ABSTRACT
The article discusses new findings on the role of the 4 human WIPI proteins at the onset of macroautophagy/autophagy. New insights revealing a circuit scaffold function of WIPI β-propellers that interconnect autophagy signaling control with appropriate autophagosome formation are summarized.
KEYWORDS: WIPI1, WIPI2, WIPI3, WIPI4, autophagy
Macroautophagy (hereafter autophagy) is positively regulated by the energy sensor AMPK, that activates catabolic pathways including autophagy, and negatively regulated by the nutrient sensor TORC1, that activates anabolic pathways, thereby inhibiting autophagy. Growth factor and amino acid signaling activate TORC1 via RHEB and RRAG/Rag GTPases at the lysosomal surface. TORC1 activation is counteracted by the TSC complex, which upon site-specific phosphorylation of TSC2 by AMPK, contributes to the displacement of TORC1 from the surface of the lysosome. Moreover, activated AMPK further phosphorylates TORC1, thereby also directly inhibiting its activity. Hence, AMPK and TORC1 signaling is tightly connected and AMPK activation leads to TSC complex-mediated inhibition of TORC1 in the lysosomal compartment. Both AMPK and TORC1 regulate autophagy through site-specific phosphorylation of autophagy-related (ATG) proteins, critically the serine threonine-specific protein kinase ULK1. AMPK-mediated phosphorylation of ULK1 activates autophagy whereas TORC1-mediated ULK1 phosphorylation inhibits autophagy. AMPK-activated ULK1 phosphorylates factors of the class III phosphatidylinositol 3-kinase (PtdIns3K) complex which in turn produces phosphatidylinositol-3-phosphate (PtdIns3P) at the endoplasmic reticulum (ER), a prerequisite step for autophagosome formation.
WIPI proteins, the human group of the PROPPIN family with the 4 members WIPI1, WIPI2, WDR45B/WIPI3 and WDR45/WIPI4, are considered to function as PtdIns3P effectors, but this function has been demonstrated unambiguously for only WIPI2, that bridges PtdIns3P production with the recruitment of the ATG16L1 complex for subsequent LC3 lipidation at the phagophore (Fig. 1). As for the other WIPI proteins, WIPI1 was considered to function upstream of LC3, and WDR45/WIPI4 downstream of LC3. But, their functions were unknown, and WDR45B/WIPI3 was entirely uncharacterized.
Figure 1.
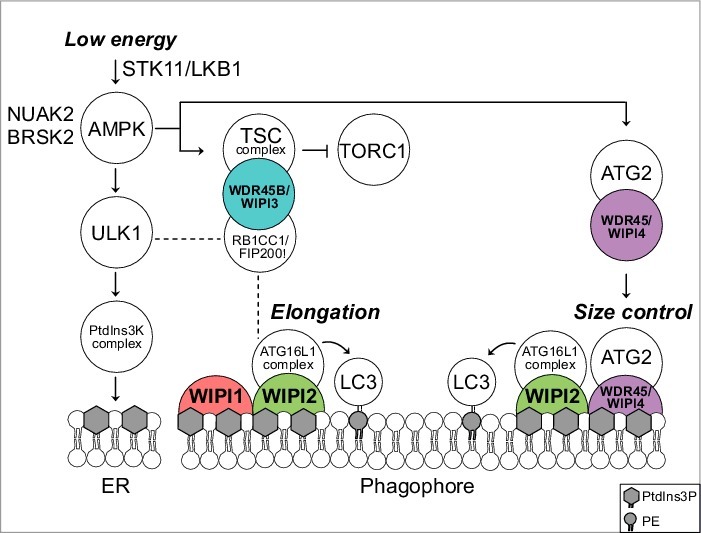
Predicted model for a functional WIPI scaffold circuit at the nascent autophagosome under the control of AMPK. Low energy stimulates the STK11/LKB1-AMPK axis to initiate catabolic pathways, thereby activating autophagy through ULK1. Downstream, the class III PtdIns3K, PIK3C3VPS34, a member of the PtdIns3K complex, produces PtdIns3P at the endoplasmic reticulum (ER). This stimulates the formation of omegasomes (not shown) and phagophores (template membranes that elongate and close to form the autophagosome) through an as yet unidentified mechanism. Phagophore elongation is achieved through the PtdIns3P effector activity of WIPI2, that recruits the ATG16L1 complex for subsequent LC3 lipidation. WIPI1 assists WIPI2 in this function. Phagophore expansion is size controlled by WDR45/WIPI4-ATG2 that dissociates from AMPK-ULK1 upon AMPK activation. TORC1 inhibition is achieved through AMPK-mediated phosphorylation of TSC2 that in complex with WDR45B/WIPI3 (and RB1CC1/FIP200) displaces TORC1 from the lysosomal surface. Both AMPK-related kinases NUAK2 and BRSK2 produce a direct signal to WDR45/WIPI4 but the details are unknown. Dashed lines indicate known interactions that have not been addressed in the study described. PE, phosphatidylethanolamine.
