Abstract
Norepinephrine (NE) is considered to exert an important modulatory influence upon the activity of gonadotropin-releasing hormone (GnRH) neurons. In the present study we used a transgenic GnRH-green fluorescent protein mouse model to examine the effects of NE on the electrical excitability of GnRH neurons in male and female mice. Gramicidin-perforated patch recordings demonstrated that NE (10-100µM) exerted a robust membrane hyperpolarization, with associated suppression of firing, in >85% of male prepubertal and adult GnRH neurons (n=25). The same hyperpolarizing action was observed in female GnRH neurons from diestrous (91%, n=11), proestrous (50%, n=14), estrous (77%, n=13) and ovariectomized mice (82%, n=11). A sub-population (<10%) of silent GnRH neurons in all groups responded to NE with hyperpolarization followed by the initiation of firing upon NE washout. The hyperpolarizing actions of NE were mimicked by α1 (phenylephrine) and β (isoproterenol) adrenergic receptor agonists, but α2 receptor activation (guanabenz) had no effect. Approximately 75% of the NE-evoked hyperpolarization was blocked by the α1 receptor antagonist prazosin, and 75% of GnRH neurons responded to both phenylephrine and isoproterenol. These findings indicate that NE acts through both α1 and β adrenergic receptors located on the soma/dendrites of GnRH neurons to directly suppress their excitability throughout the estrous cycle and following ovariectomy. These data force a re-analysis of existing models explaining the effects of NE on gonadotropin secretion.
Introduction
Investigations undertaken over many years have implicated norepinephrine (NE) as being one of the key neurotransmitters within the “GnRH neuronal network”. Pioneering studies by Sawyer and colleagues showed that the administration of adrenergic blockers prevented ovulation in the rabbit in the 1940s (1) and subsequent investigations have indicated roles for NE in the regulation of luteinizing hormone (LH) secretion in multiple species, including primates (2–5).
It is proposed that NE modulates the activity of gonadotropin-releasing hormone (GnRH) neurons directly to regulate LH release. A solid body of tract-tracing evidence has shown that brainstem NE neurons of the A1, A2 and A6 cell groups provide species-specific, inputs to brain regions where GnRH neuron soma are found (6–9). Early electron microscopic studies identified tritiated NE-containing nerve terminals synapsing on GnRH neurons in the rat (10), although supporting evidence for the direct regulation of GnRH neurons by NE (11) has been slow to emerge in this species. Recent studies in the mouse, however, have shown that that i) A2 and A6 neurons provide direct inputs to GnRH neurons (12), ii) dopamine-β-hydroxylase–immunoreactive terminals form synapses on GnRH neuron dendrites (13), and iii) adult GnRH neurons express transcripts for α1, α2 and β1 adrenergic receptors (14). Together, these observations indicate that NE acts directly upon GnRH neurons in the mouse.
The effects of NE on LH secretion have been assessed by both acute and chronic adrenergic receptor manipulations. In ovariectomized (OVX) rats, the acute infusion of NE (15–17) or the activation of ascending NE tracts (18), results in the suppression of LH pulse frequency. Interestingly, adrenergic receptor antagonists also suppress pulsatile LH secretion (19), suggesting that a set window of adrenergic receptor activation is essential for pulsatile LH secretion to occur. Importantly, other investigations have shown that pulsatile LH secretion can recover over time following the complete lesioning of NE pathways and inputs 20–22). Together, these studies suggested that NE exerted a permissive role in the regulation of pulsatile GnRH and LH secretion, whereby a set tone of adrenergic receptor activation is necessary for pulse generation but that this can be replaced under pathological situations (5). A further complexity to the issue of NE actions, is that OVX rats treated with estradiol and progesterone (OVX+E+P), respond to NE administration with an increase in LH secretion on the afternoon of the expected LH surge (16, 17). This suggests that gonadal steroids modulate the effects of NE on neural mechanisms regulating gonadotropin secretion.
The recent advent of GnRH transgenic mouse models has enabled the cellular and molecular features of adult GnRH neurons to be examined in situ (23, 24). Whereas the effects of adrenergic receptor manipulations on LH secretion are well characterized, there is presently no information on what actions NE may exert on adult GnRH neurons themselves. In an effort to provide clarity to the precise mechanisms through which NE modulates LH secretion, we have examined here the effects of adrenergic receptor activation on GnRH neuron excitability in male as well as diestrous, proestrous, estrous and OVX female mice.
Materials & Methods
Animals
All experiments were approved by the University of Otago Animal Welfare and Ethics Committee. Male and female GnRH-GFP mice (25) were housed under 12 h light/dark cycles (lights on at 7:00 A.M.) with ad libitum access to food and water. Male (32-100 days) and post-pubertal female (>35 days) mice were used for experiments. Vaginal smears were performed to determine the estrous cycles stage for females. One group of adult female mice were ovariectomized under Halothane anesthesia and used for experimentation 2 weeks later. Animals were killed between 10:00 and noon, and recordings made during the afternoon up to 19:00h.
Brain slice preparation and electrophysiology
Brains were prepared and recordings made as reported previously (25). Brains were rapidly removed and placed in ice-cold bicarbonate-buffered artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 118 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 11 D-glucose, 10 HEPES, 25 NaHCO3 (pH 7.4 when gassed with 95% O2 and 5% CO2). Brains were blocked and glued with cyanoacrylate to the chilled stage of a vibratome (VT1000S, Leica, Nussloch, Germany) and 150 to 200 µm-thick coronal slices containing the rostral preoptic area were cut. The slices were then incubated in oxygenated ACSF at room temperature for at least 1 hour before recording.
