Abstract
O-glycosylation is probably one of the most varied sets of post-translational modifications across all organisms, but amongst the most refractory to analyse. In animals, O-xylosylation of serine residues represents the first stage in the synthesis of glycosaminoglycans, whose repeat regions are generally analysed as fragments resulting from enzymatic or chemical degradation, whereas their core regions can be isolated by β-elimination or endo-β-xylosidase digestion. In the present study, we show that hydrazinolysis can be employed for release of glycosaminoglycan-type oligosaccharides from nematodes prior to fluorescent labelling with 2-aminopyridine. While various [HexNAcHexA]nGal2Xyl oligosaccharides were isolated from the model organism Caenorhabditis elegans, more unusual glycosaminoglycan-type glycans were found to be present in the porcine parasite Oesophagostomum dentatum. In this case, as judged by MS/MS before and after hydrofluoric acid or β-galactosidase digestion, core sequences with extra galactose and phosphorylcholine residues were detected as [(±PC)HexNAcHexA]n(±PC)Galβ3-(±Galβ4)Galβ4Xyl. Thus, hydrazinolysis and fluorescent labelling can be combined to analyse unique forms of O-xylosylation, including new examples of zwitterionic glycan modifications.
Keywords: glycosaminoglycan-like O-glycans, hydrazinolysis, mass spectrometry, nematode
Introduction
Glycosaminoglycans (GAGs), based on repeats of N-acetylhexosamine and hexuronic acid residues, are a hallmark of the metazoa and play key roles in animal development. In mammals, they are primarily classified as chondroitin, dermatan, heparan, hyaluronan or keratan sulphates, depending on the exact sequence of the repeating units and the type (or lack thereof) of the covalent linkage to protein (Li et al., 2012; Prydz, 2015). However, glycosaminoglycan chains are challenging to analyse due to their heterogeneous and anionic nature. Rarely are intact glycosaminoglycans analysed, exceptions being bikunin and decorin (Ly et al., 2011; Yu et al., 2017). Most commonly individual endoglycosidase-generated repeat units or, in the case of chondroitin and heparan, the GlcAβ3Galβ3Galβ4XylβOSer tetrasaccharide core are examined (Kon et al., 1991; Li et al., 2012); also chondroitinase-treated chains, trimmed down to a residual hexasaccharide but still attached to the core protein, have been analysed (Noborn et al., 2018). Phosphorylation and sulphation of core xylose and galactose residues are known (Tone et al., 2008), but other modifications of this region are rare; however, Siaα2,3Galβ1,4Xyl sequences can be generated in mammalian cells if glycosaminoglycan biosynthesis is impaired (Bai et al., 1999; Wen et al., 2014).
In nematodes, it has been shown that the chondroitin/heparan common tetrasaccharide core is conserved in the model nematode Caenorhabditis elegans, but also that the chondroitin chains are primarily not sulphated in this organism (Dierker et al., 2016; Guerardel et al., 2001; Izumikawa et al., 2016; Yamada et al., 1999). On the other hand, C. elegans has been valuable in elucidating the role of glycosaminoglycans in animal development as each of the sqv (squashed vulva) mutants affects a different gene required for chondroitin sulphate biosynthesis, including formation of the tetrasaccharide core (Hwang et al., 2003). There is apparently little information regarding glycosaminoglycans of nematodes other than C. elegans, even though many are parasitic species. In contrast, there is relatively rich information available on N-glycomes from this phylum, whereby we have recently reported novel N-glycan structures from C. elegans (Yan et al., 2015a; Yan et al., 2015b; Yan et al., 2018) as well as from Oesophagostomum dentatum (Jimenez-Castells et al., 2017). As part of these studies, we employed hydrazinolysis (Jimenez-Castells et al., 2017; Yan et al., 2018). This method is primarily used for release of N-glycans, but in case of altered conditions can be applied to prepare mucin-type O-glycans (Fukuda et al., 1976; Patel et al., 1993).
Unexpectedly, upon applying ‘N-glycan-specific’ hydrazinolysis conditions on nematode glycopeptides, we also released a set of structures which, due to the fragmentation pattern, were concluded to be based on xylose as the reducing terminus. Thereby, we could analyse short glycosaminoglycan-type O-glycans from both C. elegans and O. dentatum by off-line LC-MS in combination with enzymatic and chemical treatments. While the core structure of the unsulphated GAG-like O-glycans in C. elegans was as expected, related oligosaccharides from O. dentatum were decorated with extra galactose and phosphorylcholine residues, which is indicative of species-specific aspects of nematode glycosylation. A selection of the resulting data is discussed in order to illustrate the difference in the GAG-like O-glycomes between C. elegans and O. dentatum.
