SUMMARY
Objective
The aim of this study was to investigate peri-ictal central apnea as a seizure semiological feature, its localizing value, and possible relationship with sudden unexpected death in epilepsy (SUDEP) pathomechanisms.
Methods
We prospectively studied polygraphic physiological responses, including inductance plethysmography, peripheral capillary oxygen saturation (SpO2), electrocardiogram, and video electroencephalogram (VEEG) in 473 patients in a multicenter study of SUDEP. Seizures were classified according the ILAE 2017 seizure classification based on the most prominent clinical signs during VEEG. The putative epileptogenic zone was defined based on clinical history, seizure semiology, neuroimaging and EEG.
Results
Complete datasets were available in 126 patients in 312 seizures. Ictal central apnea (ICA) occurred exclusively in focal epilepsy (51/109 patients [47%] and 103/312 seizures [36.5%]) (p<0.001). ICA was the only clinical manifestation in 16/103 (16.5%) seizures, and preceded EEG seizure onset by 8+4.9 seconds, in 56/103 [54.3%] seizures. ICA > 60 seconds was associated with severe hypoxemia (SpO2 <75%). Focal onset impaired awareness (FOIA) motor onset with automatisms and FOA non-motor onset semiologies were associated with ICA presence (p<0.001), ICA duration (p=0.002) and moderate/severe hypoxemia (p=0.04). Temporal lobe epilepsy was highly associated with ICA in comparison to extratemporal epilepsy (p=0.001) and frontal lobe epilepsy (p=0.001). Isolated post-ictal central apnea was not seen; in 3/103 seizures (3%), ICA persisted into the post-ictal period.
Significance
ICA is a frequent, self-limiting semiological feature of focal epilepsy, often starting before surface EEG onset, and may be the only clinical manifestation of focal seizures. However, prolonged ICA (>60 seconds) is associated with severe hypoxemia and may be a potential SUDEP biomarker. ICA is more frequently seen in temporal than extratemporal seizures, and in typical temporal seizure semiologies. ICA rarely persists after seizure end. ICA agnosia is typical, and thus ICA may remain unrecognized without polygraphic measurements that include breathing parameters.
Keywords: apnea, breathing, seizures, SUDEP, temporal epilepsy
INTRODUCTION
Hypoventilation and hypoxemia are typically seen in focal to bilateral tonic clonic seizures (FBTCS) and generalized tonic clonic seizures (GTCS), 1, 2,3 and severe alteration of breathing patterns after such seizures has been suggested as a possible mechanism of Sudden Unexpected Death in Epilepsy (SUDEP) 1. However, oxygen desaturations are also found in 30–60% of focal seizures without generalized convulsions 2. Desaturations are more commonly seen with temporal lobe than extratemporal seizures 2, 4. Electrical stimulation of mesial temporal structures, consistently elicits central apnea, potentially explaining this observation 5, 6. Ictal apnea has also been noted in 44–48 % of non-generalizing focal seizures, 2, 4, 7, 8 and has been reported as the main manifestation of focal seizures in a few case reports9, 10. Ictal and post-ictal central apnea has been suggested as a potential mechanism in some SUDEP11 and near-SUDEP12 cases. However, the role of ictal and post-ictal central apnea in SUDEP remains to be definitively demonstrated. We set out to examine the phenomenology, localizing value, and impact of ictal and post-ictal central apnea in patients with intractable epilepsy, in the epilepsy monitoring unit setting.
METHODS
Patients and clinical settings.
All patients were prospectively consented and recruited participants in the NINDS Center for SUDEP Research’s Autonomic and Imaging Biomarkers of SUDEP project (U01-NS090407). Patients with epilepsy aged >16 years undergoing video electroencephalogram (EEG) evaluation were studied in the epilepsy monitoring units (EMUs) of University Hospitals Cleveland Medical Center, University of Iowa, Northwestern University, New York University, Thomas Jefferson University, University of California at Los Angeles, University College London, and Columbia University. Inclusion criteria were patients in whom inductance plethysmography (abdominal and/or thoracic belts) and video EEG recording were carried out during the evaluation in the epilepsy unit. Exclusion criteria were movement or electrical artifacts obscuring plethysmographic signal, or obstructed or unavailable video.
Cardiorespiratory monitoring and VEEG monitoring.