Slices were transferred to the recording chamber, held submerged, and continuously superfused with ACSF at a rate of 4-5 ml/min. The slices were viewed with an upright microscope (BX51WI, Olympus, Tokyo, Japan) and fluorescent GnRH neurons identified at 10x- and 40x-objective magnification by brief fluorescence illumination and then viewed and patched under Nomarski differential interference contrast optics. Patch pipettes were pulled from thin-wall borosilicate glass-capillary tubing (PG52151-4, WPI, Sarasota, USA) on a Flaming puller (P-97; Sutter Instruments Co., Novato, CA). The pipette solution was passed through a disposable 0.22 µm filter and contained (in mM): 130 KCl, 5 NaCl, 0.4 CaCl2, 1 MgCl2,10 HEPES, 1.1 EGTA (pH 7.3 with KOH). Gramicidin (Sigma, St. Louis, USA) was first dissolved in dimethylsulfoxide (Sigma) to a concentration of 2.5-5 mg/ml and then diluted in the pipette solution just before use to a final concentration of 2.5-5 µg/ml and sonicated for 15 minutes. Before backfilling the electrode with the gramicidin-containing solution, the tip of the electrode was loaded with a small volume of gramicidin-free pipette solution. The tip resistance of the electrodes was 4-7 MΩ. In initial experiments, access resistance was monitored and experiments begun when resistance stabilized at 50-90 Mohm. This typically took 15–20 min following giga-seal formation and always corresponded to the resting membrane potential of the cell reaching a stable level below -45 mV. In all subsequent cells, experiments were begun when the resting membrane potential reached a stable level below -45 mV. Spontaneous rupture of the seal was evident by a sudden over-shooting of action potentials above 0 mV. The junction potential between the patch pipettes and bath solution was nulled before giga-seal formation. Spontaneous activities were sampled online using a Digidata 1322A interface (Axon Instruments) connected to an IBM personal computer. Signals were filtered (10 kHz, Bessel filter of Multiclamp 700A) before digitizing at a rate of 1 kHz. Acquisition and subsequent analysis of the acquired data were performed using the Clampex9 suite of software (Axon Instruments). Any GnRH neuron that displayed a shift in resting membrane potential of > 2.0 mV was considered to have responded. Traces were plotted using the Origin7 software (MicroCal Software, Northampton, MA). All recordings were made at room temperature.
NE, phenylephrine (PE), guanabenz, DL-2-amino-5-phosphonovaleric acid (APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and isoproterenol were obtained from Sigma and dissolved directly in the ACSF. Prazosin and picrotoxin were obtained from Sigma and were first dissolved in dimethylsulfoxide and then diluted by 103 in ACSF before use. Tetrodotoxin (TTX) was obtained from Tocris and dissolved directly in the ACSF. Drugs were tested on cells held at their resting membrane potential by applying to the bathing solution 1or 2 min.
Statistical analysis
Experimental data were expressed as mean ± SEM. As more than one acceptable recording was only occasionally recorded from the same mouse brain, the N number for GnRH neurons is equivalent to the animal N number. Mann-Whitney U-test was used to examine differences between two experimental groups. One-way ANOVA was performed to test changes in membrane potential and resting membrane potential differences between >2 experimental groups. Changes in membrane potential were calculated by comparing pre-test levels (30s prior to drug) with the 30s period at the time of maximum hyperpolarization. The Chi-Square test was used to examine differences in the percentage of responding cells from different groups. A level of P < 0.05 was considered to be significant.
Results
In total, perforated-patch recordings were made from 88 GnRH neurons of which 80 were located in the rostral preoptic area and 8 in the medial septum (MS). Under perforated-patch, resting membrane potentials (RMP; mV) were -59.4±1.0 (males, N=39), -55.5±1.5 (diestrous females, N=11), -59.3±1.2 (proestrous females, N=14), -55.6±2.5 (estrous females, N=13) and -58.8±1.1 (OVX females, N=11).
NE hyperpolarizes GnRH neurons in male mice
In the first series of experiments, the effects of 1, 10, 30 and 100 µM NE on GnRH neuron excitability were examined in male mice. At 1µM, NE had no effect on 5 of 6 cells (RMP, -56.8±1.4 mV) with the remaining cell responding with a 3 mV hyperpolarization. At 10µM, NE generated a robust 6.7±1.0 mV hyperpolarization in 9 of 10 (90%; RMP, -58.0±1.1 mV)) GnRH neurons (Fig.1A,B). The remaining cell did not respond. The 30µM dose of NE was examined on two cells and both showed a 6-8mV hyperpolarization (Fig.1A). At 100µM, NE generated a similar 6.6±0.6 mV hyperpolarization in 17 of 20 GnRH neurons (85%; RMP, -60.2±1.3 mV)(Fig.1A,B), with one neuron exhibiting a depolarizing response and the other two not responding. Following rupture of the membrane, indicated by the sudden overshoot of the action potential, NE failed to evoke a response in adult male GnRH neurons. No differences in RMP existed between the GnRH neurons tested with the different concentrations of NE (ANOVA) and no relationship was found between the RMP of a cell and the degree of hyperpolarization evoked by NE (r=-0.297; p=0.13; linear regression analysis; n=27). In GnRH neurons exhibiting spontaneous firing, the NE hyperpolarization was associated with a cessation or marked reduction in firing rate with a return to the pre-test pattern of firing upon washout (Fig.1A). Three MS GnRH neurons were recorded and 2 of these were hyperpolarized by NE.