Results
Hydrazinolysis as a method to release glycosaminoglycan chains
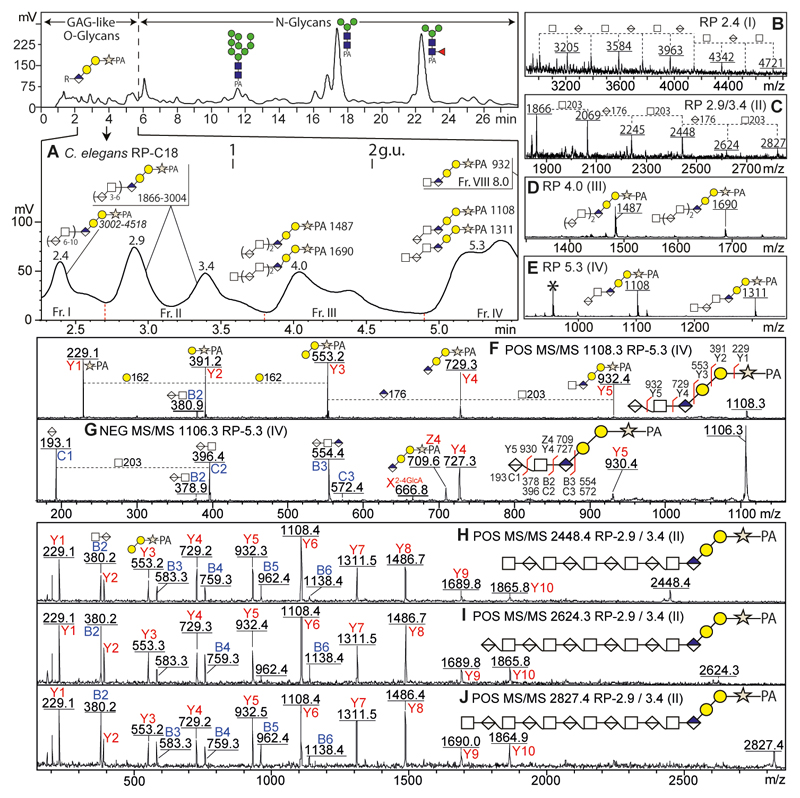
As part of our ongoing studies on the N-glycosylation of the model nematode C. elegans, we performed hydrazinolysis under ‘N-glycan-specific’ conditions at 100 °C for 5 h (Yan et al., 2018), which is not recommended for mucin-type O-glycans due to ‘peeling’ of the reducing terminus. After hydrazinolysis and pyridylamination, we employed an off-line HPLC-MS approach to analyse the released glycans; thereby, we were surprised that we identified some glycans in the early fractions with a fragmentation pattern indicative of a reducing-terminal pentose (m/z 229; i.e., 132 + PA-label) as opposed to N-acetylglucosamine (m/z 300; i.e., 203 + PA-label) occurring on 2-aminopyridine-labelled N-glycans. Thus we surmised that GAG-like O-glycans could also be released by hydrazinolysis at 100 °C and that these could be easily separated from N-glycans by RP-HPLC. Indeed, MALDI-TOF MS and MS/MS of all fractions suggested that the former eluted between 2-5 minutes (< 2.5 g.u.; Figure 1 A) while the latter were isolated in the subsequent fractions (7-35 minutes; > 2.5 g.u.). Like other chemical glycan-release methods, hydrazinolysis is known to result in peeling (loss of the reducing-terminal residue), but these artefactual products are indistinguishable from those of endogenous endoglycosidases. However, either are easily identified due to intense positive-mode MS/MS Y-ions at m/z 300 and 462 (Man0-1GlcNAc1-PA for N-glycans lacking one reducing-terminal GlcNAc) and 259, 421 and 597 (GlcA0-1Gal1-2-PA for GAG-like O-glycans lacking the xylose residue), which contrasts with the typical Y1-ion at m/z 229 (Xyl1-PA) indicative of an intact glycosaminoglycan reducing terminus. On this basis, we presume a minor degree of peeling, but the majority of the GAG-like structures is concluded to be intact (Supplementary Figure 1).
Figure 1. RP-HPLC and MALDI-TOF-MS analyses of GAG-like O-glycans from Caenorhabditis elegans.
(A) After hydrazinolysis and pyridylamination, glycans from C. elegans were separated by an Agilent Hypersil ODS-C18 column; the zoom shows the region in which GAG-like O-glycans elute. (B-E) MS of fractions I-IV show increments of m/z 176 (HexA) and 203 (HexNAc) compatible with the basic sequence [HexNAcn,n-1GlcAβ3n]Galβ3Galβ4Xyl-PA; shown are negative (B) or positive MS (C-E). (F-J) Selected positive (F, H-J; [M+H]+) or negative (G; [M-H]-) MS/MS. Fragments in positive mode are annotated for m/z 229 (Y1-ion, Xyl1-PA), 391 (Y2-ion, Gal1Xyl1-PA), 553 (Y3-ion, Gal2Xyl1-PA), 729 (Y4-ion, GlcA1Gal2Xyl1-PA), 932 (Y5-ion, HexNAc1GlcA1Gal2Xyl1-PA) and subsequent Y-ions with serial addition of GlcA and HexNAc. The fragments in negative mode of the m/z 1106 glycan showed specific B/C and Y/Z-ions associated with the HexA residue. MS/MS and glycan annotations ( HexNAc;
HexNAc;  HexA;
HexA;  GlcA;
GlcA;  Gal;
Gal;  Xyl) are based on standard nomenclatures (Domon and Costello, 1988; Varki et al., 2015). Calibration is in terms of glucose units (g.u.). Elution positions of major N-glycans between 6-24 min are annotated (Yan et al., 2018); for further and wider-range MS and MS/MS analyses of C. elegans GAG-liked glycans, refer to Supplementary Figures 1 and 2.
Xyl) are based on standard nomenclatures (Domon and Costello, 1988; Varki et al., 2015). Calibration is in terms of glucose units (g.u.). Elution positions of major N-glycans between 6-24 min are annotated (Yan et al., 2018); for further and wider-range MS and MS/MS analyses of C. elegans GAG-liked glycans, refer to Supplementary Figures 1 and 2.
Screening of glycosaminoglycan-like O-glycans from C. elegans
The GAG-like O-glycans are based on a GlcAβ3Galβ3Galβ4Xyl tetrasaccharide core sequence common to all chondroitin and heparan sulphates and has been previously found in C. elegans (Yamada et al., 2002). A closer examination of the relevant MS and MS/MS spectra indeed revealed that [HexNAcn,n-1HexAn]GlcA1Gal2Xyl1-PA sequences (m/z 932 to 4721) were eluted within the first five minutes of the RP-HPLC run, with the larger oligosaccharides tending to elute earlier due to an increasing number of hexuronic acid residues (Figure 1A). All GAG-like O-glycans from C. elegans exhibited intense core-sequence Y-ions in positive MS/MS mode (Figure 1 F and 1 H-J) at m/z: 229 (Y1; Xyl-PA), 391 (Y2; Gal1Xyl-PA), 553 (Y3; Gal2Xyl-PA), 729 (Y4; GlcA1Gal2Xyl-PA), and subsequent serial addition of N-acetylhexosamine (HexNAc, +203 Da) and hexuronic acid (HexA, +176 Da); B ions were also observed in positive mode at m/z 380 to 1138 (B2-B6; HexNAc1-3GlcA1-3; Figure 1 H-J). However, B-ions were relatively more intense in negative mode with the largest fragments being B16 for the m/z 3584 species (Figure 1G and Supplementary Figure 2).
