ABSTRACT
IFNG (interferon gamma)-induced autophagy plays an important role in the elimination of intracellular pathogens, such as Mycobacterium tuberculosis (Mtb). However, the signaling cascade that leads to the increase in autophagy flux in response to IFNG is poorly defined. Here, we demonstrate that HMOX1 (heme oxygenase 1)-generated carbon monoxide (CO) is required for the induction of autophagy and killing of Mtb residing in macrophages in response to immunomodulation by IFNG. Interestingly, IFNG exposure of macrophages induces an increase in intracellular calcium levels that is dependent on HMOX1 generated CO. Chelation of intracellular calcium inhibits IFNG-mediated autophagy and mycobacterial clearance from macrophages. Moreover, we show that IFNG-mediated increase in intracellular calcium leads to activation of the phosphatase calcineurin (PPP3), which dephosphorylates the TFEB (transcription factor EB) to induce autophagy. PPP3-mediated activation and nuclear translocation of TFEB are critical in IFNG-mediated mycobacterial trafficking and survival inside the infected macrophages. These findings establish that IFNG utilizes the PPP3-TFEB signaling axis for inducing autophagy and regulating mycobacterial growth. We believe this signaling axis could act as a therapeutic target for suppression of growth of intracellular pathogens.
KEYWORDS: autophagy, calcineurin, calcium signaling, carbon monoxide, heme oxygenase-1, interferon-gamma, TFEB, tuberculosis pathogenesis
Abbreviations
- ATG5
autophagy- related 5
- CFU
colony-forming units
- CO
carbon monoxide
- CORM II
tricarbonyldichlororuthenium (II) dimer
- CsA
cyclosporin A
- CEBPB
CCAAT/enhancer binding protein (C/EBP), beta
- DAPK1
death associated protein kinase 1
- DCFDA
2’,7’-dichlorofluorescin diacetate
- EIF2AK4/GCN2
eukaryotic translation initiation factor 2 alpha kinase 4
- HMOX1
heme oxygenase 1
- IFNG
interferon gamma
- IRGM
Immunity-related GTPase family M
- LAMP1
lysosomal-associated membrane protein 1
- MAPK
mitogen-activated protein kinase
- MDC
monodansylcadaverine
- MOI
multiplicity of infection
- Mtb
Mycobacterium tuberculosis
- MTOR
mechanistic target of rapamycin
- PPP3/calcineurin
protein phosphatase 3
- tfLC3
tandem fluorescent tagged LC3
- SnPP
Tin protoporphyrin IX
- SQSTM1
sequestosome 1
- TB
tuberculosis
- TFEB
transcription factor EB
- ZnPP
zinc protoporphyrin IX
Introduction
IFNG (interferon gamma) orchestrates innate and adaptive immune responses against intracellular pathogens. Exposure to IFNG affects several cellular processes in macrophages, such as pathogen surveillance, phagocytic activity, generation of reactive oxygen species and reactive nitrogen intermediates, processing and presentation of microbial antigens, microbicidal activity, immunomodulation, proliferation, and cell death [1,2]. IFNG plays a critical role in controlling growth of mycobacteria in macrophages and infected animals [3,4] however molecular events initiated by IFNG that ultimately lead to elimination of intracellular pathogens such as Mycobacterium tuberculosis (Mtb) remain unknown. Recent studies have demonstrated that IFNG induces macroautophagy (hereafter referred to as autophagy) to eliminate intracellular pathogens such as Mtb [5]. Autophagy is an evolutionarily conserved adaptive process that facilitates the regeneration of cellular resources through the selective degradation of damaged cellular components and organelles [6]. This process is also exploited by antigen-presenting cells such as macrophages for the trafficking of pathogens to lysosomes and thus their clearance [7,8]. In addition to promoting the elimination of pathogens through phagolysosome fusion, autophagy also enhances the presentation of processed antigens by MHC-II molecules to induce adaptive immunity [7,8].
The IFNG-induced signaling pathways that modulate the induction of ROS (reactive oxygen species) and RNI (reactive nitrogen intermediates) generation, class I and class II antigen presentation, antiproliferative effects and apoptotic cell death are well defined. However, the signaling axis that regulates IFNG-induced autophagy remains poorly understood. IFNG is believed to induce autophagy and eliminate intracellular pathogens via upregulation of immunity-related p47 guanosine triphosphatases (IRGs) [9,10]. In response to Mtb infection, IRGM moves to the mitochondria and induces depolarization that leads to autophagy [11]. Interestingly, the IRGM-like human ortholog of murine IRGs directly binds to the autophagy proteins ULK1 and BECN1/Beclin 1 to recruit the autophagy initiation complexes [12]. Importantly, a polymorphism in IRGM is associated with tuberculosis (TB) and leprosy [13,14], suggesting an important role of IRGM-mediated autophagy in these mycobacterial diseases. However, IRGM is not responsive to IFNG in human cells [10]. Nevertheless, IFNG induces autophagy in human monocytes and provides protection against TB [15], suggesting the presence of IRGM-independent IFNG-induced autophagy pathways. Furthermore, IFNG can induce autophagy in macrophages derived from irgm1−/− mice [16]. This induction of autophagy is dependent on the MAPK14/p38 alpha signaling pathway and plays an important role in the clearance of intracellular pathogens [17]. Additionally, a number of other mechanisms have been proposed to explain IFNG-induced autophagy, such as IFNG-induced depletion of intracellular arginine coupled to induction of EIF2AK4/GCN2 (eukaryotic translation initiation factor 2 alpha kinase 4) signaling pathway [18], induction of IRF1 (interferon regulatory factor 1) [19], and the induction of DAPK1 (death associated protein kinase 1) via the ATF6 (activating transcription factor 6)-CEBPB (CCAAT/enhancer binding protein [C/EBP], beta) signaling pathway [20]. The above literature suggests that although IFNG can induce autophagy through multiple pathways, a clear understanding of the primary pathway used by IFNG to induce autophagy remains to be established.
TFEB (transcription factor EB) has been described as a master regulator of lysosomal biogenesis and autophagy [21,22]. TFEB-mediated autophagy induction is regulated through its compartmentalization. The phosphorylated form localizes in the cytoplasm, and the dephosphorylated form translocate to the nucleus to induce lysosome biogenesis and autophagy. TFEB is phosphorylated by the mechanistic target of rapamycin complex 1 (MTORC1) [23–26] and MAPK1/ERK2 (mitogen-activated protein kinase 1) [22] at serine 211 and serine 142 residues, respectively. Importantly, in response to stress, the levels of cytoplasmic calcium are increased through MCOLN1 (mucolipin 1). This increase in cytoplasmic calcium activates the phosphatase PPP3/calcineurin a heterodimeric phosphatase, to dephosphorylate TFEB and thus induce autophagy [27].
HMOX (heme oxygenase) catalyzes the oxidative degradation of heme into carbon monoxide (CO), molecular iron (Fe2+) and biliverdin [28]. Two isomeric forms of HMOX are known. Hmox1 is an inducible form, whereas Hmox2 is constitutively expressed [29]. Recently, HMOX1 has been implicated in the modulation of autophagy [30–32]. In the proximal tubule cells, HMOX1 inhibits cisplatin-induced autophagy [30], whereas in primary mouse hepatocytes and macrophages [31,32], the induction of autophagy is dependent on HMOX1. These observations suggest that HMOX1 may induce or inhibit autophagy in a cell line-specific and signal-specific manner. Whether HMOX1 modulates autophagy in Mtb-infected macrophages in response to IFNG is not known. Steyn and coworkers have demonstrated that Mtb infection leads to the upregulation of HMOX1 transcript, protein and activity levels at the site of infection [33]. They have further demonstrated that HMOX1-generated CO leads to the induction of the Dos/dormancy regulon and thus mycobacterial persistence [33,34]. Furthermore, recent studies have established that HMOX1 protects the host against the Mtb infection in animals [35–37]. But, whether HMOX1 regulates IFNG-induced autophagy to control tuberculosis has not been analyzed. In the present study, we utilized pharmacological modulators of HMOX1, as well as knockout and knockdown of Hmox1 to study the role of HMOX1 and CO in the regulation of autophagy upon exposure to IFNG and its effect on mycobacterial survival in macrophages. We further studied the signaling axis that plays a critical role in IFNG-induced autophagy.
Results
HMOX1 is required for IFNG-induced autophagy
IFNG induces autophagy [5] and also leads to the induction of HMOX1 (Figure S1) [38]. Recent studies suggest that HMOX1 can either inhibit [30], or play an essential role in the induction of autophagy [31,32], Consequently, we hypothesized that HMOX1 modulates autophagic induction by IFNG. To test this hypothesis, RAW 264.7 macrophage cells were transiently transformed with a plasmid (ptfLC3) coding for the tandem mammalian red fluorescent protein (RFP)-enhanced green fluorescent protein (EGFP)- MAP1LC3B reporter (ptfLC3) [39]. This reporter system is considered a gold standard for measuring autophagy and monitors the rate of autophagic flux and the maturation of autophagosomes simultaneously [39]. When autophagosomes mature and fuse with lysosomes to form autolysosomes, EGFP degrades under the acidic environment of autolysosomes, whereas RFP is stable. Thus, the presence of yellow puncta indicates autophagosome formation (or autophagic flux), and the formation of red puncta depicts autophagosome maturation and formation of autolysosomes. RAW 264.7 macrophage cells were treated with IFNG for 3 h, and autophagy flux and maturation were analyzed. The level of autophagy was measured by enumerating the number of puncta/cell and the number of cells with > 5 autolysosomes/100 cells counted. Treatment with IFNG-induced autophagosome formation and maturation ( Figure 1A to C). Interestingly, abolishing the function of HMOX1 using the pharmacological inhibitor zinc protoporphyrin IX (ZnPP) led to reduction of formation and maturation of autophagosomes in response to IFNG (Figure 1A to C). In contrast, exogenous CO (one of the reaction products of HMOX1) produced by the CO donor tricarbonyl dichloro ruthenium (II) dimer (CORM II) restored the induction of autophagy in response to IFNG in the presence of the pharmacological inhibitor of HMOX1 (Figure 1A to C). In fact, exposure to exogenous CO facilitated higher levels of autophagy compared with IFNG alone. Importantly, the pharmacological concentrations of ZnPP and knockdown used in this study did not affect the viability of the macrophages. Staining the similar panel of cells with the commercially available reagent CYTO-ID Green substantiated these data (Figure S2). CYTO-ID Green specifically stains autophagic vacuoles, which are visible as green puncta-like structures within macrophages. Regulation of the formation of autophagic vacuoles by HMOX1-generated CO was further confirmed by staining with the lysosomotropic dye monodansylcadaverine (MDC) [40] (Figure S3A) and acridine orange (Figure S3B). These results were supported by western blot analysis of the lipidated and autophagosome-associated LC3B-II form of LC3B (Figure 1G) and suggest that HMOX1-generated CO is indispensable for IFNG-induced autophagy. The role of HMOX1 in induction of LC3 was also confirmed using the peritoneal macrophages isolated from hmox1 knockout mice and their littermates with the wild-type Hmox1 allele (Figure 1H) [41]. It was observed that IFNG exposure does not increase LC3B-II protein levels in the peritoneal macrophages harvested from hmox1−/− mice whereas in the macrophages isolated from the wild-type animals, IFNG increased LC3B-II levels.
Figure 1.
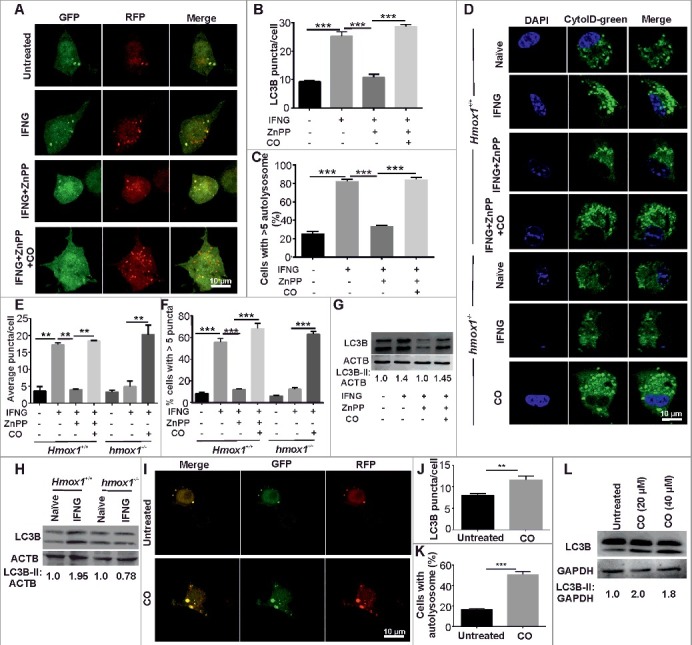
HMOX1 is required for IFNG-induced autophagy. (A) RAW 264.7 macrophages (0.5 × 106) were transiently transfected with the ptfLC3 plasmid and independently treated with ZnPP (5 μM) and ZnPP (5 μM) along with CO (20 μM) for 2 h followed by IFNG (200 units/ml) for 3 h. These cells were fixed with 4% PFA, and slides were prepared and viewed under a confocal microscope. Representative confocal microscopy images are shown. The LC3 puncta/cell (B) and the percentage of cells with >5 autolysosomes (C) were calculated. (D) Primary macrophages (peritoneal macrophages) were harvested from hmox1−/−and Hmox1+/+ mice and were treated with ZnPP (5 μM) and CO (20 μM) for 2 h followed by IFNG (200 units/ml) for 3 h. The cells were stained with CytoID green detection dye and fixed with 4% PFA; slides were prepared and observed under a confocal microscope. The CytoID-stained vacuoles were quantified by calculating the average number of puncta/cell (E) and the number of cells with more than 5 puncta/100 cells (F). (G) Western blot analysis of panel of cells prepared as described in panel (A). Numbers below lanes indicate the fold change calculated using the densitometric analysis of LC3B-II relative to the ACTB signal using ImageJ software (https://imagej.nih.gov/ij/). (H) Peritoneal macrophages were isolated from the Hmox1+/+ and hmox1−/− mice, exposed to IFNG for 3 h, the cells were lysed in RIPA buffer with protease inhibitor cocktail and the lysate was subjected to western blotting. The numbers below the blot are the densitometric analysis of LC3B-II relative to ACTB using ImageJ software. (I) RAW 264.7 macrophages (0.5 × 106) were transiently transfected with the ptfLC3 plasmid and treated with 20 µM CO for 2 h. After the treatment, cells were fixed using 4% PFA, slides were prepared and observed under the confocal microscope. The figure shows the representative images from 3 independent experiments. The LC3 puncta per cell (J) and the percentage cells with >5 autolysosomes (K) were calculated. (L) The same panel of cells were subjected to western blot analysis and the blot was probed with LC3 and GAPDH antibody. Numbers below lanes indicate the fold change calculated using the densitometry analysis of LC3B-II relative to GAPDH. Data in panels B, C, E, F, J, and K represent the mean±SEM from 3 independent experiments performed in triplicate. Statistical significance was determined using the Student t test. ** indicates a P value < 0.01, *** indicates a P value < 0.001.