All patients had prolonged surface video EEG monitoring using the 10–20 International Electrode System. EEG and electrocardiogram (EKG) were acquired using the Nihon Kohden (Tokyo, Japan), Micromed (Modigliani Veneto, Italy) and Xltek (Natus) acquisition platforms. Peripheral capillary oxygen saturation (SpO2) and heart rate were monitored using pulse oximetry (Nellcor OxiMax N-600x [Convidien], Masimo Radical-7 [Irvine] and SenTec Digital Monitoring System [Therwil BL]). Chest and abdominal excursions were recorded using inductance plethysmography (Ambu [Ballerup, Denmark] Sleepmate and Perfect Fit 2 [Dymedix]). Oxygen desaturations were classified as mild (SpO2 of 90–94%), moderate (75–89%) and severe (< 75%). We defined central apnea as cessation of breathing movements lasting for > 10 seconds in the absence of generalized tonic or clonic movements, since such movements invariably produced movement artifact in breathing channels. Tachycardia and bradycardia were defined as heart rate >100 beats per minute and <60 beats per minute respectively, or a >20% deviation from baseline. Seizures were classified according to the ILAE 2017 seizure classification13 based on the most prominent clinical signs: focal onset impaired awareness (FOIA) motor onset with automatisms, FOIA non-motor onset (dialepsis), FOIA motor onset with hyperkinesis, focal onset aware (FOA) motor onset tonic and/or clonic, focal onset to bilateral tonic-clonic seizures (FBTCS) and FOA non-motor onset (auras). Cognitive seizures, where the main clinical manifestation was aphasia, were further classified as aphasic seizures. Electrographic seizures were defined as seizures where the sole clinical manifestation was ictal central apnea. Generalized onset non-motor typical seizures were classified as absence seizures, and generalized onset tonic clonic seizures of primary generalized epilepsy were classified as GTCS = generalized tonic-clonic seizures (diagnosis based on history and EEG findings). The putative epileptogenic zone was defined based on clinical history, seizure semiology, neuroimaging and scalp EEG.
Statistical analysis.
Statistical analysis was performed using Statistical Package for Social Science (SPSS - IBM, corp. version 24). Summary statistics were reported as mean+SD (median, range). Chi-square test and binary logistic regressions were used to assess the association between dichotomous variable apnea (yes/no), with other variables and combinations. Since the 103 apneic seizures were not normally distributed, non-parametric testing (Kurskal-Wallis test) was used to assess apnea duration with other variables.
RESULTS
473 patients underwent polygraphic study of seizures. Reliable inductance plethysmography recordings and unobstructed seizure videos for the assessment of breathing responses were available in 312 seizures in 126 patients (77 female). Mean age was 40.09+14.71 years (median 38.5; 16–77). 109 patients had focal epilepsy and 17 patients had primary generalized epilepsy. Mean epilepsy duration was 17.8+13.5 years (17; 0–52).
A). Ictal central apnea (ICA) incidence and duration.
ICA was found in 103/312 (36.5%) seizures in 51/126 (40.5%) patients (29 female). Mean ICA duration was 28+18.8 (22; 10–97) seconds (s). Oxygen saturation data was available in 227/312 seizures overall, and in 79/103 seizures with ICA. In the remaining seizures, data was rendered unreliable because of dislodged sensors and movement. Prolonged ICA (> 60 seconds) occurred in eight patients and was associated with severe hypoxemia (SpO2 <75%) in six. Seizure and epilepsy details of patients with and without ICA are shown in table 1.
Table 1.
Seizure and epilepsy characteristics in 126 patients, with and without apnea
| Total Number | Apnea | No apnea | |
|---|---|---|---|
| Total of seizures | 312 | 103 | 209 |
| Type of seizures Focal Generalized |
285 27 |
103* 0 |
182 27 |
| State at the seizure onset Awake Sleep |
146 166 |
50 53 |
96 113 |
| Epileptogenic zone Temporal Frontal Parietal Occipital Insula Generalized Unknown |
156 79 11 24 2 30 10 |
84* 10 0 4 2 0 3 |
72 69 11 20 0 30 7 |
| Brain MRI (number of patients) Negative (84) Mesial temporal sclerosis (8) Hippocampus atrophy (2) Focal cortical dysplasia (6) Gray matter heterotopia (3) Tumor (11) Encephalomalacia (3) Schizencephaly (1) Encephalocele (1) Arterial venous malformation (2) Corpus callosal dysgenesis (1) MRI unavailable/normal CT head (4) |
312 197 17 6 28 6 38 5 1 3 3 2 6 |
103 72 3 5 2 1 7 3 1 3 2 2 2 |
209 125 14 1 26 5 31 2 0 0 1 0 4 |
| Seizure semiology** FOIA motor onset with automatisms FOIA non-motor onset (dialepsis) FOA non-motor onset (auras) FOIA motor onset hyperkinetic or FOA motor onset tonic/clonic Aphasic FBTCS Absence GTCS Electrographic seizures |
63 34 28 99 17 22 7 15 27 |
45* 19* 3 10 0 10 0 0 16 |
18 15 25 89 17 12 7 15 11 |
| EEG seizure onset Temporal Frontal Parietal Occipital Non-lateralizable Obscured by artifact |
132 51 6 14 64 45 |
71* 10 0 2 6 14 |
61 41 6 12 58 31 |
| EEG seizure discharges at or after ICA onset Unilateral Right Unilateral Left Non-lateralizable Obscured by artifact |
94 120 59 39 |
34* 51* 7 11 |
60 69 52 28 |
Significant values (p<0.05)
According to ILAE seizure classification13
FOIA = Focal onset impaired awareness
FOA = Focal onset aware
FBTCS = Focal to bilateral tonic-clonic seizures (of focal epilepsy)
GTCS = Generalized tonic-clonic seizures (of primary generalized epilepsy)
ICA=Ictal central apnea
Influence of type and duration of epilepsy, age and gender on apnea.