Considering the presence of hexuronic acid (primarily assumed to be glucuronic acid) in GAG-like chains, the relevant RP-HPLC fractions were also subject to MALDI-ToF MS and MS/MS in negative ion mode. As demonstrated for HexNAc1HexA2Gal2Xyl-PA (m/z 1108/1106), these spectra revealed specific BC and YZ-ions associated with its HexA residues including an intense C1-ion at m/z 193 (HexA+H2O, Figure 1 F). The MS/MS data are thereby in accordance with previously-published data demonstrating isolation from C. elegans of unsulphated chondroitin-like tetra- and pentamers following β-elimination (Guerardel et al., 2001), but here we actually observed up to 25 saccharide units.
Screening of glycosaminoglycan-like O-glycans from O. dentatum
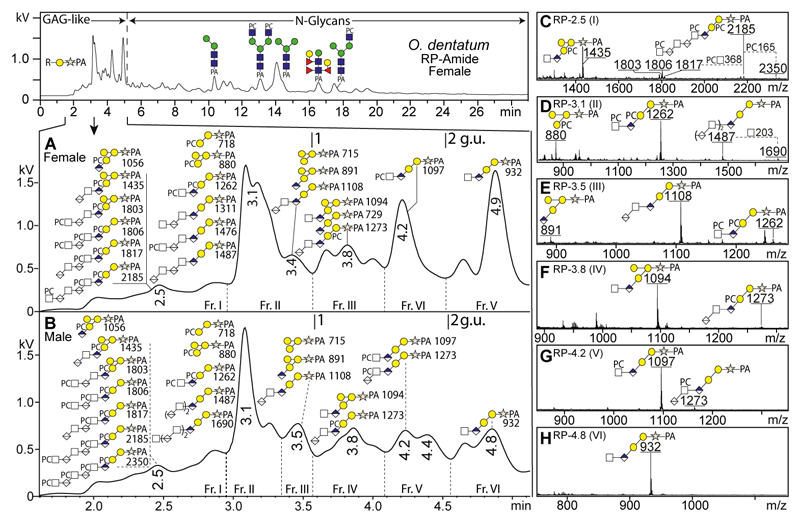
We recently also employed hydrazinolysis to release N-glycans from O. dentatum (Jimenez-Castells et al., 2017), which is a common parasite of pigs with the severity of the symptoms depending on the parasite load. The workflow was similar as for C. elegans, but the glycans were separated on a fused core RP-amide column, as well as on ODS-C18 and AS11 columns (Supplementary Figure 3). Again, a range of glycans with m/z 229 Y1-fragments was detected in early-eluting fractions within the first five minutes (< 2.5 g.u.; Figure 2). However, the range of putative glycosaminoglycan-like O-glycans from O. dentatum contrasted with those from C. elegans as there were Δm/z values suggestive of additional hexose and/or phosphorylcholine residues. Indeed, MS/MS fragmentation of GAG-like O-glycans from O. dentatum confirmed that several structures were decorated by phosphorylcholine and/or an additional hexose, thus forming novel sequences of PC0-4HexNAcn/n-1GlcAnHex3Xyl1. Therefore, we also treated selected GAG-containing fractions with exoglycosidases and hydrofluoric acid to elucidate further aspects of their structure.
Figure 2. RP-HPLC and MALDI-TOF-MS analyses of GAG-like O-glycans from Oesophagostomum dentatum.
(A and B) After hydrazinolysis and pyridylamination, glycans from male and female O. dentatum were separated on an RP-amide column; the zoom of the chromatograms from females (A) and males (B) focuses on the region in which GAG-like O-glycans elute. (C-H) Positive mode MS of male fractions I-VI is compatible with the basic sequence of [HexNAcn,n-1-GlcAβ3n]-Galβ3-Galβ4-Xyl-PA as well as unprecedented core decoration with phosphorylcholine (PC) and/or galactose. Proposed structures (all [M+H]+) were confirmed by MS/MS as well as by chemical or enzymatic digestions. Calibration is in terms of glucose units (g.u.). Elution positions of major N-glycans between 5-24 min are annotated (Jimenez-Castells et al., 2017). For the ODS-C18 RP-HPLC and HIAX NP-HPLC chromatograms of O. dentatum GAG-like O-glycans, refer to Supplementary Figure 3.
Anionic and neutral glycosaminoglycan-like O-glycans from O. dentatum
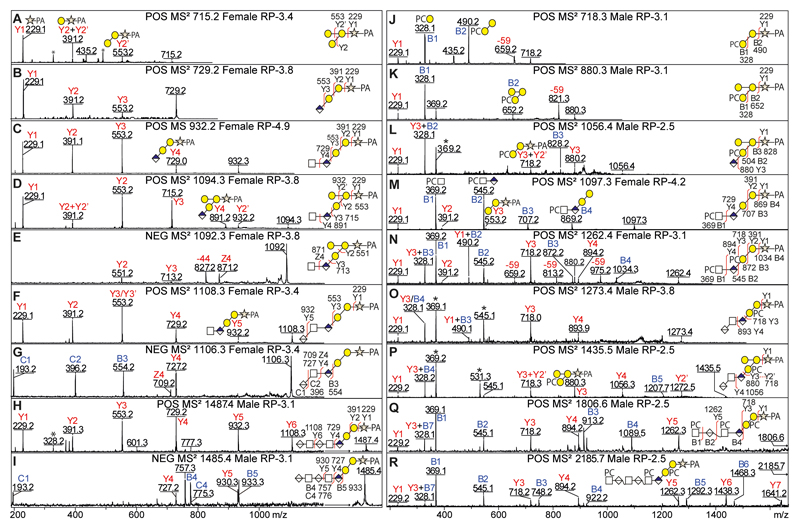
The basic core sequences were investigated in both O. dentatum male and female O-glycomes by positive and negative MS/MS (Figure 3 and Supplementary Figure 4) modes to confirm the presence of GAG-like O-glycans. Their fragmentation patterns were rather similar to those observed in C. elegans in terms of the Y/B-ion intensities and distributions. However, the maximum number of HexNAc1HexA1 repeat units detected for O. dentatum male and female (n = 3) was lower than for C. elegans (n = 10).
