Abstract
Purpose
We sought to determine the role of epithelium-produced thymic stromal lymphopoietin (TSLP) and its underlying mechanisms in corneal innate immune defense against Pseudomonas (P.) aeruginosa keratitis.
Methods
The expression of TSLP and TSLPR in cultured human corneal epithelial cells (HCECs) and mouse corneas was determined by PCR, Western, and/or ELISA. Cellular localization of TSLP receptor (TSLPR) was determined by whole mount confocal microscopy. TSLP-TSLPR signaling was downregulated by neutralizing antibodies and/or small interfering (si)RNA; their effects on the severity of P. aeruginosa–keratitis and cytokine expression were assessed using clinical scoring, bacterial counting, PMN infiltration, and real-time PCR. The role of dendritic cells (DCs) in corneal innate immunity was determined by local DC depletion using CD11c-DTR mice.
Results
P. aeruginosa–infection induced the expression of TSLP and TSLPR in both cultured primary HCECs and in C57BL/6 mouse corneas. While TSLP was mostly expressed by epithelial cells, CD11c-positive cells were positive for TSLPR. Targeting TSLP or TSLPR with neutralizing antibodies or TSLPR with siRNA resulted in more severe keratitis, attributable to an increase in bacterial burden and PMN infiltration. TSLPR neutralization significantly suppressed infection-induced TSLP and interleukin (IL)-17C expression and augmented the expression of IL-23 and IL-17A. Local depletion of DCs markedly increased the severity of keratitis and exhibited no effects on TSLP and IL-23 expression while suppressing IL-17A and C expression in P. aeruginosa–infected corneas.
Conclusions
The epithelium-expressed TSLP plays a protective role in P. aeruginosa keratitis through targeting of DCs and in an IL-23/IL-17 signaling pathway-related manner.
Keywords: bacterial keratitis, TSLP, dendritic cells, innate immunity, IL-23-IL-17 pathway
Bacterial keratitis is a common infectious ocular disease and a leading cause of ocular morbidity and blindness worldwide.1 The Gram-negative pathogen, P. aeruginosa is a major corneal pathogen, representing nearly 38% of infectious keratitis cases.1 P. aeruginosa keratitis is generally thought to be more severe at presentation, more difficult to treat, and results in worse visual outcomes than other forms of bacterial keratitis.2 Although it is a ubiquitous pathogenic bacterium, P. aeruginosa can only invade the cornea to cause opportunistic infection when the corneal epithelium barrier is impaired. Like other mucosal linings, corneal epithelial cells express pathogen recognizing receptors and play an important role in the innate immune response by sensing the presence of pathogens and by expressing inflammatory cytokines such as IL-1β, IL-8 and CXCL2, and antimicrobial peptides (AMPs) such as β-defensins and CRAMP.3 In addition to epithelial cells, the epithelial layer contains a rich network of sensory nerves and sparsely-distributed intraepithelial dendritic cells. These cells form a functional unit with a coordinated response to environmental challenges, including infectious pathogens. The molecules involved in the coordination of this defense network remain largely to be elucidated.
TSLP is an IL-7 like cytokine expressed mainly by epithelial cells and is known to activate STAT3, STAT5, and JAK2 pathways, which control processes such as cell proliferation and development of the hematopoietic system.4 Originally shown to promote the growth and activation of B cells, it is now known to have wide-ranging impacts on both hematopoietic and nonhematopoietic cell lineages, including dendritic cells, basophils, eosinophils, mast cells, CD4(+), CD8(+) and natural killer T cells, B cells and epithelial cells.4 TSLP-induced Th2 responses are associated with the pathogenesis of allergic inflammatory diseases, including atopic dermatitis, asthma, and rhinitis. It can directly or indirectly promote Th2 and Treg responses, and inhibit Th1 and Th17 responses through limiting the expression of pro-inflammatory cytokines such as IL-17 and IFN-gamma.5 Based on recent findings in humans and mouse models, TSLP might also be involved in the pathogenesis of inflammatory bowel disease and progression of cancer.6 The receptor of TSLP, mainly found in dendritic cells, is a heterodimeric complex that consists of TSLP receptor (TSLPR) and IL-7Rα. Activation of this complex results in STAT3 and STAT5 activation.7,8 Evidence shows that epithelial cells increasingly express TSLP mRNA and protein upon stimulation by microbial products.9 For example, the colon of TSLPR−/− mice displays exaggerated Th1/Th17 responses and reduced Treg cell activation even in the presence of a limited and benign bacterial community.10 In addition, an alternatively spliced short form of human TSLP has been shown to act as a potent antimicrobial peptide.11,12 Expression of TSLP in the skin, oral and GI mucosa is part of the defense barrier that aids in the control of both commensal and pathogenic microbes.11 In the cornea, epithelium-derived TSLP has been linked to experimental mouse allergic conjunctivitis.13,14 and Aspergillus fumigatus keratitis in in vitro cell culture models.15,16 The involvement of TSLP in bacterial keratitis in vivo remains unknown.
We addressed here whether TSLP has a role in regulating P. aeruginosa keratitis. In this study, we investigated the expression of TSLP and TSLPR in C57BL/6 mouse corneas in response to P. aeruginosa infection and demonstrated that targeting the TSLP-TSLPR signaling axis significantly increased the severity of keratitis including leading to a high bacterial burden. Our data suggests that TSLP plays a protective role in the cornea from P. aeruginosa infection through activation of DCs.
Materials and Methods
Animals
Wild-type C57BL/6 mice (8 weeks; female) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). B6-DTR mice, which express simian diphtheria toxin receptor (DTR)–enhanced green fluorescent protein fusion protein under the control of the CD11c promoter, were originally purchased from the Jackson Laboratory and breaded in house at a Wayne State University animal housing facility. All animal procedures were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of Wayne State University.
