Abstract
The VEGFR2 receptor tyrosine kinase regulates vascular physiology and animal development. The mechanism underlying VEGFR2 membrane trafficking is not well understood. Herein, we show that VEGFR2 undergoes membrane recycling in both vascular and non-vascular cells. In primary human endothelial cells, VEGFR2 normally distributes between the plasma membrane and early endosomes undergoing endocytosis and recycling. This pathway is independent of VEGFR tyrosine kinase activity and occurs constitutively, similar to other integral membrane proteins such as the transferrin receptor and β1 integrin. Expression of a VEGFR2-EGFP hybrid protein in non-vascular cells revealed plasma membrane and endosome distribution. The VEGF-A ligand stimulated phosphorylation of residue Y1175 on VEGFR2-EGFP which is a key hallmark of receptor activation. Live cell imaging and quantitative analysis showed that activated VEGFR2-EGFP displayed reduced mobility linked to endocytosis and recycling between the plasma membrane and endosomes. Total internal reflection microscopy and kinetics indicates that VEGFR2 undergoes recycling between the plasma membrane and peripheral endosomes proximal to the membrane bilayer. We thus provide evidence that the VEGFR2 receptor tyrosine kinase undertakes a constitutive recycling pathway between the peripheral endosomes and cell surface and this exists in both vascular and non-vascular cells.
Keywords: VEGF-A, VEGFR2, endothelial, endosome-to-plasma membrane recycling
Introduction
Vascular endothelial growth factor A (VEGF-A) is essential for vasculogenesis and angiogenesis in mammals, playing a key role in new blood vessel growth in cancer, diabetic retinopathy, macular degeneration and ischemic heart disease [1]. The VEGF-A gene produces both pro-angiogenic and anti-angiogenic isoforms [2]. VEGF-A165 (termed VEGF-A) is the major secreted pro-angiogenic isoform and binds two different receptor tyrosine kinases, VEGFR1 (Flt-1) and VEGFR2 (KDR) [3]. Although VEGFR2 has lower affinity for VEGF-A, it triggers key intracellular signaling events in endothelial cells [4]. VEGF-A binding to VEGFR2 on endothelial cells triggers activation of both p42/44 mitogen-activated protein kinase (MAPK, ERK1/2) and the serine/threonine protein kinase c-Akt which in turn phosphorylate different cellular proteins that regulate vascular physiology [5–7].
VEGFR2 signaling has been intensively studied but how this is co-ordinated with receptor trafficking is not clear. Whilst receptor tyrosine kinase endocytosis can attenuate signaling output, it has been noted that signaling outputs may differ depending on location within the endocytic pathway [8, 9]. Such receptors usually encounter one of two possible fates: recycling back to the plasma membrane or degradation within the endosome-lysosome pathway. VEGFR2 undergoes ligand-stimulated activation, phosphorylation and ubiquitination linked to proteasome and lysosome-linked degradation [9, 10]. Recycling of activated VEGFR2 has also been observed [11] but neither the mechanism(s) nor how this is co-ordinated with ligand receptor activation are understood. We investigated VEGFR2 recycling in primary human vascular cells and its dependence on tyrosine kinase activity. Furthermore the intrinsic membrane trafficking properties encoded by the VEGFR2 protein were analyzed using a VEGFR2-EGFP hybrid protein which enabled live cell imaging of transfected non-vascular cells.
Materials and Methods
Materials and cell culture
Biochemicals were from Sigma-Aldrich (Poole, UK) unless otherwise stated. Antibodies, primary human endothelial and human HEK-293T cell culture preparation, growth and media have been previously described elsewhere [10, 12, 13]. Goat anti-VEGFR2 antibodies (R&D Systems, Minneapolis, USA) were used with HRP-conjugated secondary antibodies (ThermoFisher, Loughborough, UK) and AlexaFluor-conjugated secondary antibodies (Invitrogen, Amsterdam, Netherlands). Non-endothelial cell culture medium and supplements were from Invitrogen (Netherlands) whereas endothelial cell growth medium and supplements were from Promocell (Heidelberg, Germany).