Figure 3. Positive and negative MS/MS of GAG-like O-glycans from Oesophagostomum dentatum.
The positive and negative MS/MS of anionic GAG-like O-glycans from O. dentatum (A-I) show data on structures from m/z 715 to 1487 (HexNAc0-2GlcA0-3Gal2-3Xyl1-PA). Most of these structures have the basic GAG-like O-glycan core sequence and share some of the same Y-fragmentation features as described in C. elegans (see Figure 1); however, a few possess a trigalactosylated core sequence (D) as indicated by a specific Y3-ion in positive mode at m/z 715 (Gal3Xyl1-PA). The positive MS/MS of phosphorylcholine-modified GAG-like O-glycans from O. dentatum (J-R) exhibit key PC-fragments at m/z 328 (PC1Hex1) and/or 369 (PC1HexNAc1) while the ion-loss of 59 Da is due to an inherent PC breakdown. The fragment signature of a PC and galactosylated core is an Y3-ion of PC1Hex3Xyl-PA at m/z 880. * Contaminant fragments derived from co-eluting PC-containing glycans; for MS/MS of m/z 1056 and 1435 in HIAX fractions lacking the m/z 369 co-fragment, refer to Supplementary Figure 4 A and D.
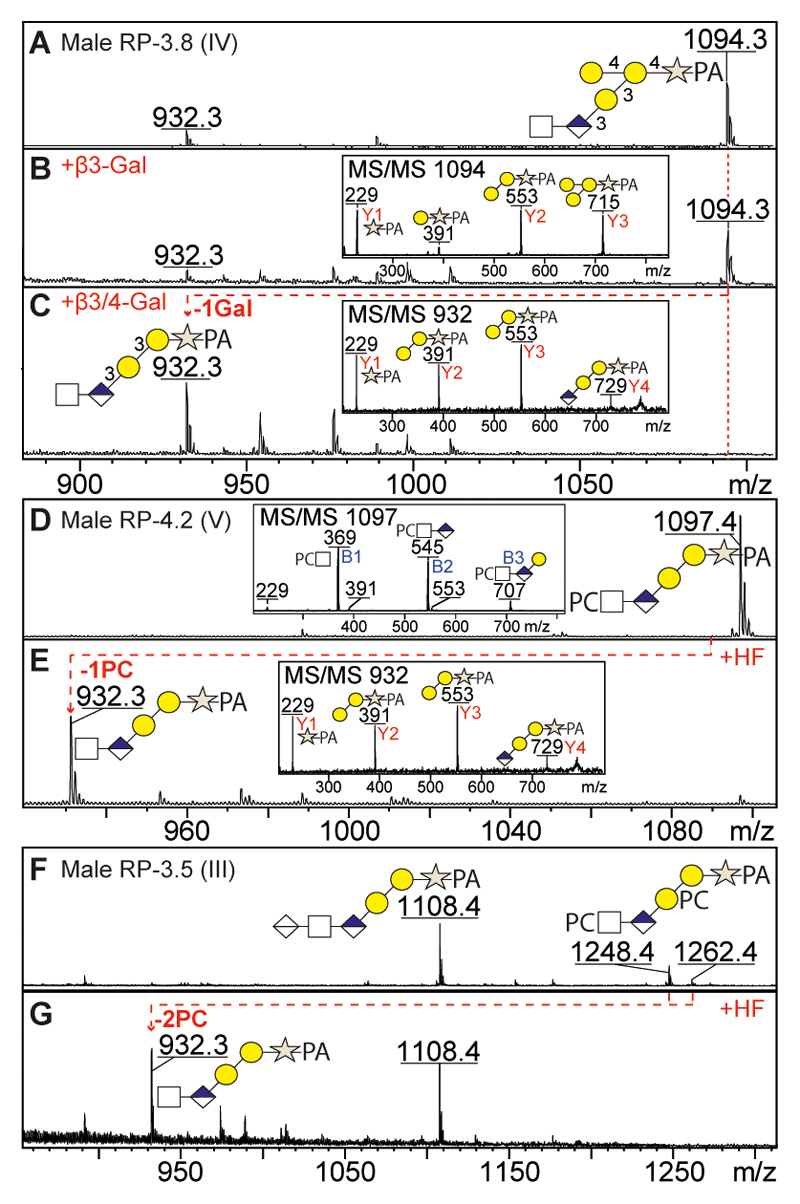
The novel hexose-substituted core sequence was observed in both O. dentatum male and female O-glycomes and correlated with an intense Y3-ion at m/z 715 corresponding to Hex3Xyl-PA (Figure 3 D). To investigate the nature and position of the additional hexose, we treated an example fraction with either β3-galactosidase, β3/4-galactosidase or jack bean α-mannosidase (Figure 4 A-C); only the β3/4-galactosidase was able to remove one hexose from HexNAc1GlcA1Hex3Xyl1-PA (m/z 1094), which suggested that the hexose branch is a β4-linked galactose. Furthermore, the positive MS/MS of this structure (Figure 3 D; m/z 1094) indicated that the branching galactose was linked to the first galactose of the core sequence as judged by the intense Y2 and Y3 ions at m/z 553 and 715 (Gal2-3Xyl1-PA), while the Y2+Y2’ ion at m/z 391 (Gal1Xyl1-PA) is much less intense as it results from a double fragmentation event. Upon de-galactosylation to HexNAc1GlcA1Gal2Xyl1-PA (m/z 932; Figure 4C), the Y2 ion at m/z 391 (Gal1Xyl1-PA) is of increased intensity as this time it results from a single fragmentation. The conclusion regarding the position of the extra hexose was further confirmed in PC-containing structures (see below).
Figure 4. Identification of new core sequences of GAG-like O-glycans from O. dentatum by enzymatic and chemical digestions (A-G).