These results were confirmed using primary macrophages (peritoneal macrophages) harvested from Hmox1+/+ and hmox1−/− littermates. The primary macrophage cells were treated with IFNG, and the level of autophagy was measured by CYTO-ID staining. Interestingly, in macrophages derived from the hmox1−/− mice, IFNG was unable to induce autophagy. The defect in autophagy induction could be fully reversed with exogenous CO (Figure 1D to F). On the contrary, IFNG-induced autophagy in peritoneal macrophages derived from Hmox1+/+ mice, the inhibition of HMOX1 using pharmacological inhibitors resulted in the reduced levels of autophagy and could be restored by supplementation with exogenous CO (Figure 1D to F). These findings were further confirmed in macrophage cells of human origin. Toward this, PMA-activated THP-1 macrophages were transfected with the GFP-LC3 plasmid using Lipofectamine 3000. We again observed that abrogation of HMOX1 function with ZnPP was sufficient to inhibit the increase in autophagy flux in response to IFNG (Figure S4). This defect could be reversed by exogenous CO (Figure S4).
The above results suggest that the induction of autophagy in response to IFNG depends on the CO generated by the induced levels of HMOX1 enzyme. Thus, we hypothesized that exogenous CO can induce autophagy in the absence of IFNG. To test this hypothesis, RAW 264.7 macrophages were exposed to exogenous CO and autophagy flux was analyzed using the tRFP-GFP-LC3 construct. Importantly, we observed that exogenous CO was capable of inducing the autophagic flux (Figure 1I to K). These results were confirmed using western blot analysis for LC3 (Figure 1L).
The survival of Mtb inside macrophages is dependent on its ability to arrest autophagosome maturation [42,43]. The autophagy inducer IFNG averts this arrest of autophagosome maturation in Mtb-infected macrophages. The above-described experiments clearly demonstrate that HMOX1-derived CO is required for the induction and autophagosome maturation, and thus the next logical step was to analyze whether HMOX1 or its reaction product CO was required for autophagy induction in Mtb-infected macrophages in response to IFNG. To test this hypothesis, RAW 264.7 macrophages were transiently transfected with the ptfLC3 plasmid and infected with Mtb H37Rv (1:5 multiplicity of infection [MOI]) for 3 h using previously established methods [44], followed by the treatments described earlier. IFNG treatment of Mtb-infected cells led to autophagosome formation and maturation, which was inhibited by ZnPP; this inhibition was further abrogated by the application of exogenous CO (Figure S5). The same panel of cells were stained with CYTO-ID that specifically stains the autophagic vacuoles (Figure S6). The observations made using RAW 264.7 macrophages were then confirmed in the primary peritoneal macrophage cells harvested from hmox1−/− and Hmox1+/+ mice using the CYTO-ID staining (Figure S7).
HMOX1 regulates IFNG-induced trafficking of Mtb to autolysosomes
Further experiments were performed to assess the effect of HMOX1-generated CO on the maturation of autophagosomes (containing Mtb) subsequent to macrophage activation by IFNG. Mtb overexpressing GFP (GFP-Mtb H37Rv) was used in these experiments to visualize the trafficking of Mtb, and LysoTracker Red was used to monitor the acidic lysosomes. IFNG could overcome the mycobacterial blockade of autophagosome lysosome fusion induced by Mtb (Figure 2A and B). A 3-fold decrease in autophagosome-lysosome fusion was observed when cells were treated with the HMOX1 inhibitor (ZnPP), which suggested that the HMOX1 is required for the maturation of autophagosomes carrying Mtb (Figure 2A and B). This inhibition of autophagy by ZnPP was reversed upon exposure to exogenous CO released by CORM-II (Figure 2A and B). These findings were also confirmed with low MOI infection of Mtb (1:1) in RAW 264.7 macrophages (Figure S8). To further confirm that HMOX1 and its reaction product CO play a critical role in IFNG-induced trafficking of Mtb cells to autolysosomes, we infected peritoneal macrophages isolated from wild-type (with Hmox1 allele) or hmox1−/− littermates. Although macrophages derived from the wild-type mice behaved similarly to RAW 264.7 macrophages, the primary macrophages isolated from hmox1−/− mice were significantly attenuated in their ability to traffic Mtb cells to autolysosomes in response to IFNG (Figure 2C and D). This attenuation was reversed by pretreatment of these macrophages with exogenous CO. To further confirm the role of HMOX1-generated CO in trafficking Mtb to the autolysosomes in infected macrophages, we exposed RAW 264.7 macrophages with exogenous CO using CORM II in absence of IFNG and then infected them with the Mtb. We observed CO exposure alone in the absence of IFNG leads to significantly more localization of Mtb in the autolysosomes compared to untreated cells (Figure 2E and F).
Figure 2.
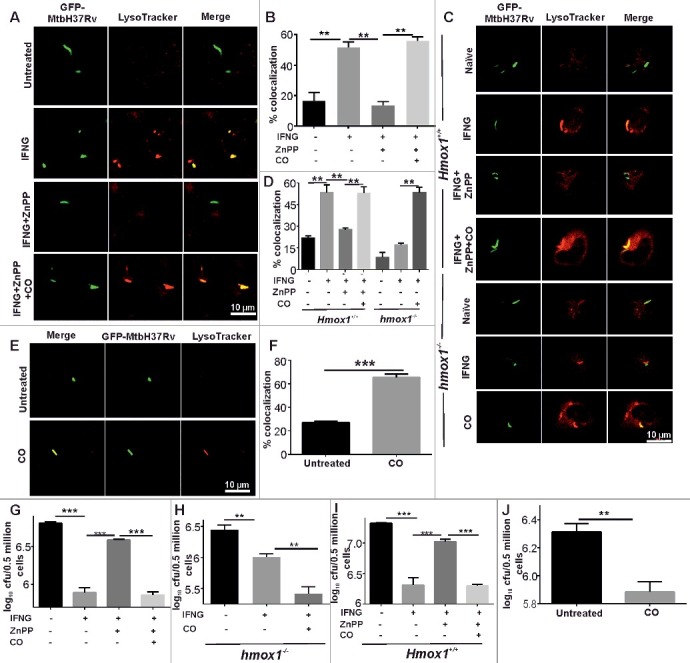
IFNG-induced trafficking of Mtb to autolysosomes is regulated by HMOX1. (A) RAW 264.7 macrophages (0.5 × 106) were infected with GFP-Mtb H37Rv (1:5 MOI) for 3 h, followed by treatments with ZnPP (5 μM) and ZnPP (5 μM) along with CO (20 μM) for 2 h and then IFNG (200 units/ml) for 3 h. The lysosomes were stained with 200 nM LysoTracker Red for 20 min, fixed using 4% PFA and analyzed using confocal microscopy. The figure shows representative images of the colocalization of Mtb (green channel) and LysoTracker Red (red channel). (B) The percentage of Mtb-containing autophagosomes colocalized with lysosomes stained with LysoTracker Red. (C) Primary macrophages (peritoneal macrophages) were harvested from Hmox1+/+ and hmox1−/- mice and were infected with GFP-Mtb H37Rv and treated as described above, followed by analysis using confocal microscope. (D) The percentage of Mtb-containing autophagosomes colocalized with LysoTracker Red (per 100 cells). (E) The same experiment was repeated with CO (20 μM) alone. Briefly, RAW 264.7 macrophages (0.5 × 106) were infected with GFP-Mtb H37Rv (1:5 MOI) for 3 h followed by CO treatment, staining with LysoTracker Red and analyzed using a confocal microscope. (F) The percentage of Mtb-containing autophagosomes colocalized with lysosomes stained with LysoTracker Red (per 100 Mtb cells). (G) RAW 264.7 macrophages were infected with Mtb (1:10 MOI) for 3 h, followed by gentamycin (100 μg/ml) treatment for 45 min. The cells were treated with ZnPP (5 μM), exogenous CO (20 μM) and IFNG when required. 24 h postinfection, the cells were lysed in 0.06% SDS after 24 h and plated on 7H11 plates. (H and I) Peritoneal macrophages isolated from hmox1−/− and Hmox1+/+ mice were treated as described above and infected with Mtb and the CFU were estimated after 24 h of infection. (J) Similarly RAW 264.7 were infected and treated with CO as mentioned earlier. 24 h postinfection, CFUs were enumerated by plating the lysate on 7H11 plates. Data in panels B, D, F, G, H, I and J represent the mean±SEM from 3 independent experiments. Statistical significance was determined using the Student t test where ** indicates a P value < 0.01, and *** indicates a P value < 0.001.
HMOX1 regulates the antimycobacterial activity of IFNG-treated macrophages via autophagy
The data from the above-mentioned studies strongly suggest that HMOX1-generated CO dictates the autophagic response of IFNG, and thus its role in the IFNG-mediated antimycobacterial activity of macrophages was examined. RAW 264.7 macrophages were infected with Mtb and then activated with IFNG in the presence of the HMOX1 inhibitor or HMOX1 inhibitor together with the exogenous CO donor CORM II. Colony-forming units (CFU) counts were determined at 24 h postinfection. An increase in bacterial viability was observed in ZnPP-treated cells compared with IFNG-treated cells (Figure 2G). Interestingly, the viability of intracellular Mtb decreased significantly upon the addition of CORM II to ZnPP-treated cells (Figure 2G), emphasizing the role of HMOX1-generated CO in the prevention of mycobacterial replication within macrophages via the upregulation of autophagy. These findings were validated using another HMOX1 inhibitor SnPP IX (Figure S9). In these experiments, the role of HMOX1 in Mtb trafficking and survival was determined. These findings were further confirmed in peritoneal macrophages harvested from hmox1−/− and Hmox1+/+ mice (Figure 2H and I). Interestingly, macrophages harvested from the hmox1−/− mice displayed a compromised ability to kill intracellular Mtb in response to IFNG (Figure 2H). However, following the treatment with exogenous CO, these cells acquired significantly increased antimycobacterial activity (Figure 2H), although previous studies have demonstrated that CO itself does not possess any antimycobacterial activity [45]. Conversely, macrophages derived from Hmox1+/+ mice displayed significant antimycobacterial activity that was dependent on HMOX1-generated CO (Figure 2I). We also analyzed if CO alone without immunomodulation with IFNG can induce mycobacterial killing inside the macrophage cells. Importantly, we also observed that treatment of RAW 264.7 macrophages with exogenous CO generated by CORM II results in decreased mycobacterial survival (Figure 2J). Interestingly, exogenous CO facilitates the killing of Mtb cells residing in the macrophages but does not either kill or inhibit Mtb growth in the synthetic medium (our own observation and reference [45]).
The HMOX1-dependent Mtb trafficking and regulation of growth of intracellular Mtb could result from modulation of multiple signaling pathways. To confirm that these effects are primarily due to modulation of autophagy, we performed experiments wherein the Hmox1 was knocked down along with Atg5 in macrophage cells and the effect of the double knockdown on mycobacterial trafficking and mycobacterial clearance was analyzed. The knockdown of Hmox1 and Atg5 was confirmed with western blotting analysis for HMOX1 and ATG5 (Figure S10). In support of our earlier observations (Figure 2), we noticed that knockdown of Hmox1 resulted in decrease in the Mtb colocalization with lysosomes (Figure 3A and B) and increased Mtb survival (Figure 3C) in IFNG treated RAW 264.7 macrophages. As expected, we also observed that Atg5 knockdown also resulted in decreased trafficking of Mtb to lysosomes (Figure 3A and B) and an increase in the survival of intracellular Mtb cells (Figure 3C). Importantly, we observed that the mycobacterial trafficking to lysosomes (Figure 3A and B) and clearance (Figure 3C) were similar in the Hmox1 knockdown and double knockdown of Hmox1 and Atg5 suggesting that the HMOX1 is upstream of the autophagy pathway and its effects on Mtb trafficking and survival are primarily exerted through modulation of autophagy.
