Abstract
Objective.
Kidney podocytes and their slit diaphragms prevent urinary protein loss. T cells from patients with systemic lupus erythematosus display increased expression of calcium/calmodulin-dependent protein kinase IV (CaMKIV). The present study was undertaken to investigate the role of CaMKIV in podocyte function in lupus nephritis (LN).
Methods.
We treated kidney podocytes with IgG derived from healthy individuals or patients with LN and then analyzed gene expression using a DNA microarray. The localization of IgG in podocytes was analyzed by immunofluorescence staining, with or without silencing of neonatal Fc receptor (FcRn). In addition, we silenced CAMK4 in podocytes and analyzed the expression of selected genes. We also examined the expression of CD86 in kidney podocytes from MRL/lpr, MRL/lpr.camkiv−/−, and MRL/MPJ mice by in situ hybridization.
Results.
We found that exposure of podocytes to IgG resulted in entry of IgG into the cytoplasm. IgG entered podocytes via the FcRn because less IgG was found in the cytoplasm of podocytes treated with FcRn small interfering RNA. DNA microarray studies of podocytes exposed to LN-derived IgG revealed up-regulation of genes related to the activation of immune cells or podocyte damage. Interestingly, CD86 expression decreased after silencing CAMK4 in podocytes. Also, in situ hybridization experiments showed that the expression of CD86 was reduced in podocytes from MRL/lpr.camkiv−/− mice.
Conclusion.
LN-derived IgG enters podocytes and up-regulates CAMK4, which is followed by increased expression of genes known to be linked to podocyte damage and T cell activation. Targeted inhibition of CAMK4 in podocytes may prove to be clinically useful in patients with LN.
Lupus nephritis (LN) is still the major cause of morbidity and mortality in patients with systemic lupus erythematosus (SLE) (1). The filtration barrier of the glomerulus is composed of fenestrated endothelial cells, the glomerular basement membrane (GBM), and the foot processes and slit diaphragms of the podocytes. Podocytes are highly differentiated epithelial cells that form part of the filtration barrier in the kidney, acting to prevent urinary protein loss. The effacement of the foot processes as a result of podocyte injury has been associated with the development of proteinuria and nephrotic syndrome (2). Little attention has been paid to the role of podocytes in human LN, and only a few studies have reported a correlation between proteinuria and diffuse effacement of podocyte foot processes in patients with LN without evidence of immune deposition (3,4).
SLE T cells express high levels of calcium/calmodulin-dependent protein kinase IV (CaMKIV), which translocates to the nucleus upon engagement of the T cell receptor–CD3 complex and accounts for decreased production of interleukin-2 (IL-2) (5). We have previously shown that a small molecule inhibitor of CaMKIV (KN-93) and deletion of the CAMK4 gene mitigate disease development in lupus-prone mice by suppressing cytokine production (6–8) and costimulatory molecule CD86 (B7-2) and CD80 (B7-1) expression (9) in lymphocytes.
Podocytes may express molecules typically found in immune cells. It was previously reported that CD80, a transmembrane protein generally expressed on the surface of antigen-presenting cells (APCs) and involved in T cell costimulation, is also expressed in podocytes following stimulation with lipopolysaccharide through Toll-like receptor 4. CD80 expression in podocytes in murine and human LN correlates with the severity of proteinuria (10). However, the expression of CD86, another costimulatory molecule, by podocytes and its role in the pathogenesis of LN remain unknown.
IgG from patients with SLE has been shown to bind with greater avidity to human mesangial cells (11) and pleural mesothelial cells (12). The neonatal Fc receptor (FcRn), an IgG and albumin transport receptor, is also expressed by podocytes and functions to internalize IgG from the GBM (13). We report herein our findings that LN-derived IgG enters podocytes via the FcRn and up-regulates CAMK4 and, through this, a number of genes involved in podocyte injury.
PATIENTS AND METHODS
Patients and controls.
We studied 15 patients who fulfilled at least 4 of the 11 American College of Rheumatology revised criteria for the classification of SLE (14) and had biopsy-proven lupus nephritis according to the International Society of Nephrology/Renal Pathology Society criteria (15) (3 with class II and 4 each with classes III-V disease). All patients were women between the ages of 20 and 64 years and had SLE Disease Activity Index scores ranging from 8 to 16. Seven normal healthy women served as controls in this study. Serum samples were collected before the treatment and were stored at −80°C until used. The protocol was approved by Institutional Review Board of the Nagasaki University Hospital.