Receptor recycling assays
Receptor biotinylation and recycling assay was adapted from another study described elsewhere [14]. This means that recycled proteins will indeed be stripped of their biotin so when biotinylated proteins are isolated, if recycling has occurred there will be a lower level of the protein following the recycling incubation than the internalization incubation. Confluent HUVECs were serum-starved for 3 hours before washing with ice-cold PBS then incubated on ice for 30 min with 0.2 mg/ml cleavable NHS-SS-Biotin (ThermoFisher). Control samples (total biotinylation) were then lysed in buffer (1% (v/v) NP-40, 50 mM Tris pH 7.5, 150 mM sodium chloride, protease inhibitor cocktail) or fixed whilst other samples were incubated at 37°C in serum-free medium for varying time periods. After incubation, cell surface biotin were removed using a reducing buffer (100 mM sodium 2-mercaptoethansulfonate (MESNA), 50 mM Tris pH 8.6, 100 mM sodium chloride, 1 mM EDTA, 0.2% (w/v) BSA) and quenched in buffer containing 120 mM iodoacetamide. For receptor recycling assay, cells were subjected to an additional 20 min incubation in serum-free medium at 37°C prior to a second wash with reducing buffer. Biotinylated proteins were isolated using neutravidin-agarose beads (ThermoFisher), washed in lysis buffer before SDS-PAGE and immunoblotting was performed as described previously [9, 13]. Endothelial cells show a similar distribution of VEGFR2 before or after serum starvation.
A different receptor recycling assay using surface antibody labelling was based on a previous method [15]. Serum-starved HUVECs were incubated for 1 h at 37°C with primary antibody or were labeled at 4°C (negative control) before antibodies bound to plasma membrane receptors were removed by an acid wash in serum-free medium (pH 2.0), followed by a wash with normal medium. Cells were incubated with secondary antibody for 1 h at 37°C and plasma membrane-bound antibodies again removed by acid washing before fixation and processing for immunofluorescence microscopy. For a protein to be detected using this assay, it must have picked up both primary and secondary antibodies which is only possible if it has visited the cell surface twice. Therefore only proteins which have recycled at least once are visible.
Microscopy and live cell imaging
HUVECs and HEK-293T cells were fixed and processed for immunofluorescence microscopy as described previously [12]. For live cell imaging, HEK-293T cells were grown on glass-bottomed dishes and transiently transfected with pCDNA3.1-VEGFR2-EGFP plasmid 48 h prior to live cell imaging using a Deltavision deconvolution microscopy system (Applied Precision Inc., Issaquah, USA). Single images were captured at 5 sec intervals over a 6 min period. Images were then exported as a movie for further analysis. Vesicle tracking was performed in Image J (NIH, Maryland, USA; http://rsbweb.nih.gov/ij/) using the Manual Tracking plugin. Fifteen vesicles from each of three cells were analysed and the velocity of movement averaged.