Figure 3.

Trafficking and clearance of Mtb in Hmox1 and Atg5 double-knockdown RAW 264.7 macrophages (A) 0.3 × 106 RAW 264.7 macrophages were transfected with siRNA specific for Hmox1 (50 nM) and Hmox1 along with Atg5 (50 nM) using DharmaFECT. Scrambled siRNA was used as a control. After 30 h, the cells were infected with GFP-Mtb H37Rv (1:5 MOI) for 3 h followed by IFNG treatment for 3 h. The lysosomes were stained with 300 nM LysoTracker Red for 20 min, fixed using 4% PFA and analyzed using confocal microscopy. The figure shows representative images of the colocalization of Mtb (green channel) and LysoTracker Red (red channel). (B) The percentage of Mtb-containing autophagosomes colocalized with lysosomes stained with LysoTracker Red (per 100 Mtb cells). (C) The hMOX1 knockdown and HMOX1 along with Atg5 double-knockdown macrophages were infected with Mtb (1:10 MOI) for 3 h, followed by gentamycin (100 μg/ml) treatment for 45 min. The cells were treated with IFNG for 3 h and the CFU plating was done 24 h postinfection. Briefly, the cells were lysed in 0.06% SDS after 24 h, dilutions were prepared and plated on 7H11 plates. Data in panels B and C represent the mean±SEM from 3 independent experiments. Statistical significance was determined using the Student t test where * indicates a P value <0.05, ** indicates a P value < 0.01, and *** indicates a P value < 0.001.
HMOX1 regulates autophagy in Mtb-infected animals
Because HMOX1 regulates the antimycobacterial activity of IFNG in macrophages via autophagy, we next assessed whether HMOX1 could regulate autophagy in animals to modulate the pathogenesis of TB. To achieve this goal, hmox1−/−, Hmox1−/+ and Hmox1+/+ mice (littermates) were infected with Mtb H37Rv via aerosol challenge. After 3 wk of infection, the left lung of the infected mice was removed and used for histological analysis. Granulomas were evaluated in 5-µm-thick paraffin-embedded sections, followed by haematoxylin and eosin (HandE) staining. The histological analysis revealed that hmox1−/− mice had a significantly greater number of granulomas compared with Hmox1−/+ and Hmox1+/+ mice (Figure 4A and B). These results correlate with the better survival of Mtb in hmox1−/− mice compared with Hmox1+/+ mice [36,46] (Figure S11 and 12). The histopathology data were scored on a scale from 0 (absent) to 3 (severe), and the differences in tissue pathology were statistically significant among hmox1−/−, Hmox1−/+ and Hmox1+/+ (Figure 4B). Furthermore, the paraffin-embedded sections were deparaffinised, and immunostaining was performed to visualize the expression of the autophagy markers, LC3 and BECN1. The tissue sections from Hmox1+/+ mice exhibited elevated level of endogenous LC3 compared with the tissue sections from hmox1−/− mice (Figure 4C and D). We also observed higher levels of autophagy marker BECN1 in Hmox1+/+ mice compared to the hmox1−/− mice (Figure 4E and F). These data suggest that HMOX1 regulates autophagy in vivo. Autophagy plays an important role in immune responses to infection and in regulating inflammation by modulating the levels of proinflammatory and antiinflammatory cytokines; therefore, we used the lung lysates from Mtb-infected mice for cytokine analysis. The differences in most of the analyzed cytokines were not significant, but the levels of the proinflammatory cytokine, IL1A, were elevated in wild-type mice lung lysates (Figure S13A to I). Additionally, significant increases in the levels of IL10 and the IL12A-IL12B/IL-12p70 heterodimer were observed in the lung lysates from hmox1 knockout mice (Figure S13A to I). Importantly, IL12A-IL12B is secreted at increased level in the absence of autophagy in response to Mtb infection [47], and IL10 inhibits the production of IL1, IL6, and TNF through the inhibition of inflammation [48,49].
Figure 4.
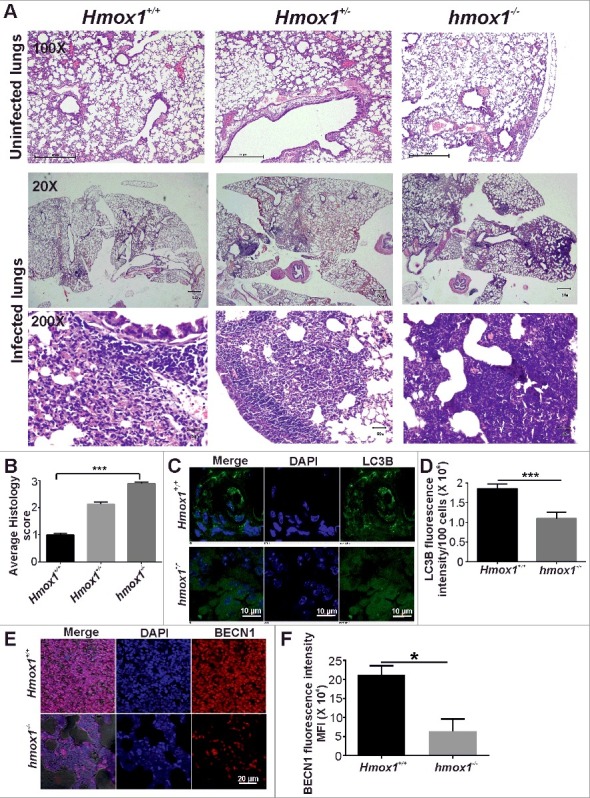
HMOX1 regulates autophagy in Mtb-infected animals. (A) Hmox1+/+, hmox1−/+ and hmox1−/− mice were infected (via aerosol challenge) with Mtb H37Rv to acquire an initial deposition of 100 to 200 CFU in mice lungs. After 4 wk of infection, the left lung was fixed in 10% formalin, and 5-μm-thick paraffin-embedded sections were obtained and stained with HandE. The hmox1−/− mice exhibited more lesions compared with the Hmox1+/+ mice. The figure represents the lower (20X) and higher (200X) magnification image of the lungs. The first panel represents the HandE staining of the uninfected lung sections at 100X magnification. (B) The histopathology was scored on a scale from 0 (zero lesions) to 3 (more than 15 lesions), revealing a significant difference between Hmox1+/+ and hmox1−/− mice (n = 3). The paraffin-embedded sections were deparaffinised and used for immunostaining to detect endogenous levels of the autophagy marker LC3 (C) and BECN1 (E). Anti-LC3B (1:500) (C), anti-BECN1 (1:500) (E) primary antibody and Alexa Fluor 488-labeled anti-rabbit (1:1500) and Alexa Fluor 561-labeled anti-rabbit (1:1500) secondary antibodies were used, respectively. The nuclei were stained with DAPI (1 μg/ml). The slides were observed under a confocal microscope, and the mean fluorescence intensity was quantified using ImageJ software (D, F). Data in panels D and F represent the mean±SEM from 3 independent experiments. Statistical significance was determined using the Student t test. * indicates a P value < 0.05 and *** indicates a P value < 0.001.
HMOX1-generated CO modulates intracellular calcium levels in response to IFNG
Recent studies have demonstrated that intracellular levels of calcium (Ca2+) modulate autophagy [27,50,51]. Consequently, we hypothesized that IFNG treatment could increase cytoplasmic Ca2+ levels to modulate autophagy. To test this hypothesis, we used Fluo-3 AM to examine the levels of cytoplasmic Ca2+ levels of RAW 264.7 macrophage in response to IFNG treatment. Interestingly, IFNG caused an increase in cytoplasmic Ca2+ levels (Figure 5A). These findings were in agreement with the observation that exposure to IFNG increases intracellular Ca2+ levels in primary human thyroid cells [52]. This increase was dependent on the function of HMOX1 because ZnPP could abrogate this response. However, exogenous CO was sufficient to substitute for functional HMOX1 to regulate intracellular Ca2+ levels (Figure 5A). To verify the role of HMOX1 in the IFNG-mediated increase of the intracellular Ca2+ levels, we isolated peritoneal macrophages from Hmox1+/+and hmox1−/− mice. These macrophages were exposed to IFNG for 3 h and the cells were stained with Fluo-3 AM for analysis of intracellular Ca2+ levels. We observed that in the macrophages isolated from wild-type mice strains, IFNG exposure resulted in a statistically significant increase in the intracellular Ca2+ levels (Figure 5B). However, in the macrophages isolated from the hmox1−/− mice, IFNG exposure does not increase the intracellular Ca2+ levels (Figure 5B). Therefore the molecular mechanism underlying the regulation of cytoplasmic Ca2+ levels by HMOX1 remains unknown.
Figure 5.

HMOX1-generated CO is required for increased intracellular calcium levels in response to IFNG. (A) RAW 264.7 macrophages (0.5 × 106) were independently treated with ZnPP (5 μM) and ZnPP (5 μM) along with CO (20 μM) for 2 h, followed by IFNG (200 units/ml) for 3 h. These cells were stained with 2 µM Fluo-3 AM for 1 h and analysis by flow cytometry. The mean fluorescence intensity plots were prepared using GraphPad Prism. (B) Peritoneal macrophages were isolated from the HMOX1+/+ and hmox1−/− mice, exposed to IFNG for 3 h and the cells were stained with FLU-3AM. The mean fluorescence intensity was plotted. Data represent the mean±SEM from 3 independent experiments. Statistical significance was determined using the Student t test. (C) RAW 264.7 macrophages overexpressing tfLC3 were activated with IFNG in the presence or absence of the Ca2+ chelator BAPTA-AM and viewed under a confocal microscope. Representative confocal microscopy images are shown. The LC3 puncta per cell (D) and the percentage cells with > 5 autolysosomes (E) were calculated. (F) The same panel of cells as in (C) without transfection were subjected to western blotting. Densitometry analysis of LC3-II relative to ACTB in terms of fold change is shown below the blot. (G) 0.5 × 106 RAW 264.7 macrophages were transfected with GFP-LC3 plasmid using Lipofectamine 3000. Calcium-free DMEM-media was added to one panel of cells, 6 h prior to IFNG treatment. After 6 h, the cells were treated with IFNG, followed by fixation with 4% PFA and observed under the confocal microscope. Images are representative of 3 biological experiments. (H) The graph shows the average number of puncta/cell per 100 cells counted. Data in panel H represent the mean±SEM from 3 independent experiments. (I) Intracellular Ca2+ levels regulate the trafficking of intracellular Mtb. RAW 264.7 macrophages (0.5 × 106) were infected with GFP-Mtb H37Rv (1:5 MOI) for 3 h, followed by treatments with IFNG and BAPTA-AM, stained with LysoTracker Red and observed using a confocal microscope. The figure shows a representative image of the colocalization of Mtb (green channel) and LysoTracker Red (red channel). (J) Percentage of Mtb-containing autophagosomes colocalizing with lysosomes stained with LysoTracker Red (per 100 Mtb cells). (K) The same panel of cells was lysed in 0.06% SDS after 24 h and plated on 7H11 plates for calculating CFUs. Data in panels A, B, D, E, H, J and K represent the mean±SEM from 3 independent experiments. Statistical significance was determined using the Student t test. * indicates a P value < 0.05, ** indicates a P value < 0.01, and *** indicates a P value < 0.001.
Increased intracellular Ca2+ is required for IFNG-induced autophagy, mycobacterial trafficking and growth regulation
We next assessed whether the IFNG-mediated increase in cytoplasmic Ca2+ levels played a critical role in the modulation of autophagy. To achieve this goal, we activated RAW 264.7 macrophages overexpressing tfLC3 with IFNG in the presence or absence of the Ca2+ chelator BAPTA-AM. The flux of IFNG-induced autophagy was significantly reduced in RAW 264.7 macrophages that were pretreated with BAPTA-AM, compared with the untreated cells (Figure 5C to E). These findings were confirmed using RAW 264.7 macrophages overexpressing EGFP-LC3B (MAP1LC3B) fusion proteins (Figure S14) and MDC staining (Figure S15). Western blot analysis also suggested that the increased levels of LC3B-II in response to IFNG were reduced in cells pretreated with BAPTA-AM (Figure 5F). The increase in the intracellular Ca2+ levels could result from increase in uptake of Ca2+ from the media or from the release of Ca2+ from intracellular stores such as sarcoplasmic reticulum [53]. To ascertain the source of Ca2+, we cultured the RAW 264.7 macrophages transfected with GFP-LC3 plasmid in Ca2+-free media or Ca2+-replete media and exposed the cells to IFNG. Analysis of GFP-LC3 puncta formation suggested that IFNG exposure in the macrophages grown in Ca2+-free media induced autophagy but the levels of increase in the autophagy flux was significantly lower than observed in macrophages cultured in Ca2+-replete media (Figure 5G and H). These data suggest that the influx of extracellular calcium plays an important role in IFNG mediated autophagy. However it must be noted that even in absence of extracellular calcium IFNG was able to induce autophagy, suggesting that release from intracellular stores also contributes to IFNG induced autophagy. The identity of the internal stores that contribute to induction of autophagy in response to IFNG remains to be established and is beyond the scope of this study.