The HexNAc1GlcA1Hex3Xyl1-PA (m/z 1094, see Figure 3D) was analysed by positive MS and MS/MS before (A) and after β3-galactosidase (B) or β3/4-galactosidase (C) digestions; only the β3/4-galactosidase was able to remove a single hexose, as also seen by the alteration in the MS/MS spectra (see insets), thus indicating that the third hexose is a branching β4-linked galactose. The PC1-2HexNAc1GlcA1Hex2Xyl1-PA glycans (m/z 1097 and 1262; see Figures 2 E/G and 3 M/N) were analysed by positive MS before (D, F) and after (E, G) hydrofluoric acid (HF) treatment which removed one or two phosphorylcholine residues to yield HexNAc1GlcA1Hex2Xyl1-PA at m/z 932; shifts in the MS/MS spectra (see insets) show the absence of the PC-containing B-ions (e.g., PC-HexNAc at m/z 369) and the dominance of the PA-containing Y-ions (e.g., Xyl-PA at m/z 229) after HF treatment. Some partial loss of methyl groups from the PC units during the release and work-up procedure resulted in the observation of ions at m/z 1248 in addition to the bona fide structure at m/z 1262 (F).
Zwitterionic glycosaminoglycan-like O-glycans from O. dentatum
Modifications with phosphorylcholine are associated with a mass increment of 165 Da and occur e.g., on N-glycans of nematodes, Lepidoptera, cestodes and a mollusc as well as on lipopolysaccharides of some bacteria and glycolipids of some invertebrates; this modification can be detected in positive MS mode and has a proven sensitivity to hydrofluoric acid treatment (Eckmair et al., 2016; Paschinger et al., 2012; Paschinger and Wilson, 2015; Yan et al., 2015c). In contrast to the ODS-C18 HPLC column, the presence of phosphorylcholine reduces retention times on the RP-amide HPLC column, an effect even more pronounced for multi-PC containing structures occurring in the 2.5 minute HPLC fractions of O. dentatum male and female glycomes (< 2 g.u.; Figure 2 A-C and Supplementary Figure 3).
The MS/MS fragmentation patterns of PC-containing structures of GAG-like O-glycans from O. dentatum demonstrated a range of structures from PC1Hex2Xyl1-PA to PC3HexNAc3GlcA3Hex2Xyl1 (m/z 718-2185; Figure 3 J-R and Supplementary Figure 4) and revealed intense PC-fragments at m/z 328 (PC1Hex1) and m/z 369 (PC1HexNAc1) which indicated that PC either substitutes a HexNAc or Gal. Also, the presence of PC on a galactosylated core sequence was deduced in the case of PC1GlcA1Gal3Xyl-PA and PC1HexA1-2HexNAc1-2GlcA1Gal3Xyl-PA (m/z 1056, 1435 and 1814; see Figure 3 L and Supplementary Figure 4D and F) which showed Y3-ions at m/z 718 (PC1Gal2Xyl-PA) and 880 (PC1Gal3Xyl-PA).
In the case of PC2HexNAc1GlcA1Hex2Xyl-PA (m/z 1262; Figure 3 N), fragments were observed at m/z 872 (B3; PC2HexNAc1GlcA1Hex1), 391 (Y2; Hex1Xyl-PA) and 718 (Y3; PC1Hex2Xyl-PA), but not at m/z 556 (theoretically PC1Hex1Xyl1-PA). Thus, the MS/MS data demonstrate that one phosphorylcholine is linked to HexNAc, while the other one substitutes the second core galactose and so it is concluded that the structure is PCHexNAcGlcAβ3(PC)Galβ3Galβ4Xyl. The PC position is similar in the case of an additional galactose (Figure 3 L and P) as judged by positive fragments at m/z 880 (Y3; PC1Gal3Xyl-PA) and 718 (Y3+ Y2’; PC1Gal2Xyl-PA), which excludes the possibility that the branching galactose is substituted with PC. Furthermore, the PC1-2HexNAc1GlcA1Gal2Xyl1-PA structures (m/z 1097-1262; Figure 4 D and F) were treated with HF which specifically removed all phosphodiester substitutions to yield HexNAc1GlcA1Gal2Xyl1-PA (m/z 932; Figure 4 E and G), thus confirming the presence of the PC residues. The products of HF treatment also displayed an MS/MS pattern dominated by Y-fragments, rather than by the comparatively very easily-ionising PC-containing B-fragments present in the case of the original glycans (compare insets in Figure 4 D and E).
Discussion
In this study, we found that short GAG-like O-glycans were efficiently co-released with N-glycans by hydrazinolysis, which enabled their subsequent labelling by pyridylamination (PA) and analysis by an off-line LC-MS strategy. In other studies, the core regions of chondroitin and heparan chains have been analysed after release by either β-elimination which excludes the possibility of subsequent labelling by reductive amination. Alternative strategies have been to use either an endo-β-xylosidase (apparently not commercially available) or lithium hydroxide, both of which enable subsequent labelling (Kon et al., 1991; Yamada et al., 2002). Previously, hydrazinolysis of proteoglycans was apparently only used to de-N-acetylate the HexNAc residues prior to nitrous acid deamination which is a method for isolation of modified forms of the individual disaccharide repeats (Shaklee and Conrad, 1986). Thus, we can demonstrate a new use for hydrazine in the study of glycosaminoglycan-like structures.
The GAG-like O-glycans isolated from C. elegans and from O. dentatum were separated from N-glycans by RP-HPLC which enabled subsequent MALDI-TOF MS and MS/MS as well as chemical or enzymatic treatments. Thereby, it is concluded that hydrazinolysis at high temperature (‘N-glycan-specific’ conditions) is able to release simultaneously GAG-like O-glycans and N-glycans, but destroys most of the mucin-type O-glycans. Although there is a degree of artefactual ‘peeling’ of the natural reducing terminal monosaccharide (i.e., loss of xylose) or demethylation of phosphorylcholine (Δm/z 14), this was probably no more than 20% in our hands when performing hydrazinolysis on these samples, but an exact figure is difficult to estimate on the basis of fluorescence and ionisation intensities of HPLC fractions containing multiple components. In terms of separation, a trend to earlier RP-HPLC elution of the longer oligosaccharides was observed, which is in keeping with the increasing number of hexuronic acid residues.