Total internal reflection fluorescence (TIRF) microscopy was carried out using a TE2000U system (Nikon, Amsterdam, Netherlands) microscope with a 100X objective. Laser illumination of a sample based on using an angle of incidence greater than the critical angle of total internal reflection results in the total internal reflection of laser light at the interface and low penetration of the beam into the cell interior. Only molecules present within an ~100 nm thickness optical slice is illuminated, allowing for imaging of fluorophores in close proximity to the cell surface or within the ~100 nm section. An argon ion laser (excitation at 488 nm, emission at 515 nm) was used to detect VEGFR2-EGFP and the angle of incidence adjusted until the critical angle was reached when all internal light is reflected i.e. no light is refracted. Images were acquired every 100 ms and reconstructed into a movie using EZC1 software (Nikon). Movies were converted into .avi files and fluorescence intensity analysed using ImageJ software.
Results
VEGFR2 undergoes endocytosis and endosome-to-plasma membrane recycling
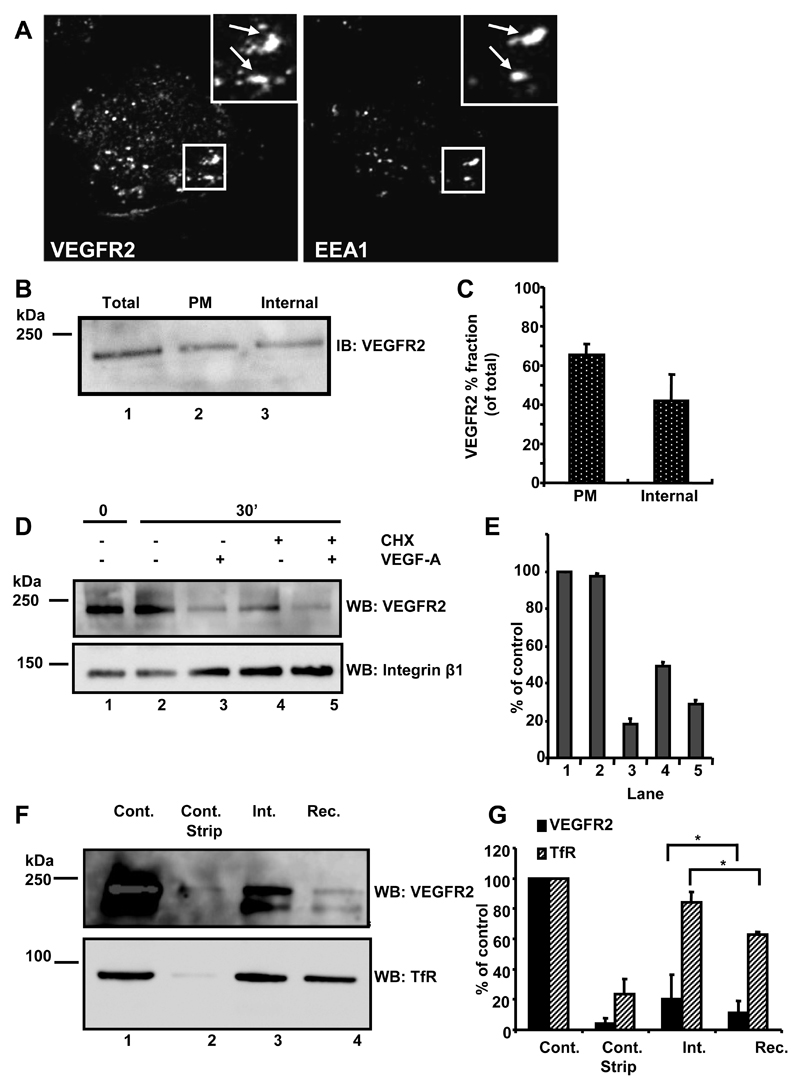
We and others have shown that VEGFR2 exhibits both plasma membrane and endosomal localization [8, 10, 11, 13]. Is this dual localization linked to recycling between these two compartments? To test this idea we combined microscopy and cell surface biotinylation assays on primary human endothelial cells (Fig. 1). Quiescent endothelial cells displayed VEGFR2 localization to large punctate vesicles resembling endosomes (Fig. 1A). VEGFR2 showed ~50% co-localization with early endosomal antigen-1, EEA1 (Fig. 1A). However, VEGFR2 did not show significant co-localization with late endosomes (CD63), late endosome and lysosomes (LAMP-1) or lysosomes (cathepsin D) (data not shown). Using a cell surface biotinylation assay, the total cellular VEGFR2 (Fig. 1B, lane 1), plasma membrane (Fig. 1B, lane 2) and internal endocytosed (Fig. 1B, lane 3) pools were compared in primary endothelial cells. Quantification showed ~40% VEGFR2 in an internal endocytosed pool with the remaining ~60% of VEGFR2 in a plasma membrane accessible pool.
Fig. 1.
VEGFR2 undergoes endocytosis and recycling. (A) Quiescent HUVECs were fixed, permeabilized and labeled with goat anti-extracellular domain VEGFR2 and rabbit anti-EEA1. Bound primary antibodies were visualized using species-specific AlexaFluor-conjugated secondary antibodies. Insets show 2-fold magnification of indicated areas and arrows indicate co-localization of both proteins. Data shown are representative of three independent experiments. (B) Biotinylated and non-biotinylated proteins from quiescent HUVECs were probed by immunoblotting (IB) for relative total VEGFR2 (total, lane 1) with parallel analysis of the biotinylated plasma membrane pool (PM, lane 2). The remaining supernatant represented intracellular endosomal VEGFR2 (internal, lane 3). (C) Quantification of cell surface biotinylation and immunoblotting (error bars denote ±SEM; n=3). (D) Starved endothelial cells were treated with cycloheximide (CHX; 2 hr) to block new protein synthesis where indicated. VEGF-A stimulation for 0 or 30 min was followed by cell surface biotinylation, lysis and immunoblotting using goat anti-VEGFR2 or mouse anti-β1 integrin. (E) Quantification of representative datasets shown in panel (D) (error bars denote ±SEM; n=3). (F) Starved quiescent endothelial cells were biotinylated at 4°C and either lysed directly (control) or subjected to reducing cleavage to strip the biotin label (control strip). Other samples were biotinylated at 4°C, and then incubated at 37°C for 10 min to allow internalisation prior to reduction and lysis (int.). For recycling analysis (rec.), samples were incubated for a further 20 min at 37°C before reduction, lysis, isolation of biotinylated proteins and immunoblotting sequentially with goat anti-VEGFR2 followed by mouse anti-TfR. (G) Quantification of representative datasets shown in panel (F) (error bars denote ±SEM; n=3).