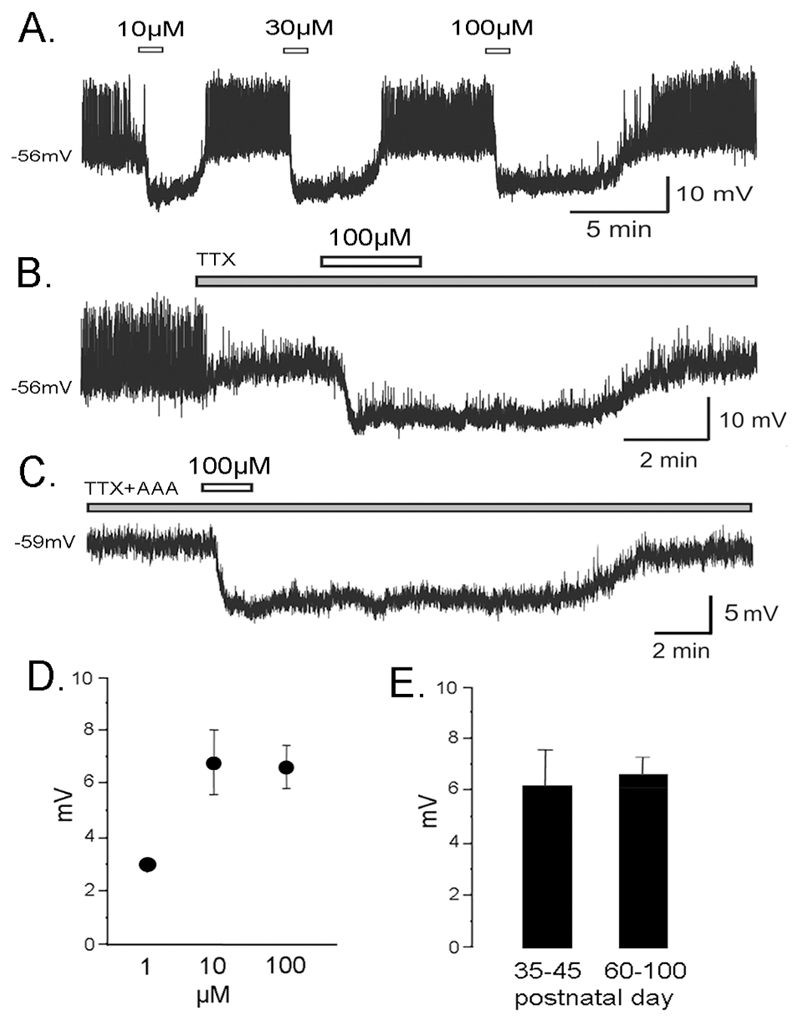
Figure 1.
NE suppresses the excitability of GnRH neurons. A. Perforated-patch recording from a GnRH neuron of a 35 day old male mouse in which three concentrations of NE were applied. B. Perforated-patch recording from a GnRH neuron of a 35 day old male mouse in which 0.5µM tetrodotoxin (TTX) was applied prior to NE. C. Perforated-patch recording from a GnRH neuron of a 45 day old male mouse in which 0.5µM tetrodotoxin (TTX) and the amino acid receptor antagonists (AAA) CNQX, APV & picrotoxin were applied prior to NE. D. Dose-response relationship showing the effect of increasing NE concentrations upon evoked membrane hyperpolarization in male mice (1µM, only one of 6 cells responded; 10µM, n=9; 100µM, n=16). E. Bar graphs show the mean+SEM membrane hyperpolarization evoked by 100µM NE in GnRH neurons of peripubertal (35-45 days old, n=5) and adult (>60 days old, n=12) male mice.
Responses to NE were short-lived, repeatable (Fig.1A) and dose-dependent. A dose-response relationship existed in terms of the % of GnRH neurons that responded to NE (1µM, 17%; 10µM, 90%; 100µM 85%; p<0.01; Chi-Square test). As only one cell responded to NE at 1µM (3mV hyperpolarization) it was not possible to test for significant differences across the NE concentrations (Fig.1D). A dose-response relationship also existed in terms of the duration of the hyperpolarization evoked by NE (Fig.1A). The duration of hyperpolarization in response to 10µM NE was 2.8±0.3 min (n=9) and 9.2±0.9 min following 100µM NE (n=11, p<0.001, Mann-Whitney U-test). In all subsequent experiments, 100µM NE was used.
The possibility that NE may exert direct actions on GnRH neurons was assessed by undertaking recordings in the presence of tetrodotoxin, a sodium channel antagonist that blocks action potential-dependent transmission. TTX quickly blocked the occurrence of action potentials, but did not inhibit the hyperpolarizing action of NE on GnRH neurons (Fig. 1B; N=3). As GnRH neurons are subjected to action potential-independent GABA (and possibly glutamate) release in the brain slice preparation (26, 27), experiments were also undertaken in the presence of TTX plus the amino acid receptor antagonsits, CNQX, APV and picrotoxin. NE continued to exert hyperpolarizing actions upon GnRH neurons under these conditions (Fig.1C; N=3).
As mice from peripubertal (postnatal days 35-45) to adult (>60 days) ages were investigated, the effects of NE on GnRH neurons from these two age groups was compared. The responses were the same in both age groups with 5 of 6 (83%; RMP, -59±1.2 mV) peripubertal GnRH neurons responding to 100µM NE with a 6.3±1.4 mV hyperpolarization compared with 12 of 14 (86%; RMP, -60.8±1.9 mV) adult GnRH neurons that displayed a 6.7±0.6 mV hyperpolarization (Fig. 1E).