We next analyzed whether the IFNG-mediated increase in cytoplasmic Ca2+ levels could regulate the trafficking of intracellular Mtb. To achieve this goal, we infected RAW 264.7 macrophages with GFP-overexpressing Mtb and analyzed its trafficking. LysoTracker Red staining was used to visualize the lysosomes. Significantly fewer mycobacterial cells colocalized with the lysosomes in BAPTA-AM-treated cells (Figure 5I and J), supporting a critical role for the IFNG-mediated increase in cytoplasmic Ca2+ levels during the maturation of Mtb-containing autophagosomes into phagolysosomes. We also analyzed the effect of Ca2+ chelation on the control of the growth of intracellular Mtb. IFNG-activated RAW 264.7 macrophages exposed to BAPTA-AM exhibited an attenuated control of intracellular Mtb replication (Figure 5K).
IFNG induces lysosomal biogenesis by modulating intracellular Ca2+ levels
IFNG is known to induce autophagy to eliminate intracellular pathogens [5] however, whether it also modulates lysosomal biogenesis is not known. To assess whether IFNG induces lysosomal biogenesis, we exposed RAW 264.7 macrophage cells to IFNG and analyzed the levels of LAMP1 (lysosomal-associated membrane protein 1) by confocal microscopy. In response to IFNG, increased levels of LAMP1 were detected in RAW 264.7 macrophages, which suggested that IFNG induces lysosomal biogenesis in murine macrophages (Figure 6A and B). This result was further confirmed by western blot analysis of genes that are upregulated upon TFEB overexpression namely, SQSTM1/p62, LAMP1, BECN1 and ATG5 (Figure 6C). These findings were validated using peritoneal macrophages from wild-type and hmox1 knockout mice (Figure 6D). It was observed that peritoneal macrophages isolated from the hmox1−/− mice were severely compromised for induction of lysosomal biogenesis in response to IFNG (Figure 6E and F). These findings were confirmed using pharmacological inhibition of HMOX1 and exogenous CO (Figure S16). We further analyzed whether lysosomal biogenesis was dependent on the IFNG-mediated increase in intracellular Ca2+ levels. To achieve this goal, RAW 264.7 macrophages were activated with IFNG in the presence of BAPTA-AM. Pretreatment of cells with the Ca2+ chelator inhibited the increase in LAMP1-positive puncta formation in response to IFNG (Figure 6G and H).
Figure 6.
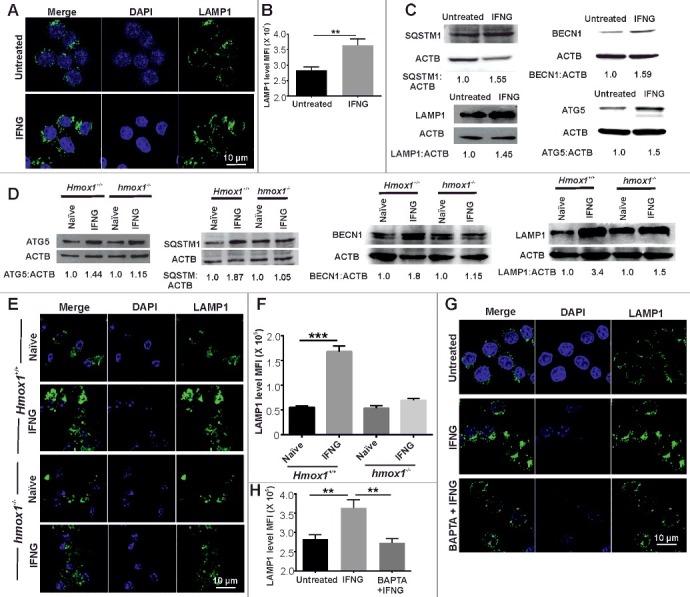
IFNG induces lysosomal biogenesis by modulating intracellular Ca2+ levels. (A) RAW 264.7 macrophages were exposed to IFNG, and the levels of LAMP1 (lysosomal-associated membrane protein 1) were analyzed using confocal microscopy. (B) The difference in fluorescence intensity was determined using ImageJ software. (C) The same panel of cells depicted in panel (A) were subjected to western blotting and probed with the antibodies which has upregulated gene expression with respect to TFEB namely, SQSTM1, BECN1, LAMP1 and ATG5. ACTB was used as an internal loading control in these experiments. Numbers below the lanes indicate the fold change calculated using the densitometry analysis of LC3-II: ACTB. (D) Peritoneal macrophages were isolated from the Hmox1+/+ and hmox1−/− mice, exposed to IFNG for 3 h and the lysate was prepared and was subjected to western blotting using antibodies for ATG5, SQSTM1, BECN1 and LAMP1. ACTB was used as an internal loading control in these experiments. Numbers below lanes indicate the fold change calculated using the densitometry analysis of LC3-II relative to ACTB. (E) Peritoneal macrophages were isolated from the Hmox1+/+ and hmox1−/− mice, exposed to IFNG, and the levels of LAMP1 were analyzed using confocal microscopy. (F) The difference in fluorescence intensity was determined using ImageJ software. (G) RAW 264.7 macrophages were activated with IFNG in the presence of BAPTA-AM, and the levels of LAMP1 were assessed by confocal microscopy to evaluate the dependence of lysosomal biogenesis on the IFNG-mediated increase in intracellular Ca2+ levels. (H) The difference in fluorescence intensity was determined using ImageJ software. Data in panels B, F and H represent the mean±SEM from 3 independent experiments. Statistical significance was determined using the Student t test. ** indicates a P value < 0.01, and *** indicates a P value < 0.001.
The IFNG-HMOX1-Ca2+ signaling axis regulates the expression and nuclear translocation of TFEB
The experiments described above demonstrated that IFNG induces autophagy and lysosomal biogenesis in and HMOX1-dependent manner. Because TFEB is the master regulator of lysosomal biogenesis and autophagy, we next analyzed whether IFNG could modulate TFEB expression or its function. Interestingly, western blot analysis revealed that the level of TFEB was significantly increased in IFNG-treated RAW 264.7 macrophages (Figure 7A). Because the ability of TFEB to induce lysosomal biogenesis is dependent on its nuclear localization, we also analyzed the cellular localization of TFEB in peritoneal macrophages from Hmox1+/+ and hmox1−/− mice following exposure to IFNG using confocal microscopy. Interestingly, confocal microscopy of endogenous TFEB revealed that, in peritoneal macrophages from Hmox1+/+ mice, TFEB primarily localized in the cytoplasm. However, following activation with IFNG, TFEB translocates into the nucleus (Figure 7B and C). Importantly, in IFNG activated peritoneal macrophages from hmox1−/− mice, TFEB did not migrated to the nucleus (Figure 7B and C). A similar effect on the translocation of TFEB was observed with the pharmacological inhibition of HMOX1 in RAW 264.7 macrophages (Figure 7D and E). Inhibition of the enzymatic activity of HMOX1 could be reversed using exogenous CO, suggesting that HMOX1-generated CO plays a critical role in IFNG-induced nuclear localization of TFEB. These findings were substantiated by western blot analysis of endogenous TFEB in whole cell lysates (Figure 7A) and the cytoplasmic and nuclear fractions from naïve and IFNG-activated RAW 264.7 macrophages (Figure 7F). Previously described experiments have suggested that IFNG leads to an increase in cytoplasmic Ca2+ levels, which facilitates autophagy and lysosomal biogenesis. Thus, we assessed whether Ca2+ chelation affected the nuclear localization of TFEB. RAW 264.7 macrophages were activated with IFNG in the presence of BAPTA-AM. We observed that Ca2+ chelation with BAPTA-AM abrogated the ability of IFNG to induce the nuclear localization of TFEB (Figure 7G and H), suggesting that cytoplasmic Ca2+ levels play an important role in IFNG-induced translocation of TFEB into the nucleus.
Figure 7.
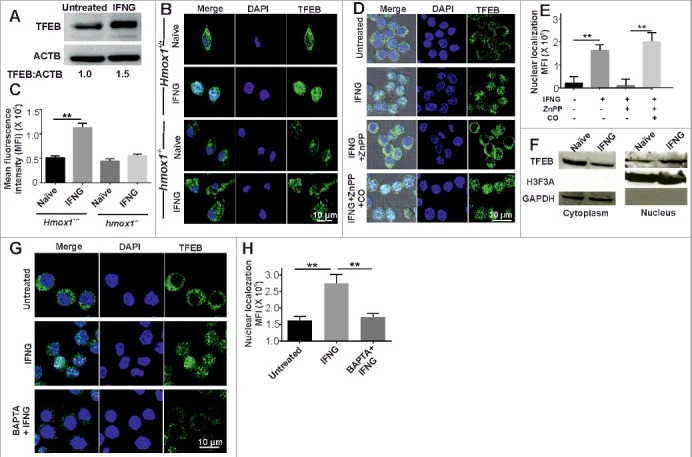
The IFNG-HMOX1-Ca2+ signaling axis regulates the expression and nuclear translocation of TFEB. (A) RAW 264.7 macrophages were exposed to IFNG and then subjected to western blotting analysis of TFEB. Densitometric analysis in terms of the fold change of LC3-II relative to ACTB is shown below the blot. (B) Peritoneal macrophages were isolated from the Hmox1+/+ and hmox1−/− mice, exposed to IFNG, and the endogenous TFEB was stained using antibody and observed under the confocal microscope. (C) The percentage of nuclear localization was determined using ImageJ software. (D) RAW 264.7 macrophages were treated with ZnPP (5 μM), exogenous CO (20 μM) and IFNG, as described previously, and endogenous TFEB was stained and observed under a confocal microscope. (E) The percentage of nuclear localization was determined using ImageJ software. (F) IFNG-activated macrophages were fractionated to isolate the nuclear and cytoplasmic fraction, and the samples were subjected to western blotting. The blots were probed with TFEB, histone H3 and GAPDH antibodies. (G) RAW 264.7 macrophages were activated with IFNG in the presence of BAPTA-AM, and the localization of endogenous TFEB was studied with the help of confocal microscopy. The percentage of nuclear localization was determined using ImageJ software (H). Data in panel C, E and H represent the mean±SEM from 3 independent experiments. Statistical significance was determined using the Student t test where ** indicates a P value < 0.01.
Earlier studies have suggested that TFEB is phosphorylated by MTOR at serine 211. This phosphorylation is required for preventing the translocation of TFEB into nucleus and thus prevents induction of autophagy. It is plausible that IFNG could inhibit MTORC1 activity and that could result in nuclear localization of TFEB into the nucleus. To analyze if the activity of MTORC1 is inhibited in response to IFNG exposure of macrophage cells, we analyzed the levels of phosphorylation of ribosomal protein RPS6KB/p70S6 kinase. RPS6KB is a substrate of MTOR and its phosphorylation levels acts as readout of the MTORC1 activity. We observed that the level of phosphorylation of RPS6KB remains unchanged in response to IFNG exposure (Figure S17A), suggesting that IFNG does not utilize the MTOR pathway for induction of autophagy.
Another IFNG-responsive regulator of autophagy is IRGM1, thus, we analyzed whether HMOX1-generated CO regulates the induction of IRGM in response to IFNG. We observed that IFNG induces the expression of IRGM but this induction was not regulated by HMOX1-generated CO (Figure S17B). These observations suggest that IRGM represents a HMOX1-independent pathway for regulation of autophagy. A number of studies have suggested that increased levels of cellular ROS promote higher autophagy flux [54,55]. In the light of this, we analyzed the levels of ROS using the cell-permeant reagent 2’, 7’ -dichlorofluorescin diacetate (DCFDA). Importantly, we observed that Mtb infection induces cytoplasmic ROS but inhibition of HMOX1 with ZnPP further induces cellular ROS levels. These levels could be significantly reduced by exogenous CO (Figure S18). Interestingly, ZnPP treatment reduces autophagy but increases ROS. In the light of this, it could be concluded that the increase in ROS levels does not correlate with the autophagy flux in Mtb-infected macrophages.
PPP3 plays an important role in IFNG-induced autophagy and mycobacterial clearance
An elegant study by Ballabio and colleagues has demonstrated that in response to stresses such as nutrient starvation, cytoplasmic Ca2+ levels are increased through MCOLN1 (mucolipin 1). This increase in intracellular Ca2+ activates phosphatase PPP3, which then dephosphorylates TFEB to facilitate its nuclear translocation [27]. Thus, we assessed whether the autophagy induced by IFNG was dependent on the phosphatase PPP3. For this, we transfected RAW 264.7 macrophages with siRNA specific for the beta isoform (Ppp3cb) of the catalytic subunit of PPP3/calcineurin or scrambled siRNA, activated the cells with IFNG and performed western blot analysis for LC3. Whereas the scrambled siRNA had no effect on LC3 II levels, the cells transfected with Ppp3cb siRNA displayed a significantly attenuated ability to induce autophagy in response to IFNG (Figure 8A and Figure S19). To further confirm these findings, we used the PPP3-specific inhibitors, cyclosporin A (CsA) and FK506 [56]. Treatment with CsA or FK506 inhibited increase in LC3-II levels in the RAW 264.7 macrophages in response to treatment with IFNG (Figure 8B). Additionally, in the presence of CsA and FK506, IFNG activation did not cause an increase in autophagic flux in the RAW 264.7 macrophages overexpressing GFP-LC3 (Figure 8C to E). We also analyzed the effect of the inhibition of PPP3 phosphatase activity on the localization of TFEB and lysosomal biogenesis in response to IFNG activation. Inhibition of PPP3 phosphatase activity using CsA and FK506 abrogated the nuclear localization of TFEB (Figure 8F and G) and lysosomal biogenesis as analyzed using LAMP1 as a marker (Figure 8H and I) in response to activation of RAW 264.7 macrophage cells by IFNG. To further confirm the role of PPP3 in the trafficking of mycobacterial cells and their survival, we infected IFNG-activated RAW 264.7 macrophages pretreated with CsA and FK506 with Mtb overexpressing GFP. Inhibition of PPP3 phosphatase activity attenuated the maturation of Mtb-carrying autophagosomes into phagolysosomes (Figure 8J and K) and improved the survival of Mtb cells in activated macrophages (Figure 8I).