Receptor tyrosine kinases can undergo ligand-independent endocytosis [16]. To test this for VEGFR2, quiescent endothelial cell plasma membrane proteins were biotinylated on ice (Fig. 1D, lane 1), then incubated for 30 min at 37ºC in the absence (Fig. 1D, lane 2) or presence (Fig. 1D, lane 3) of VEGF-A. This was compared with cells which were treated with cycloheximide to block protein synthesis prior to a 30 min incubation at 37ºC in the absence (Fig. 1D, lane 4) or presence (Fig. 1D, lane 5) of VEGF-A. Blocking protein synthesis caused a significant, ~50%, decrease in the plasma membrane VEGFR2 pool independent of VEGF-A stimulation (Fig. 1E). Addition of exogenous VEGF-A further stimulated VEGFR2 internalization both in the presence or absence of cycloheximide (Fig. 1D, lanes 3 and 5). In contrast, levels of a cell surface adhesion receptor (integrin β1 subunit) remained relatively constant (Fig. 1D, lanes 1-5).
Can constitutively endocytosed VEGFR2 recycle from the endosome-to-plasma membrane? A further biotinylation experiment addressed this question. The total pool of labeled plasma membrane proteins contained both VEGFR2 and Transferrin receptor (TfR), another recycling receptor (Fig. 1F, lane 1). An immediate reducing wash subsequent to cell surface biotinylation prevented both VEGFR2 and TfR being isolated and detected (Fig. 1F, lane 2). A 30 min internalization at 37ºC showed VEGFR2 and TfR still present within an internal pool of biotinylated membrane proteins (Fig. 1F, lane 3). Incubation at 37°C for an additional 30 min revealed decreased biotinylated VEGFR2 and TfR levels (Fig. 1E, lane 4) indicating recycling of both membrane receptors back to the plasma membrane. Quantification showed different proportions of the VEGFR2 (~25%) or TfR (~85%) plasma membrane pools undergo endocytosis (Fig. 1G). Additionally, different proportions of the internal VEGFR2 (~50%) or TfR (~30%) pools are recycled back to the plasma membrane from early endosomes (Fig. 1G).
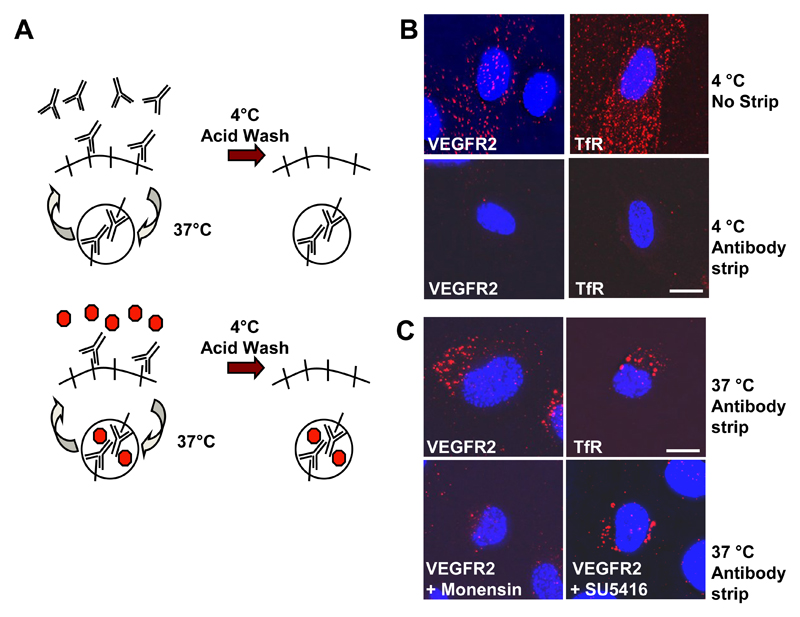
To further test VEGFR2 endosome-to-plasma membrane recycling, we used an antibody uptake-based assay (Fig. 2A). Labeling cell surface receptors alone revealed homogeneous staining for control TfR or VEGFR2 that could be removed by an acid wash (Fig. 2B). Antibody binding to cell surface receptors followed by a 37ºC incubation showed receptor-antibody complex accumulation within punctate perinuclear structures containing both transferrin receptor and VEGFR2 (Fig. 2C) indicating constitutive receptor recycling. Monensin is an ionophore that inhibits endosome-to-plasma membrane recycling. Monensin substantially inhibited appearance of VEGFR2-containing punctate structures (Fig. 2C) indicating that receptor recycling is blocked by endosomal perturbation. SU5416 (semaxanib) is a tyrosine kinase inhibitor that inhibits VEGFR2 trafficking to late endosomes but did not affect perinuclear VEGFR2 accumulation (Fig. 2C), suggesting internalization is independent of VEGFR kinase activity. Cell surface biotinylation and recycling experiments show that ~40% VEGFR2 was present in an internal endosome pool with the remaining ~60% in a plasma membrane pool (data not shown). Comparison to a constitutively recycling membrane protein (human TfR) showed that different proportions of the internal VEGFR2 (~50%) or TfR (~70%) pools are recycled back to the plasma membrane from early endosomes (data not shown).