Bacterial Preparation
P. aeruginosa strain ATCC 19660 (cytotoxic) was used in this study. This strain is a standard laboratory strain that provides a reproducible inflammatory response in the cornea in the B6 mouse.17–19 A colony of bacteria was picked and grown overnight at 37°C in tryptic soy broth (TSB) with constantly shaking. The next morning, 20 μL of P. aeruginosa–containing TSB was transferred to 4 mL fresh medium and allowed to grow for 4 hours with OD600 less than 1. After the concentration of bacteria in TSB was determined (1 OD600 = × 108 P. aeruginosa/mL), the bacteria were washed and resuspended in PBS and then either used to infect mouse corneas or placed in a boiling water bath for 5 minutes to generate heat-killed P. aeruginosa. Heat-killed bacteria were diluted with growth factor-free and antibiotic-free keratinocyte basic medium (KBM; Lonza, Basel, Switzerland).
Cell Culture
Primary human corneal epithelial cells (HCECs) were isolated from human donor corneas obtained from Eversight Michigan (Ann Arbor, MI, USA) using a previously described method.20 P4 of the primary HCECs were used for the experiments. Before treatment, cells were starved overnight in KBM. Subsequently, cells were challenged with heat-killed P. aeruginosa (1:50 multiplicity of infection) for the indicated times.
Mouse Model of P. aeruginosa Keratitis
Mice, five in each group, were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) before surgical procedures. Mouse corneas were scratched gently with a sterile 26-gauge needle to create three 1-mm incisions to break the epithelial barrier and inoculated with 1.0 × 104 colony-forming units (CFUs) of P. aeruginosa in 5 μL PBS. The number of bacteria used to inoculate the mouse corneas were 100-fold less than that used by others.21
The Application of siRNA or Neutralizing Antibody
TSLP-specific siRNA reagents (SMARTpool: a mixture of 4 siRNAs; ON-TARGETplus) and negative control nonspecific siRNAs were designed by and purchased from GE Dharmacon Company (Lafayette, CO, USA). Mice were subconjunctivally injected twice with siRNAs (10 μM, 5 μL) over 2 days at −24 and −4 hours prior to P. aeruginosa infection (time 0). To apply neutralizing antibody, mice were subconjunctivally injected with rabbit-anti-TSLP (#4023, Prosci, Poway, CA, USA) and goat-anti-TSLPR (#AF546, 200 ng/5 μL; R&D Systems, Minneapolis, MN, USA) 4 hours before the inoculation with P. aeruginosa on the corneas.
Clinical Examination, Bacteria Load Determination, MPO Measurement, and ELISA
Clinical examination was performed with corneal photographs and clinical scoring as described previously,22 five mice in each group. We used our previously modified methods that allowed all three assays (bacteria load, MPO determination, and cytokine ELISA measurement) to be performed with a single mouse cornea. Briefly, the corneas, five in each group, were excised, minced, and homogenized in 100 μL PBS with a micro tissue grinder (Dounce; Wheaton, Milville, NJ, USA). The homogenates were divided into two. The first part was subjected to plate bacterium counting. Aliquots (50 μL) of serial dilutions were plated onto pseudomonas isolation agar plates in triplicates, and colonies were counted the next day. The results were expressed as the mean number of CFU/cornea ± standard error. The second part of the homogenates was mixed with 5 μL of 1% SDS and 10% Triton X-100. For MPO assay, 30 μL homogenates was mixed with 270 μL of hexadecyltrimethylammonium bromide (HTAB) buffer (0.5% HTAB in 50 mM phosphate buffer, pH 6.0). The samples were then subjected to three freeze-thaw cycles, followed by centrifugation at 16,000g for 20 minutes. A total of 20 μL of the supernatant was mixed with 180 μL of 50 mM phosphate buffer (pH 6.0) containing 16.7 mg/mL O,O-dianisidine hydrochloride and 0.0005% hydrogen peroxide at a 1:30 ratio in a 96-well plate. The change in absorbance at 460 nm was monitored continuously for 5 minutes in a microplate reader (Synergy2; BioTek). The results were expressed in units of MPO activity/cornea. For ELISA, 20 μL cell lysates was used. The ELISA was performed according to the manufacturer's instructions (#MTLP00; R&D Systems) with minimal three samples for each condition.
Semiquantitative and Quantitative PCR
Total RNA was extracted with an RNA extraction kit (RNeasy Mini Kit; Qiagen, Valencia, CA, USA) following the manufacturer's instructions. RNA was reversed-transcribed to cDNA with a first-strand synthesis system (DNA Thermal Cycler 480; Derkin Elmer Letus, Norwalk, CT, USA). For semiquantitative PCR, cDNA was amplified with TaqMan technology (Promega, Madison, WI, USA). PCR products were subjected to electrophoresis on 2% agarose gels containing ethidium bromide. For quantitative PCR, cDNA from three corneas was amplified using a Real-Time PCR system (StepOnePlus; Applied Biosystems, University Park, IL, USA) with a PCR Master Mix (SYBR Green; Applied Biosystems). Data were analyzed by using ΔΔCT method with β-actin or GAPDH as the internal control.
Western Blot
Mouse corneal samples were lysed with RIPA buffer. The lysates were centrifuged to obtain supernatant. Protein concentration was determined by BCA assay with a Protein Assay Kit (Micro BCA; Pierce Biotechnology, Rockford, IL, USA). The protein samples were separated by SDS-PAGE and electrically transferred onto nitrocellulose membranes (Bio-Rad Laboratories; Hercules, CA, USA). The membranes were blocked with 3% BSA and incubated with primary antibodies overnight at 4°C. After several washes, the membranes were incubated with horseradish peroxidase–conjugated secondary antibodies. Signals were visualized using a chemiluminescent substrate (SuperSignal West Pico; Thermo Fisher Scientific, Pittsburgh, PA, USA). β-actin was used as the loading control. Antibodies: rabbit-anti-TSLP and goat-anti-TSLPR. For each condition, two samples were shown to indicate similar band intensity.