NE hyperpolarizes GnRH neurons in ovariectomized and cycling female mice
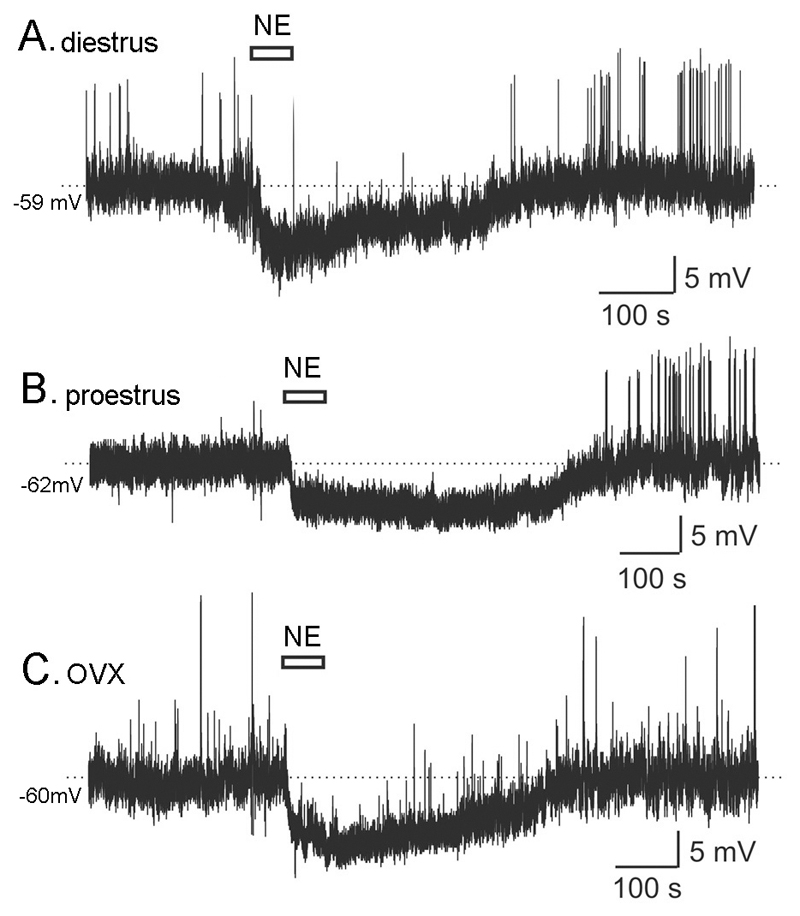
The effects of NE in the female were examined by making recordings from GnRH neurons obtained from diestrous, proestrous, estrous and ovariectomized mice. NE was found to exert hyperpolarizing effects similar to those recorded from male mice in each of the female groups (Figs.2 & 3). Ten of 11 (91%; RMP, -56.3±1.7 mV) diestrous GnRH neurons responded to 100µM NE with a mean membrane hyperpolarization of 6.8±0.5 mV (Fig.3) with the remaining cell not responding. Seven of 14 (50%; RMP, -61.4±1.1 mV) proestrous GnRH neurons responded to 100µM NE with a mean membrane hyperpolarization of 7.6±1.2 mV (Fig.3) with the remaining cells (RMP, -59.9±2.3 mV) not responding. Ten of 13 (77%; RMP, -56.0±2.1 mV) estrous GnRH neurons responded to 100µM NE with a mean membrane hyperpolarization of 6.9±0.9 mV (Fig.3) with the remaining cells not responding. Nine of 11 (82%; RMP, -58.8±1.1 mV) GnRH neurons in ovariectiomized mice responded to 100µM NE with a mean membrane hyperpolarization of 7.0±1.1 mV (Fig.3) with the remaining cells not responding. Spontaneously active neurons stopped firing during the NE-induced hyperpolarization and then continued their pattern of firing upon washout (Fig. 2A,C).
Figure 2.
NE suppresses the excitability of GnRH neurons throughout the cycle and following ovariectomy (OVX) in female mice. Representative perforated-patch recordings from GnRH neurons of diestrous (A), proestrous (B) and OVX (C) female mice showing the hyperpolarizing action of 100 µM NE.
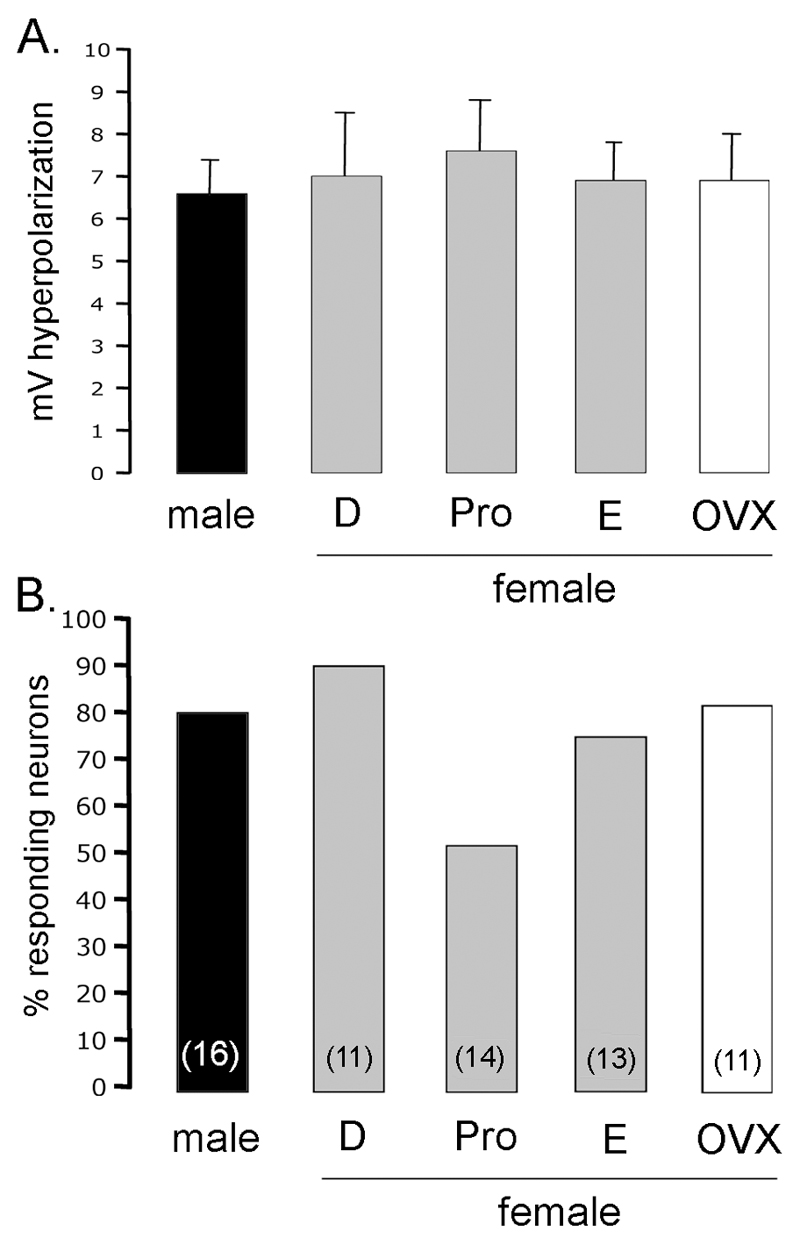
Figure 3.