Fig. 2.
VEGFR2 recycling is independent of tyrosine kinase activity. (A) Schematic outline of an antibody-based assay to monitor receptor recycling between the plasma membrane and internal compartment(s). (B) Endothelial cells were labeled with goat anti-VEGFR2 or mouse anti-transferrin receptor (TfR) for 1 h at 4°C. Bound cell surface antibodies were either stripped by acid wash (antibody strip) or left bound to the protein (no strip). Cells were subsequently labeled for a further 1 h at 4°C with AlexaFluor-594-conjugated anti-sheep or Texas Red-conjugated anti-mouse IgG. Accessible cell surface antibody complexes were again removed from stripped samples by acid wash and samples were fixed and labeled with a DNA stain (DAPI; blue) before microscopy. Bar, 10 μm. (C) Direct recycling assay for cells treated with anti-VEGFR2 or anti-TfR antibodies at 37°C for 30 min. Cells were also pre-treated with either 20 μM monensin (endosome-to-plasma membrane recycling inhibitor) or 5 μM SU5416 (VEGFR tyrosine kinase inhibitor)and labeled with anti-VEGFR2. Results are representative of three independent experiments. Bar, 10 μm.
VEGFR2 trafficking in living cells
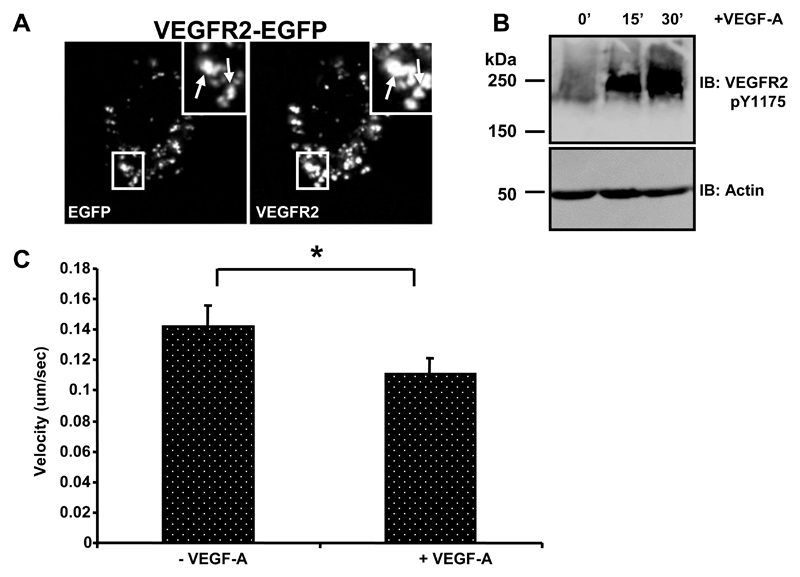
What is the nature of VEGFR2 trafficking in living cells? We constructed a VEGFR2-EGFP hybrid protein and transfected into both vascular and non-vascular cells. However, transfection and expression in endothelial cells was low and complicated by the presence of the endogenous VEGFR2 (data not shown). We tested expression of this VEGFR2-EGFP plasmid construct in the highly transfectable non-vascular human cell line (HEK-293T) which does not express endogenous VEGFR2 (Fig. 3). HEK-293T transfected cells showed VEGFR2-EGFP expression with localization of this hybrid protein to plasma membrane and punctate structures resembling endosomes (Fig. 3A, arrows). The VEGFR2-EGFP protein also showed co-localization with EEA1, a key early endosome marker protein (data not shown). To further evaluate the properties of this VEGFR2-EGFP protein, transfected HEK-293T cells were subjected to a time course of VEGF-A stimulation (Fig. 3B). Immunoblotting using a phosphotyrosine-specific antibody revealed that a ~250 kDa species corresponding to VEGFR2-EGFP containing the pY1175 epitope appeared in a time-dependent manner (Fig. 3B). Thus the VEGFR2-EGFP hybrid protein displayed biochemical properties characteristic of endothelial VEGFR2.
Fig. 3.
VEGF-A-dependent phosphorylation of a VEGFR2-EGFP hybrid protein. (A) A human non-endothelial cell line (HEK-293T) was transiently transfected to express VEGFR2-EGFP, fixed and labeled with goat anti-VEGFR2 and rabbit anti-GFP followed by AlexaFluor-conjugated secondary antibodies. Arrows in panel insets denote punctate structures showing co-labeling for VEGFR2 and EGFP. (B) Cells subjected to VEGF-A (0, 15 or 30 min) were lysed and immunoblotted using anti-VEGFR2 pY1175 VEGFR2 or anti-actin antibodies. Results shown are representative of three independent experiments. (C) Quantification of velocity of VEGFR2-EGFP-labeled vesicles in living HEK-293T cells. Fifteen vesicles per cell were tracked to determine the average velocity of movement in each of three cells per condition. Error bars denote ±SEM (n = 3; * denotes significance, p<0.05).