ICA was seen exclusively in focal epilepsy (36.5% of all partial seizures; p<0.001); none of the 17 primary generalized epilepsy patients (15 primary generalized tonic-clonic seizures [GTCS] and 7 absence seizures), had ICA. None of the 15 GTCS were preceded by central apnea. In the focal epilepsy group, 10/22 focal to bilateral tonic-clonic seizures (FBTCS) had preceding ICA; ICA was the sole clinical manifestation in all ten before the onset of tonic-clonic activity. We found that older age was significantly associated with ICA presence (p<0.001), but not with ICA duration or hypoxemia severity (p=0.6). There were no gender differences in ICA incidence (p=0.2). Although longer duration of epilepsy was not significantly associated with the presence of ICA (p=0.3), it was associated with longer ICA duration (p<0.001). Examples of presence or absence of ICA are shown in figures 1 and 2.
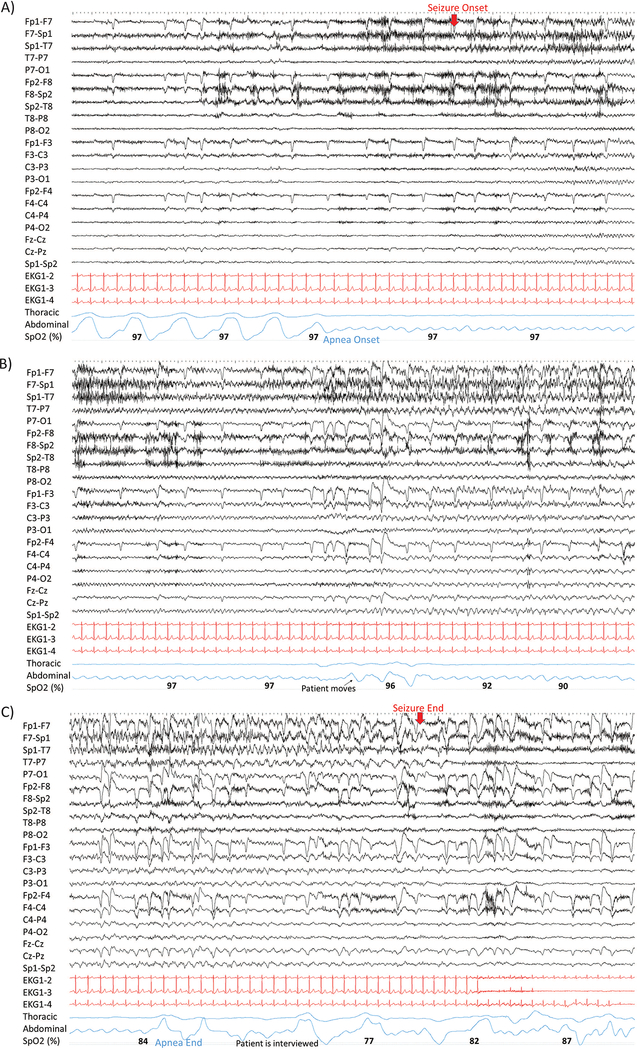
Figure 1.
A left temporal lobe seizure is shown in three consecutive 30 second-pages, in polygraphic detail. In A), the patient is awake before seizure onset. Breathing movements’ cessation was noted six seconds before epileptiform discharges began. In B), during a 50 second apnea period, complete absence of breathing movement is seen, along with oxygen desaturation, with only pulse artifacts identifiable in the plethysmography signal. In C), the patient re-starts breathing 15 seconds before seizure end, when he is interviewed by nurses. The patient was apnea agnostic.