NE exerts a similar effect on GnRH neurons across the estrous cycle. A. Bar graphs showing the mean+SEM hyperpolarization evoked by 100µM NE from GnRH neurons in male and female diestrous, proestrous, estrous and ovariectomized (OVX) mice. B. Bar graphs showing the % of GnRH neurons responding to NE in male and female diestrous, proestrous, estrous and OVX mice. The total number of GnRH neurons tested is given in brackets for each group. No significant differences were detected.
Across the four female groups, no significant differences were detected in the RMP (p>0.05, ANOVA), % of GnRH neurons responding to NE (p = 0.10; Chi-Square), degree of membrane hyperpolarization evoked by NE (p>0.05, ANOVA; Fig.3A), or the duration of the NE-induced hyperpolarization (p>0.05, ANOVA; diestrus 11.0±1.7 min; proestrus 10.1±0.4 min; estrus 10.6±1.4 min; OVX 9.6±1.4 min; n=11-14 per group).
In both males and females, a small sub-population of silent GnRH neurons was found that responded to NE with hyperpolarization followed by phasic or continuous firing after NE washout (Fig.2B). In total, 9 of 88 (10%) GnRH neurons responded in this way and were evenly distributed amongst the experimental groups (% silent cells responding to NE in this way was 25% males; 22% diestrus; 20% proestrus, and 28% estrus). The RMP of silent GnRH neurons that were activated following NE hyperpolarization (-60.6±1.2 mV; n=9) was not different to the RMP of silent GnRH neurons that remained silent after NE hyperpolarization (-57.6±1.2 mV; n=20; p>0.05; Mann-Whitney U-test). However, silent GnRH neurons with rebound activation exhibited a significantly greater NE-evoked hyperpolarization (8.2±0.5 mV; n=9) compared with silent GnRH neurons that remained silent following NE washout (6.0±0.4 mV; n=20; p<0.01; Mann-Whitney U-test).
The hyperpolarizing actions of NE are mediated by α1 and β adrenergic receptors
The adrenergic receptor types underlying the effects of NE were examined using a range of agonists and antagonists. In each case, a GnRH neuron was tested with NE (100 µM) followed by the α1-adrenergic receptor agonist PE (30-100 µM), α2-adrenergic receptor agonist guanabenz (10 or 20 µM), or β-adrenergic receptor agonist isoproterenol (100 µM). If a good quality recording was still possible after this, tests were made examining the presence of multiple adrenergic receptors and/or the effects of prazosin the α1-aderenergic receptor antagonist (10 µM). The concentrations of agonists and antagonists used in this study have been proven to be effective in previous electrophysiolgical experiments (28, 29).
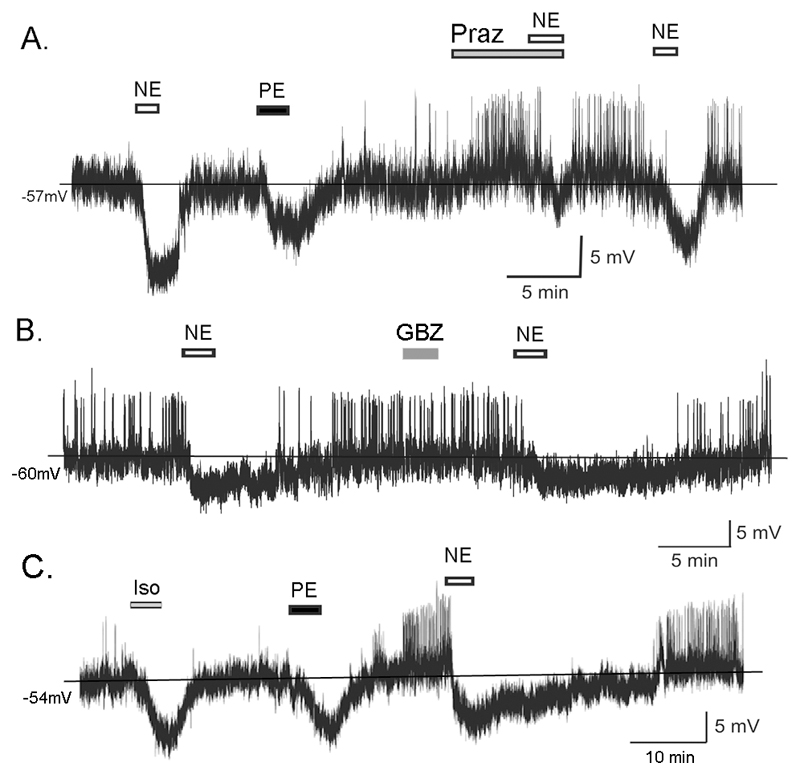
Thirteen of 21 (62%) GnRH neurons responding to NE also responded to PE with membrane hyperpolarization (5.5±0.6 mV)(Fig.4A & Table). The 8 other cells did not respond. It was possible to test three NE- and PE-sensitive GnRH neurons further with prazosin. Prazosin alone was found to increase cell excitability in 2 of the 3 cells, and significantly attenuate the NE-induced hyperpolarization by 75% (pre-prazosin 8.6±0.5 mV, during prazosin 2.0±0.3 mV; p<0.01, paired t-test) in all 3 GnRH neurons (Fig.4A).