Figure 8.
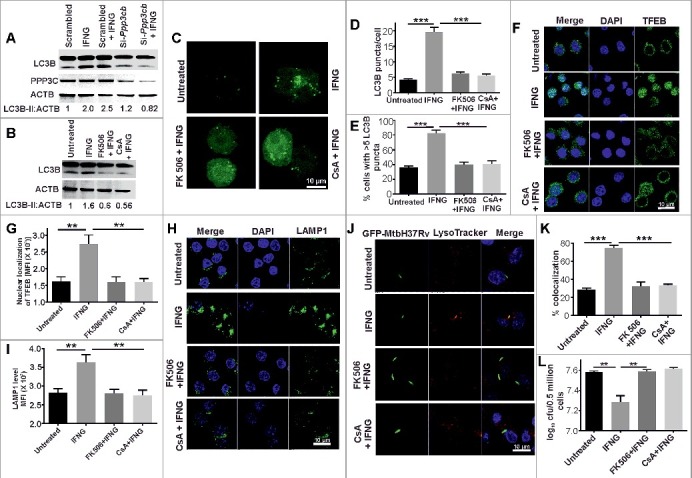
PPP3 plays an important role in IFNG-induced autophagy and mycobacterial clearance. (A) RAW 264.7 macrophages (0.5 × 106) were transiently transfected with Ppp3cb-specific or scrambled siRNAs and then activated them with IFNG and subjected to SDS-PAGE and western blot analysis. (B) The above findings were confirmed using the PPP3 -specific inhibitors cyclosporin A (CsA) and FK506. Densitometry analysis of LC3-II relative to ACTB is shown below the blot in panel A and B. (C) RAW 264.7 macrophages (0.5 × 106) were transiently transfected with GFP-LC3 plasmid and independently treated with cyclosporin A (CsA) (10 µM) and FK506 (5 µM) for 1 h, followed by IFNG for 3 h and viewed under a confocal microscope. Representative confocal microscopy images are shown. The LC3 puncta per cell (D) and the percentage of cells with >5 LC3 puncta (E) were calculated. Similar panel of cells was stained with TFEB (F) and LAMP1 (H), and the nuclear localization of TFEB (G and fluorescence intensity of LAMP1 (I) were determined using ImageJ software. (J) RAW 264.7 macrophages (0.5 × 106) were infected with GFP-Mtb H37Rv (1:5 MOI) for 3 h, followed by treatments with IFNG, CsA and FK506 as described and observed using a confocal laser scanning microscope. The figure shows representative images of the colocalization of Mtb (green channel) and LysoTracker Red (red channel). (K) Percentage of Mtb-containing autophagosomes colocalizing with lysosomes stained with LysoTracker Red (per 100 cells). (L) The same panel of cells were lysed in 0.06% SDS after 24 h of infection and plated on 7H11 to calculate CFUs. Data in panels D, E, G, I, K and L are mean±SEM from 3 independent experiments. ** indicates a P value < 0.01, and *** indicates a P value < 0.001.
Discussion
IFNG plays a critical role in shaping the immune response against intracellular pathogens. IFNG exposure activates autophagy in antigen-presenting cells and this autophagy plays a critical role in clearance of intracellular Mtb; however, the molecular basis of this activation is poorly understood. In the present study, we demonstrated that the increase in autophagic flux and mycobacterial killing by macrophages in response to IFNG was dependent on HMOX1-generated CO. We also demonstrated that IFNG exposure resulted in a HMOX1-generated CO-dependent increase in the cytoplasmic calcium levels that activate the phosphatase PPP3. Activated PPP3 then dephosphorylates TFEB to facilitate its localization into the nucleus and thereby induces lysosomal biogenesis and increases the autophagic flux (depicted in the model presented in Figure 9). Furthermore, we demonstrated that this molecular pathway played a critical role in the clearance of intracellular Mtb from infected macrophages and animals. These results provide novel insights into the mechanism underlying IFNG-induced autophagy, independent of previously conceived pathways such as the MTORC1, IRGM and MAPK14 signaling pathways.
Figure 9.
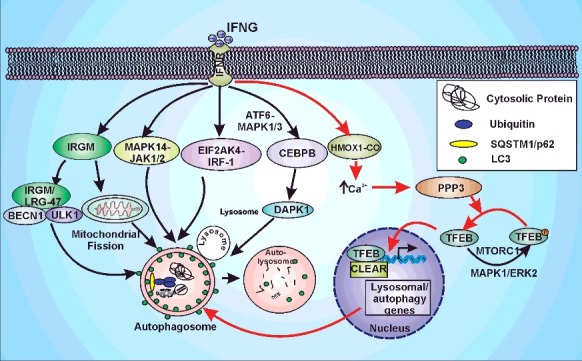
IFNG-induced autophagy is regulated by HMOX1. IFNG can induce autophagy through multiple pathways namely: IRGM/LRG-47, MAPK14, JAK1/2, EIF2AK4, IRF1 (interferon regulatory factor 1) or CEBPB. The model depicts the autophagic induction by IFNG through above-mentioned pathways and highlights the new signaling axis identified in the current study (Pathway depicted with red arrows). IFNG leads to the induction of HMOX1 and hence its product CO. The HMOX1-generated CO leads to the increase in cytoplasmic calcium levels that activates PPP3. This phosphatase dephosphorylates TFEB which leads to the transport of TFEB into the nucleus and hence the increase in autophagic flux and lysosomal biogenesis.
IFNG-mediated autophagy is critical for the clearance of intracellular Mtb by macrophages. Numerous mechanisms have been proposed to explain the increase in autophagy flux upon activation of macrophages by IFNG. These mechanisms include modulation of the MAPK14 signaling pathway [16,17], the interaction of IFNG-induced IRGM with the autophagy proteins ULK1 and BECN1 [12], induction of the EIF2AK4 signaling pathway [18], and upregulation the of Interferon regulatory factor 1 [19], ATF6-CEBPB pathway [20], among others. Importantly, IFNG is known to induce iNOS and thus NO in macrophage cells [57]. NO activates HMOX1 expression through mitogen-activated protein kinases MAPK/ERK and MAPK14 [58,59]. However, it must be noted that HMOX1 is also induced by a NOS2/iNOS-independent pathway in response to IFNG [10]. Prior to this study, the modulation of TFEB by IFNG via HMOX1 and its implications in the modulation of autophagy and lysosomal biogenesis have not been described to the best of our knowledge. The present results established that immunomodulation of macrophages with IFNG leads to translocation of TFEB into nucleus to induce the expression of genes involved in autophagy and lysosomal biogenesis. Importantly, translocation of TFEB requires HMOX1-generated CO. Multiple lines of evidence supports this conclusion. We used confocal microscopy to visualize endogenous TFEB and demonstrated that TFEB resides primarily in the cytoplasm in naïve macrophages, whereas in response to macrophage activation by IFNG, TFEB moves into the nucleus. These findings were further supported by experiments in which the nuclear and cytoplasmic fractions were separated and the levels of TFEB in these cellular compartments were analyzed. Additionally, the expression level of TFEB regulated proteins was analyzed. Given that the IFNG is unable to induce autophagic flux in the presence of the calcium chelator, BAPTA, or the PPP3 inhibitors, FK506 and CsA or the genetic ablation of PPP3, and the observation that TFEB is a master regulator of autophagy [21,22], we think that the pathway described in this study is the primary pathway involved in the increase in autophagy in response to IFNG. However, it must be noted that IFNG utilizes other pathways besides autophagy for killing intracellular pathogens such as generation of bactericidal levels of oxygen species and reactive nitrogen intermediates [1,2]. Importantly, we have observed that IFNG was able to restrict the growth of Mtb inside macrophages obtained from hmox1 knockout mice although less efficiently than the macrophages obtained from the wild-type controls.
Furthermore, we observed that hmox1 knockout mice are attenuated for controlling the replication of Mycobacteria, but this attenuation is not as severe as that of the interferon gamma knockout strain [4]. These observations suggest that besides autophagy other antimycobacterial mechanisms are also modulated by IFNG. We have validated our findings that HMOX1 is required for increase in the autophagy flux in response to IFNG using hmox1 knockout animals. We observed that the level of LC3 and BECN1 was higher in the wild-type animals compared the ones that are lacking Hmox1. The levels of these proteins were analyzed at 4 wk postinfection, because it is at 3 w postinfection that IFNG is produced (Figure 4C-F). We have also analyzed cytokines at this time point as autophagy can influence cytokine production. Cytokine IL1A, IL17A were significantly less produced in hmox1 knockout animals whereas IL12 and IL10 were more abundant. IL1A and IL17 are proinflammatory cytokines that help in the control of tuberculosis infection [60,61]. Importantly, autophagy levels are influenced by the levels of IL1A [62], and IL17 levels [63]. IL10 is a potent anti-inflammatory cytokine that is known to inhibit autophagy [64]. The low level of autophagy in the Mtb-infected hmox1 knockout mice could be due to high levels of IL10. IL10 gene polymorphism is associated with TB susceptibility as well [65]. These observations support the role of HMOX1 regulation of autophagy in vivo.
HMOX1 modulates pathogenesis of a number of microbial infections. It has protective effects in microbial infections and diseases such as cerebral and noncerebral malaria [66,67], Pseudomonas aeruginosa [68], colitis [69], polymicrobial sepsis [70], HIV [71], dengue virus [72], M. avium [36], methicillin-resistant Staphylococcus aureus,73 etc. Conversely, HMOX1 promotes susceptibility towards infections from Burkholderia pseudomallei [74], M. abscessus [75], Salmonella Typhimurium [76], etc. Thus, more studies are required for understanding the role of HMOX1 in microbial pathogenesis. This study has delineated the role of HMOX1 in Mtb survival inside the IFNG-activated macrophages. HMOX1 has been previously described to protect against mycobacterial infections [35–37]. However, recent studies have debated the role of HMOX1 in protection against the Mtb infection [77,78]. Both studies have exploited prolonged treatment of macrophage cells or animals with the HMOX1 inhibitors for long durations [77,78]. It is well known that prolonged exposure to HMOX1 inhibitors leads to cellular toxicity due to off-target effects and generates large quantities of reactive oxygen species through photochemical activation [79–82]. We think that perhaps the contrasting findings between the studies using genetic knockout mice (this study and refs [36,37,46]) and those using HMOX1 inhibitors may be due to off-target effects of HMOX1 inhibitors [77,78]. Note that our results on the role of HMOX1 in antimycobacterial activity in macrophages contrast with the observations of Shiloh and coworkers [78]. To validate our results, we have used multiple approaches such as ablation of HMOX1 function using genetic knockout or knockdown approaches and different pharmacological inhibitors. In all cases, we observed that ablation of HMOX1 in IFNG activated macrophages resulted in increased survival of Mtb. The difference in our observations from those of Shiloh and coworkers could be due to the use of activated macrophages in our study and naïve macrophages by Shiloh and coworkers [78]. Activated macrophages and naïve macrophages differ tremendously in their capability to control the growth of intracellular Mtb [3]. Furthermore, the time points used for accessing the antimycobacterial activity in the macrophages were different in the 2 studies (24 h in this study, while 168 h in the other study). Another explanation for these contrasting findings is that HMOX1 may have a dual function and thus both protect the host and facilitate the Mtb growth. As a consequence, the outcome of Mtb infection depends on the function of the HMOX1 in particular cellular context. We believe that HMOX1 could act as a double-edged sword in Mtb infections and further research using clinical samples will provide better insights into the role of HMOX1 in TB pathogenesis.
In this study, we demonstrated that IFNG increases intracellular Ca2+ levels. These findings are in agreement with previous studies [83,84]. However, the implications of the IFNG-induced increase in cytoplasmic calcium levels for macrophage function are poorly understood. We also demonstrated that HMOX1-generated CO regulates the increase in intracellular Ca2+. This observation is supported by earlier studies wherein the HMOX1 reaction product, CO, can increase cytoplasmic calcium levels in rat pancreatic acinar cells though the PLC-IP3-IP3 receptor cascade [85]. On the contrary, another study has suggested that CO inhibits cytoplasmic calcium levels by inhibiting T-type Ca2+ channels [86]. Interestingly, some earlier studies have suggested that cytoplasmic calcium levels induce HMOX1 expression [87,88], and activity [89]. This discrepancy could be due to use of different cell lines in these studies. Interestingly, infection with live Mtb inhibits the increase in intracellular Ca2+ level that is observed with opsonized Mtb cells or killed Mtb cells [90,91]. Here, we observed that activation of macrophages with IFNG reverts this inhibition. Furthermore, this reversion is mediated by the HMOX1 generated CO. Importantly Mtb infection is also known to induce HMOX1 expression [33], but not to the levels induced by IFNG. These findings indicate that the level of HMOX1-generated CO fine-tunes the outcome of the Mtb infection. Nevertheless, the role of CO in intracellular Ca2+ homeostasis remains poorly understood, and additional research is needed to conclusively establish the function of CO in intracellular Ca2+ homeostasis.