We then evaluated the kinetics of VEGFR2-EGFP expressed in living HEK-293T cells using time-lapse fluorescence microscopy. A single optical section of a HEK-293T cell was monitored over a 6 min period using time-lapse fluorescence microscopy with images taken every 5 sec. VEGFR2-EGFP positive vesicles could be seen to move both within the focal plane and also in and out of focus of this single optical section indicating movement in all three xyz planes through the cell (Supplemental Data Movie 1). In order to determine the dynamics of movement, vesicles were tracked for movement between frames. These tracks showed multi-directional intracellular movement (Supplemental Data Movie 1).
Time lapse fluorescence microscopy was also used to monitor VEGFR2-EGFP membrane dynamics in living HEK-293T cells following stimulation with VEGF-A. Incubation with VEGF-A for 30 sec revealed VEGFR2-EGFP-labeled vesicles moving both within and in and out of the focal plane (Supplemental Data Movie 2). Tracking these movements showed multi-directional movement of VEGFR2-EGFP-containing vesicles continued during VEGF-A stimulation. Tracking multiple vesicles containing VEGFR2-EGFP showed average velocity of ~0.14 μm/sec in controls contrasting with a decrease in mobility to ~0.11 μm/sec in the presence of VEGF-A (Fig. 3C). Thus VEGF-A stimulation could modulate dynamics of VEGFR2-EGFP-labeled punctate structures resembling endosomes.
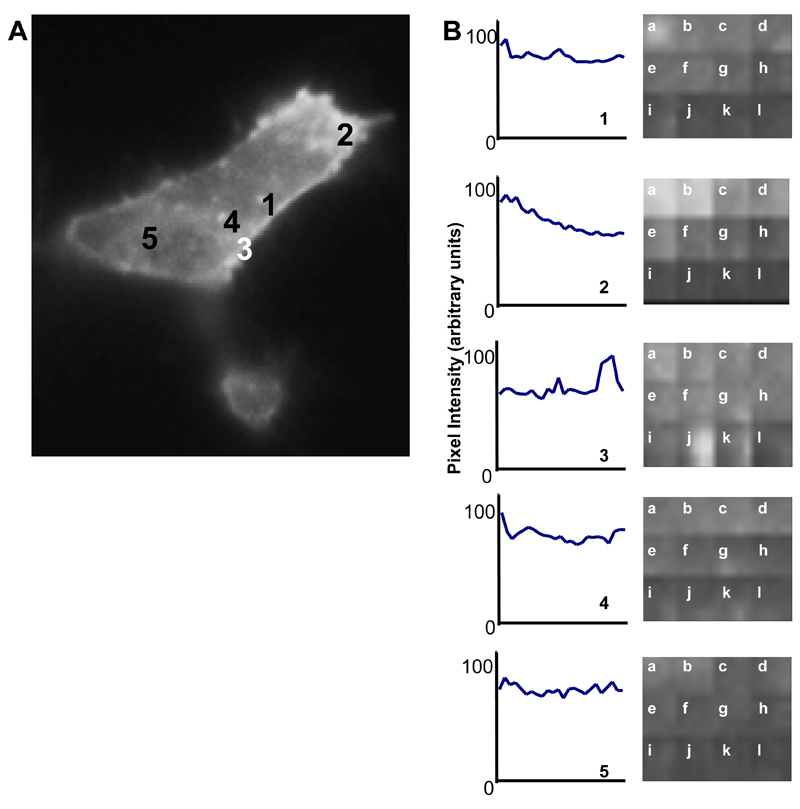
A complementary approach for studying membrane dynamics in living cells is total internal reflection fluorescence (TIRF) microscopy that enables imaging of events at or close to the plasma membrane. Using TIRF, fluorescence emission by the VEGFR2-EGFP hybrid protein in single transfected cells was monitored over an 8 min period with VEGF-A added after 120 sec. Images were captured every 0.01 sec and over time, photo-bleaching occurred due to the continual laser beam excitation of the fluorophore within the illuminated cell. However, VEGFR2-EGFP vesicles could be seen both appearing and disappearing from the plasma membrane (Supplemental Data Movie 3). This was investigated further by looking at the fluorescence intensity of five regions of each cell after correcting for photo-bleaching (Fig. 4). The majority of regions showed internalization of VEGFR2-EGFP indicated by an overall decrease in fluorescence intensity (Fig. 4, regions 1, 2, 5). However, some regions also showed the appearance of vesicles at the cell surface denoted by a peak in fluorescence intensity (Fig. 4, regions 3, 4). All peaks indicate a change in the cell surface intensity of staining although smaller peaks probably represent movement within the plane of the membrane. Larger peaks likely represent movement of protein into the plane of the membrane and thus representative of translocation from an internal compartment e.g. endosome. This approach thus shows that VEGFR2-EGFP-labeled punctate structures appeared and disappeared rapidly at the plasma membrane, indicating discrete endocytosis and recycling events.