IgG purification and antibody labeling.
IgG purification kits (Dojindo Molecular Technologies) were used for isolation and purification of IgG from SLE and normal individuals according to the manufacturer’s protocol. Purity was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Output from the column was used for non-IgG binding samples. Each sample of purified IgG was fluorescence labeled with an Alexa Fluor 488 monoclonal antibody labeling kit (Invitrogen). Fluorescence labeled IgG was used for localization of IgG in podocytes by immunofluorescence staining and flow cytometry.
Cell culture.
Conditionally immortalized human podocytes (AB 8/13) were kindly provided by Dr. Moin A. Saleem (University of Bristol, Bristol, UK) and maintained at 33°C in RPMI 1640 supplemented with 10% fetal calf serum and 1% ITS Premix (BD Biosciences) and then shifted to 37°C for 10–14 days for differentiation (16).
Immunofluorescence staining.
Double-immunofluorescence staining of tissue sections and cells was performed as described below. Briefly, frozen sections (4 μm) were fixed for 3 minutes in cold (−20°C) acetone and then air dried. Sections were incubated overnight at 4°C with primary antibodies, guinea pig anti-human nephrin (Invitrogen), and mouse anti-human CaMKIV (BD Biosciences). Subsequently, sections were washed 3 times in phosphate buffered saline (PBS) and incubated for 1 hour at room temperature with Alexa Fluor 488–labeled goat anti–guinea pig IgG or Alexa Fluor 568–labeled goat anti-mouse IgG (Invitrogen). After 3 washes with PBS, Vectashield antifade mounting medium (Vector Laboratories) was applied.
Cultured podocytes (0.2 × 105) were seeded onto type I collagen 4-well culture slides (BD Biosciences) and exposed for 24 hours to fluorescence-labeled IgG or nonlabeled IgG (10 μg/ml) from LN patients and healthy individuals. After 30 minutes of incubation with RPMI 1640 medium supplemented with 2% bovine serum albumin (BSA), the cells were washed once with PBS and fixed for 20 minutes with 4% paraformaldehyde. The cells were permeabilized for 5 minutes with 0.1% Triton X-100 in PBS, followed by blocking for 30 minutes with PBS containing 2% BSA. Cells were then stained for 1 hour at room temperature with anti-FcRn antibody (1:100 dilution; Santa Cruz Biotechnology). After washing 3 times with PBS, the cells were stained with Alexa Fluor 568 anti-rabbit IgG (highly cross-absorbed) as the secondary antibody (Invitrogen). The stained cover glasses were mounted on a glass slide with 10 μl of DAPI Fluoromount-G (SouthernBiotech) and sealed with nail polish. The stained samples were inspected and photographed using a Biorevo BZ-9000 fluorescence microscope (Keyence).
Western blot analysis.
For Western blot analysis, treated human podocyte lysates were separated on 4–12% SDS-PAGE gels, transferred to nitrocellulose membranes, and then incubated with primary antibody. After washing, the membranes were incubated with horseradish peroxidase–conjugated IgG and developed using enhanced chemiluminescence (GE Healthcare). The following antibodies were used for immunoblot assays: mouse anti-CaMKIV (BD Biosciences), goat anti-CD86 (Santa Cruz Biotechnology), and rabbit anti-actin (Sigma).
RNA extraction and polymerase chain reaction (PCR).
Human podocytes were cultured for 24 or 48 hours with IgG purified from the sera of normal individuals and LN patients and levels of messenger RNA (mRNA) for CAMK4 were determined by real-time PCR. Podocytes were homogenized and total RNA was extracted using an RNeasy Mini kit (Qiagen). Complementary DNA was produced using random primers from an equal amount of RNA. The following primers were obtained from Takara Bio: for CaMKIV, 5′-GTGCTCATGAAGACAGTATGTGGAA-3′ (forward) and 5′-CACCTCAGGTCCATAGGCACAA-3′ (reverse); for CD86, 5′-TGGCCTAGGGTACAGGCAACA-3′ (forward) and 5′-GCCCAGATAGAAGTGGCTCCAG-3′ (reverse); and for 18S ribosomal RNA, 5′-ACTCAACAC-GGGAAACCTCA-3′ (forward) and 5′-AACCAGACAAATC-GCTCCAC-3′ (reverse).