The results for both organisms were surprisingly different with larger and unmodified core sequences being present in C. elegans, while male and female O. dentatum expressed GAG-like structures with new core sequences with an additional galactose and/or phosphorylcholine. Furthermore, no significant difference was observed between male and female in O. dentatum samples in terms of quality and quantity of GAG-like glycans. Based on previous studies, including data on the single pentasaccharide containing xylose at the reducing terminus identified by NMR after β-elimination (Guerardel et al., 2001), we presume that we have isolated unsulphated chondroitin chains based on GalNAcβ4GlcAβ3Galβ3Galβ4Xyl. On the other hand, the strategy of using hydrazinolysis followed by fluorescent labelling allowed us to isolate and identify more and larger chondroitin-like O-glycans than previously found in nematodes (up to almost 5 kDa in C. elegans; Figure 1), but structures larger than 25 saccharide units were not observed. Furthermore, chondroitin in its sulphated form is rare in C. elegans and possibly represents less than 1% of total GAG in this organism (Izumikawa et al., 2016); thus, it would be a challenge to detect such chains.
Several glycosyltransferases genes (sqv-1, -2, -3 and -8) are required to synthesise the core sequence of GAG-like O-glycans in C. elegans, all of which are essential for its development (Hwang et al., 2003). However, the biosynthetic origin of the additional β4-linked galactose and phosphorylcholine residues on the O. dentatum [PCGalNAcβ4GlcAβ3]nPCGalβ3(Galβ4)Galβ4Xyl structures is unknown. On the other hand, similar to its N-glycans (Jimenez-Castells et al., 2017), the degree of phosphorylcholinylation on O. dentatum glycosaminoglycan-like sequences is relatively high as compared to the total of the identified structures, but also represents the first zwitterionic modification to be detected on this class of glycan. Thus, our study is another indication of the high variability of glycoconjugate structures in invertebrates, which can only be uncovered by the use of an extended methodological portfolio and in-depth analysis of the glycomic data.
Materials and Methods
Nematode culture and isolation
C. elegans (wild-type N2 strain) was cultivated in our laboratory using standard conditions with Escherichia coli strain OP50 (i.e., maintained on nematode growth medium agar plates, grown in larger scale in liquid S complete medium and isolated by sucrose density centrifugation prior to freezing at -80 °C). O. dentatum adults (OD-Hann strain) were recovered from the large intestines of infected pigs by agar-gel migration and visually separated into males and females prior to washing in 0.9 % NaCl at 38°C, sedimentation, snap freezing and storage at -80°C until use (Slotved et al., 1996).
Hydrazinolysis of nematode glycopeptides
Worms were homogenised and subject to proteolysis with pepsin (overnight at 37 °C; for C. elegans) or thermolysin (2 hours at 70 °C; for O. dentatum) prior to hydrazinolysis and pyridylamination; based on previous procedures (Jimenez-Castells et al., 2017; Patel et al., 1993). Briefly, 1 mg protease per gram of wet weight material was employed to prepare glycopeptides, which were purified by cation exchange (50W×8 Dowex, BioRad; elution with 0.5 M ammonium acetate, pH 6) and gel filtration (G25, GE Healthcare; elution with 0.5% acetic acid) prior to transfer into a glass reaction tube and drying overnight. Hydrazinolysis was then performed in 500 μL of anhydrous hydrazine (prepared from hydrazine monohydrate, Sigma) at 100°C for 5 h and removal of unreacted reagent by centrifugal evaporation. Glycans were re-N-acetylated using 450 μL of 100 mM sodium bicarbonate and 21 μL of acetic anhydride at 0°C for 1 h. Then, the reducing ends were liberated using 600 μL of 5% trifluoroacetic acid at 4°C for 1 h. Thereafter, the free glycans were purified by cation exchange (50W×8 Dowex; eluted with 2% acetic acid), non-porous graphitized carbon (eluted with 40% acetonitrile containing 0.1% TFA) and C18 columns (eluted with water). Finally, glycans were fluorescently labelled using 2-aminopyridine (Hase et al., 1984). For a fuller description of these methods, refer to the Supplement.
HPLC purification of O-glycans
Oligosaccharides with xylose at the reducing terminus were separated from N-glycans by RP-HPLC using a Shimadzu Nexera HPLC system equipped with a fluorescence detector (RF 20 AXS) and reversed phase columns (either Ascentis Express RP-Amide, Sigma-Aldrich, or Hypersil C18, Agilent). Glycans were eluted using a buffer system of 100 mM ammonium acetate (pH 4.0) and 30% (v/v) methanol at a flow rate of either 0.8 or 1.5 ml/min (Hykollari et al., 2017). Glycans were detected by fluorescence with excitation/emission wavelengths of 320/400 nm. RP-HPLC columns were calibrated daily in terms of glucose units using a pyridylaminated dextran hydrolysate; the order of elution of the standards was confirmed by MALDI-TOF MS of collected calibrant fractions. In the case of O. dentatum, the glycans eluting early from the RP-amide column were re-pooled and applied to an AS11 HIAX/NP-HPLC column using a set of oligomannosidic glycans for calibration (see Supplement).
MALDI TOF MS analysis
Glycans in individual RP-HPLC fractions were analysed by MALDI-TOF MS (Autoflex Speed, Bruker Daltonics, Germany) in positive and negative ion mode using FlexControl 3.4 software and 6-aza-2-thiothymine (ATT) as matrix; MS/MS to confirm the composition of all proposed structures was performed by laser-induced dissociation (precursor ion selector was generally set to ±0.6%). The detector voltage was typically 1977 V for MS and 2133 V for MS/MS; 1000-3000 shots from different regions of the sample spots were summed. Spectra were processed with the manufacturer’s software (Bruker Flexanalysis 3.3.80) using the SNAP algorithm with a signal/noise threshold of 6 for MS (unsmoothed) and 3 for MS/MS (four-times smoothed). All MS and MS/MS spectra were manually interpreted on the basis of the mass fragmentation pattern and results of chemical and enzymatic treatments; O-xylose-based glycans were distinguished from N-glycans by the respective presence in positive MS/MS of Y1-ions at m/z 229 (Xyl1-PA) or 300 (HexNAc1-PA).