Fig. 4.
Analysis of VEGFR2-EGFP membrane dynamics using TIRF microscopy. HEK-293T cells were transiently transfected to express VEGFR2-EGFP and VEGF-A (50 ng/ml) added to cells at 120 sec. (A) Fluorescence intensity of 5 regions of a transfected cell footprint was monitored through the time course. (B) Traces of intensity of the different regions indicated in panel A was normalized to background photo-bleaching. Letters denote the time course starting at 0 sec (a) and ending at 480 sec (l) with 40 sec intervals. Peaks on traces indicate VEGFR2-EGFP labeled profiles which have translocated to the cell surface.
Discussion
VEGFR2 is a key vascular regulator but the influence of membrane trafficking on such VEGFR2 signaling is unclear. Here, we provide evidence that endothelial VEGFR2 undergoes constitutive recycling between the plasma membrane and endosomes. This occurs via a constitutive pathway present in both vascular and non-vascular human cells. VEGFR2 can localize to both the plasma membrane and intracellular vesicles in endothelial cells. Approximately 40% VEGFR2 resides within early endosomes whilst the remaining fraction is present at the plasma membrane agreeing with previous studies. VEGFR2 undergoes constitutive endocytosis of ~2.5% of the plasma membrane pool per min. Whilst VEGFR2 displays a lower internalization rate than the constitutively endocytosed (~8% per min), this is still a relatively high level of constitutive endocytosis. VEGFR2 has been noted to associate with lipid rafts which can modulate endocytosis [17]. Increased VEGFR2 endocytosis triggered by VEGF-A could be linked to VEGFR2 exit from plasma membrane caveolae or lipid rafts to facilitate receptor-mediated endocytosis. Although plasma membrane VEGFR2 internalization increased ~1.5-fold upon VEGF-A stimulation (HMJ and SP, unpublished observations), this does not account for increased endosome-plasma membrane recycling.
Live cell imaging showed VEGFR2 loss from the plasma membrane consistent with ligand-independent endocytosis. Our findings suggest this pathway mediates constitutive VEGFR2 endocytosis in both non-endothelial and endothelial cells. The saltatory (moving by leaps or jumps) properties of VEGFR2-EGFP-containing vesicles or profiles was altered upon VEGF-A stimulation. Non-stimulated or quiescent VEGFR2-EGFP movement was decreased by ~30% following VEGF-A stimulation. Although saltatory movement of endosomes can reach ~0.3 μm/sec, analysis of another receptor tyrosine kinase EGFP fusion (c-Kit-EGFP) showed saltatory movements ranging from 0.05 μm/sec to 0.2 μm/sec [18]. Thus VEGFR2 exhibits saltatory movement resembling that of c-kit, another member of the RTK superfamily. Interestingly, the SU5416 compound (semaxanib) does not block VEGFR2 endocytosis indicating that this event does not require VEGFR tyrosine kinase activity. One feature of the VEGFR2-EGFP hybrid protein is that it undergoes sustained activation as shown by the persistence of the pY1175 epitope in transfected non-endothelial cells in contrast to endothelial VEGFR2. In addition, VEGFR2-EGFP displays an increased half-life suggesting that the endogenous VEGFR2 protein undergoes more rapid turnover in primary endothelial cells (HMJ and SP, unpublished observations).
What is the physiological relevance of our studies to endogenous VEGFR2 trafficking in vascular tissues? Although we and others have reported endogenous VEGFR2 localization to plasma membrane and endosomes [8, 10, 11, 13], there are a paucity of studies using VEGFR fluorescent hybrid protein fusions. However, one study has reported that a VEGFR2-GFP hybrid protein was localized to the nucleus when expressed in endothelial cells [19]. We could not address this issue as expression of our VEGFR2-EGFP could not be detected in endothelial cells. However, in non-vascular cells lacking VEGFR2 (i.e. HEK-293T), VEGFR2-EGFP expression was evident and localized to plasma membrane and endosomes in a pattern resembling native VEGFR2 in vascular cells. These findings suggest that expression of wild-type or mutant VEGFR2 proteins could have significant effects on protein and cell function and such studies need controls and careful interpretation of such data.