Whole-Mount Confocal Microscopy
Mice were euthanized, and the entire cornea plus the limbus was excised under the operating microscope. Excised corneas were fixed in 4% paraformaldehyde and stored at 4°C until further processing. Before staining, radial incisions were made to produce six pie-shaped wedges. Corneas were washed in PBS, incubated in 20 mmol/L pre-warmed EDTA for 30 minutes at 37°C, and incubated with a 0.2% solution of Triton X-100 in PBS plus 1% bovine serum albumin (BSA) with Fc block for 20 minutes at room temperature. After blocking, the corneas were incubated overnight at 4°C with 100 L of hamster CD11c antibody (BD Pharmingen, San Diego, CA, USA) and goat TSLPR antibody (AF546; R&D Systems) diluted in PBS with 1% BSA. The tissues were then washed five times in PBS. Corneas were then incubated with 100 L AlexaFluor 594-conjugated anti-goat antibody and FITC-conjugated anti-Armenian hamster antibody diluted in PBS with 1% BSA for 1 hour at room temperature. This was followed by five washes in PBS. Stained tissue whole mounts were placed in mounting medium (Vectashield; Vector Laboratories, Burlingame, CA, USA) onto glass slides and coverslipped. Corneal whole mounts were examined using confocal microscopy (TCSSP2; Leica, Wetzlar, Germany) for immunopositive cells in the cornea.
Depletion of DCs
CD11c-DTR and WT B6 mice were injected with 100 ng of DT in 100 μL of PBS intraperitoneally (IP). Twenty-four hours after DT injection, the corneas were incubated with 1.0 × 104 CFU of P. aeruginosa; the progress of infection was monitored for up to 3 days postinjection (dpi). This procedure was shown to deplete DCs systematically of CD11c-DTR but not WT B6 mice.23
Statistical Analysis
Data were presented as mean values ± SD. For between-group comparisons, an unpaired, 2-tailed Student t-test was performed, and for three or more conditions 1-way ANOVA with Bonferroni Correction were performed to determine statistical significance of differences in fungal counts, cytokine ELISA findings, and the MPO assay. A nonparametric Mann–Whitney U test was performed to determine the statistical significance of differences in clinical scores. Experiments with five mice for each condition were repeated at least twice to ensure reproducibility, and differences were considered statistically significant at values of P < 0.05. The results from one experiment were not compared with those of a repeated experiment because the severity of keratitis may differ among experiments. Each experiment was performed with 5 WT, untreated B6 mice as the control.
Results
TSLP Expression in Primary Human CECs in Response to P. aeruginosa Challenge
We first investigated whether HCECs express TSLP in response to P. aeruginosa challenge in vitro. Primary HCECs (passage 4) were challenged with heat-killed P. aeruginosa. TSLP at the mRNA and protein levels were assessed by qPCR and Western blotting (Fig. 1). TSLP expression at the mRNA levels was significantly increased at both 1 and 2 hours postchallenge (Fig. 1A), followed by a rapid decline to a level slightly, but significantly higher than that in naïve cells (set as a value of 1) at 4 and 8 hours post infection (hpi; Fig. 1A). At the protein level, while TSLP was detected in the control (0 hours) and 3-hour time point, the band intensity increased drastically at 8 hours post P. aeruginosa challenge in cultured HCEC lysates (Fig. 1B). Interestingly, TSLP protein can be detected in the culture media of primary HCECs at 3 hours post P. aeruginosa challenge, suggesting that TSLP induced at this time point was primarily secreted (Fig. 1B).
Figure 1.

TSLP expression and secretion in primary human corneal epithelial cells in response to P. aeruginosa challenge. HCECs (p4) grown overnight in KBM were treated with heat-killed P. aeruginosa (MOI = 50) for different times. Cell lysates as well as culture media were collected and subjected to PCR and Western blotting analyses. (A) TSLP expression in cultured HCECs challenged with heat-killed P. aeruginosa for 1, 2, 4, and 8 hours was analyzed by real-time PCR. The results are representative of two independent experiments, each with three corneas. *P < 0.05. **P < 0.01 (1-way ANOVA, with Bonferroni Correction). (B) The steady-state expression of TSLP in HCEC cell lysates at indicated times were analyzed by Western blot. (C) TSLP secretion was analyzed by Western blotting of the concentrated culture media of HCECs challenged with heat-killed P. aeruginosa. The data shown are representative of two independent experiments.
TSLP Expression in Mouse Corneas in Response to P. aeruginosa Infection
The induction of TSLP expression has been linked to allergic conjunctivitis.13,14 To determine whether microbial infection also causes TSLP upregulation in the cornea, we inoculated B6 mouse corneas with 1.0 × 104 CFU of P. aeruginosa (strain ATCC 19660) and assessed the expression of TSLP and TSLPR in CECs at different times after bacterial inoculation. RT-PCR revealed that no TSLP mRNA can be detected in naïve and 2 hpi corneal CECs (Fig. 2A) while TSLPR was detected in the naïve condition, with a slight increase in P. aeruginosa–challenged CECs at 2 hpi. At 6 hpi, strong bands were seen for TSLP while TSLPR levels remained relatively high. The expression patterns of TSLP and TSLPR revealed by RT-PCR were quantitated by qPCR (Figs. 2B, 2C). Infection induced more than a 20-fold increase in TSLP transcripts at 6 hpi (Fig. 2B) whereas the expression of TSLPR increased gradually during 2 to 6 hpi (Fig. 2B). ELISA analysis revealed a sharp increase in the levels of TSLP at 6 hpi, followed by a decrease but still significantly higher that the naïve corneas at 9 hpi (Fig. 2D). Hence, P. aeruginosa infection of the cornea results in an upregulation of TSLP in CECs starting after 3 hpi.
Figure 2.
TSLP and TSLPR expression in P. aeruginosa–infected mouse corneal epithelial cells. The centers of B6 mouse corneas were gently scratched with 26-gauge needles (1 mm long; 3 lines) and incubated with 1.0 × 104 CFU of P. aeruginosa. At the indicated times, mouse CECs were collected and subjected to (A) RT-PCR, (B, C) Real-time PCR for TSLP and TSLPR expression in naïve and P. aeruginosa infected mouse CECs, respectively. (D) TSLP expression in mouse CECs was assessed using ELISA. Error bars show standard deviations. The results are representative of two independent experiments, each with 3 corneas. *P < 0.05. **P < 0.01 (1-way ANOVA, with Bonferroni correction).