Figure 4.
NE acts through α1 and β adrenergic receptors to suppress GnRH neuron excitability. A. Perforated-patch recording from a GnRH neuron of a 33 day old male mouse showing hyperpolarizing responses to NE (100µM) and phenylephrine (PE; 30µM) followed by a partial suppression of the NE hyperpolarization by prazosin (Praz; 10µM) and then recovery of the NE action. B. Perforated-patch recording from a GnRH neuron of a 60 day old male mouse showing lack of effect of guanabenz (GBZ; 10µM). C. Perforated-patch recording from a GnRH neuron of a 42 day old female mouse showing hyperpolarizing responses to Iso (100µM), PE (30µM) and NE (100µM).
Table.
Summary showing numbers of GnRH neurons hyperpolarized by α1 (phenylephrine), α2 (guanabenz) and β (isoproterenol) adrenergic receptor agonists. “Male” and “Female” rows give total numbers of GnRH neurons tested with different drugs. “NE-positive” shows numbers of GnRH neurons responsive to NE that were then tested with different adrenergic agonists.
| phenylephrine | isoproterenol | guanabenz | |
|---|---|---|---|
| Male | 34/50 (68%) |
5/8 (63%) |
0/4 |
| Female | 11/17 (65%) |
7/8 (87%) |
0/3 |
| NE-positive | 13/21 (62%) |
7/12 (58%) |
0/7 |
None of seven NE-sensitive GnRH neurons responded to the selective α2-adrenergic receptor agonist guanabenz (Fig.4B & Table).
Seven of 12 (58%) NE-sensitive GnRH neurons also responded to isoproterenol with a membrane hyperpolarization (6.1±0.7 mV)(Fig.4C & Table). Eight GnRH neurons were tested with NE, PE and isoproterenol and 6 were found to respond to all 3 agonists (Fig.4C), the remaining two cells responded to NE and PE but not isoproterenol, or NE and isoproterenol but not PE.
The hyperpolarizing responses of male and female GnRH neurons to agonists, either alone or following NE treatment, were similar (Table).
Discussion
We show here that GnRH neurons respond directly to NE. These effects are mediated by both α1 and β adrenergic receptors on the soma and/or dendrites and generate a robust suppression of excitability and firing in the great majority of GnRH neurons. The effects of NE persist in the presence of TTX and amino acid receptor antagonists indicating that NE acts directly upon GnRH neurons. This corroborates tract-tracing (12), immunocytochemical (13, 30) and receptor profiling (14) studies that have all suggested a direct NE input to GnRH neurons in the mouse. We found no differences in the effects of NE on GnRH neurons recorded from males or females across the cycle or following ovariectomy. Together, these results demonstrate that direct adrenergic receptor activation exerts a potent, gonadal steroid-independent, suppressive influence upon GnRH neurons.
Eighty-90% of GnRH neurons were found to respond to NE, and the majority (75%) tested with PE and isoproterenol responded to both agonists suggesting the co-existence of α1 and β adrenergic receptors. Further, the hyperpolarizing action of NE was significantly reduced, but not completely abolished, by the α1 receptor antagonist prazosin, suggesting co-operation between the two receptors in hyperpolarizing GnRH neurons. This indicates a unique scenario in which either or both α1 or β adrenergic receptors function to hyperpolarize GnRH neurons. Whereas β adrenergic receptors are known to mediate the suppression of neuronal excitability, for example in hypothalamic paraventricular neurons (31), to our knowledge, this is the first time an α1 receptor-mediated hyperpolarizing response has been recorded from central neurons. To date, we have been unable to establish the signaling pathway underling this unique response.
Approximately 20% of “silent” GnRH neurons responding to NE, were noted to fire in a rebound manner following the NE-induced hyperpolarization. The observation that those GnRH neurons showing rebound firing were significantly more hyperpolarized by NE than those silent cells that remained silent, suggests the involvement of an intrinsic hyperpolarization-activated conductance. Previous studies have indicated that a sub-population of GnRH neurons exhibit the hyperpolarization-activated cation current (32) that is known mediate rebound activation (33). However, it is important to note that the number of GnRH neurons showing this response was small, representing less than 10% of all recorded GnRH neurons, and that they had a similar incidence in males and females throughout the cycle.
To examine the possibility that gonadal steroids modulated the effects of NE on GnRH neurons, we evaluated GnRH neurons taken from diestrous, proestrous, estrous and OVX mice. Surprisingly, considering the clear steroid dependency of NE actions on LH secretion (16, 17), we found that NE exerted a consistent suppressive effect on GnRH neuron excitability throughout the cycle and in OVX mice. Whereas the robust suppression of LH release by NE activation in OVX rats (16, 17) is compatible with the present electrophysiological data, the stimulatory effects of NE on LH are not explained by the direct effects NE on GnRH neurons. There are several explanations for this apparent incongruence. It is possible that the NE component of the GnRH neuronal network operates differently in the mouse compared with the rat, where the LH secretion data have been obtained. Another explanation, is that the reported effects NE on LH secretion result from widespread activation of adrenergic receptors throughout the GnRH neuronal network, rather than reflecting actions solely at the GnRH neuron soma/dendrites. Also, it is important to recognize that this switch from an inhibitory LH response in OVX animals to a stimulatory LH response in OVX+E+P rats has been observed with a wide range of neurotransmitters (see (34)). Thus, it is very likely that the switch to a stimulatory action reflects more the state of the GnRH neurons in OVX+E+P rats rather than any special feature of the individual neurotransmitter or receptor (34).