Structural elucidation using exoglycosidases and chemical treatment
Exoglycosidase treatment was performed using either recombinant β3/4-galactosidase from Aspergillus niger prepared in house (Dragosits et al., 2014) or recombinant β3-galactosidase from Xanthomonas manihotis from New England Biolabs (Wong-Madden and Landry, 1995). In general, a 1-2 μl aliquot of a lyophilysed and re-dissolved HPLC fraction into 10 μL was incubated with 0.2 μl exoglycosidase and 0.5 μl 100 mM ammonium acetate solution, pH 5.0, overnight at 37 °C. For removal of phosphorylcholine, aliquots of O-glycan fractions were dried in a Speed-Vac and then incubated with 3 μl of 48% (w/v) hydrofluoric acid (HF) on ice for 24 hours prior to drying again (Dennis et al., 1998). Chemically or enzymatically treated glycans were re-analysed by MALDI-TOF MS and MS/MS without further purification.
Supplementary Material
Acknowledgements
This work was supported by the Austrian Science Fund (FWF) and by the European Union; S.Y. and K.P. are FWF fellows (grants P30021 and P25058); J.V. was an experienced researcher within the Glycopar Initial Training Network (PITN-GA-2013-608295). We also thank the staff of Ludger Ltd for an introduction to hydrazinolysis, especially Radoslaw Kozak and Daniel Spencer, as well as Markus Blaukopf for further help with hydrazinolysis and Bärbel Ruttkowski for nematode collection.
Footnotes
Note:
The authors declare that they have no conflict of interest.
References
- Bai X, Wei G, Sinha A, Esko JD. Chinese hamster ovary cell mutants defective in glycosaminoglycan assembly and glucuronosyltransferase I. J Biol Chem. 1999;274(19):13017–24. doi: 10.1074/jbc.274.19.13017. [DOI] [PubMed] [Google Scholar]
- Dennis RD, Lochnit G, Geyer R. Strategies for preliminary characterization of novel amphoteric glycosphingolipids. Methods Mol Biol. 1998;76:197–212. doi: 10.1385/0-89603-355-4:197. [DOI] [PubMed] [Google Scholar]
- Dierker T, Shao C, Haitina T, Zaia J, Hinas A, Kjellen L. Nematodes join the family of chondroitin sulfate-synthesizing organisms: Identification of an active chondroitin sulfotransferase in Caenorhabditis elegans. Sci Rep. 2016;6 doi: 10.1038/srep34662. 34662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon B, Costello CE. A Systematic Nomenclature for Carbohydrate Fragmentations in Fab-Ms Ms Spectra of Glycoconjugates. Glycoconj J. 1988;5(4):397–409. [Google Scholar]
- Dragosits M, Pflugl S, Kurz S, Razzazi-Fazeli E, Wilson IBH, Rendić D. Recombinant Aspergillus β-galactosidases as a robust glycomic and biotechnological tool. Appl Microbiol Biotechnol. 2014;98(8):3553–67. doi: 10.1007/s00253-013-5192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmair B, Jin C, Abed-Navandi D, Paschinger K. Multi-step fractionation and mass spectrometry reveals zwitterionic and anionic modifications of the N- and O-glycans of a marine snail Mol Cell Proteomics. 2016;15:573–597. doi: 10.1074/mcp.M115.051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kondo T, Osawa T. Studies on the hydrazinolysis of glycoproteins. Core structures of oligosaccharides obtained from Porcine thyroglobulin and pineapple stem bromelain. J Biochem. 1976;80(6):1223–32. doi: 10.1093/oxfordjournals.jbchem.a131393. [DOI] [PubMed] [Google Scholar]
- Guerardel Y, Balanzino L, Maes E, Leroy Y, Coddeville B, Oriol R, Strecker G. The nematode Caenorhabditis elegans synthesizes unusual O-linked glycans: identification of glucose-substituted mucin-type O-glycans and short chondroitin-like oligosaccharides. Biochem J. 2001;357(Pt 1):167–82. doi: 10.1042/0264-6021:3570167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase S, Ibuki T, Ikenaka T. Reexamination of the pyridylamination used for fluorescence labelling of oligosaccharides and its application to glycoproteins. J Biochem (Tokyo) 1984;95:197–203. doi: 10.1093/oxfordjournals.jbchem.a134585. [DOI] [PubMed] [Google Scholar]
- Hwang HY, Olson SK, Brown JR, Esko JD, Horvitz HR. The Caenorhabditis elegans genes sqv-2 and sqv-6, which are required for vulval morphogenesis, encode glycosaminoglycan galactosyltransferase II and xylosyltransferase. J Biol Chem. 2003;278(14):11735–8. doi: 10.1074/jbc.C200518200. [DOI] [PubMed] [Google Scholar]
- Hykollari A, Paschinger K, Eckmair B, Wilson IBH. Analysis of Invertebrate and Protist N-Glycans. Methods Mol Biol. 2017;1503:167–184. doi: 10.1007/978-1-4939-6493-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa T, Dejima K, Watamoto Y, Nomura KH, Kanaki N, Rikitake M, Tou M, Murata D, Yanagita E, Kano A, et al. Chondroitin 4-O-Sulfotransferase Is Indispensable for Sulfation of Chondroitin and Plays an Important Role in Maintaining Normal Life Span and Oxidative Stress Responses in Nematodes. J Biol Chem. 2016;291(44):23294–23304. doi: 10.1074/jbc.M116.757328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Castells C, Vanbeselaere J, Kohlhuber S, Ruttkowski B, Joachim A, Paschinger K. Gender and developmental specific N-glycomes of the porcine parasite Oesophagostomum dentatum. Biochim Biophys Acta. 2017;1861(2):418–430. doi: 10.1016/j.bbagen.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon A, Takagaki K, Kawasaki H, Nakamura T, Endo M. Application of 2-aminopyridine fluorescence labeling to glycosaminoglycans. J Biochem. 1991;110(1):132–5. doi: 10.1093/oxfordjournals.jbchem.a123531. [DOI] [PubMed] [Google Scholar]