TSLPR is Mostly Colocalized With CD11c Positive Cells
Having shown epithelial expression of TSLP, we next assessed the target cells of TSLP using whole mount confocal microscopy, with a focus on DCs in B6 mouse corneas. In a naïve cornea (Fig. 3A), cells in the limbal region were both TSLPR (red) and CD11c (green) positive whereas in the peripheral region of the cornea, dendriform DCs24 (arrowheads) were CD11c-positive but TSLPR-negative. In this region, a few non-dendriform DCs were found to be both CD11c and TSLPR positive. In infected corneas at 6 hpi, many CD11c-positive cells migrated into the cornea with a relatively high density near the limbus (Fig. 3B). Most cells in this region had similar morphology as those in the limbus and were positive for both CD11c and TSLPR (arrows). We conclude that except for some residential DCs in the epithelium, most of the corneal DCs express TSLPR in response to P. aeruginosa infection.
Figure 3.
Expression and distribution of TSLPR in residential and infiltrated DCs in naïve and P. aeruginosa infected mouse corneas. B6 mouse corneas (n = 5 for each condition) were inoculated with P. aeruginosa as described in Figure 2. Naïve (A) or P. aeruginosa infected, 6 hpi (B) corneas were collected for whole mount confocal microscopy and stained with TSLPR (red) and CD11c (green). Note: The CD11c-positive cells in naïve corneas can be divided into two different phenotypes: dendriform DCs (arrowheads) and oval ones (arrows). L, the limbus; C, the cornea. While dendriform DCs were TSLPR negative, Oval DCs in the limbus and naïve and infected corneas were all TSLPR positive.
Inhibition of TSLP/TSLPR Signaling Increases the Severity of P. aeruginosa Keratitis
TSLP signals through IL-7R and a unique TSLP receptor (TSLPR).25 To assess the effects of TSLP signaling on P. aeruginosa keratitis, we used TSLP neutralizing antibody, TSLPR-specific siRNA, and TSLPR neutralizing antibody in the B6 mouse model. Both TSLP neutralizing antibody and TSLPR siRNA treatments augmented the severity of keratitis at 1 dpi (data not shown). Figure 4 shows the effects of TSLPR neutralizing antibody on the severity of keratitis at 1, 2, and 3 dpi. The control corneas were opacified with a clearly-visible dense area covering: 20% at 1 dpi, 70% at 2 dpi, and almost 100% at 3 dpi. In TSLPR neutralizing antibody-treated corneas, the opacification covered more than 70% of the cornea with a dense uneven opacity at 1 dpi. At 2 dpi, the entire cornea was covered with heavy opacification with a ring of relatively light opacity in between the central and peripheral region. At 3 dpi, heavy opacity covered the entire cornea with corneal ulceration, melting ring, and neovascularization (reddish color), pathologies usually seen in the control corneas at day 5.18,26 The clinical scores revealed significantly higher disease severity in the TSLPR neutralizing antibody-treated group than that observed in the IgG injected control group (6.2 vs. 11.6 at 3 dpi; Fig. 4A). Micrographs showing pathology at 1, 2, 3 dpi from all 5 corneas, from which the clinical scores were derived, were presented as supplemental Figure S1. At 3 dpi, most corneas treated with TSLPR neutralizing antibody were about to be perforated (Supplementary Fig. S1). TSLPR neutralization resulted in a significantly higher bacterial burden (3.37 ± 1.84 × 106 vs. 3.31 ± 2.28 × 107 CFU, P = 0.0198), neutrophil infiltration (84.57 ± 39.08 vs. 319.35 ± 28.04 units/cornea) and the expression of IL-1β at the protein levels (811.1 vs. 3870.5 pg/μg protein) when compared to the control, IgG injected corneas at 3 dpi (Figs. 4B, 4C, 4D).
Figure 4.
Effects of TSLPR neutralization mediated downregulation on the severity of P. aeruginosa keratitis. Mouse corneas were subconjunctivally injected 5 μL control IgG or anti-TSLP at −4 hours. At 0 hours, the corneas were inoculated with 1.0 × 104 CFU of P. aeruginosa. (A) Micrographs and clinical scores of the control (IgG) and TSLPR neutralizing antibody treated corneas at 1, 2, and 3 dpi. Note the increased opacification in anti-TSLPR–treated corneas (right panels). The infected corneas were excised, homogenized, and subjected to (B) bacteria counting, (C) MPO determination (units/cornea), and (D) ELISA determination for IL1β. The results are representative for two independent experiments. **P < 0.01, (t-test).
Blockade of TSLP/TSLPR Signaling Differentially Alters the Expression of IL-23 and IL-17 Cytokines in P. aeruginosa–Infected Corneas
Having shown that TSLP plays a protective role in P. aeruginosa keratitis, we next investigated whether TSLP signaling affects the expression of IL-23/IL-17 cytokines at an earlier stage, 1 dpi since at this time point the host responses are mostly innate immunity which has grout influence on the outcome of P. aeruginosa keratitis. We used TSLPR neutralizing antibody to block TSLP signaling and observed, similar to what we had observed using TSLPR siRNA and TSLP neutralizing antibody, a significant increase in P. aeruginosa keratitis as assessed by clinical scoring, with a score of 3 in the control IgG treated and 7.5 in TSLPR neutralized corneas (Fig. 5A) at 1 dpi. As shown in Figure 5B, the infection-induced expression of TSLP was suppressed to a level lower than that in naïve corneas (from 3.1-fold increase lowered to 55.4% of the control set value as 1, a 6-fold decrease). The expression of IL-23 and its downstream cytokine IL-17A were induced by P. aeruginosa infection and further augmented by TSLPR neutralization (5.11- to 16.68- and 4.59- to 10.84-fold increase for IL-23 and IL-17A, respectively). Interestingly, TSLPR neutralization greatly downregulated IL-17c expression (from 8.48- lowered to 2.46-fold increase over the naïve corneas as the control).
Figure 5.
The expression of TSLP and IL-23/IL-17 in P. aeruginosa–infected mouse corneas with or without TSLPR neutralization. Mouse corneas were subconjunctivally injected TSLPR neutralizing antibody at −4 hours. At 0 hours, the corneas were incubated with 1.0 × 104 CFU of P. aeruginosa. (A) Micrographs and clinical scores of the control (IgG) and TSLPR neutralizing antibody treated corneas at 1 dpi. (B) Corneas (3 in each group) were excised from the enucleated eyes at 1 dpi and subjected to Real-time PCR analysis of TSLP, IL-23, IL-17A, and IL-17C. The results are presented as fold increase with the levels of naïve corneas set as 1. The Figure is representative of two independent experiments, each with five corneas. *P < 0.05. **P < 0.01 (1-way ANOVA, with Bonferroni correction).