What cytosolic factors may be involved in regulating VEGFR2 recycling? Members of the Rab family of small GTPases such as Rab4a and Rab11a are notable regulators of receptor recycling from endosomes [20–23]. Early and recycling endosomes resemble membrane mosaics containing different regulatory proteins including Rab GTPases. Divalent Rab effector proteins further regulate the specificity of protein-protein and protein-lipid interactions within these specialized membrane domains. However, another study suggests a role for the c-Src tyrosine kinase in regulating VEGFR2 recycling via a Rab-independent pathway from endosomes to the plasma membrane [11]. Nonetheless, our studies argue that VEGFR2 undertakes a recycling pathway from peripheral early endosomes back to the plasma membrane but the specific regulatory molecules involved remain unclear. Further elucidation of the mechanism and regulators involved in this process will allow us to better understand the endothelial response to cytokines such as VEGF-A.
Supplementary Material
Acknowledgments
This work was supported by grants from the British Heart Foundation (S.P.), the Wellcome Trust (S.P., N.G.) and a BBSRC DTA PhD studentship (H.M.J.). We thank members of the Endothelial Cell Biology Unit for help and advice.
References
- [1].Zachary I, Morgan RD. Therapeutic angiogenesis for cardiovascular disease: biological context, challenges, prospects. Heart. 2011;97:181–189. doi: 10.1136/hrt.2009.180414. [DOI] [PubMed] [Google Scholar]
- [2].Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bruns AF, Bao L, Walker JH, Ponnambalam S. VEGF-A-stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co-ordinated trafficking and proteolysis. Biochem Soc Trans. 2009;37:1193–1197. doi: 10.1042/BST0371193. [DOI] [PubMed] [Google Scholar]
- [4].Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- [5].Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- [6].Rahimi N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp Eye Res. 2006;83:1005–1016. doi: 10.1016/j.exer.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- [8].Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bruns AF, Herbert SP, Odell AF, Jopling HM, Hooper NM, Zachary IC, Walker JH, Ponnambalam S. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic. 2010;11:161–174. doi: 10.1111/j.1600-0854.2009.01001.x. [DOI] [PubMed] [Google Scholar]
- [10].Ewan LC, Jopling HM, Jia H, Mittar S, Bagherzadeh A, Howell GJ, Walker JH, Zachary IC, Ponnambalam S. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7:1270–1282. doi: 10.1111/j.1600-0854.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- [11].Gampel A, Moss L, Jones MC, Brunton V, Norman JC, Mellor H. VEGF regulates the mobilization of VEGFR2/KDR from an intracellular endothelial storage compartment. Blood. 2006;108:2624–2631. doi: 10.1182/blood-2005-12-007484. [DOI] [PubMed] [Google Scholar]
- [12].Howell GJ, Herbert SP, Smith JM, Mittar S, Ewan LC, Mohammed M, Hunter AR, Simpson N, Turner AJ, Zachary I, Walker JH, et al. Endothelial cell confluence regulates Weibel-Palade body formation. Mol Membr Biol. 2004;21:413–421. doi: 10.1080/09687860400011571. [DOI] [PubMed] [Google Scholar]
- [13].Jopling HM, Odell A, Hooper NM, Zachary IC, Walker JH, Ponnambalam S. Rab GTPase Regulation of VEGFR2 Trafficking and Signaling in Endothelial Cells. Arterioscler Thromb Vasc Biol. 2009;29:1119–1124. doi: 10.1161/ATVBAHA.109.186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- [15].Mitchell H, Choudhury A, Pagano RE, Leof EB. Ligand-dependent and - independent transforming growth factor-β receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell. 2004;15:4166–4178. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- [17].Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jahn T, Seipel P, Coutinho S, Urschel S, Schwarz K, Miething C, Serve H, Peschel C, Duyster J. Analysing c-kit internalization using a functional c-kit-EGFP chimera containing the fluorochrome within the extracellular domain. Oncogene. 2002;21:4508–4520. doi: 10.1038/sj.onc.1205559. [DOI] [PubMed] [Google Scholar]
- [19].Santos SC, Miguel C, Domingues I, Calado A, Zhu Z, Wu Y, Dias S. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp Cell Res. 2007;313:1561–1574. doi: 10.1016/j.yexcr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- [20].Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de Renzis S, Sonnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol. 2002;4:124–133. doi: 10.1038/ncb744. [DOI] [PubMed] [Google Scholar]
- [22].Ward ES, Martinez C, Vaccaro C, Zhou J, Tang Q, Ober RJ. From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol Biol Cell. 2005;16:2028–2038. doi: 10.1091/mbc.E04-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.