Abstract
Werner syndrome (WS) is a rare autosomal recessive disorder characterized by systemic accelerated aging. It is caused by pathogenic variants of the WRN gene that encodes a nuclear helicase. In this report, we describe 4 newly identified WS cases among those referred to the Japanese Werner Consortium, Chiba University, Japan. All 4 cases were compound heterozygotes of the Japanese founder mutation, c.3139-1G>C, and a novel null pathogenic variant, c.1587G>A, c.2448+1G>A, or c.3233+1G>T, or an amino acid substitution variant, c.1720G>A, p.Gly574Arg. These 3 null pathogenic variants were not previously described. The p. Gly574Arg was previously reported in a European patient, and the identification of the second p. Gly574Arg case, with classical WS features, further confirmed the pathogenic nature of this variant. For the case with c.3233+1G>T, we determined the phase of 2 disease-causing mutations and demonstrated that they are on different chromosomes. This assay would be particularly important for those cases with ambiguous clinical diagnosis.
keywords: Mendelian disorder, Progeroid syndrome, Werner syndrome, WRN
Segmental progeroid syndromes are a group of genetic disorders in which affected individuals exhibit progressive systemic deteriorations characterized by accelerated aging [Hisama et al., 2016]. The best-known example is Werner syndrome (WS; OMIM 277700) caused by biallelic pathogenic variants of the WRN gene [Goto et al., 2013; Oshima et al., 2016, 2017]. WS patients develop an aged appearance and age-related disorders such as ocular cataracts, graying and loss of hair, atrophic skin, osteoporosis, atherosclerosis, and malignancies at earlier age. The most common causes of death are myocardial infarction and cancer at a median age of 54 years [Huang et al., 2006; Goto et al., 2013]. The Japanese Werner Consortium, Chiba University, in Chiba, Japan, proposed diagnostic criteria consisting of 6 cardinal symptoms: progeroid change of hair, cataracts, changes of skin including intractable skin ulcer, soft-tissue calcification, and a bird-like abnormal face [Takemoto et al., 2013].
The WRN gene encodes a nuclear DNA helicase with exonuclease activity which participates in DNA repair during various DNA transactions [Croteau et al., 2014; Shamanna et al., 2017]. More than 80 different WRN pathogenic variants have been reported from all over the world [Huang et al., 2006; Yokote et al., 2017]. Due to the presence of founder mutations, Japan and the region of Sardinia in Italy are among countries with the highest frequencies of WS [Satoh et al., 1999; Masala et al., 2007; Goto et al., 2013]. Another potential founder mutation has been reported in India/Pakistani patients, although WS may be underdiagnosed due to the relatively low awareness of this disorder [Saha et al., 2013]. A recent report showed that the increase of compound heterozygotes and decrease of homozygotes among those cases referred to the Japanese Werner patients likely reflects the social trend of a decrease in consanguineous marriages [Yamaga et al., 2017]. Here, we report the results of mutation analyses of WRN loci in 4 newly identified WS patients referred to the Japanese Werner Consortium. All 4 patients were compound heterozygotes of the Japanese founder mutation and the other pathological variant, 3 of which have not been described previously.
Materials and Methods
Patient Recruitment
Japanese WS patients were anonymously referred to the Japanese Werner Consortium by physicians who requested a molecular confirmation of a WRN mutation. Consent forms obtained by the physicians follow local regulations. After enrollment, blood samples collected from the patients were shipped to us for genetic testing.
Genetic Analysis
Blood samples were processed as described previously [Huang et al., 2006]. Genomic DNA was subjected to Sanger sequencing of 36 WRN exons as described before.
For the phase study of a compound heterozygote, the region of intron 25–26 of the WRN gene was amplified from the patient's DNA. The PCR product was then subcloned into pBlueScriptII-KS linearized with XhoI and HindIII using the Gibson assembly system according to the manufacturer's instruction (New England Biolabs, Cata#E2611) [Gibson et al., 2009]. Primers used for the amplification of WRN exon 26 for Gibson assembly were: I25-F: ACGACTCACTATAGGGCGAATTGGAGCTCGGTAAACAGTGTAGGAGTCTG; I26-R: CCTCGAGGTCGACGGTATCGATAAGCTTCTTGTGAGAGGCCTATAAACT. The underlines indicate the sequences in the WRN gene, and the regions without underlines are the sequence of pBlueScript linearized with XhoI and HindIII. The assembled DNA was used for Escherichia coli transformation, and the plamids isolated from 8 bacterial colonies were analyzed by Sanger sequencing.
