ABSTRACT
Target of rapamycin complex 1 (TORC1) is an evolutionarily conserved protein kinase complex, whose activation in response to nutrients suppresses autophagy. In mammalian cells, amino-acid stimuli induce lysosomal translocation and activation of MTORC1 through the RRAG GTPase heterodimer, which is tethered to the surface of lysosomes by the Ragulator complex. Our recent study demonstrated that the fission yeast Schizosaccharomyces pombe also has a Ragulator complex that anchors the Gtr1-Gtr2 Rag GTPase heterodimer to the vacuole, a lysosome-like organelle. Unexpectedly, however, neither vacuolar localization nor activation of TORC1 is dependent on the Rag-Ragulator complex, which instead plays a critical role in attenuating TORC1 signaling. Our findings suggest dual functionality of the Rag GTPase in both activation and inactivation of TORC1.
KEYWORDS: autophagy, GATOR1, proliferation, Rag GTPase, Ragulator, TOR, TORC1
Mechanistic target of rapamycin kinase complex 1 (MTORC1) is responsive to nutrients, growth factors, and environmental stress, and integrates such diverse extracellular signals to control multiple facets of cellular metabolism. MTORC1 promotes cellular anabolic processes, such as protein synthesis through phosphorylation of RPS6KB1/S6K1 (ribosomal protein S6 kinase B1) and EIF4EBP1/4E-BP1 (eukaryotic translation initiation factor 4E binding protein 1). Conversely, MTORC1 inhibits catabolic processes, including autophagic protein degradation through phosphorylation of ULK1 and ATG13, the essential components of the protein complex required for initiation of autophagy.
The signaling mechanisms that control MTORC1 activity in response to extracellular stimuli have been extensively researched in recent years. Growth factor signals regulate MTORC1 via the TSC1-TSC2 complex, which harbors GTPase-activating protein (GAP) activity towards the RHEB GTPase, an essential activator of MTORC1. In contrast, nutrients, in particular amino acids, induce MTORC1 activation through the RRAG GTPase heterodimer consisting of RRAGA or RRAGB bound to RRAGC or RRAGD. The RRAG heterodimer is tethered to the lysosomal surface by a pentameric protein complex called Ragulator, which is composed of LAMTOR1 to LAMTOR5. When RRAGA/B is in the GTP-bound form, the RRAG heterodimer can bind MTORC1, resulting in lysosomal recruitment of MTORC1 and its activation by RHEB (Figure 1A). In the absence of amino acids, the GATOR1 complex composed of DEPDC5, NPRL2 and NPRL3 functions as a GAP for RRAGA/B to hydrolyze its bound GTP to GDP, leading to the release of MTORC1 from lysosomes, and thus termination of its activation by RHEB (Figure 1B).
Figure 1.
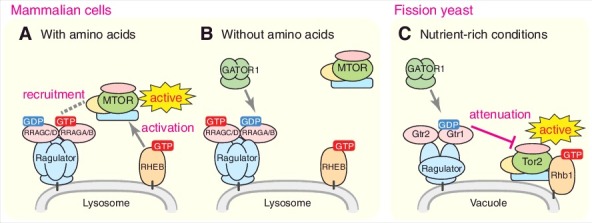
TORC1 regulation by the RRAG/Rag heterodimer in mammals and fission yeast. (A) In amino acid-stimulated mammalian cells, GATOR1 undergoes inhibition, yielding GTP-bound RRAGA/B within the RRAG heterodimer, which can then bind and recruit MTORC1 to the lysosomal membranes so that MTORC1 activation by the RHEB GTPase takes place. (B) Upon amino acid starvation, the GAP activity of the GATOR1 complex induces hydrolysis of GTP bound to RRAGA/B. As a consequence, the RRAG heterodimer releases MTORC1 to the cytosol, abrogating the MTORC1 activation by RHEB. (C) TORC1 attenuation by the Rag-Ragulator complex in fission yeast. Fission yeast TORC1 is targeted to vacuolar membranes and activated by the Rhb1 GTPase in a manner independent of the Gtr1-Gtr2 heterodimer and the Ragulator complex. Even under nutrient-rich conditions, the GAP activity of GATOR1 is required to generate GDP-bound Gtr1, which attenuates TORC1 activity to levels optimal for cell proliferation.