Another important finding of this study was that the IFNG-mediated increase in cytoplasmic Ca2+ was sensed by PPP3 to modulate the localization of TFEB into the nucleus, thus increasing the autophagy flux mediating the clearance of intracellular Mtb. These findings build upon a recently published elegant study by Ballabio and colleagues [51], which demonstrated that PPP3 is a regulator of TFEB and autophagy. However, our findings regarding the role of PPP3 in Mtb clearance contradict an earlier study, wherein activation of PPP3 by Coronin1 blocks the maturation of phagosomes into lysosomes [92]. These different observations could have arisen from the use of a virulent strain of Mtb in our study compared with M. bovis BCG by Jayachandran et al. Another explanation could be the use of IFNG-activated macrophages in our analysis; in contrast, Jayachandran et al. use naïve macrophages. The role of TFEB in TB pathogenesis has been described in only a few studies. In one study, silencing of the microRNA Mir33 and its passenger strand Mir33* leads to TFEB activation and autophagy-mediated clearance of Mtb [93]. Additionally, NR1D1 (nuclear receptor subfamily 1, group D, member 1) regulates the expression of TFEB to modulate autophagy and eliminates the intracellular Mtb [94]. Interestingly, ROS has been shown to activate lysosomal MCOLN1 channels that facilitate lysosomal Ca2+ release to induce autophagy and lysosomal biogenesis through TFEB [95]. Because IFNG also induces ROS generation, it is plausible that MCOLN1 channels are also involved in Ca2+ release from lysosomes to facilitate the induction of autophagy, but this hypothesis requires confirmation. Nonetheless, the identity of direct targets of CO that lead to increases in cytoplasmic calcium levels remains unknown and is beyond the scope of this study. In summary, we established a new signaling pathway in which IFNG activates TFEB through PPP3 in an HMOX1-dependent manner.
Materials and methods
Chemicals and reagents
Zinc protoporphyrin IX (282820), tricarbonylruthenium (II) dimer (288144), anti-LC3B antibody (L7543), ACTB antibody (A5316), ATG5 antibody (A0731), thioglycollate (T0632), monodansylcadaverine (30432), acridine orange (A6014), BAPTA-AM (A1076), and pepstatin A (P5318) were procured from Sigma Aldrich. E-64D (BML-PI-107) and CytoID autophagy detection kit (ENZ-51031) were procured from Enzo Life Science. LysoTracker Red DND 99 (L7528), Flu-3AM (F1242) and Lipofectamine 3000 (1565386) were procured from Invitrogen. Histone H3 (9715), PPP3CA (2614), SQSTM1 (13121S) and BECN1 (3738S) antibodies were obtained from Cell Signaling Technology. TFEB antibody (sc-48784) was obtained from Santa Cruz Biotechnology. LAMP1 (ab24170) and IRGM (ab63230) antibodies were purchased from Abcam. Recombinant-IFNG (285-IF) was procured from RandD systems. CsA (tlrl-cycA) and FK506 (tlrl-fk5) were purchased from Invivogen. H2DCFDA (D6883) was procured from Sigma Aldrich.
Plasmid, siRNA, cell lines and mice
The plasmids for the tandem mRFP-GFP-LC3 (tfLC3) and for GFP-LC3 were obtained from Addgene (21074 and 21073; deposited by T. Yoshimori) [39]. RAW 264.7 murine macrophages were obtained from ATCC (ATCC® TIB-71™) and cultured in DMEM (Dulbecco Modified Eagle Medium- 10569010) with 10% fetal bovine serum (10082147) from Gibco laboratories. RAW 264.7 murine macrophages were transiently transfected with the tfLC3 plasmid using Lipofectamine 3000 (Invitrogen, L3000-008). Briefly, the DNA and lipofectamine complex was prepared in Opti-MEM (11058021, Gibco laboratories) in 1:2 ratio and added to the cell in dropwise manner. The media was changed after 6 h and the cells were rested for 18 h.The hmox1−/− mice used in the study were kindly provided by Dr. Anupam Agarwal (University of Alabama, Birmingham, AL, USA). Murine peritoneal macrophages were isolated and maintained as previously described [96]. Ppp3cb siRNA (5'-GAGUGUGUCUUAUAUUUA-3’), Atg5 and Hmox1 siRNA were obtained from Dharmacon, and transfection was performed using Lipofectamine 3000 according to the manufacturer's protocol. Briefly, the siRNA and lipofectamine complex was prepared in Opti-MEM and added to the cells in dropwise manner and the media was changed after 30 h.
Western blotting
Following the treatments, the cells were washed with DPBS (Gibco, 14190144) and the lysate was prepared in RIPA buffer (Cell Signaling Technology, 9806) with protease inhibitor cocktail (Roche, 5892970001). The protein concentration was estimated using BCA (Sigma Aldrich, B9643-1L) for protein estimation, and the samples were subjected to SDS-PAGE followed by transfer to a PVDF (polyvinylidene fluoride) membrane (mdi Membrane Technologies, svfx831). Blots were developed using peroxidase-conjugated secondary antibodies (Santacruz Biotechnology, sc-2004) and visualized using enhanced chemiluminescence (ECL)-Luminata forte (Merck Millipore, WBLUF0500). The digital images were acquired using ImageQuant LAS 4000 (GE healthcare bioscience AB, 0712440, using Image quant LAS 4000 software). GAPDH (Abcam, ab9485) and ACTB were used as internal controls, and densitometry was performed using ImageJ software.
Endogenous staining
After the completion of treatment, the cells were fixed with 4% paraformaldehyde (PFA; Sigma Aldrich, 158127-500G) in PBS (NaCl [Fisher Scientific, BP-358-1], KCl [Sigma Aldrich, 60128-1KG], Na2HPO4 [Sigma Aldrich, S3264-1KG], KH2PO4 [Sigma Aldrich, P5655-500G], pH 7.4) followed by permeabilization with 0.5% Triton X-100 (Sigma Aldrich, X100). Blocking was performed in 2% normal goat serum (MP Biomedicals, 864292) + 2% BSA (HiMedia labs, GRM105) in PBS for 1 h at room temperature. The cells were washed 3 times with PBS and incubated with primary antibody (1:500) overnight at 4°C prepared in blocking buffer. The cells were washed with PBS followed by staining with Alexa Fluor 488 (Thermo Fisher Scientific, A-11008) anti-rabbit secondary antibody for 1 h at room temperature. The nuclei were then stained with DAPI (1 μg/ml; Sigma Aldrich D9542). The slides were prepared using antifade (Invitrogen, S36936) and viewed under a NIKON A1R Laser scanning confocal microscope.at CSIR- IMTech, Chandigarh.
Autophagy staining
The macrophages were treated with ZnPP, ZnPP along with CO and IFNG when required. For CytoID Green staining the cells were stained with 2 µl CytoID Green detection reagent and 0.5 µg/ml DAPI for 30 min followed by washing with PBS. The slides were prepared and observed under a confocal microscope. For monodansylcadaverine staining (MDC), after the above mentioned treatments the cells were stained with 10 µg/ml MDC for 30 min followed by washing with PBS and slide preparation. For acridine orange staining, the cells were stained with 10 µg/ml acridine orange followed by washing with PBS and slide preparation. Finally for LysoTracker Red DND 99, after the treatment the cells were stained with 200 nM of Lysotracker Red DND99 for 30 min followed by washing with PBS and slide preparation. 488 nm, 561 nm and 405 nm lasers were used for detecting CytoID green, Lysotracker Red and MDC staining respectively. Acridine orange was observed at 561 and 488 nm.
Bacterial strain and growth conditions
Mtb H37Rv (ATCC 27294) and GFP-Mtb H37Rv were cultured in 7H9 medium (Becton Dickinson Difco, 271310) with 0.05% Tween 80 (Sigma Aldrich, P1379) and 10% Middlebrook OADC enrichment (Becton Dickinson BBL, B12351) until the optical density at 600 nm reached 0.8 to 1.0. The culture was then centrifuged at 3220 × for 10 min, and the pellet was suspended in freezing medium (complete 7H9 medium + 20% glycerol [Fisher Scientific, 15457]). CFU plating was performed to estimate the number of viable cells, and the stocks were aliquoted and stored at −80°C. These stocks were used for all the experiments. All experiments involving Mtb were conducted in a BSL-3/A-BSL-3 facility (CSIR-IMTech, Chandigarh).
Colony-forming unit assay
RAW 264.7 macrophages (5 × 105) were plated in a 6-well plate and incubated in a CO2 incubator at 37°C. The cells were infected with Mtb H37Rv (1:10 MOI) for 3 h, treated with 100 μg/ml gentamycin (Sigma Aldrich, G1272) for 45 min and then stimulated with 200 units/ml of IFNG, 5 μM ZnPP and 20 μM CO according to the protocol. The cells were lysed with 0.06% SDS (Sigma Aldrich, L5750) after 24 h, serially diluted and plated on Middlebrook 7H11(Becton Dickinson Difco, 283810) plates with OADC. The plates were incubated at 37°C, and colonies were counted after 2 to 3 wk.
Animal experiments and ethics statement
Mice experiments were approved by the Institutional Animal Ethics Committee of Council of scientific and Industrial Research-Institute of Microbial Technology (Approval no IAEC/11/10). These experiments were performed according to the guidelines issued by the Committee for the Purpose of Supervision of Experiments on Animals (No.55/1999/CPCSEA) under the Prevention of Cruelty to Animals Act 1960 and amendments introduced in 1982 by Ministry of Environment and Forest, Govt. of India. Mice were maintained and bred under specific pathogen-free conditions in the animal house facility of CSIR-IMTech. The mice strain resulted from the cross of C57BL/6 and FVB mice. The hmox1 knockout mice display partial prenatal lethality [96] although mice are healthy upon birth. Due to the partial prenatal lethality, hmox1−/− males were mated with Hmox1+/− female mice, and around 25% of the progeny were hmox1−/−. In all experiments, littermate knockout animals were compared with wild-type or heterzygous (for Hmox1 allele) animals. Animal infections and subsequent studies were performed at the Animal BSL-3 facility of CSIR-IMTech. Mice were infected through the aerosol route with Mtb H37Rv using a nebulizer (Glas-col, Terre Haute, IN, USA) to achieve a bacterial deposition in the lungs of 100 to 200 CFU. The mice were sacrificed at specific time points, and the lungs were dissected, homogenized and plated on 7H11 plates with 10% OADC and antibiotics [vancomycin (10 μg/ml; MP Biochemicals, 195540), cycloheximide (20 μg/ml; MP Biochemicals, 100183), carbenicillin (50 μg/ml: Gold Biotech, C-103-100), amphotericin B (20 μg/ml; MP Biochemicals, 195043), polymyxin B (10 μg/ml; HiMedia labs, TC0033) and trimethoprim (20 μg/ml; Sigma Aldrich, 92131)].
Histopathology
For histopathology, the left lung of the Mtb-infected mice was removed and immersed in 10% formalin (Sigma Aldrich, HT401128) for 12 h. Granulomas were evaluated in 5-μm-thick paraffin-embedded sections followed by haematoxylin and eosin (HandE) staining. Histopathology was manually evaluated and scored for pathology on a scale from 0 (absent) to 3 (severe). Leukocyte infiltration, perivascular and peribronchiolar cuffing and airway epithelial cell injury were analyzed to score the pathology.
Nuclear and cytoplasmic fractionation
In a 10-cm dish, 80% confluent RAW 264.7 macrophages were treated with IFNG and fractionated. The cells were washed with PBS (NaCl [Fisher Scientific, BP-358-1], KCl [Sigma Aldrich, 60128-1KG], Na2HPO4 [Sigma Aldrich, S3264-1KG], KH2PO4 [Sigma Aldrich, P5655-500G], pH 7.4) and lysed in 0.5 Triton X-100 buffer (50 mM Tris-HCl [Fisher Scientific, 77-86-1], pH 7.4, 0.5% Triton X-100, 10% glycerol, 5 mM EDTA [Ambion, AM9260G], 150 mM NaCl, supplemented with protease inhibitor cocktail). After 15 min, the lysate was centrifuged (720x g, 5 min). The pellet represented the nuclear fraction, while the supernatant represented the cytoplasmic fraction. The pellet was washed twice with Triton X-100 buffer, suspended in Triton X-100 buffer with 0.5% SDS and sonicated.
Statistical analysis
Results were expressed as the mean±SEM from at least 3 independent experiments. GraphPad Prism v6.04 (GraphPad software Inc., USA) was used and the statistical significance was calculated by applying Student t test. Significance was accepted at a P value less than 0.05, where * indicates a P value <0.05, ** indicates a P value < 0.01, and *** indicates a P value < 0.001.
Supplementary Material
Funding Statement
Department of Biotechnology, Ministry of Science and Technology (DBT) [grant no. BT/PR15097/MED/29/237/2011]
Disclosure of potential conflicts of interest
Authors declare that they do not have any conflict of interest.
Acknowledgements
The hmox1 knockout and Hmox1 wild-type mice used in this study were kindly provided by Dr. Anupam Agarwal, University of Alabama, Birmingham. We also thank Sharmila Basu-Modak for help with the transfer of mice. Funding from the Department of Biotechnology, Government of India (BT/PR15097/MED/29/237/2011), supported this work. AK is supported by DST, India (DST/INT/AUS/GCP-7/13 and SR/SO/BB-0037/2013) and CSIR (BSC0210G, BSC0119F and BSC0211E) India. NS, PK, NB and ZA are grateful to the CSIR for funding JRF and/or SRF.