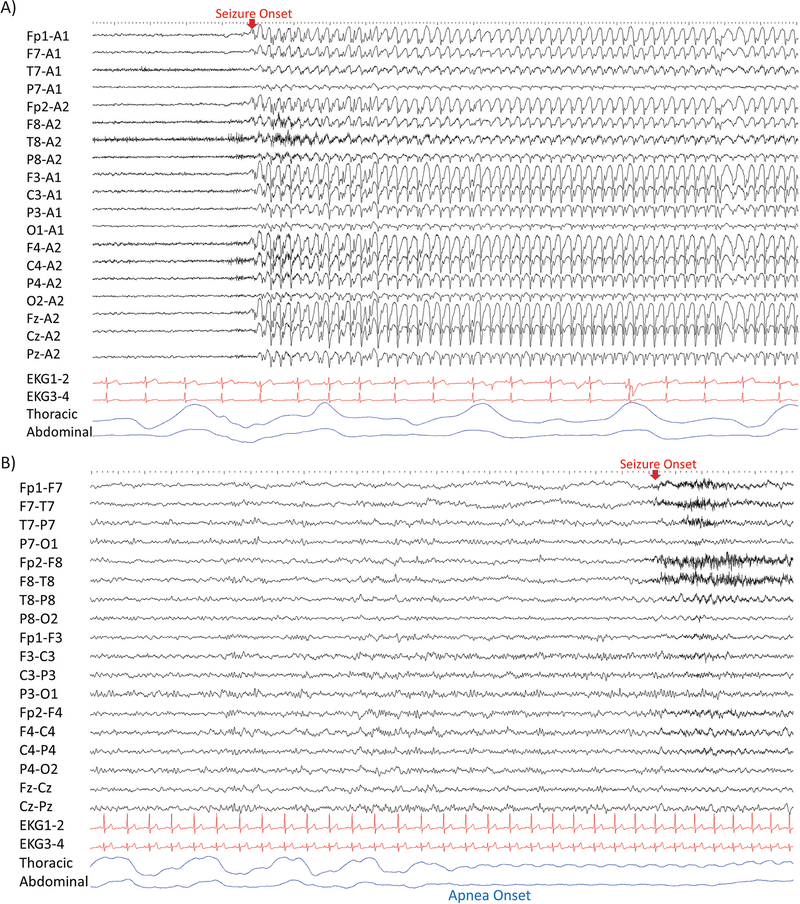
Figure 2.
Differences in polygraphy studies are represented in a typical A), generalized seizure with 3 Hz spike and wave’s discharges where no apnea is observed and B), focal epilepsy and right temporal lobe seizure where central apnea is clearly seen.
Epileptogenic and ictal onset zones and seizure semiology.
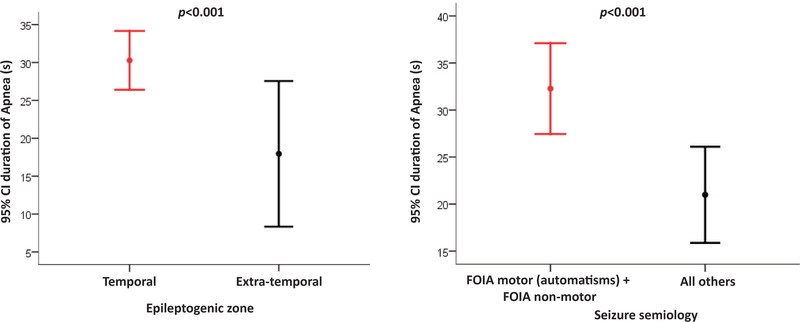
Temporal lobe epilepsy was highly associated with ICA presence in comparison to extratemporal epilepsy (odds ratio (OR) 10.1; 95% CI (5.5–18.5); P=0.001) and to frontal lobe epilepsy (OR 8.3; 95% (4–17.3); P=0.001) (Figure 3). Temporal lobe ictal onset zone was accordingly significantly associated with ICA (p<0.001). We then assessed whether the first ictal discharges at or after ICA onset involved one or both hemispheres. The ictal discharge was unilateral in 85/103 apneic seizures (87%), non-lateralizable in 7/103 (7%) and obscured by artifact at ICA onset in 11/103 (11%). ICA was significantly more likely to be associated with unilateral (left or right) ictal discharge at apnea onset compared to non-lateralizable EEG seizure onset (p<0.001). Temporal lobe epilepsy (p<0.001) and unilateral temporal lobe EEG ictal onset (p=0.001) were both significantly associated with longer ICA duration. There was a higher incidence of ICA in FOIA motor onset with automatisms (71.4%) and FOIA non-motor onset (dialepsis) (55.9%) compared to other seizure types (figure 3). Both were highly associated with ICA presence (p<0.001), as well as ICA duration (p=0.002) and severe hypoxemia (p=0.04).
Figure 3.