TORC1 is ubiquitously conserved among eukaryotes as a pivotal component of the nutrient-responsive signaling pathway, including in the fission yeast S. pombe. This unicellular model organism has 2 TOR kinases, one of which, Tor2, serves as the catalytic subunit of TORC1. The TSC protein complex is also conserved in this yeast species, acting as a GAP for Rhb1, a RHEB-ortholog essential for TORC1 activation. The Gtr1 and Gtr2 GTPases are S. pombe counterparts of RRAGA/B and RRAGC/D, respectively, and form a heterodimer that localizes on the surface of the vacuole, a lysosome equivalent in yeast. Through biochemical isolation of proteins that associate with the Gtr1-Gtr2 heterodimer, we identified a Ragulator-like complex composed of the Lam1 to Lam4 proteins. Despite the limited sequence similarity between the S. pombe Lam proteins and mammalian LAMTORs, the vacuolar Lam complex is essential for the localization of Gtr1-Gtr2 to the surface of vacuoles, indicating that the Lam complex is a Ragulator equivalent in fission yeast.
Consistent with the idea that the Rag GTPase heterodimer and Ragulator form a functional module, fission yeast strains lacking Gtr1, Gtr2 or the Lam proteins exhibit indistinguishable growth defects. Unexpectedly, however, a very similar phenotype is observed in a mutant strain where Gtr1 is constitutively in the GTP-bound form, whereas the GDP-locked mutant of Gtr1 allows normal proliferation. Therefore, the GDP-bound Gtr1 (Gtr1GDP), rather than its GTP-bound form (Gtr1GTP), is crucial for proliferation of fission yeast, showing a stark contrast with mammalian RRAGA/B whose GTP-bound form mediates MTORC1 activation and hence, cell growth (Figure 1A). We confirmed this unpredicted functionality of Gtr1GDP by identifying GATOR1 in fission yeast, a complex of Iml1, Npr2 and Npr3 with GAP activity toward Gtr1. Mutational inactivation of GATOR1 is expected to result in the loss of Gtr1GDP; indeed, the GATOR1-defective phenotype is very similar to that caused by the GTP-locked Gtr1 and complemented by the expression of GDP-locked Gtr1.
What is the function of Gtr1GDP that is important for growth of fission yeast? We propose that the Gtr1GDP-Gtr2 heterodimer attenuates TORC1 to prevent its cytotoxic hyperactivation (Figure 1C), based on the following observations: The TORC1 inhibitor rapamycin, as well as hypomorphic mutations of the Tor2 subunit of TORC1, can rescue the growth defects of the mutants lacking functional Gtr1-Gtr2, Ragulator or GATOR1. In those mutants, the TORC1-dependent phosphorylation of Sck1, an ortholog of Sch9 in budding yeast, is elevated, and the mutants also fail to promptly quench TORC1 activity upon starvation. Thus, without Gtr1GDP-Gtr2 anchored to vacuolar membranes by Ragulator, TORC1 appears to be hyperactivated. Congruously, fission yeast TORC1 is localized to vacuolar membranes and activated by the Rhb1 GTPase even in the absence of the Rag-Ragulator complex, which in mammals mediates the lysosomal activation of MTORC1 by RHEB (Figure 1A). We also observed that amino-acid transporters fail to localize to the plasma membrane in strains lacking Gtr1GDP-Gtr2 or Ragulator. This mutant phenotype is also indicative of TORC1 hyperactivation, as TORC1 signaling induces internalization of the plasma membrane amino-acid transporters in fission yeast.
Whereas the RRAG heterodimer has been recognized as part of an activation machinery for TORC1 in mammals and other organisms, our findings have shed light on its novel functionality in the negative regulation of TORC1. In the currently prevailing model, the RRAGA/BGDP-RRAGC/DGTP heterodimer is merely an inactive form incapable of MTORC1 recruitment to lysosomes (Figure 1B); therefore, it would be of great interest to delve into a possible role of this form of the RRAG heterodimer in attenuating MTORC1 signaling. Intriguingly, our genetic analysis in fission yeast suggests that the Rag-Ragulator complex can suppress TORC1 activity independently of the TSC-Rhb1 pathway. Future study of its molecular mechanism is expected to further our understanding of the TORC1 regulation in autophagy induction, cell proliferation, and longevity.
Funding Statement
This work was supported by JSPS KAKENHI [grant number 26840069 (TF)], [grant number 17K07330 (TF)] and [grant number 26291024 (KS)] and by Suzuken Memorial Foundation [grant number 14-099 (TF)].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.