Results
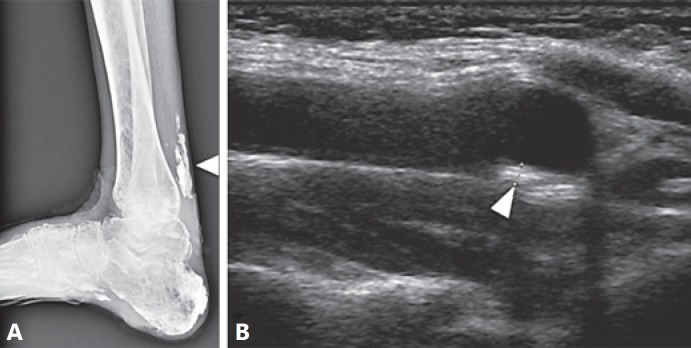
The first case is a 62-year-old Japanese woman. Her medical history included bilateral cataract at 42 years of age, diabetes mellitus at age 53, and calcification in the left Achilles tendon at age 55. She subsequently developed a refractory skin ulcer and underwent left leg amputation. At her first visit to our hospital, she exhibited short statue, a high-pitched voice, a bird-like facial appearance, thin extremities, and refractory skin ulcers on her right foot. Her height was 1.46 m, weight was 35.2 kg, and her BMI was 14.4 kg/m2. Her diabetes was controlled by intensive insulin therapy, the HbA1c was maintained less than 7.0%. X-ray examination revealed massive calcification in the right Achilles tendon, which is highly characteristic of WS [Takemoto et al., 2013], and ultrasound demonstrated 1.6 mm atherosclerotic plaque in the left carotid artery (Fig. 1).
Fig. 1.
Radiographic findings of a newly identified Werner patient. A Calcification in the right Achilles tendon. B Atherosclerotic plaque in the left carotid artery.
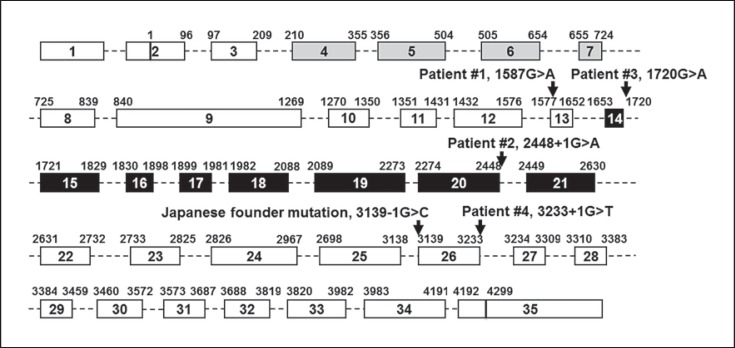
Sanger sequencing of WRN exons in the abovementioned case revealed a novel heterozygous variant, c.1587G>A, p.Trp529* in exon 13, and a heterozygous Japanese founder mutation, c.3139-1G>C, which results in the deletion of exon 26 (r.3139_3233del95) followed by the premature termination, p.Gly1047Phefs*14 (Fig. 2).
Fig. 2.
WRN mutations found in newly identified Werner patients. A diagram of the WRN gene is shown with the locations of mutations described in this study. Boxes indicate 35 exons and dotted lines indicate introns. Gray and black boxes are exonuclease and helicase regions, respectively. Lengths of introns and exons do not show the actual nucleotide lengths.
The second case was referred to us with a clinical diagnosis of WS, and the sequencing analysis showed a heterozygous Japanese founder mutation and a novel heterozygous variant, c.2448+1G>A, which results in the skipping of exon 20 (r.2274_2448del175) followed by the premature termination, p.Ser759Valfs*3 (Fig. 2).
The third case was referred to us with a clinical diagnosis of WS, presenting with all 6 cardinal symptoms of the syndrome proposed by the Japanese Werner Consortium. This patient carried a heterozygous Japanese founder mutation and a compound missense variant, c.1720G>A, p.Gly574Arg. The p. Gly574Arg was previously reported in a single German patient as a compound heterozygous mutation [Tadokoro et al., 2013; Yokote et al., 2017]. Although biochemical studies had already demonstrated the abrogation of enzymatic activities in a recombinant WRN protein with p.Gly574Arg [Tadokoro et al., 2013], the identification of the second p. Gly574Arg case further strengthens the notion of the pathogenicity of this variant and the loss of enzymatic activity as the cause of the WRN mutation.
