ABSTRACT
Phagocytes cope with the threat of living bacteria via detection of vita-PAMPs, a specific class of pathogen-associated molecular patterns (PAMPs) that denotes microbial viability and trigger a commensurate innate response. Prokaryotic mRNA and cyclic-di-adenosine monophosphate (c-di-AMP) serve as vita-PAMPs for Gram-negative and Gram-positive bacteria, respectively, and elicit heightened proinflammatory responses not warranted for dead bacteria. The innate sensor TMEM173/STING detects c-di-AMP produced by internalized live Gram-positive bacteria, and quickly mobilizes interdependent pre-formed cell-autonomous responses including endoplasmic reticulum (ER) stress, MTOR inactivation, and reticulophagy. In turn, reticulophagy serves a dual role in restoring phagocyte homeostasis and orchestrating a type I IFN response. ER-stress induced macroautophagy/autophagy sequesters stressed ER, resolves ER stress and prevents apoptosis in response to live bacteria. Reticulophagy relocalizes ER-resident TMEM173/STING to phagophores, which then act as TMEM173/STING-signaling compartments. Here, we discuss our findings in the context of innate immunity and cell homeostasis.
KEYWORDS: autophagy, c-di-AMP, cell-autonomous innate immunity, ER stress, ER-phagy, Gram-positive bacteria, MTOR, STING, type-I interferon, vita-PAMP
Innate immunity relies on the recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRR). Vita-PAMPs, a subset of PAMPs expressed specifically by live microorganisms, are the signature of microbial viability. Vita-PAMPs trigger highly inflammatory responses to face the heightened threat live microbes pose compared to their dead counterparts. Cell-autonomous responses, either interferon-inducible or constitutive (pre-existing), help neutralize microorganisms. Constitutive cell-autonomous responses depend on pre-formed homeostatic and defense processes including autophagy, yet the mechanisms by which these responses are orchestrated are poorly understood.
We found that a series of integrated constitutive cell-autonomous responses is mobilized by live Gram-positive bacteria. We characterized cyclic-di-adenosine monophosphate (c-di-AMP), a bacterial second messenger, as a novel vita-PAMP. Engagement of the ER-associated PRR TMEM173/STING by c-di-AMP triggers endoplasmic reticulum (ER) stress, which inactivates the mechanistic target of rapamycin kinase complex 1 (MTORC1) and induces canonical autophagy. This canonical autophagy functions as reticulophagy; it resolves ER stress by sequestering stressed ER membrane proteins, including the ER stress sensors EIF2AK3/PERK and ERN1/IRE1α. By doing so, reticulophagy prevents ER stress-induced apoptosis. Furthermore, reticulophagy relocalizes TMEM173/STING to phagophores thus enabling the heightened IFN-I response to live Gram-positive bacteria. c-di-AMP availability, TMEM173/STING expression, ER stress, MTORC1 inhibition and autophagy, are all required for this IFN-I response. Therefore, vita-PAMP sensing by a PRR engages complex and multilayered constitutive cell-autonomous responses, which preserve phagocyte homeostasis and initiate IFN-inducible immunity (Figure 1).
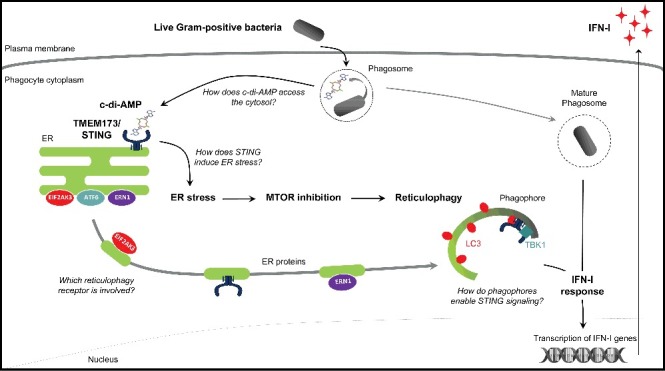
Our findings comprise the second example of a distinct innate response mobilized uniquely to bacterial viability and irrespective of the expression of bacterial virulence factors. In addition to bacterial mRNA, the vita-PAMP for Gram-negative bacteria that elicits activation of the NLRP3 inflammasome and heightened IFN-I response, we identified c-di-AMP for live Gram-positive bacteria. The innate response to live Gram-positive bacteria also differs from that to Gram-negative bacteria, because it does not involve inflammasome activation. Therefore, the responses to microbial viability and the spectrum of cytokines produced vary according to the nature of the vita-PAMP detected. c-di-AMP is produced via the bacterial di-adenylyl cyclase by many bacteria including Staphylococcus aureus or Listeria monocytogenes, where it plays an essential role in ion transport, cell wall homeostasis, membrane lipid homeostasis and sensing of DNA damage. Besides bacterial mRNA and c-di-AMP, other vita-PAMPs likely exist and may result in various viability-specific innate immune responses depending on the PRR engaged. vita-PAMPs most likely activate cytosolic PRR upon their release from bacteria-containing phagosomes and access to the cytosol. Guanylate-binding proteins, a family of interferon-inducible GTPases, destabilize the Salmonella-containing vacuole and induce the release of phagosomal contents, therefore promoting innate immune detection of PAMPs. The molecular mechanisms by which vita-PAMPs access the cytosol, either via guanylate-binding proteins or other molecules such as channels or selective transporters, have not been assessed so far.