Dendritic Cells Are Required for Corneal Innate Defense Against P. aeruginosa
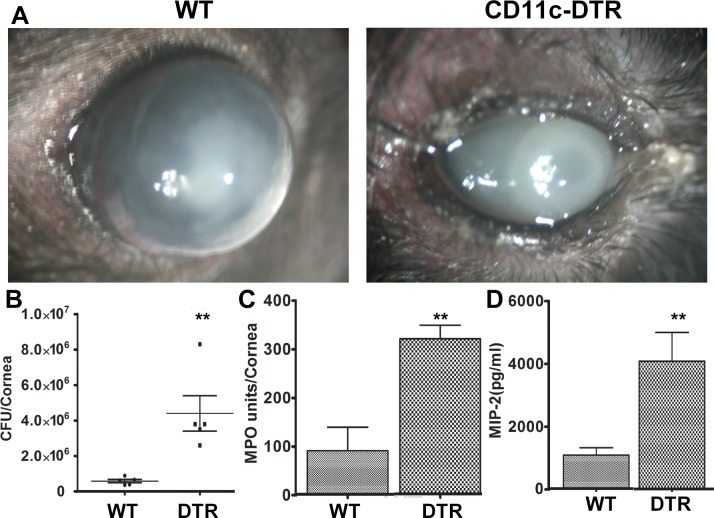
Having shown that DCs are the target of TSLP, we sought to determine if DCs are required for the innate immune response for the cornea in controlling P. aeruginosa, using CD11c-DTR mice. We previously showed that subconjunctival injections of DT sufficiently depleted DCs in the cornea, resulting in significant delays in epithelial wound closure.27 Our preliminary study revealed that local depletion of DCs had small effects on the outcomes of P. aeruginosa keratitis. We then used whole body depletion of DCs by 100 ng IP in 100 μi PBS. Our previous study showed this treatment depleted DCs from the spleen and cornea.27 Figure 6 shows that the depletion of DCs resulted in severe keratitis, including an increase in the bacterial load (5.84 × 105 vs. 4.40 × 106 CFU at 3 dpi, 13.27-fold increase), in neutrophil infiltration as determined by MPO assay (90.0 vs. 319.35 units/cornea), and in the expression of MIP-2 (1.72 vs. 4.7 ng/cornea). The DC-depleted cornea shown at 3 dpi was about to be perforated (Fig. 6), a process normally requiring a minimum of 5 days in WT B6 mice.
Figure 6.
Depletion of dendritic cells and the severe P. aeruginosa keratitis. CD11c-DTR and WT mice were injected with 100 ng of DT in 100 μL of PBS IP 24 hours after DT injection, the corneas were incubated with 1.0 × 104 CFU of P. aeruginosa; the progress of infection was monitored for 3 days. (A) Micrographs of infected corneas at 3 dpi. Note that the DC depleted corneas (CD11c-DTR) were perforating (clinical score 12) while the control cornea (WT) remained intact with center covered by opacification. The corneas were excised and subjected to (B) a standard plate count to determine the number of bacteria in each cornea; (C) MPO determination (units/cornea); and (D) ELISA determination for MIP-2 (CXCL2). The results are representative for two independent experiments. **P < 0.01 (t-test).
The corneas with or without DC depletion were also collected at 1 dpi and subjected to qPCR analysis (Fig. 7). Unlike TSLPR neutralization which decreased TSLP and increased IL-23 expression, DC depletion had no detectable effects on infection-induced TSLP and IL-23 expression. Interestingly, the infection-induced expression of both IL-17A and -17C was significantly dampened by DC depletion.
Figure 7.
The expression of TSLP and IL-23/IL-17 in DC depleted, P. aeruginosa–infected B6 mouse corneas. CD11c-DTR and WT mice were injected with 100 ng of DT in 100 μL of PBS IP 24 hours before 1.0 × 104 CFU of P. aeruginosa inoculation of the cornea. (A) Micrographs of the WT or CD11c-DTR mice treated with DT and inoculated P. aeruginosa at 1 dpi. Note that the DC depleted corneas had much high average clinical score (n = 5). (B) The corneas were excised from the enucleated eyes, homogenized, and subjected to real-time PCR analysis of TSLP, IL-23, IL-17A, and IL-17C. The results are presented as fold increase with the levels of naïve corneas (NL) set as 1. The figure is representative of two independent experiments. *P < 0.05. **P < 0.01 (1-way ANOVA, with Bonferroni correction).
Discussion
Using the mouse P. aeruginosa–keratitis model and primary HCECs, we tested the hypothesis that TSLP plays a role in antibacterial innate immune defense. Our results demonstrate that the inoculation of P. aeruginosa stimulates both human and mouse CECs to produce and/or secrete TSLP. Downregulation of TSLP signaling with TSLPR neutralization aggravated the severity of keratitis, including great elevations of inflammation (increasing corneal opacification, clinical scores, PMN infiltration, and the expression of pro-inflammatory cytokine IL-1β) and a moderate increase in bacterial burden, suggesting a protective role of TSLP in mediating host response to P. aeruginosa infection. Moreover, we demonstrated that most CD11c-positive cells expressed TSLPR in the infected corneas. In addition, TSLP differentially affected the expression of Th17 cytokines in B6 mouse corneas: the inhibition of TSLPR induced an up-regulation of infection-induced IL-17A but downregulated IL-17C. Depletion of DCs resulted in severe keratitis and greatly down-regulated expression of IL-17A and C. Taken together, our results suggest that TSLP plays a protective role in P. aeruginosa keratitis by activating DCs and by altering the expression of IL-23/IL-17 cytokines in the cornea.