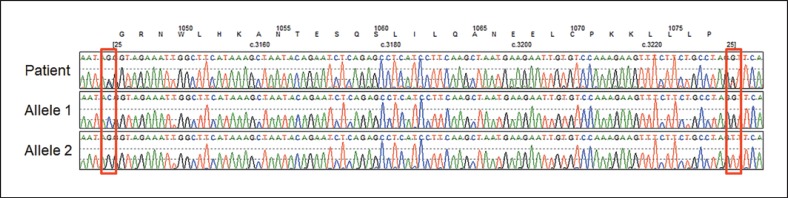
The fourth case also presented with classical features of WS and was referred to us for genetic testing. This patient carried a heterozygous Japanese founder mutation, c.3139-1G>C, and a novel heterozygous variant, c.3233+1G>T (Fig. 3). The c.3139-1G>C is located at 5′ of exon 26, and c.3233+1G>T is located at 3′ of exon 26, both are expected to cause a deletion of exon 26. Parental samples were unable to be obtained to determine the phase of 2 pathogenic variants. We therefore opted to separate 2 alleles by PCR subcloning using Gibson assembly. Following the bacterial transformation and plasmid isolation, 4 out of 8 clones had the c.3139-1G>C change, but not c.3233+1G>T (allele 1 in Fig. 3); 3 out of 8 clones had c.3233+1G>T, but not c.3139-1G>C (allele 2 in Fig. 3), and 1 did not have the insert, roughly falling into the expected 1:1 ratio of 2 alleles. This confirms the compound heterozygous status of WRN mutations in this patient.
Fig. 3.
Phase determination of the compound heterozygous mutations, c.3233+1G>T and c.3139-1G>C. Top lines are amino acid number and sequence of the WRN protein and nucleotide number of coding exon 25 (exon 26). Sequence results of patient DNA and each of 2 alleles are shown with locations of pathogenic variants in red squares.
Discussion
We described 3 newly identified null WRN pathogenic variants and a previously reported amino acid substitution variant found in Japanese WS patients. All were found as one of the compound heterozygous changes in combination with the Japanese founder mutation. It has been noted that the proportion of compound heterozygotes increased from 14.2% in 1997 to 31.8% in 2017 in Japanese WS patients. Reciprocally, the homozygotes of the Japanese founder mutation decreased from 73.2 to 63.6% during the same 20-year period [Yamaga et al., 2017]. Considering the trend in Japan to fewer consanguineous marriages, an increase in compound heterozygotes and corresponding decrease in homozygotes is to be expected [Nalls et al., 2009].
Most of the pathogenic variants of the WRN gene were null mutations, either splicing, stop codon, or small indels [Yokote et al., 2017]. Amino acid substations within the exonuclease domain found in a German patient causes protein instability [Huang et al., 2006]; thus, they were also functionally null. There have been only 2 likely pathogenic missense variants, namely p.Arg637>Trp and p.Gly574Arg [Uhrhammer et al., 2006; Tadokoro et al., 2013; Yokote et al., 2017], both of which were found in the compound heterozygotes with null mutations. Of those, only p.Gly574Arg was pathogenic [Tadokoro et al., 2013]. Our patient with p.Gly574Arg had all 6 cardinal symptoms, indicating that the combination with a null mutation is sufficient to develop typical WS features.
When a patient presents with classical features of WS, the presence of 2 heterozygous WRN mutations is generally considered sufficient to make a diagnosis of WS. We, however, felt it is necessary to determine the phase of 2 heterozygous mutations, c.3233+1G>T and c.3139-1G>C, because both of them result in the skipping of exon 26. Strictly speaking, this should be done for all compound heterozygotes when technically feasible. In fact, recommendation of the description of sequence variation (www.hgvs.org/mutnomen/recs-DNA.html#DNA) clearly distinguishes compound heterozygosity with known phases, e.g., c.[3233+1G>T];[3139-1G>C], versus unknown phases, e.g., c.[3233+1G>T(;)3139-1G>C]. Such assay is necessary for the cases with ambiguous clinical diagnosis and may become a part of routine procedure in the future, as technology progresses.
Statement of Ethics
The study was conducted according to the Declaration of Helsinki. Written informed consent was obtained prior to clinical procedures. This study was approved by the Internal Review Board of the Chiba University, Japan.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgments
We thank Ms. Julia S. Appelbaum for her editorial assistance. This work was supported by the grants NIH/NCI R01CA210916 and JSPS KAKENHI 17H04037 to J.O. as well as 17H01558 to K.Y, and the grants from KAKENHI on Innovative Areas “Stem Cell Aging and Disease 26115009,” from AMED 17bm0804016h0001 and 17ek0109126h0003, the Health and Labor Sciences Research 15545420, and from the Project for Elucidating and Controlling Mechanisms of Aging and Longevity, AMED, all to K.Y.