Plot with error bars of ictal central apnea duration in seconds by epileptogenic zone (A) and seizure semiology (B), showing 95% confident intervals (CI) for temporal compared to extra-temporal (p<0.001) (A) and FOIA motor onset with automatisms and FOIA non-motor (dialepsis), compared to other seizure semiologies (p<0.001) (B).
FOIA= focal onset impaired awareness.
Awake and sleep states.
Mean duration of ICA in seizures during the awake state was 27.87+19.38 seconds and 28.13+18.43 seconds during the non-REM (rapid-eye movement) sleep state; the awake/sleep states at seizure onset did not significantly impact either ICA presence or ICA duration (p=0.6). No seizures arose during REM sleep.
Apnea-induced oxygen desaturation of hemoglobin (SpO2).
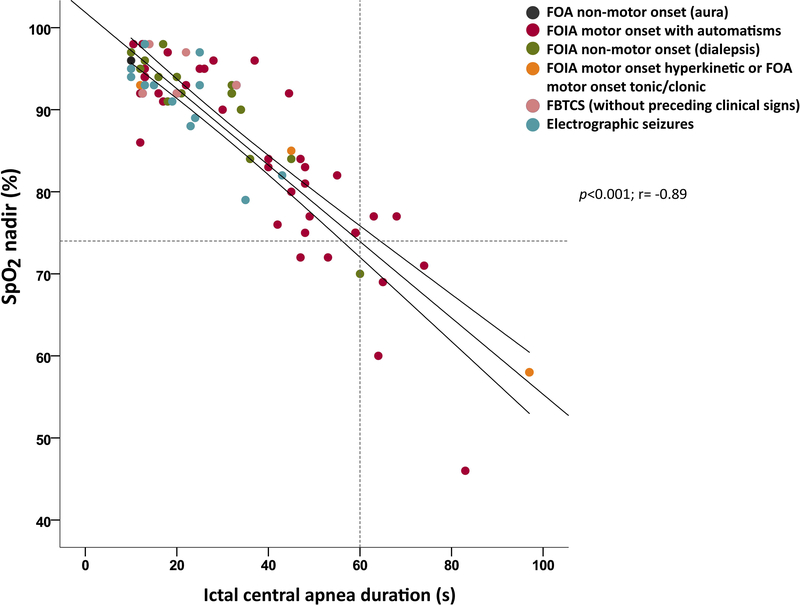
Ictal hypoxemia was present in 56/79 (70.8%); desaturation was mild in 26/56 (46%) seizures (mean 92.5+1.2 [93; 90–94]), moderate in 22/56 (39%) (mean 81.5+4.0 [82.5; 75–89]) and severe in 8/56 (14%) (mean 64.7+9.3 [69.5; 46–72]). Mean oxygen desaturation nadir was 87.7+10.3 (92; 46–98). Duration of ICA was significantly negatively correlated with SpO2 nadir (r=−0.89; p<0.001) (Figure 4). 53% of FOIA motor onset with automatism seizures, had moderate or severe hypoxemia compared to all other semiologies combined (p=0.04). Temporal lobe seizures were more likely to have moderate or severe ictal hypoxemia compared to other epileptogenic zones (p=0.03).
Figure 4.
Peripheral capillary oxygen saturation (SpO2) nadir and ictal central apnea (ICA) duration. The abscissa is apnea duration (in seconds) and the ordinate is the SpO2 at apnea end. The robust simple linear regression line and 95% confidence intervals are shown. Dashed lines show that apnea duration of 60 seconds approximately correlates with SpO2 <75%.
FOA= focal onset aware, FOIA= focal onset impaired awareness, FBTCS= focal to bilateral tonic-clonic seizure, s= seconds.
Ictal apnea characteristics and relationship with EEG onset/clinical onset.
In 16/103 (16.5%) of seizures, ICA was the only clinical manifestation during the entirety of the seizure. In 56/103 (54.3%) seizures, ICA occurred before unambiguous EEG seizure onset (mean 8+4.9 [7.7; 1–29] seconds). In 15/103 (14.5%), EEG onset and ICA onset were simultaneous, and in 32/103 (31%) EEG seizure onset preceded ICA (mean 7.7+7.9 [7.7; 1–28] seconds). In 61/103 seizures (68.5%), ICA onset occurred before clinical onset (mean 12.3+9.7[10; 1–50] seconds). These ICA onsets were simultaneous in 15/103 (16.8%), and in 13 seizures (14.6%), clinical onset preceded ICA onset (mean 13.6+9.6[12; 1–33] seconds). ICA always occurred in the expiratory phase and all patients were agnostic to their apneas, confirmed by questioning.
Apnea and bradycardia.
During ICA periods, heart rate increased in all 103 seizures; it was always seen at or after EEG seizure onset, rather than with ICA onset. Peri-ictal bradycardia was not observed in any seizure.