References
- [1].Stark GR, Kerr IM, Williams BR, et al. How cells respond to interferons [Review]. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. PubMed PMID: 9759489; eng. [DOI] [PubMed] [Google Scholar]
- [2].Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions [Review]. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. PubMed PMID: 14525967; eng. [DOI] [PubMed] [Google Scholar]
- [3].Flesch I, Kaufmann SH. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis [Comparative Study Research Support, Non-U.S. Gov't]. J Immunol. 1987;138(12):4408—4413. PubMed PMID: 3108389; eng. [PubMed] [Google Scholar]
- [4].Flynn JL, Chan J, Triebold KJ, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–2254. PubMed PMID: 7504064; PubMed Central PMCID: PMC2191274. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119(6):753–766. doi: S0092867404011067 [pii] 10.1016/j.cell.2004.11.038. PubMed PMID: 15607973; eng. [DOI] [PubMed] [Google Scholar]
- [6].Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 9015 [pii]. PubMed PMID: 11099404; PubMed Central PMCID: PMC2732363. eng. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deretic V, Levine B. Autophagy, immunity, and microbial adaptations [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Cell Host Microbe. 2009;5(6):527–549. doi: 10.1016/j.chom.2009.05.016. PubMed PMID: 19527881; PubMed Central PMCID: PMC2720763. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paludan C, Schmid D, Landthaler M, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy [Research Support, Non-U.S. Gov't]. Science. 2005;307(5709):593–596. doi: 10.1126/science.1104904. PubMed PMID: 15591165; eng [DOI] [PubMed] [Google Scholar]
- [9].MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47 [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. Science. 2003;302(5645):654–659. doi: 10.1126/science.1088063. PubMed PMID: 14576437; eng. [DOI] [PubMed] [Google Scholar]
- 10.Singh SB, Davis AS, Taylor GA, et al Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313(5792):1438–1441. doi: 1129577 [pii] 10.1126/science.1129577. PubMed PMID: 16888103; eng. [DOI] [PubMed] [Google Scholar]
- [11].Singh SB, Ornatowski W, Vergne I, et al. Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Nat Cell Biol. 2010;12(12):1154–1165. doi: 10.1038/ncb2119. PubMed PMID: 21102437; PubMed Central PMCID: PMC2996476. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chauhan S, Mandell MA, Deretic V. IRGM governs the core autophagy machinery to conduct antimicrobial defense [Research Support, N.I.H., Extramural]. Mol Cell. 2015;58(3):507–521. doi: 10.1016/j.molcel.2015.03.020. PubMed PMID: 25891078; PubMed Central PMCID: PMC4427528. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Intemann CD, Thye T, Niemann S, et al. Autophagy gene variant IRGM -261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains [Research Support, Non-U.S. Gov't] . PLoS Pathog. 2009;5(9):e1000577. doi: 10.1371/journal.ppat.1000577. PubMed PMID: 19750224; PubMed Central PMCID: PMC2735778. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang D, Chen J, Shi C, et al. Autophagy gene polymorphism is associated with susceptibility to leprosy by affecting inflammatory cytokines [Research Support, Non-U.S. Gov't]. Inflammation. 2014;37(2):593–598. doi: 10.1007/s10753-013-9773-1. PubMed PMID: 24264476; eng. [DOI] [PubMed] [Google Scholar]
- [15].Rovetta AI, Pena D, Hernandez Del Pino RE, et al. IFNG-mediated immune responses enhance autophagy against Mycobacterium tuberculosis antigens in patients with active tuberculosis [Research Support, Non-U.S. Gov't]. Autophagy. 2014;10(12):2109–2121. doi: 10.4161/15548627.2014.981791. PubMed PMID: 25426782; PubMed Central PMCID: PMC4502660. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matsuzawa T, Kim BH, Shenoy AR, et al. IFN-gamma elicits macrophage autophagy via the p38 MAPK signaling pathway [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. J Immunol. 2012;189(2):813–818. doi: 10.4049/jimmunol.1102041. PubMed PMID: 22675202; PubMed Central PMCID: PMC3392356. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matsuzawa T, Fujiwara E, Washi Y. Autophagy activation by interferon-gamma via the p38 mitogen-activated protein kinase signaling pathway is involved in macrophage bactericidal activity [Research Support, Non-U.S. Gov't]. Immunology. 2014;141(1):61–69. doi: 10.1111/imm.12168. PubMed PMID: 24032631; PubMed Central PMCID: PMC3893850. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xia XJ, Gao YY, Zhang J, et al. Autophagy mediated by arginine depletion activation of the nutrient sensor GCN2 contributes to interferon-γ-induced malignant transformation of primary bovine mammary epithelial cells [Article]. Cell Death Discovery. 2016; 2:15065. doi: 10.1038/cddiscovery.2015.65. PubMed PMID: 27551491; PubMed Central PMCID: PMC4979444. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li P, Du Q, Cao Z, et al. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1) [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Cancer Lett. 2012;314(2):213–222. doi: 10.1016/j.canlet.2011.09.031. PubMed PMID: 22056812; PubMed Central PMCID: PMC3487386. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gade P, Ramachandran G, Maachani UB, et al. An IFN-gamma-stimulated ATF6-C/EBP-beta-signaling pathway critical for the expression of Death Associated Protein Kinase 1 and induction of autophagy [Research Support, N.I.H., Extramural]. Proc Natl Acad Sci U S A. 2012;109(26):10316–1-321. doi: 10.1073/pnas.1119273109. PubMed PMID: 22699507; PubMed Central PMCID: PMC3387052. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function [Research Support, Non-U.S. Gov't]. Science. 2009;325(5939):473–477. doi: 10.1126/science.1174447. PubMed PMID: 19556463; eng. [DOI] [PubMed] [Google Scholar]
- [22].Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. TFEB links autophagy to lysosomal biogenesis [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't ]. Science. 2011;332(6036):1429–1433. doi: 10.1126/science.1204592. PubMed PMID: 21617040; PubMed Central PMCID: PMC3638014. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martina JA, Chen Y, Gucek M, et al. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB [Research Support, N.I.H., Intramural]. Autophagy. 2012;8(6):903–914. doi: 10.4161/auto.19653. PubMed PMID: 22576015; PubMed Central PMCID: PMC3427256. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, et al. Regulation of TFEB and V-ATPases by mTORC1 [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] . Embo J. 2011;30(16):3242–3258. doi: 10.1038/emboj.2011.257. PubMed PMID: 21804531; PubMed Central PMCID: PMC3160667. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Roczniak-Ferguson A, Petit CS, Froehlich F, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Sci Signal. 2012;5(228):ra42. doi: 10.1126/scisignal.2002790. PubMed PMID: 22692423; PubMed Central PMCID: PMC3437338. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Settembre C, Zoncu R, Medina DL, et al. A lysosome-to-nucleus signaling mechanism senses and regulates the lysosome via mTOR and TFEB [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Embo J. 2012;31(5):1095–1108. doi: 10.1038/emboj.2012.32. PubMed PMID: 22343943; PubMed Central PMCID: PMC3298007. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Medina DL, Di Paola S, Peluso I, et al. Lysosomal calcium signaling regulates autophagy through calcineurin and TFEB [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Nat Cell Biol. 2015;17(3):288–299. doi: 10.1038/ncb3114. PubMed PMID: 25720963; PubMed Central PMCID: PMC4801004. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61(2):748–755. PubMed PMID: 4386763; eng. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1029–L1037. PubMed PMID: 11076792; eng. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- [30].Bolisetty S, Traylor AM, Kim J, et al. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol. 2010;21(10):1702–1712. doi: ASN.2010030238 [pii] 10.1681/ASN.2010030238. PubMed PMID: 20705711; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Carchman EH, Rao J, Loughran PA, et al. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53(6):2053–62. doi: 10.1002/hep.24324. PubMed PMID: 21437926; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Waltz P, Carchman EH, Young AC, et al. Lipopolysaccaride induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway [Research Support, U.S. Gov't, Non-P.H.S.]. Autophagy. 2011;7(3):315–320. doi: 14044 [pii] 10.4161/auto.7.3.14044. PubMed PMID: 21307647; eng. [DOI] [PubMed] [Google Scholar]
- [33].Kumar A, Deshane JS, Crossman DK, et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283(26):18032–18039. doi: M802274200 [pii] 10.1074/jbc.M802274200. PubMed PMID: 18400743; PubMed Central PMCID: PMC2440631. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kumar A, Toledo JC, Patel RP, et al. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc Natl Acad Sci U S A. 2007;104(28):11568–11573. doi: 0705054104 [pii] 10.1073/pnas.0705054104. PubMed PMID: 17609369; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Regev D, Surolia R, Karki S, et al. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria [Research Support, N.I.H., Extramural]. Lab Invest. 2012;92(11):1541–1552. doi: 10.1038/labinvest.2012.125. PubMed PMID: 22964851; PubMed Central PMCID: PMC4017357. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Silva-Gomes S, Appelberg R, Larsen R, et al. Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection [Research Support, Non-U.S. Gov't]. Infect Immun. 2013;81(7):2536–2545. doi: 10.1128/IAI.00251-13. PubMed PMID: 23630967; PubMed Central PMCID: PMC3697604. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Surolia R, Karki S, Wang Z, et al. Attenuated heme oxygenase-1 responses predispose the elderly to pulmonary nontuberculous mycobacterial infections. Am J PhysiolLung Cell Mol Physiol. 2016;311(5):L928–L940. doi: 10.1152/ajplung.00397.2015. PubMed PMID: 27694475; PubMed Central PMCID: PMC5504405. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Malaguarnera L, Imbesi R, Di Rosa M, Scuto A, Castrogiovanni P, Messina A, Sanfilippo S. Action of prolactin, IFN-gamma, TNF-alpha and LPS on heme oxygenase-1 expression and VEGF release in human monocytes/macrophages [Research Support, Non-U.S. Gov't]. Int Immunopharmacol. 2005;5(9):1458–1469. doi: 10.1016/j.intimp.2005.04.002. PubMed PMID: 15953572; eng. [DOI] [PubMed] [Google Scholar]
- [39].Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3 [Research Support, Non-U.S. Gov't]. Autophagy. 2007;3(5):452–460. PubMed PMID: 17534139; eng. [DOI] [PubMed] [Google Scholar]
- [40].Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995 Jan;66(1):3–14. PubMed PMID: 7750517; eng. [PubMed] [Google Scholar]
- [41].Xu Y, Jagannath C, Liu XD, et al. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Immunity. 2007;27(1):135–144. doi: 10.1016/j.immuni.2007.05.022. PubMed PMID: 17658277; PubMed Central PMCID: PMC2680670. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. Cell Microbiol. 2009;11(8):1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. PubMed PMID: 19438516; PubMed Central PMCID: PMC3170014. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rohde KH, Abramovitch RB, Russell DG. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues [Research Support, N.I.H., Extramural]. Cell Host Microbe. 2007 Nov 15;2(5):352–364. doi: 10.1016/j.chom.2007.09.006. PubMed PMID: 18005756; eng. [DOI] [PubMed] [Google Scholar]
- [44].Dey B, Dey RJ, Cheung LS, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Nat Med. 2015;21(4):401–406. doi: 10.1038/nm.3813. PubMed PMID: 25730264; PubMed Central PMCID: PMC4390473. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Cell Host Microbe. 2008;; 3(5):323–30. doi: 10.1016/j.chom.2008.03.007. PubMed PMID: 18474359; PubMed Central PMCID: PMC2873178. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Regev D, Surolia R, Karki S, et al. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria [Research Support, N.I.H., Extramural]. Laboratory Investigation; A Journal Of Technical Methods And Pathology. 2012;92(11):1541–1552. doi: 10.1038/labinvest.2012.125. PubMed PMID: 22964851; PubMed Central PMCID: PMC4017357. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Castillo EF, Dekonenko A, Arko-Mensah J, et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation [Research Support, N.I.H., Extramural]. Proc Natl Acad Sci U S A. 2012;109(46):E3168–E3176. doi: 10.1073/pnas.1210500109. PubMed PMID: 23093667; PubMed Central PMCID: PMC3503152. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schottelius AJ, Mayo MW, Sartor RB, et al. Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. J Biol Chem. 1999;274(45):31868–31874. PubMed PMID: 10542212; eng. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- [49].Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia–the good, the bad, and the ugly [Review]. Kidney Int. 2005;67(4):1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. PubMed PMID: 15780075; eng. [DOI] [PubMed] [Google Scholar]