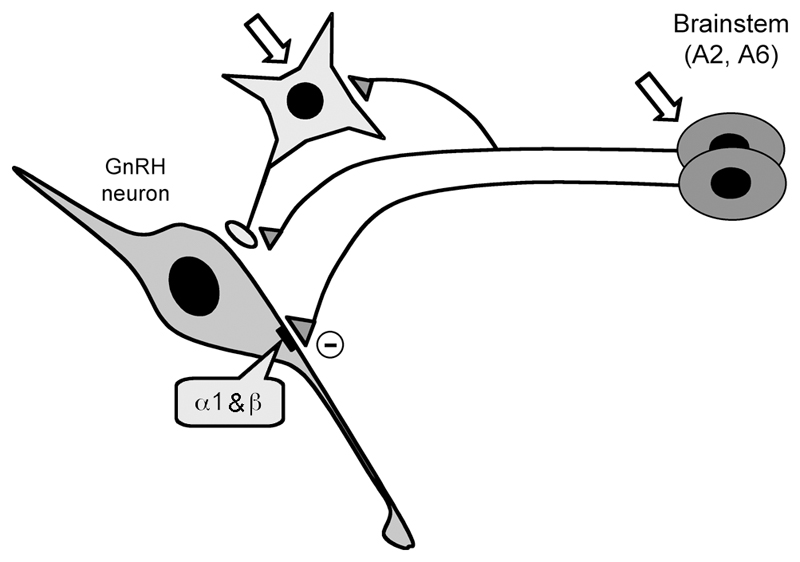
Brought together, we propose that, under physiological situations, direct inputs from brainstem NE neurons to GnRH neuron soma/dendrites suppress firing throughout the estrous cycle in females, and in males. We find no evidence that gonadal steroid status influences the functioning of adrenergic receptors expressed by GnRH neurons. However, other actions of NE within the GnRH neuronal network may modulate, and even over-ride, this direct suppressive influence. As such, we speculate that that gonadal steroid influences occur either indirectly through the regulation of adrenergic receptor activity on afferent neurons within the network, or through the modulation of NE release (Fig.5).
Figure 5.
Schematic diagram showing proposed NE connections within the GnRH neuronal network of the mouse. Direct NE inputs from brainstem A2 and A6 cell groups suppress GnRH neuronal firing through α1 and β adrenergic receptors. Indirect modulation through the regulation of afferent inputs (light grey) is also likely. Open arrows indicate possible sites through which gonadal steroids may act to modulate NE influences within the network.
In summary, we provide here evidence that NE evokes a direct and robust suppressive influence on GnRH neuron firing in male as well as female mice throughout the estrous cycle. These actions are mediated through both α1 and β adrenergic receptors on the GnRH neuron soma and/or dendrite. The suppressive influence of α1 adrenergic receptor activation on GnRH neurons is unique within the brain. Coming many years after the description of the effects of NE on LH, the identification here that NE potently inhibits GnRH neuron firing calls for a re-assessment of current models explaining NE’s actions within the brain to regulate LH secretion.
Acknowledgments
The authors thank Drs. Grattan, Jasoni, Lee and Liu of the Centre for Neuroendocrinology for helpful comments on the draft of the manuscript. Work was supported by The Wellcome Trust (UK) and the Health Research Council of New Zealand.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Sawyer CH, Markee JE, Hollinshead WH. Inhibition of ovulation in the rabbit by the adrenergic-blocking agent dibenamine. Endocrinology. 1947;41:395–402. doi: 10.1210/endo-41-5-395. [DOI] [PubMed] [Google Scholar]
- 2.Sawyer CH. The seventh Stevenson Lecture. Brain amines and pituitary gonadotrophin secretion. Canadian journal of physiology and pharmacology. 1979;57:667–680. doi: 10.1139/y79-102. [DOI] [PubMed] [Google Scholar]
- 3.Barraclough CA, Wise PM. The role of catecholamines in the regulation of pituitary luteinizing hormone and follicle-stimulating hormone secretion. Endocr Rev. 1982;3:91–119. doi: 10.1210/edrv-3-1-91. [DOI] [PubMed] [Google Scholar]
- 4.Spies HG, Pau KY, Yang SP. Coital and estrogen signals: a contrast in the preovulatory neuroendocrine networks of rabbits and rhesus monkeys. Biol Reprod. 1997;56:310–319. doi: 10.1095/biolreprod56.2.310. [DOI] [PubMed] [Google Scholar]
- 5.Herbison AE. Noradrenergic regulation of cyclic GnRH secretion. Reviews in Reproduction. 1997;2:1–6. doi: 10.1530/ror.0.0020001. [DOI] [PubMed] [Google Scholar]
- 6.Wright DE, Jennes L. Origin of noradrenergic projections to GnRH perikarya-containing areas in the medial septum-diagonal band and preoptic area. Brain Research. 1993;621:272–278. doi: 10.1016/0006-8993(93)90116-5. [DOI] [PubMed] [Google Scholar]
- 7.Tillet Y, Batailler M, Thibault J. Neuronal projections to the medial preoptic area of the sheep, with special reference to monoaminergic afferents: immunohistochemical and retrograde tract tracing studies. Journal of Comparative Neurology. 1993;330:195–220. doi: 10.1002/cne.903300205. [DOI] [PubMed] [Google Scholar]
- 8.Simonian SX, Spratt DP, Herbison AE. Identification and characterization of estrogen receptor a-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol. 1999;411:346–358. doi: 10.1002/(sici)1096-9861(19990823)411:2<346::aid-cne13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Rawson JA, Scott CJ, Pereira A, Jakubowsak A, Clarke IJ. Noradrenergic projections from the A1 field of preoptic area in the brain of the ewe and Fos reponses to oestrogen in the A1 cells. Journal of Neuroendocrinology. 2001;13:129–138. doi: 10.1046/j.1365-2826.2001.00588.x. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T, Nakai Y. Electron microscopic cytochemistry of catecholaminergic innervation of LHRH neurons in the medial preoptic area of the rat. Archivum histologicum Japonicum. 1987;50:103–112. doi: 10.1679/aohc.50.103. [DOI] [PubMed] [Google Scholar]
- 11.Hosny S, Jennes L. Identification of alpha1B adrenergic receptor protein in gonadotropin releasing hormone neurones of the female rat. J Neuroendocrinol. 1998;10:687–692. doi: 10.1046/j.1365-2826.1998.00256.x. [DOI] [PubMed] [Google Scholar]