B). Post-ictal central apnea.
Spontaneous restoration of breathing before seizure end was seen in 100/103 (97%). In 3 FOIA motor onset with automatism seizures (3%), in 2/126 patients (2%), apnea persisted into the postictal period for 16–22 seconds (total peri-ictal apnea periods were 46–97 seconds). None had apnea beginning exclusively in the post-ictal period.
DISCUSSION
This study suggests that ICA is a semiological feature exclusive to focal epilepsy, most commonly starts before unambiguous surface EEG onset, and can be the only clinical manifestation of focal seizures. Temporal lobe epilepsy and frontal lobe epilepsy accounted for the majority of focal epilepsies associated with apnea; temporal lobe epilepsy not only had an eightfold greater association with apnea than frontal lobe epilepsy, it was also significantly more likely to be associated with longer apneas and more severe hypoxemia. FOIA motor onset with automatisms and FOIA non-motor onset seizure semiologies, typical of temporal lobe epilepsy, were similarly much more likely to produce ICA, and longer ICA durations. Thus, ICA presence and ICA duration may not only help distinguish focal epilepsies, they may enhance localization to the temporal lobe. Temporal lobe symptomatogenicity for ICA is in concordance with direct electrical cortical stimulation studies in humans that point to highly reproducible apneic responses with 14, 15low intensity, unilateral (left or right) amygdalar and hippocampal stimulation. 5, 6, 16 Although seizure spread to bilateral temporal structures has been considered necessary to produce ICA, these unilateral stimulation experiments, and the focal, unilateral ictal discharges at the time of ICA in many of our patients, suggest that such spread is not always the case.
It is likely that seizure discharges impair involuntary suprapontine (amygdalo-hippocampal) breathing control, resulting in ICA. Since ICA occurred after expiration in all our patients, it is likely that inspiration is immediately inhibited by seizure discharge, whereas expiration is mostly passive and allowed to occur to completion. The most likely downstream driver for ICA is seizure induced inhibition or disruption of brainstem inspiratory neuronal function. Descending amygdala projections to the parabrachial structures which exert critical roles in phase switching from expiration to inspiration have been described in cats single pulse amygdala stimulation triggers inspiratory onset17. Hippocampal activity increases before apnea termination in cats 18 and some hippocampal and amygdalar neurons phase-lock with the respiratory cycle in humans19, 20. Thus, the relatively frequent occurrence of ICA in temporal lobe epilepsy patients is unsurprising. Whether ICA in extratemporal epilepsy patients reflects spread to amygdalohippocampal structures (anterior cingulate, orbitofrontal and anterior insular regions, are extratemporal sites with amygdalar connections, that have also been implicated in cortical breathing control, 16), or whether this implies involvement of symptomatogenic extra-temporal breathing control structures is uncertain; shorter duration of apnea in these patients may indicate the involvement of breathing network nodes that are distinct from those involved in temporal lobe seizures.
The apnea agnosia described in stimulation studies, 5, 6 appeared to be true of ICA in our patients, and cessation of apnea in partial seizures was not followed by breathing distress, air hunger or dyspnea despite significant oxygen desaturations. This may explain why ICA has largely gone unrecognized, aside from few case series and case reports10, 21. An additional explanation is that plethysmography is not commonly used in EMUs. Breathing resumption in ICA patients, prior to seizure end, was the rule (97%) with few exceptions (3%). Lack of ICA awareness may only be dangerous in prolonged apnea. We observed that ICA cessation did not appear to be driven by hypoxemia. Although hypercarbia cannot be commented upon here since carbon dioxide was not measured, ictal central apneas are not reversed by augmentations in ventilatory drive from increasing carbon dioxide in previous human2 and animal22 studies and similar observations have been made in stimulation experiments5. ICA durations were highly varied, and hence changes in seizure discharge intensity in breathing control structures are a more likely explanation.
In our patients, the complete absence of ictal bradycardia with ICA, reported in a minority of seizures in one series,7 is surprising since bradycardia is a normal response to hypoventilation. Asphyxia in animal models results in heart rate decline and cardiac arrest in approximately 5 minutes23. Consistent with the literature, 12, 24 even in the rare, prolonged ICA epochs (up to 97 seconds), no bradycardia was observed in our study. Seizure-driven tachycardia is common25, and may conceivably have overcome any physiological tendency to bradycardia in these patients. Combined peri-ictal apnea and bradycardia, therefore, appears rare, but when it does occur, may comprise a potentially deleterious, high vagal tone, phenotype in seizure patients in the SUDEP context; the observed tachycardia apnea combination in this study, may reflect a more benign, self-limiting seizure manifestation.