TSLP is a cytokine that is mostly epithelium-expressed, and DCs are its primary target.28 At the ocular surface, the expression of TSLP was linked to allergic conjunctivitis in BALB/c mice as well as patients with various types of allergic conjunctivitis in a Th2-response related manner.13,14,29 In vitro, Aspergillus fumigatus stimulated the expression of TSLP in cultured human CECs in a TLR2/MyD88 dependent manner.15,16 In this study, we showed that challenging primary HCECs with heat-killed P. aeruginosa resulted in the upregulation of TSLP at mRNA levels within the first 2 hours, and at the protein levels at up to 8 hours. TSLP can also be detected in the culture media at 3 hpi. In vivo, P. aeruginosa infection stimulated TSLP expression between 2 and 6 hpi and peaked at the protein level at 6 hpi, followed by a decline at 9 hpi. Hence, unlike the IL-1 family of cytokines that are expressed rapidly (within first 3 hours) and continue to rise with the pathogenesis of microbial keratitis,30 TSLP expression was relatively late in mouse corneas in response to P. aeruginosa infection. Importantly, the expression of TSLPR was also increased even before TSLP up-regulation in CECs, suggesting autocrine signaling of TSLP in the epithelia in response to P. aeruginosa infection.
Using antibody neutralization, we showed that downregulating TSLP-TSLPR signaling significantly augmented corneal opacification, clinical scores, bacterial burden, PMN infiltration, and the expression of IL-1β at 3 dpi. The bacterial plate counts for the controls of P. aeruginosa toxic strain at 3 dpi are within the ranges reported in the literature31,32 as well as our previous studies,18,26 even though the number of bacteria used to inoculate the mouse corneas were 100-fold less than that used by others.21,31 The differences in the detected bacterial burden between the control and TSLPR-neutralized corneas at 3 dpi (∼10 fold with P = 0.0198) is comparable to our previous study of the corneas treated with recombinant IL-24.26 Together, these parameters—opacification shown in micrograph, clinical scoring, bacterial plate counting, MPO measurement, and proinflammatory cytokine detection—revealed a significant increase in the severity of keratitis in B6 mice. In the literature, TSLP has been shown to enhance neutrophil killing of methicillin-resistant Staphylococcus aureus without affecting TH2 responses during an in vivo skin infection.33 On the other hand, overproduction of TSLP by intestinal epithelial cells negatively regulated IL-22 production by group 3 innate lymphoid cells and impaired innate immunity to Citrobacter rodentium infection.34 Our study indicated a protective role of TSLP-TSLPR signaling in corneal innate immune defense against P. aeruginosa infection in B6 mice similar to that observed in the skin.33 Disruption of TSLPR signaling resulted in a marked reduction of TSLP mRNA levels in infected corneas, suggesting a positive feedback loop of regulation of the epithelial cytokine. A positive feedback loop that drives TSLP transcription in a STAT-dependent manner was reported recently in intestinal and skin epithelial cells.35 TSLP has also been known to both induce Th2 differentiation and to be induced by activated Th2 cells, resulting in a positive feedback loop to enhance allergic inflammatory conditions.36 Hence, the positive feedback loop should amplify the protective effects of TSLP in the corneas in response to P. aeruginosa infection.
We assessed the effects of TSLPR signaling blockade on the expression of cytokines with a focus on IL-23/IL17 pathways in the B6 mouse corneas. This is because TSLP targets DCs and DCs are known to be a major source of IL-23 under many homeostatic and pathologic conditions.37 Moreover, TSLP was shown to induce the production of copious amounts of IL23 in primary human DCs.38 Importantly, the IL-23/IL-17 immune axis has been recognized as a critical element of innate immune defense against different pathogens.39 For example, IL-17 is known as a strong inducer of antimicrobial peptides, particularly β-defensins40 and S100A8/A9,41 which can prevent infection at mucosal surfaces and in the skin.42 IL-17A is the best-characterized member of the IL-17 family to date. Here we have shown that like IL-23, the expression of IL-17A, also referred to as IL-17 in the literature, was greatly upregulated when TSLPR signaling was disrupted. This could also suggest that TSLP plays an anti-inflammatory role in P. aeruginosa keratitis. Indeed, TSLP was found to inhibit IL-12p40, a subunit of IL-23, as well as IL-17 production in the intestine in response to Heligmosomoides polygyrus infection43 and the gut-dwelling parasite Trichuris muris44 to play a protective role in the mouse intestine. Our results that TSLP plays a protective and anti-inflammatory role in P. aeruginosa keratitis are consistent with the report that topical neutralization of IL-17 (IL-17A) promoted bacterial clearance and reduced the pathology of keratitis.45 Surprisingly, the expression of IL-17C, a novel member of the IL-17 cytokine family, was markedly suppressed by TSLP neutralizing antibody. In human gastric mucosa, Helicobacter pylori infection was associated with a significant increase in IL-17C expression in gastric mucosa, and its role is still undetermined.46 Our study suggests that the ability to alter the expression of IL-17 may represent a mechanism underlying the anti-infection and protective role of TSLP in the cornea, and IL-17C, known to be expressed mostly by epithelia, may play a role in TSLP-TSLPR-mediated innate protection in the cornea.
DCs are known as the main target of TSLP47 and TSLP acts as a link between epithelial cells and DCs.48,49 In the current study, we showed that DCs expressed TSLPR in the cornea. Using a well-established transgenic mouse line, we transiently depleted DCs and showed that DC depletion resulted in a much more severe keratitis. This result is in sharp contrast of a report that showed temporary depletion of CD11c positive cells triggered phagocyte accumulation in the spleen and enhances their innate bacterial killing capacity in an experimental infection model of Yersinia enterocolitica, a bacterium that causes food-borne acute and chronic gastrointestinal and systemic diseases in both humans and mice.50 The ablation of DCs was also shown to enhance resistance to intranasal challenge with Pneumococcal pneumonia.51 As an immune privileged site, DCs are the only immune cells residing in the corneal epithelium, which lacks γδ-T cells,52 while macrophages are present in the posterior corneal stroma.53 The infiltrated γδ T cells were shown to directly stimulate epithelial secretion of CXCL1, a chemokine that promotes recruitment of neutrophils54,55 and promotes a IL-17 response at the ocular surface.56 Hence, lack of DCs may lead to the deficiency of the timely infiltration of γδ T cell from the limbal region, resulting in delayed PMN infiltration, and the deficiency of IL-17 responses in the cornea,57 resulting in its rapid clinical deterioration as seen at 3 dpi of DC-depleted corneas (Fig. 7). Indeed, unlike in TSLPR-neutralized corneas, DC-depletion resulted in the suppression of both IL-17A and 17C, although levels of TSLP and IL-23 was not affected. Although DCs are thought to be the major source of IL-23, it can also be produced by epithelial cells in response to injury, allergic challenge, and infection.58–60 This may explain why a high level of IL-23 mRNA was detected in DC-depleted corneas. Our study is the first to link DCs in an infected tissue to the expression of IL-17 isoforms. It is important to note that the majority of IL-17 is associated with cells of the innate immune system such as neutrophils, γδT cells, and innate lymphoid cells61 and that epithelia express and secrete IL-23 to regulate intestinal epithelial cell homeostasis to limit mucosal damage.60 The function of IL-17 as a whole, and of the individual isoforms IL-17A and IL-17C in the pathogenesis of P. aeruginosa keratitis is currently under investigation in our laboratory.