- Li L, Ly M, Linhardt RJ. Proteoglycan sequence. Mol Biosyst. 2012;8(6):1613–25. doi: 10.1039/c2mb25021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Leach FE, 3rd, Laremore TN, Toida T, Amster IJ, Linhardt RJ. The proteoglycan bikunin has a defined sequence. Nat Chem Biol. 2011;7(11):827–33. doi: 10.1038/nchembio.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noborn F, Gomez Toledo A, Nasir W, Nilsson J, Dierker T, Kjellen L, Larson G. Expanding the chondroitin glycoproteome of Caenorhabditis elegans. J Biol Chem. 2018;293(1):379–389. doi: 10.1074/jbc.M117.807800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Gonzalez-Sapienza GG, Wilson IBH. Mass spectrometric analysis of the immunodominant glycan epitope of Echinococcus granulosus antigen Ag5. Int J Parasitol. 2012;42:279–285. doi: 10.1016/j.ijpara.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Wilson IBH. Two types of galactosylated fucose motifs are present on N-glycans of Haemonchus contortus. Glycobiology. 2015;25:585–590. doi: 10.1093/glycob/cwv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Bruce J, Merry A, Bigge C, Wormald M, Jaques A, Parekh R. Use of hydrazine to release in intact and unreduced form both N- and O-linked oligosaccharides from glycoproteins. Biochemistry. 1993;32(2):679–693. doi: 10.1021/bi00053a037. [DOI] [PubMed] [Google Scholar]
- Prydz K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules. 2015;5(3):2003–22. doi: 10.3390/biom5032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklee PN, Conrad HE. The disaccharides formed by deaminative cleavage of N-deacetylated glycosaminoglycans. Biochem J. 1986;235(1):225–36. doi: 10.1042/bj2350225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotved HC, Barnes EH, Bjørn H, Christensen CM, Eriksen L, Roepstorff A, Nansen P. Recovery of Oesophagostomum dentatum from pigs by isolation of parasites migrating from large intestinal contents embedded in agar-gel. Vet Parasitol. 1996;63(3–4):237–45. doi: 10.1016/0304-4017(95)00916-7. [DOI] [PubMed] [Google Scholar]
- Tone Y, Pedersen LC, Yamamoto T, Izumikawa T, Kitagawa H, Nishihara J, Tamura J, Negishi M, Sugahara K. 2-O-phosphorylation of xylose and 6-O-sulfation of galactose in the protein linkage region of glycosaminoglycans influence the glucuronyltransferase-I activity involved in the linkage region synthesis. J Biol Chem. 2008;283(24):16801–7. doi: 10.1074/jbc.M709556200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T, et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology. 2015;25(12):1323–1324. doi: 10.1093/glycob/cwv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Xiao J, Rahdar M, Choudhury BP, Cui J, Taylor GS, Esko JD, Dixon JE. Xylose phosphorylation functions as a molecular switch to regulate proteoglycan biosynthesis. Proc Natl Acad Sci U S A. 2014;111(44):15723–8. doi: 10.1073/pnas.1417993111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Madden ST, Landry D. Purification and characterization of novel glycosidases from the bacterial genus Xanthomonas. Glycobiology. 1995;5:19–28. doi: 10.1093/glycob/5.1.19. [DOI] [PubMed] [Google Scholar]
- Yamada S, Okada Y, Ueno M, Iwata S, Deepa SS, Nishimura S, Fujita M, Van Die I, Hirabayashi Y, Sugahara K. Determination of the Glycosaminoglycan-Protein Linkage Region Oligosaccharide Structures of Proteoglycans from Drosophila melanogaster and Caenorhabditis elegans. J Biol Chem. 2002;277(35):31877–31886. doi: 10.1074/jbc.M205078200. [DOI] [PubMed] [Google Scholar]
- Yamada S, van Die I, van den Eijnden DH, Yokota A, Kitagawa H, Sugahara K. Demonstration of glycosaminoglycans in Caenorhabditis elegans. FEBS Lett. 1999;459:327–331. doi: 10.1016/s0014-5793(99)01286-7. [DOI] [PubMed] [Google Scholar]
- Yan S, Brecker L, Jin C, Titz A, Dragosits M, Karlsson NG, Jantsch V, Wilson IB, Paschinger K. Bisecting Galactose as a Feature of N-Glycans of Wild-type and Mutant Caenorhabditis elegans. Mol Cell Proteomics. 2015a;14(8):2111–25. doi: 10.1074/mcp.M115.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Jin C, Wilson IB, Paschinger K. Comparisons of Caenorhabditis Fucosyltransferase Mutants Reveal a Multiplicity of Isomeric N-Glycan Structures. J Proteome Res. 2015b;14(12):5291–305. doi: 10.1021/acs.jproteome.5b00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Vanbeselaere J, Jin C, Blaukopf M, Wols F, Wilson IBH, Paschinger K. Core Richness of N-Glycans of Caenorhabditis elegans: A Case Study on Chemical and Enzymatic Release. Anal Chem. 2018;90(1):928–935. doi: 10.1021/acs.analchem.7b03898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Wilson IBH, Paschinger K. Comparison of RP-HPLC modes to analyse the N-glycome of the free-living nematode Pristionchus pacificus. Electrophoresis. 2015c;36(11–12):1314–29. doi: 10.1002/elps.201400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Duan J, Leach FE, 3rd, Toida T, Higashi K, Zhang H, Zhang F, Amster IJ, Linhardt RJ. Sequencing the Dermatan Sulfate Chain of Decorin. J Am Chem Soc. 2017;139(46):16986–16995. doi: 10.1021/jacs.7b10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.