Is ictal apnea a SUDEP biomarker? The majority of patients had a brief, self-limiting apnea with mild or moderate hypoxemia, suggesting that ICA poses no danger in most cases. Mean and median ICA durations in this cohort were 28 and 22 seconds respectively; the sheer frequency of ICA in this cohort, their short durations, and cessation before seizure end, suggest that in the majority of cases, ICA is self-limiting and unlikely to be a SUDEP concern. However, prolonged ICA (>60 seconds) was associated with severe hypoxemia (SpO2<75%) (Figure 4) and hence this combination may prove to be a biomarker of SUDEP that deserves prospective study. Indeed, two non-fatal ICA durations of 57 and 58 seconds, with SpO2 of 68% and 62%, respectively were recorded in a previously reported patient who subsequently died of SUDEP at home. 12 Longer duration of epilepsy is a known SUDEP risk factor26. Our observation of a positive correlation between duration of epilepsy and ICA duration is intriguing, and suggests potential plasticity in respiratory circuitry that may render relatively benign, short duration ICA into potentially lethal, longer duration ICA that may predispose to SUDEP. For example, functional neuroplasticity in the nucleus of the tractus solitarius, as evidenced by long-term changes in glutamate release and GABAergic neuronal activity, has been shown to occur with epileptogenesis in mice with acquired temporal lobe epilepsy27.
Post-ictal apnea appears to be a rare phenomenon. Only 3% of ICA persisted (for 16–22 seconds) beyond electroclinical seizure end in this study. None had isolated post-ictal central apnea beginning exclusively after seizure end. Duration of apnea continuance beyond seizure end was short (16–22 seconds) with total peri-ictal apnea periods between 46 and 97 seconds. Post-ictal apneic bradycardia, frequently reported in the post-ictal, agonal phases of MORTEMUS SUDEP cases, and near-SUDEP cases, after a partial seizure11 or GTCS28, did not occur in any of our patients. The persistent apnea observed into the post-ictal period may not have been truly post-ictal, as epileptiform discharges can persist in deep regions, such as amygdala or hippocampus, and not been seen on scalp EEG. However, persistent apnea could also represent a phenomenon similar to Todd’s paralysis or postictal aphasia, due to dysfunction or “exhaustion” in the major breathing control sites in in the human brainstem.
Some limitations of our study need to be considered. Our conclusions are based on a relatively small number of seizures in the primary generalized epilepsy group. Additionally, by considering GTCS or FBTCS onset as ICA end, we may have underestimated ICA duration since central apnea may conceivably commence in or continue into the tonic-clonic phase. The invariable loss of plethysmographic breathing signal due to movement artifact and the contribution of respiratory muscle spasm to hypoxia render comment on ICA difficult. The exclusion of data which is contaminated by artifact, may also underestimate ICA in extra-temporal seizures, since these may be more likely to induce vigorous movements (for example hypermotor movements) than temporal seizures.
CONCLUSION
ICA is a frequent, self-limiting semiological feature in focal epilepsy and can be its only clinical manifestation. However, prolonged ICA and severe hypoxemia together may comprise a potential biomarker of SUDEP. ICA is ten times more frequently seen at the beginning of temporal than extratemporal seizures; and in typical temporal (FOIA motor onset with automatisms and FOIA non-motor onset) seizure semiologies. The apnea is frequently seen before unambiguous scalp EEG or clinical seizure onset. ICA rarely persists after seizure end. Without polygraphic monitoring, including pulse oximetry and breathing plethysmography during VEEG, ICA may go unrecognized, since patients are agnostic to the apnea.
Key points:
Ictal central apnea (ICA) is frequent in focal seizures and may be their first clinical sign.
Patients are apnea agnostic and hence polygraphic monitoring of respiration during seizures is necessary for diagnosis.
ICA is ten times more often seen in temporal than extratemporal seizures.
Prolonged ICA (>60 seconds) is associated with severe hypoxemia.
Acknowledgments
We would like to thank the Universitat Autonoma de Barcelona for supporting NL’s Doctoral thesis in Medicine and Shailen Nandy, PhD (Cardiff University, UK) for his assistance with the statistical analysis.