We identified TMEM173/STING as the PRR responsible for the induction of the cell-autonomous response to live Gram-positive bacteria, starting with induction of ER stress, which then controls MTORC1 inactivation and reticulophagy. Our assays reveal 3 positive readouts of ER stress including autophosphorylation of the ER stress sensors EIF2AK3/PERK and ERN1/IRE1α, induction of the ER stress targets HSPA5/BIP and DDIT3/CHOP, and dilation of the ER. ER stress in response to live Gram-positive bacteria is abrogated in tmem173/sting–/– phagocytes. Our study characterized the first example of PRR-induced ER stress. Yet, the precise mechanisms by which TMEM173/STING leads to ER stress remain to be explored. Interestingly, TMEM173/STING is an ER-resident molecule, anchored to ER membranes via 4 transmembrane domains. We speculate that the conformational changes that TMEM173/STING undergoes upon activation by c-di-AMP might disrupt the process of protein folding in the ER lumen. Alternatively, TMEM173/STING activation might trigger proteins involved in phosphorylation of the ER stress sensors, and whose identities are unknown so far.
Beginning with ER stress, a multilayered integrated cell-autonomous stress response is engaged, leading to MTORC1 inhibition and culminating in autophagy and an IFN-I response. Unlike the well-characterized inhibition of autophagy by MTORC1, the links between MTOR and ER stress are more complex, bidirectional and context-dependent. We showed that MTORC1 inactivation by ER stress is EIF2AK3/PERK dependent. The fast nature of the cell-autonomous response we observed, resulting in autophagy between 1–2 h following infection, excludes a role for autophagy-related gene transcription and protein translation. Rather, ER stress-dependent autophagy is mediated via MTORC1 inactivation, which we found is required to activate canonical autophagy.
We showed that reticulophagy also enables the heightened IFN-I response to live Gram-positive bacteria. TMEM173/STING is enriched in the phagophores, and interacts with endogenous LC3 together with TBK1 in an autophagy-dependent manner. TMEM173/STING relocalization from the ER was previously identified as a prerequisite for TMEM173/STING signaling, activation of the TBK1-IRF3 axis and expression of IFN-I genes. Compartments where TMEM173/STING signaling is active have not been properly characterized. TMEM173/STING colocalizes with several autophagy-related proteins such as LC3 or ATG9A (involved both in autophagy and vesicle trafficking), yet phagophores have not been identified as a TMEM173/STING-signaling compartment. In our study, we propose that TMEM173/STING relocalization to phagophores, together with TBK1, enables expression of IFN-I genes. Phagophores could act as TMEM173/STING-signaling compartments presumably by favoring the recruitment of molecules required for IFN signaling. However, the precise mechanisms would have to be determined in futures studies.
We have characterized a complex cell-autonomous response where TMEM173/STING co-opts reticulophagy, a pre-existing homeostatic cellular response, to link constitutive cell-autonomous responses to IFN-inducible immunity (Figure 1). Such a cooperative mechanism among ER stress, MTOR and reticulophagy is of particular interest beyond the context of infected phagocytes. Many loss-of-function autophagy variants have been associated with inflammatory bowel disease (most notably Atg16l1 variants in Crohn disease), and similarly loss of function variants in XBP1, a key player in the ER stress response, promote intestinal inflammation. Both ER stress and autophagy are critical for optimal Paneth cell function, and impaired autophagy in the face of ER stress is detrimental to these highly secretory cell types. ER stress and autophagy have also been associated with cell malignancy in many types of solid tumors, as well-established mechanisms to control tumor cell survival or death. Therefore, the complex bidirectional relationship we have described between ER stress and autophagy in phagocytes may be relevant to the function of other specialized cell types. Eventually, understanding the molecular mechanisms underlying this relationship might open new therapeutic strategies to prevent inflammation or control cell malignancy.
Funding Statement
This work was supported by the Burroughs Wellcome Fund; Leukemia and Lymphoma Society; National Institute of Allergy and Infectious Diseases [grant number AI127658]; National Institute of Allergy and Infectious Diseases [grant number AI123284]; National Institute of Diabetes and Digestive and Kidney Diseases [grant number DK111862].
Disclosure statement
The authors are unaware of any potential conflicts of interest.