In summary, in this study we assessed the expression and function of an epithelium-produced cytokine TSLP in mediating corneal innate immune response to P. aeruginosa infection. We demonstrated a supportive role TSLP through its receptor TSLPR and its target DCs in the cornea. This protective role may be related to IL-23/IL-17 pathways and may require the participation of residential and infiltrating cells. While the requirement for neutrophils, macrophages, NK cells, and DCs have been documented, the involvement of other innate immune cells, such as γδT cells and innate lymphoid cells, in mediating the expression of IL-17 and in the innate immune defense against microbial keratitis are expected but have yet to be proven. The results shown in this study provide a link for future study to define the role IL-23/IL-17 and the involvement of innate immune cells in the pathogenesis of bacterial keratitis.
Supplementary Material
Acknowledgments
The authors thank all the members of the Yu laboratories for assistance and comments on the work. The authors also thank Patrick Lee for critical reading of the manuscript.
Supported by NIH/NEI R01-EY010869, R01-EY017960, P30-EY004068 grants, Research to Prevent Blindness, the National Natural Science Foundation of China (81700807). Xinhan Cui was supported by the China Scholarship Council for 19m study at Wayne State University (No. 201406100092).
Disclosure: X. Cui, None; N. Gao, None; R. Me, None; J. Xu, None; F.-S.X. Yu, None
References
- 1.Fong CF, Tseng CH, Hu FR, Wang IJ, Chen WL, Hou YC. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol. 2004;137:329–336. doi: 10.1016/j.ajo.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Sy A, Srinivasan M, Mascarenhas J, et al. Pseudomonas aeruginosa keratitis: outcomes and response to corticosteroid treatment. Invest Ophthalmol Vis Sci. 2012;53:267–272. doi: 10.1167/iovs.11-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Wu XY, Yu FS. Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr Eye Res. 2005;30:527–534. doi: 10.1080/02713680590968150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Zhang J, Wu Y, Li J. The regulation of thymic stromal lymphopoietin in gut immune homeostasis. Dig Dis Sci. 2011;56:2215–2220. doi: 10.1007/s10620-011-1587-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res. 2012;52:211–223. doi: 10.1007/s12026-012-8264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 8.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 9.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosconi I, Geuking MB, Zaiss MM, et al. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunology. 2013;6:1157–1167. doi: 10.1038/mi.2013.12. [DOI] [PubMed] [Google Scholar]
- 11.Bjerkan L, Sonesson A, Schenck K. Multiple functions of the new cytokine-based antimicrobial peptide thymic stromal lymphopoietin (TSLP) Pharmaceuticals (Basel) 2016;9:E41. doi: 10.3390/ph9030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonesson A, Kasetty G, Olin AI, et al. Thymic stromal lymphopoietin exerts antimicrobial activities. Exp Dermatol. 2011;20:1004–1010. doi: 10.1111/j.1600-0625.2011.01391.x. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Ma P, de Paiva CS, et al. TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2010;51:3076–3082. doi: 10.1167/iovs.09-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun W, Sheng Y, Chen J, Xu D, Gu Y. Down-regulation of mir-146a expression induces allergic conjunctivitis in mice by increasing TSLP level. Med Sci Monit. 2015;21:2000–2007. doi: 10.12659/MSM.894563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Wang L, Wu X. Aspergillus fumigatus promotes T helper type 2 responses through thymic stromal lymphopoietin production by human corneal epithelial cells. Clin Exp. Ophthalmol. 2016;44:492–501. doi: 10.1111/ceo.12706. [DOI] [PubMed] [Google Scholar]
- 16.Ren X, Wang L, Wu X. A potential link between TSLP/TSLPR/STAT5 and TLR2/MyD88/NFkappaB-p65 in human corneal epithelial cells for Aspergillus fumigatus tolerance. Mol Immunol. 2016;71:98–106. doi: 10.1016/j.molimm.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Yin J, Zhang J, Yu FS. Modulation of corneal epithelial innate immune response to pseudomonas infection by flagellin pretreatment. Invest Ophthalmol Vis Sci. 2007;48:4664–4670. doi: 10.1167/iovs.07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Gao N, Standiford TJ, Gallo RL, Yu FS. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect. 2010;12:978–989. doi: 10.1016/j.micinf.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun. 2008;76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon GS, Dong C, Gao N, Kumar A, Standiford TJ, Yu FS. Interferon regulatory factor-1 in flagellin-induced reprogramming: potential protective role of CXCL10 in cornea innate defense against Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 2013;54:7510–7521. doi: 10.1167/iovs.13-12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kernacki KA, Goebel DJ, Poosch MS, Hazlett LD. Early TIMP gene expression after corneal infection with Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1998;39:331–335. [PubMed] [Google Scholar]
- 22.Jie Z, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15:155–168. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]
- 23.Mott KR, Ghiasi H. Role of dendritic cells in enhancement of herpes simplex virus type 1 latency and reactivation in vaccinated mice. Clin Vaccine Immunol. 2008;15:1859–1867. doi: 10.1128/CVI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao N, Lee P, Yu F-S. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci Rep. 2016;6:36414. doi: 10.1038/srep36414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong J, Sharma J, Raju R, et al. TSLP signaling pathway map: a platform for analysis of TSLP-mediated signaling. Database (Oxford) 2014;2014:bau007. doi: 10.1093/database/bau007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross BX, Gao N, Cui X, Standiford TJ, Xu J, Yu FX. IL-24 Promotes pseudomonas aeruginosa keratitis in C57BL/6 mouse corneas. J Immunol. 2017;198:3536–3547. doi: 10.4049/jimmunol.1602087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao N, Yin J, Yoon GS, Mi QS, Yu FS. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am J Pathol. 2011;179:2243–2253. doi: 10.1016/j.ajpath.