Footnotes
Disclosure:
Samden Lhatoo is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405 and NIH/NINDS U01-NS090407. Bilal Zonjy, Sandhya Rani, Anita Zaremba, Rup Sainju, Brian K. Gehlbach, Stephan Schuele, Daniel Friedman, Maromi Nei, Ronald Harper, Luke Allen, Beate Diehl, John Millichap, Lisa Bateman and George B. Richerson are funded by NIH/NINDS U01-NS090407. Orrin Devinsky is funded by Center for SUDEP Research NIH/NINDS UO1-NS090415 and NIH/NINDS U01-NS090407. Stephan Schuele is on the speaker’s bureau for Sunovion and Eisai. Nuria Lacuey, Johnson D. Hampson, Mark A. Granner and Deidre N. Dragon report no disclosures.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 2.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 2008;131:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James MR, Marshall H, Carew-McColl M. Pulse oximetry during apparent tonic-clonic seizures. Lancet 1991;337:394–395. [DOI] [PubMed] [Google Scholar]
- 4.Blum AS, Ives JR, Goldberger AL, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia 2000;41:536–541. [DOI] [PubMed] [Google Scholar]
- 5.Lacuey N, Zonjy B, Londono L, et al. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology 2017;88:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. J Neurosci 2015;35:10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nashef L, Walker F, Allen P, et al. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996;60:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Regan ME, Brown JK. Abnormalities in cardiac and respiratory function observed during seizures in childhood. Dev Med Child Neurol 2005;47:4–9. [DOI] [PubMed] [Google Scholar]
- 9.L HW, H SB, T WS, et al. Partial seizures manifesting as apnea only in an adult. Epilepsia 1999;40:1828–1831. [DOI] [PubMed] [Google Scholar]
- 10.Tezer FI, Remi J, Noachtar S. Ictal apnea of epileptic origin. Neurology 2009;72:855–857. [DOI] [PubMed] [Google Scholar]
- 11.So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia 2000;41:1494–1497. [DOI] [PubMed] [Google Scholar]
- 12.Schuele SU, Afshari M, Afshari ZS, et al. Ictal central apnea as a predictor for sudden unexpected death in epilepsy. Epilepsy Behav 2011;22:401–403. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RS. The New Classification of Seizures by the International League Against Epilepsy 2017. Curr Neurol Neurosci Rep 2017;17:48. [DOI] [PubMed] [Google Scholar]
- 14.Pool JL, Ransohoff J. Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol 1949;12:385–392. [DOI] [PubMed] [Google Scholar]
- 15.Chapman WP, Livingston RB, Livingston KE. Frontal lobotomy and electrical stimulation of orbital surface of frontal lobes; effect on respiration and on blood pressure in man. Arch Neurol Psychiatry 1949;62:701–716. [DOI] [PubMed] [Google Scholar]
- 16.Kaada BR, Jasper H. Respiratory responses to stimulation of temporal pole, insula, and hippocampal and limbic gyri in man. AMA Arch Neurol Psychiatry 1952;68:609–619. [DOI] [PubMed] [Google Scholar]
- 17.Harper RM, Frysinger RC, Trelease RB, et al. State-dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Res 1984;306:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Poe GR, Rector DM, Harper RM. Hippocampal reflected optical patterns during sleep and waking states in the freely behaving cat. J Neurosci 1994;14:2933–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frysinger RC, Harper RM. Cardiac and respiratory correlations with unit discharge in epileptic human temporal lobe. Epilepsia 1990;31:162–171. [DOI] [PubMed] [Google Scholar]
- 20.Zelano C, Jiang H, Zhou G, et al. Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J Neurosci 2016;36:12448–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HW, Hong SB, Tae WS, et al. Partial seizures manifesting as apnea only in an adult. Epilepsia 1999;40:1828–1831. [DOI] [PubMed] [Google Scholar]
- 22.St-John WM, Rudkin AH, Homes GL, et al. Changes in respiratory-modulated neural activities, consistent with obstructive and central apnea, during fictive seizures in an in situ anaesthetized rat preparation. Epilepsy Res 2006;70:218–228. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Mabrouk OS, Liu T, et al. Asphyxia-activated corticocardiac signaling accelerates onset of cardiac arrest. Proc Natl Acad Sci U S A 2015;112:E2073–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyal M, Bateman LM, Albertson TE, et al. Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia 2010;51:1359–1364. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Guo CL, Zhang PS, et al. Heart rate changes in partial seizures: analysis of influencing factors among refractory patients. BMC Neurol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesdorffer DC, Tomson T, Benn E, et al. Combined analysis of risk factors for SUDEP. Epilepsia 2011;52:1150–1159. [DOI] [PubMed] [Google Scholar]
- 27.Derera ID, Delisle BP, Smith BN. Functional Neuroplasticity in the Nucleus Tractus Solitarius and Increased Risk of Sudden Death in Mice with Acquired Temporal Lobe Epilepsy. eNeuro 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin L, Zhang Y, Wang XL, et al. Postictal apnea as an important mechanism for SUDEP: A near-SUDEP with continuous EEG-ECG-EMG recording. J Clin Neurosci 2017;43:130–132. [DOI] [PubMed] [Google Scholar]