- [50].Decuypere JP, Bultynck G, Parys JB. A dual role for Ca(2+) in autophagy regulation [Research Support, Non-U.S. Gov't Review]. Cell Calcium. 2011;50(3):242–250. doi: 10.1016/j.ceca.2011.04.001. PubMed PMID: 21571367; eng. [DOI] [PubMed] [Google Scholar]
- [51].Medina DL, Ballabio A. Lysosomal calcium regulates autophagy [Review]. Autophagy. 2015;11(6):970–971. doi: 10.1080/15548627.2015.1047130. PubMed PMID: 26000950; PubMed Central PMCID: PMC4502748. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kung AW, Lau KS, Wong NS. Interferon-gamma increases intracellular calcium and inositol phosphates in primary human thyroid cell culture [Research Support, Non-U.S. Gov't]. Endocrinology. 1995;136(11):5028–5033. doi: 10.1210/endo.136.11.7588238. PubMed PMID: 7588238; eng. [DOI] [PubMed] [Google Scholar]
- [53].Clapham DE. Calcium signaling [Review]. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. PubMed PMID: 18083096; eng. [DOI] [PubMed] [Google Scholar]
- [54].Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species [Research Support, Non-U.S. Gov't Review]. Antioxid Redox Signal. 2011;14(11):2215–31. doi: 10.1089/ars.2010.3554. PubMed PMID: 20874258; Eng. [DOI] [PubMed] [Google Scholar]
- [55].Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment [Research Support, Non-U.S. Gov't Review]. Antioxid Redox Signal. 2009;11(4):777–90. doi: 10.1089/ARS.2008.2270. PubMed PMID: 18828708; Eng. [DOI] [PubMed] [Google Scholar]
- [56].Liu J, Farmer JD Jr., Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Cell. 1991;66(4):807–815. PubMed PMID: 1715244; eng. doi: 10.1016/0092-8674(91)90124-H. [DOI] [PubMed] [Google Scholar]
- [57].Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177(6):1779–84. PubMed PMID: 7684434; PubMed Central PMCID: PMC2191051. eng. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chen K, Maines MD. Nitric oxide induces heme oxygenase-1 via mitogen-activated protein kinases ERK and p38 [Research Support, U.S. Gov't, P.H.S.]. Cell Mol Biol (Noisy-le-grand). 2000;46(3):609–17. PubMed PMID: 10872747; eng. [PubMed] [Google Scholar]
- [59].Takahashi K, Hara E, Suzuki H, et al. Expression of heme oxygenase isozyme mRNAs in the human brain and induction of heme oxygenase-1 by nitric oxide donors [Research Support, Non-U.S. Gov't]. J Neurochem. 1996;67(2):482–9. PubMed PMID: 8764571; eng. doi: 10.1046/j.1471-4159.1996.67020482.x. [DOI] [PubMed] [Google Scholar]
- [60].Di Paolo NC, Shafiani S, Day T, et al. Interdependence between Interleukin-1 and tumor necrosis factor regulates TNF-Dependent control of mycobacterium tuberculosis Infection [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Immunity. 2015; 43(6):1125–1136. doi: 10.1016/j.immuni.2015.11.016. PubMed PMID: 26682985; PubMed Central PMCID: PMC4685953. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Okamoto Yoshida Y, Umemura M, Yahagi A, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung [Research Support, Non-U.S. Gov't]. J Immunol. 2010;184(8):4414–4422. doi: 10.4049/jimmunol.0903332. PubMed PMID: 20212094; eng. [DOI] [PubMed] [Google Scholar]
- [62].Harris J. Autophagy and IL-1 Family Cytokines. Front Immunol. 2013;4:83. doi: 10.3389/fimmu.2013.00083. PubMed PMID: 23577011; PubMed Central PMCID: PMC3617358. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Orosz L, Papanicolaou EG, Seprenyi G, et al. IL-17A and IL-17F induce autophagy in RAW 264.7 macrophages [Research Support, Non-U.S. Gov't]. Biomed Pharmacother. 2016;77:129–134. doi: 10.1016/j.biopha.2015.12.020. PubMed PMID: 26796276; eng. [DOI] [PubMed] [Google Scholar]
- [64].Park HJ, Lee SJ, Kim SH, et al. IL-10 inhibits the starvation induced autophagy in macrophages via class I phosphatidylinositol 3-kinase (PI3K) pathway [Research Support, Non-U.S. Gov't]. Mol Immunol. 2011;48(4):720–727. doi: 10.1016/j.molimm.2010.10.020. PubMed PMID: 21095008; eng. [DOI] [PubMed] [Google Scholar]
- [65].Gao X, Chen J, Tong Z, et al. Interleukin-10 promoter gene polymorphisms and susceptibility to tuberculosis: a meta-analysis [Meta-Analysis Research Support, Non-U.S. Gov't]. PLoS One. 2015;10(6):e0127496. doi: 10.1371/journal.pone.0127496. PubMed PMID: 26030829; PubMed Central PMCID: PMC4452516. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Seixas E, Gozzelino R, Chora A, et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria [Research Support, Non-U.S. Gov't] . Proc Natl Acad Sci U S A. 2009;106(37):15837–15842. doi: 10.1073/pnas.0903419106. PubMed PMID: 19706490; PubMed Central PMCID: PMC2728109. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pamplona A, Ferreira A, Balla J, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria [Comparative Study Research Support, Non-U.S. Gov't]. Nat Med. 2007;13(6):703–710. doi: 10.1038/nm1586. PubMed PMID: 17496899; eng. [DOI] [PubMed] [Google Scholar]
- [68].Tsuburai T, Kaneko T, Nagashima Y, et al. Pseudomonas aeruginosa-induced neutrophilic lung inflammation is attenuated by adenovirus-mediated transfer of the heme oxygenase 1 cDNA in mice [Research Support, Non-U.S. Gov't]. Hum Gene Ther. 2004;15(3):273–285. doi: 10.1089/104303404322886129. PubMed PMID: 15018736; eng. [DOI] [PubMed] [Google Scholar]
- [69].Onyiah JC, Sheikh SZ, Maharshak N, et al. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Gastroenterology. 2013;144(4):789–798. doi: 10.1053/j.gastro.2012.12.025. PubMed PMID: 23266559; PubMed Central PMCID: PMC3608700. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chung SW, Liu X, Macias AA, et al. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice [Research Support, N.I.H., Extramural]. J Clin Invest. 2008;118(1):239–247. doi: 10.1172/JCI32730. PubMed PMID: 18060048; PubMed Central PMCID: PMC2104480. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol. 2006;176(7):4252–7. PubMed PMID: 16547262; eng. [DOI] [PubMed] [Google Scholar]
- [72].Tseng CK, Lin CK, Wu YH, et al. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci Rep. 2016;6:32176. doi: 10.1038/srep32176. PubMed PMID: 27553177; PubMed Central PMCID: PMC4995454. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gahlot S, Nasreen N, Johnson JA, et al. Heme Oxygenase-1 Deficiency Diminishes Methicillin-Resistant Staphylococcus aureus Clearance Due to Reduced TLR9 Expression in Pleural Mesothelial Cells. PLoS One. 2017;12(1):e0169245. doi: 10.1371/journal.pone.0169245. PubMed PMID: 28052108; PubMed Central PMCID: PMC5215390. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Stolt C, Schmidt IH, Sayfart Y, et al. Heme Oxygenase-1 and Carbon Monoxide Promote Burkholderia pseudomallei Infection. J Immunol. 2016;197(3):834–846. doi: 10.4049/jimmunol.1403104. PubMed PMID: 27316684; eng. [DOI] [PubMed] [Google Scholar]
- [75].Abdalla MY, Ahmad IM, Switzer B, et al. Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in human macrophages-like THP-1 cells [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.]. Redox Biol. 2015;4:328–339. doi: 10.1016/j.redox.2015.01.012. PubMed PMID: 25638774; PubMed Central PMCID: PMC4326180. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mitterstiller AM, Haschka D, Dichtl S, et al. Heme oxygenase 1 controls early innate immune response of macrophages to Salmonella Typhimurium infection. Cell Microbiol. 2016;18(10):1374–1389. doi: 10.1111/cmi.12578. PubMed PMID: 26866925; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Costa DL, Namasivayam S, Amaral EP, et al. Pharmacological Inhibition of Host Heme Oxygenase-1 Suppresses Mycobacterium tuberculosis Infection In Vivo by a Mechanism Dependent on T Lymphocytes [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, N.I.H., Intramural Research Support, U.S. Gov't, Non-P.H.S.]. MBio. 2016;7(5). doi: 10.1128/mBio.01675-16. PubMed PMID: 27795400; PubMed Central PMCID: PMC5080384. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Scharn CR, Collins AC, Nair VR, et al. Heme Oxygenase-1 Regulates Inflammation and Mycobacterial Survival in Human Macrophages during Mycobacterium tuberculosis Infection [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. J Immunol. 2016;196(11):4641–4649. doi: 10.4049/jimmunol.1500434. PubMed PMID: 27183573; PubMed Central PMCID: PMC4875857. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hintz SR, Vreman HJ, Stevenson DK. Mortality of metalloporphyrin-treated neonatal rats after light exposure. Dev Pharmacol Ther. 1990;14(3):187–192. PubMed PMID: 2364856; eng. [PubMed] [Google Scholar]
- [80].Keino H, Nagae H, Mimura S, et al. Dangerous effects of tin-protoporphyrin plus photoirradiation on neonatal rats [Research Support, Non-U.S. Gov't]. Eur J Pediatr. 1990;149(4):278–279. PubMed PMID: 2303077; eng. [DOI] [PubMed] [Google Scholar]
- [81].Fort FL, Gold J. Phototoxicity of tin protoporphyrin, tin mesoporphyrin, and tin diiododeuteroporphyrin under neonatal phototherapy conditions. Pediatrics. 1989;84(6):1031-=1037. PubMed PMID: 2531365; eng. [PubMed] [Google Scholar]
- [82].Land EJ, McDonagh AF, McGarvey DJ, et al. Photophysical studies of tin(IV)-protoporphyrin: potential phototoxicity of a chemotherapeutic agent proposed for the prevention of neonatal jaundice [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Proc Natl Acad Sci U S A. 1988;85(14):5249–5253. PubMed PMID: 3393537; PubMed Central PMCID: PMC281727.eng. doi: 10.1073/pnas.85.14.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Aas V, Larsen K, Iversen JG. Interferon-gamma elicits a G-protein-dependent Ca2+ signal in human neutrophils after depletion of intracellular Ca2+ stores [Research Support, Non-U.S. Gov't]. Cell Signal. 1999; 11(2):101–110. PubMed PMID: 10048787; eng. doi: 10.1016/S0898-6568(98)00040-0. [DOI] [PubMed] [Google Scholar]
- [84].Miyake M, Fuchimoto S, Orita K. Differences in intracellular calcium mobilization by interferon-beta and interferon-gamma in RPMI-4788 cells. Exp Cell Biol. 1989;57(2):67–72. PubMed PMID: 2504627; eng. [DOI] [PubMed] [Google Scholar]
- [85].Moustafa A, Habara Y. A novel role for carbon monoxide as a potent regulator of intracellular Ca2+ and nitric oxide in rat pancreatic acinar cells [Research Support, Non-U.S. Gov't]. Am J Physiol Cell Physiol. 2014 Dec 1;307(11):C1039–C1049. doi: 10.1152/ajpcell.00252.2014. PubMed PMID: 25252950; eng. [DOI] [PubMed] [Google Scholar]
- [86].Duckles H, Boycott HE, Al-Owais MM, et al. Heme oxygenase-1 regulates cell proliferation via carbon monoxide-mediated inhibition of T-type Ca2+ channels [Research Support, Non-U.S. Gov't]. Pflugers Arch. 2015;467(2):415–427. doi: 10.1007/s00424-014-1503-5. PubMed PMID: 24744106; PubMed Central PMCID: PMC4293494. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Terry CM, Clikeman JA, Hoidal JR, et al. TNF-alpha and IL-1alpha induce heme oxygenase-1 via protein kinase C, Ca2+, and phospholipase A2 in endothelial cells [Research Support, Non-U.S. Gov't]. Am J Physiol. 1999;276(5 Pt 2):H1493–H1501. PubMed PMID: 10330231; eng. [DOI] [PubMed] [Google Scholar]
- [88].Gissel C, Doutheil J, Paschen W. Activation of heme oxygenase-1 expression by disturbance of endoplasmic reticulum calcium homeostasis in rat neuronal cell culture [Research Support, Non-U.S. Gov't]. Neurosci Lett. 1997;231(2):75–78. PubMed PMID: 9291144; eng. doi: 10.1016/S0304-3940(97)00528-4. [DOI] [PubMed] [Google Scholar]
- [89].Boehning D, Sedaghat L, Sedlak TW, et al. Heme oxygenase-2 is activated by calcium-calmodulin [Research Support, U.S. Gov't, P.H.S.]. J Biol Chem. 2004;279(30):30927–30930. doi: 10.1074/jbc.C400222200. PubMed PMID: 15175337; eng. [DOI] [PubMed] [Google Scholar]
- [90].Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca(2+) signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages [Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. J Exp Med. 2000;191(2):287–302. PubMed PMID: 10637273; PubMed Central PMCID: PMC2195750. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Malik ZA, Iyer SS, Kusner DJ. Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages [Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.]. J Immunol. 2001;166(5):3392–33401. PubMed PMID: 11207296; eng. [DOI] [PubMed] [Google Scholar]
- [92].Jayachandran R, Sundaramurthy V, Combaluzier B, et al. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin [Research Support, Non-U.S. Gov't]. Cell. 2007;130(1):37–50. doi: 10.1016/j.cell.2007.04.043. PubMed PMID: 17632055; eng. [DOI] [PubMed] [Google Scholar]
- [93].Ouimet M, Koster S, Sakowski E, et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. Nat Immunol. 2016;17(6):677–686. doi: 10.1038/ni.3434. PubMed PMID: 27089382; PubMed Central PMCID: PMC4873392. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chandra V, Bhagyaraj E, Nanduri R, et al. NR1D1 ameliorates Mycobacterium tuberculosis clearance through regulation of autophagy. Autophagy. 2015;11(11):1987–1997. doi: 10.1080/15548627.2015.1091140. PubMed PMID: 26390081; PubMed Central PMCID: PMC4824569. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang X, Cheng X, Yu L, et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun. 2016;7:12109. doi: 10.1038/ncomms12109. PubMed PMID: 27357649; PubMed Central PMCID: PMC4931332. eng. doi: 10.1038/ncomms12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. Proc Natl Acad Sci U S A. 1997;94(20):10919–10924. PubMed PMID: 9380735; PubMed Central PMCID: PMC23531. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