References
- Croteau DL, Popuri V, Opresko PL, Bohr VA. Human RecQ helicases in DNA repair, recombination, and replication. Annu Rev Biochem. 2014;83:519–552. doi: 10.1146/annurev-biochem-060713-035428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Goto M, Ishikawa Y, Sugimoto M, Furuichi Y. Werner syndrome: a changing pattern of clinical manifestations in Japan (1917∼2008) Biosci Trends. 2013;7:13–22. [PubMed] [Google Scholar]
- Hisama FM, Oshima J, Martin GM. How research on human progeroid and antigeroid syndromes can contribute to the longevity dividend initiative. Cold Spring Harb Perspect Med. 2016;6 doi: 10.1101/cshperspect.a025882. a025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Lee L, Hanson NB, Lenaerts C, Hoehn H, et al. The spectrum of WRN mutations in Werner syndrome patients. Hum Mutat. 2006;27:558–567. doi: 10.1002/humu.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masala MV, Scapaticci S, Olivieri C, Pirodda C, Montesu MA, et al. Epidemiology and clinical aspects of Werner's syndrome in North Sardinia: description of a cluster. Eur J Dermatol. 2007;17:213–216. doi: 10.1684/ejd.2007.0155. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Simon-Sanchez J, Gibbs JR, Paisan-Ruiz C, Bras JT, et al. Measures of autozygosity in decline: globalization, urbanization, and its implications for medical genetics. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000415. e1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima J, Martin GM, Hisama FM, Werner Syndrome . GeneReviews® [Internet] (University of Washington, Seattle 1993–2018) In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, et al., editors. Initial posting: Dec 2, 2002. last update: Sept 29, 2016. [PubMed] [Google Scholar]
- Oshima J, Sidorova JM, Monnat RJ., Jr Werner syndrome: clinical features, pathogenesis and potential therapeutic interventions. Ageing Res Rev. 2017;33:105–114. doi: 10.1016/j.arr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B, Lessel D, Nampoothiri S, Rao AS, Hisama FM, et al. Ethnic-specific WRN mutations in South Asian Werner syndrome patients: potential founder effect in patients with Indian or Pakistani ancestry. Mol Genet Genomic Med. 2013;1:7–14. doi: 10.1002/mgg3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Imai M, Sugimoto M, Goto M, Furuichi Y. Prevalence of Werner's syndrome heterozygotes in Japan. Lancet. 1999:353–1766. doi: 10.1016/S0140-6736(98)05869-3. [DOI] [PubMed] [Google Scholar]
- Shamanna RA, Croteau DL, Lee JH, Bohr VA. Recent advances in understanding Werner syndrome. F1000Res. 2017:6–1779. doi: 10.12688/f1000research.12110.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro T, Rybanska-Spaeder I, Kulikowicz T, Dawut L, Oshima J, et al. Functional deficit associated with a missense Werner syndrome mutation. DNA Repair (Amst) 2013;12:414–421. doi: 10.1016/j.dnarep.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M, Mori S, Kuzuya M, Yoshimoto S, Shimamoto A, et al. Diagnostic criteria for Werner syndrome based on Japanese nationwide epidemiological survey. Geriatr Gerontol Int. 2013;13:475–481. doi: 10.1111/j.1447-0594.2012.00913.x. [DOI] [PubMed] [Google Scholar]
- Uhrhammer NA, Lafarge L, Dos Santos L, Domaszewska A, Lange M, et al. Werner syndrome and mutations of the WRN and LMNA genes in France. Hum Mutat. 2006;27:718–719. doi: 10.1002/humu.9435. [DOI] [PubMed] [Google Scholar]
- Yamaga M, Takemoto M, Takada-Watanabe A, Koizumi N, Kitamoto T, et al. Recent trends in WRN gene mutation patterns in individuals with Werner syndrome. J Am Geriatr Soc. 2017;65:1853–1856. doi: 10.1111/jgs.14906. [DOI] [PubMed] [Google Scholar]
- Yokote K, Chanprasert S, Lee L, Eirich K, Takemoto M, et al. WRN mutation update: mutation spectrum, patient registries, and translational prospects. Hum Mutat. 2017;38:7–15. doi: 10.1002/humu.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]