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsilingiri K, Fornasa G, Rescigno M. Thymic stromal lymphopoietin: to cut a long story short. Cell Mol Gastroenterol Hepatol. 2017;3:174–182. doi: 10.1016/j.jcmgh.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Yao J, Li B. Expression of TSLP and downstream molecules IL-4, IL-5, and IL-13 on the eye surface of patients with various types of allergic conjunctivitis. J Ophthalmol. 2016;2016:5072781. doi: 10.1155/2016/5072781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao N, Me R, Dai C, Seyoum B, Yu FX. Opposing Effects of IL-1Ra and IL-36Ra on innate immune response to Pseudomonas aeruginosa infection in C57BL/6 mouse corneas. J Immunol. 2018;201:688–699. doi: 10.4049/jimmunol.1800046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekanayaka SA, McClellan SA, Barrett RP, Kharotia S, Hazlett LD. Glycyrrhizin reduces HMGB1 and bacterial load in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2016;57:5799–5809. doi: 10.1167/iovs.16-20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Karmakar M, Roy S, et al. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West EE, Spolski R, Kazemian M, Yu ZX, Kemper C, Leonard WJ. A. TSLP-complement axis mediates neutrophil killing of methicillin-resistant Staphylococcus aureus. Sci Immunol. 2016;1:eaaf8471. doi: 10.1126/sciimmunol.aaf8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacomin PR, Moy RH, Noti M, et al. Epithelial-intrinsic IKKalpha expression regulates group 3 innate lymphoid cell responses and antibacterial immunity. J Exp Med. 2015;212:1513–1528. doi: 10.1084/jem.20141831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganti KP, Mukherji A, Surjit M, Li M, Chambon P. Similarities and differences in the transcriptional control of expression of the mouse TSLP gene in skin epidermis and intestinal epithelium. Proc Natl Acad Sci U S A. 2017;114:E951–E960. doi: 10.1073/pnas.1620697114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewas C, Chen X, Honda T, et al. TSLP expression: analysis with a ZsGreen TSLP reporter mouse. J Immunol. 2015;194:1372–1380. doi: 10.4049/jimmunol.1400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsby I, Goriely S. Regulation of interleukin-23 expression in health and disease. Adv Exp Med Biol. 2016;941:167–189. doi: 10.1007/978-94-024-0921-5_8. [DOI] [PubMed] [Google Scholar]
- 38.Volpe E, Pattarini L, Martinez-Cingolani C, et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J Allergy Clin Immunol. 2014;134:373–381. doi: 10.1016/j.jaci.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archer NK, Adappa ND, Palmer JN, et al. Interleukin-17A (IL-17A) and IL-17F are critical for antimicrobial peptide production and clearance of Staphylococcus aureus nasal colonization. Infect Immun. 2016;84:3575–3583. doi: 10.1128/IAI.00596-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J, Wang J, Wan J, et al. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect Immun. 2010;78:830–837. doi: 10.1128/IAI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massacand JC, Stettler RC, Meier R, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S.A. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 45.Zaidi TS, Zaidi T, Pier GB, Priebe GP. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect Immun. 2012;80:3706–3712. doi: 10.1128/IAI.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka S, Nagashima H, Cruz M, et al. Interleukin-17C in human Helicobacter pylori gastritis. Infect Immun. 2017;85:e00389-17. doi: 10.1128/IAI.00389-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Xing F. Human TSLP-educated DCs. Cell Mol Immunol. 2008;5:99–106. doi: 10.1038/cmi.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu YJ, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 49.Ito T, Liu YJ, Arima K. Cellular and molecular mechanisms of TSLP function in human allergic disorders--TSLP programs the “Th2 code” in dendritic cells. Allergol Int. 2012;61:35–43. doi: 10.2332/allergolint.11-RAI-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Autenrieth SE, Warnke P, Wabnitz GH, et al. Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis. PLoS Pathog. 2012;8:e1002552. doi: 10.1371/journal.ppat.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosendahl A, Bergmann S, Hammerschmidt S, Goldmann O, Medina E. Lung dendritic cells facilitate extrapulmonary bacterial dissemination during pneumococcal pneumonia. Front Cell Infect Microbiol. 2013;3:21. doi: 10.3389/fcimb.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z, Burns AR, Rumbaut RE. Smith CW. γδ T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am J Pathol. 2007;171:838–845. doi: 10.2353/ajpath.2007.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 54.Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol. 2011;178:1106–1116. doi: 10.1016/j.ajpath.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Burns AR, Miller SB, Smith CW. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.St Leger AJ, Desai JV, Drummond RA, et al. An ocular commensal protects against corneal infection by driving an interleukin-17 response from mucosal gammadelta T cells. Immunity. 2017;47:148–158.e5. doi: 10.1016/j.immuni.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suryawanshi A, Veiga-Parga T, Rajasagi NK, et al. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aden K, Rehman A, Falk-Paulsen M, et al. Epithelial IL-23R signaling licenses protective IL-22 responses in intestinal inflammation. Cell Reports. 2016;16:2208–2218. doi: 10.1016/j.celrep.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HS, Park DE, Lee JW, et al. IL-23 secreted by bronchial epithelial cells contributes to allergic sensitization in asthma model: role of IL-23 secreted by bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2017;312:L13–L21. doi: 10.1152/ajplung.00114.2016. [DOI] [PubMed] [Google Scholar]
- 60.Macho-Fernandez E, Koroleva EP, Spencer CM, et al. Lymphotoxin beta receptor signaling limits mucosal damage through driving IL-23 production by epithelial cells. Mucosal Immunol. 2015;8:403–413. doi: 10.1038/mi.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keijsers RR, Joosten I, van Erp PE, Koenen HJ, van de Kerkhof PC. Cellular sources of IL-17 in psoriasis: a paradigm shift? Exp Dermatol. 2014;23:799–803. doi: 10.1111/exd.12487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.