We characterized the 4 human WIPI proteins by comparative functional assessments and by combining a proteomics approach with a lentiviral-based kinome screening using automated imaging of autophagy.1 First, we demonstrated that WDR45B/WIPI3 and WDR45/WIPI4, as previously shown for WIPI1 and WIPI2, fold into 7-bladed β-propeller proteins that specifically bind PtdIns3P and colocalize at nascent autophagosomes (marked by ATG14, ZFYVE1/DFCP1, ATG12, LC3, SQSTM1/p62) upon starvation. However, functional impairment of individual WIPI members results in distinct phenotypes where WIPI2 knockdown impairs phagophore formation upstream of LC3, and WDR45B/WIPI3 and WDR45/WIPI4 knockdown blocks appropriate autophagosome formation downstream of LC3. Our proteomics approach revealed the WIPI protein interactome demonstrating that all 4 WIPI proteins associate in a distinct and nonredundant protein network. Next, we followed up the characterization of identified WIPI interacting partners with ATG proteins and the known autophagy regulators appearing in our WIPI interactome network. We confirmed that WIPI2 recruits the ATG16L1 complex and found that WIPI1 assists WIPI2 in efficient recruitment. Further, we found that WDR45B/WIPI3 associates with both the TSC complex and RB1CC1/FIP200. WDR45B/WIPI3 associates with the TSC complex for MTOR regulation in the lysosomal compartment, and with RB1CC1/FIP200 at the nascent autophagosome. Moreover, we showed that the WDR45/WIPI4 complex contains ATG2, AMPK and ULK1 under fed conditions. Upon starvation WDR45/WIPI4-ATG2 dissociates and translocates to nascent autophagosomes (Fig. 1). The specific WDR45/WIPI4-ATG2 interaction was mapped to show the critical involvement of the amino acids N15 and D17 in WDR45/WIPI4, and the amino acid D113 in WDR45/WIPI4 that is required for functional interaction with AMPK. Interestingly, silencing WDR45/WIPI4 and ATG2 provokes massive accumulations of WIPI1- and LC3-positive membranes in both size and numbers, in line with the appearance of elongated phagophores upon WDR45/WIPI4 knockdown. Hence we suggested that WDR45/WIPI4-ATG2 function in AMPK-mediated size control of phagophores (Fig. 1). Moreover, our kinome screen revealed that not only AMPK, but also the AMPK-related kinases, NUAK2 and BRSK2, all of which function under the control of STK11/LKB1, stimulate WDR45/WIPI4 at the onset of autophagy (Fig. 1).
Although glucose starvation induces autophagy through AMPK-mediated ULK1 phosphorylation upstream of WIPI1 and WIPI2, neither WIPI1 nor WIPI2 were found to respond to glucose starvation. However, our recent work highlights that glucose starvation signals via the STK11/LKB1-AMPK/AMPK-related kinase network to WDR45/WIPI4-ATG2, which in response controls phagophore expansion (Fig. 1). Also, WDR45B/WIPI3 is under the control of AMPK because we found that WDR45B/WIPI3 associates with the AMPK-activated TSC complex in the lysosomal compartment and we suggested that WDR45B/WIPI3 takes an essential part in scaffolding AMPK-triggered TORC1 inhibition via the TSC complex (Fig. 1). Moreover, we found that WDR45B/WIPI3 also associates with RB1CC1/FIP200, previously shown to be an essential component of the ULK1 complex, but to also localize in the lysosomal compartment and to further associate with ATG16L1 (dashed lines in Fig. 1). Indeed, we found that WDR45B/WIPI3 colocalizes with RB1CC1/FIP200 in the lysosomal compartment und further at the nascent autophagosome upon starvation. As WDR45B/WIPI3 knockdown provokes the appearance of elongated phagophores, WDR45B/WIPI3 should, similar to WDR45/WIPI4, also function in controlling appropriate phagophore elongation.
Based on our findings,1 both WDR45B/WIPI3 and WDR45/WIPI4 function upstream of PtdIns3P production and WIPI1-WIPI2, but also downstream of LC3 in controlling the size of nascent autophagosomes, with WDR45/WIPI4 acting in association with ATG2 (Fig. 1) and WDR45B/WIPI3 in association with RB1CC1/FIP200. Moreover, our study revealed that the 4 human WIPI β-propellers function as a scaffold circuit, interconnecting autophagy signal control with appropriate autophagosome formation. Our work provides a framework for the differential contributions of WIPI β-propellers during autophagosome formation (Fig. 1) that warrant further investigations in molecular detail in future studies.
Funding Statement
Deutsche Forschungsgemeinschaft (DFG) FOR2625 (project 1) and SFB/TR (project B02).
Reference
- 1.Bakula D, Müller AJ, Zuleger T, Takacs Z, Franz-Wachtel M, Thost AK, Brigger D, Tschan MP, Frickey T, Robenek H, et al. WIPI3 and WIPI4 β-propellers are scaffolds for LKB1-AMPK-TSC signalling circuits in the control of autophagy. Nat Commun. 2017;8:15637. doi: 10.1038/ncomms15637. PMID:28561066 [DOI] [PMC free article] [PubMed] [Google Scholar]