- 12.Campbell RE, Herbison AE. Definition of brainstem afferents to gonadotropin-releasing hormone (GnRH) neurons in the mouse using conditional viral tract tracing. 2007 doi: 10.1210/en.2007-0854. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MM, Zhu L. Ovariectomy and age alter gonadotropin hormone releasing hormone-noradrenergic interactions. Neurobiol Aging. 1995;16:613–625. doi: 10.1016/0197-4580(95)00044-f. [DOI] [PubMed] [Google Scholar]
- 14.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Leipheimer RE, Gallo RV. Medial preoptic area involvement in norepinephrine-induced suppression of pulsatile luteinizing hormone release in ovariectomized rats. Neuroendocrinology. 1985;40:345–351. doi: 10.1159/000124097. [DOI] [PubMed] [Google Scholar]
- 16.Gallo RV, Drouva SV. Effect of intraventricular infusion of catecholamines on luteinizing hormone release in ovariectomized and ovariectomized steroid-primed rats. Neuroendocrinology. 1979;29:149–162. doi: 10.1159/000122917. [DOI] [PubMed] [Google Scholar]
- 17.Leung PCK, Whitmoyer DI, Arendash GW, Sawyer CH. Differential effects of central adrenoceptor agonists on luteinizing hormone release. Neuroendocrinology. 1982;34:207–214. doi: 10.1159/000123301. [DOI] [PubMed] [Google Scholar]
- 18.Leung PC, Arendash GW, Whitmoyer DI, Gorski RA, Sawyer CH. Electrical stimulation of mesencephalic noradrenergic pathway: effects on luteinizing hormone levels in blood of ovariectomized and ovariectomized, steroid-primed rats. Endocrinology. 1981;109:720–728. doi: 10.1210/endo-109-3-720. [DOI] [PubMed] [Google Scholar]
- 19.Jarry H, Leonhardt S, Wuttke W. A norepinephrine-dependent mechanism in the preoptic/anterior hypothalamic area but not in the mediobasal hypothalamus is involved in the regulation of the gonadotropin-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology. 1990;51:337–344. doi: 10.1159/000125358. [DOI] [PubMed] [Google Scholar]
- 20.Clifton DK, Sawyer CH. LH release and ovulation in the rat following depletion of hypothalamic norepinephrine: chronic vs. acute effects. Neuroendocrinology. 1979;28:442–449. doi: 10.1159/000122893. [DOI] [PubMed] [Google Scholar]
- 21.Leonhardt S, Jarry H, Falkenstein G, Palmer J, Wuttke W. LH release in ovariectomized rats is maintained without noradrenergic neurotransmission in the preoptic/anterior hypothalamic area: extreme functional plasticity of the GnRH pulse generator. Brain Res. 1991;562:105–110. doi: 10.1016/0006-8993(91)91193-5. [DOI] [PubMed] [Google Scholar]
- 22.Clifton DK, Steiner RA. Recovery of pulsatile luteinizing hormone secretion following permanent disruption of the ascending noradrenergic fiber tract in the ovariectomized rat. Biol Reprod. 1985;33:808–814. doi: 10.1095/biolreprod33.4.808. [DOI] [PubMed] [Google Scholar]
- 23.Spergel DJ, Kruth U, Shimshek DR, Sprengel R, Seeburg PH. Using reporter genes to label selected neuronal populations in transgenic mice for gene promoter, anatomical, and physiological studies. Prog Neurobiol. 2001;63:673–686. doi: 10.1016/s0301-0082(00)00038-1. [DOI] [PubMed] [Google Scholar]
- 24.Herbison AE, Pape JR, Simonian SX, Skynner MJ, Sim JA. Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Mol Cell Endocrinol. 2001;185:185–194. doi: 10.1016/s0303-7207(01)00618-9. [DOI] [PubMed] [Google Scholar]
- 25.Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- 26.Sim JA, Skynner MJ, Pape J-R, Herbison AE. Late postnatal reorganization of GABAA receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 27.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002;87:2287–2296. doi: 10.1152/jn.2002.87.5.2287. [DOI] [PubMed] [Google Scholar]
- 29.Honda E, Ono K, Kataoka S, Inenaga K. Activation of subfornical organ neurons in rats through pre- and postsynaptic alpha-adrenoceptors. American journal of physiology. 2006;290:R1646–1653. doi: 10.1152/ajpregu.00801.2004. [DOI] [PubMed] [Google Scholar]
- 30.Turi GF, Liposits Z, Moenter SM, Fekete C, Hrabovszky E. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology. 2003;144:4967–4974. doi: 10.1210/en.2003-0470. [DOI] [PubMed] [Google Scholar]
- 31.Daftary SS, Boudaba C, Tasker JG. Noradrenergic regulation of parvocellular neurons in the rat hypothalamic paraventricular nucleus. Neuroscience. 2000;96:743–751. doi: 10.1016/s0306-4522(00)00003-8. [DOI] [PubMed] [Google Scholar]
- 32.Sim JA, Skynner MJ, Herbison AE. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci. 2001;21:1067–1075. doi: 10.1523/JNEUROSCI.21-03-01067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly MJ, Wagner EJ. GnRH neurons and episodic bursting activity. Trends Endocrinol Metab. 2002;13:409–410. doi: 10.1016/s1043-2760(02)00698-7. [DOI] [PubMed] [Google Scholar]
- 34.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego: Academic Press; 2006. pp. 1415–1482. [Google Scholar]