 2003;181:395–401. doi: 10.2214/ajr.181.2.1810395. [DOI] [PubMed] [Google Scholar]
- 49.Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707–711. doi: 10.1148/radiology.199.3.8637992. [DOI] [PubMed] [Google Scholar]
- 50.Baek JH, Lee JM, Kim SH, Kim SJ, Kim SH, Lee JY, Han JK, Choi BI. Small (≤3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010;257:97–106. doi: 10.1148/radiol.10092089. [DOI] [PubMed] [Google Scholar]
- 51.Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, Chung JJ, Yoo HS, Lee JT, Kim KW. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187:W178–186. doi: 10.2214/AJR.05.0569. [DOI] [PubMed] [Google Scholar]
- 52.Yu MH, Lee JY, Kim MA, Kim SH, Lee JM, Han JK, Choi BI. MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR Am J Roentgenol. 2010;195:1324–1332. doi: 10.2214/AJR.10.4452. [DOI] [PubMed] [Google Scholar]
- 53.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006;93:733–737. doi: 10.1002/bjs.5334. [DOI] [PubMed] [Google Scholar]
- 55.Chung YE, Kim MJ, Choi JY, Lim JS, Hong HS, Kim YC, Cho HJ, Kim KA, Choi SY. Differentiation of benign and malignant solid pseudopapillary neoplasms of the pancreas. J Comput Assist Tomogr. 2009;33:689–694. doi: 10.1097/RCT.0b013e31818f2a74. [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Yu JS, Kim H, Kim JK, Kim TH, Kim KW, Park MS, Kim JH, Kim YB, Park C. Solid pseudopapillary carcinoma of the pancreas: differentiation from benign solid pseudopapillary tumour using CT and MRI. Clin Radiol. 2008;63:1006–1014. doi: 10.1016/j.crad.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Lin XZ, Wu ZY, Li WX, Zhang J, Xu XQ, Chen KM, Yan FH. Differential diagnosis of pancreatic serous oligocystic adenoma and mucinous cystic neoplasm with spectral CT imaging: initial results. Clin Radiol. 2014;69:1004–1010. doi: 10.1016/j.crad.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Cho CS, Russ AJ, Loeffler AG, Rettammel RJ, Oudheusden G, Winslow ER, Weber SM. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol. 2013;20:3112–3119. doi: 10.1245/s10434-013-2986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher WE, Hodges SE, Yagnik V, Morón FE, Wu MF, Hilsenbeck SG, Raijman IL, Brunicardi FC. Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB (Oxford) 2008;10:483–490. doi: 10.1080/13651820802291225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Jong K, van Hooft JE, Nio CY, Gouma DJ, Dijkgraaf MG, Bruno MJ, Fockens P. Accuracy of preoperative workup in a prospective series of surgically resected cystic pancreatic lesions. Scand J Gastroenterol. 2012;47:1056–1063. doi: 10.3109/00365521.2012.674970. [DOI] [PubMed] [Google Scholar]
- 61.Jang DK, Song BJ, Ryu JK, Chung KH, Lee BS, Park JK, Lee SH, Kim YT, Lee JY. Preoperative diagnosis of pancreatic cystic lesions: the accuracy of endoscopic ultrasound and cross-sectional imaging. Pancreas. 2015;44:1329–1333. doi: 10.1097/MPA.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 62.Jones MJ, Buchanan AS, Neal CP, Dennison AR, Metcalfe MS, Garcea G. Imaging of indeterminate pancreatic cystic lesions: a systematic review. Pancreatology. 2013;13:436–442. doi: 10.1016/j.pan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Grieser C, Heine G, Stelter L, Steffen IG, Rothe JH, Walter TC, Fischer C, Bahra M, Denecke T. Morphological analysis and differentiation of benign cystic neoplasms of the pancreas using computed tomography and magnetic resonance imaging. Rofo. 2013;185:219–227. doi: 10.1055/s-0032-1325551. [DOI] [PubMed] [Google Scholar]
- 64.Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am J Roentgenol. 2007;189:648–656. doi: 10.2214/AJR.07.2365. [DOI] [PubMed] [Google Scholar]
- 65.Visser BC, Muthusamy VR, Yeh BM, Coakley FV, Way LW. Diagnostic evaluation of cystic pancreatic lesions. HPB (Oxford) 2008;10:63–69. doi: 10.1080/13651820701883155. [DOI] [PMC free article] [PubMed] [Google Scholar]