Transfection with small interfering RNA (siRNA).
Human podocytes were cultured for 24 hours with IgG purified from the sera of normal individuals and LN patients and were then transfected with CAMK4 siRNA, FCRN siRNA, or control siRNA (Thermo Scientific) using INTERFERin transfection reagent (Polyplus Transfection) according to the manufacturer’s protocol. After 24 or 48 hours of incubation, the cells were collected for RNA extraction or were stained for immunofluorescence analysis.
Transfection with gene expression plasmids.
Human podocytes were cultured for 10 days for differentiation. Two million cells were transiently transfected with 5 μg of human CAMK4 expression vectors (CaMKIV expression plasmid under the control of the cytomegalovirus [CMV] promoter) or empty vector (pCMV-sport6) from The Plasmid Information Database (PlasmID; http://plasmid.hms.harvard.edu). Transfections were performed using Lipofectamine 3000 according to the manufacturer’s protocol.
Gene expression analysis.
Briefly, cultured human podocytes exposed for 24 hours to IgG purified from the sera of normal individuals and LN patients were collected for RNA isolation. Following reverse transcription, complementary RNAs were labeled with Cy5-Streptavidin (GE Healthcare Biosciences). Hybridization of whole-genome human genes included in Code-Link bioarrays (Applied Microarrays) was performed for 18 hours at 37°C in a shaker incubator. The hybridization reactions were performed in duplicate. Microarrays were read with a Gene-Pix 4400A laser scanner (Molecular Devices) and were quantified and normalized using CodeLink software v5.0 (Applied Microarrays). The fluorescence intensity for each spot was expressed as the net intensity, which was calculated as the difference between the background and the raw intensity. Scan data images were analyzed using the Microarray Data Analysis Tool software package (Filgen), a comprehensive program that also produced the gene ontology and Z score reports.
Upon concluding this process, the raw gene expression values were obtained for each of the samples. During these processes, the probes with detection P values that were not significant (P > 0.1) in all samples were removed from the analysis. Lists of significantly changed genes were then compiled (> 1.5-fold difference and a false discovery rate–adjusted P value [called a q value] of <0.05). Ontologies included biologic processes, molecular functions, and cellular components and were organized according to the guidelines of the Gene Ontology Consortium (17). This data set has been made publicly available at the Gene Expression Omnibus GEO database (18) with submission number GSE 55768.
Mice.
Female MRL/lpr, Camk4tmlTch/J and MRL/MpJ mice were purchased from The Jackson Laboratory. MRL/lpr.camkiv−/− mice were generated on an MRL/lpr background as described previously (7,19). Experiments were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Kidneys from 16-week-old MRL/MpJ, MRL/lpr, and MRL/lpr.camkiv−/− mice were formalin-fixed, paraffin-embedded [FFPE] sections that were assessed for in situ hybridization.
In situ hybridization.
In situ hybridization was performed by GeneticLab Company, using a QuantiGene View-RNA FFPE assay kit with Panomics protocols (Affymetrix). Tissue sections (4 μm) were prepared from paraffin-embedded kidneys, deparaffinized, boiled in pretreatment solution, and digested with proteinase QF. Sections were hybridized for 2 hours at 40°C with a designed probe against the mouse CD86 and Nphs1 (Affymetrix). Hybridized probes were amplified using PreAmp and Amp molecules. Thereafter, multiple labeled probe oligonucleotides conjugated to alkaline phosphatase were added, and Fast Red substrate was used to produce signals (red fluorophore). Nuclei were stained with bisbenzimide (Hoechst 33258). Contiguous sections were also stained with hematoxylin and eosin. A series of high-resolution monochromatic images were captured using a confocal laser fluorescence microscope (LSM-510; Carl Zeiss). The brightness of each image file was uniformly enhanced using Adobe Photoshop Elements 6, followed by analysis using Image J software (National Institutes of Health). Image files (.tiff) were inverted and opened in grayscale mode. The CD86 content in the nephrin-positive area index was calculated using the following formula:
where the staining density is indicated by a number from 0 to 256 in grayscale (20,21).
Statistical analysis.
All results are expressed as the mean ± SD. The Kruskal-Wallis test with post hoc comparisons using the Scheffe’s test was used for intergroup comparisons of multiple variables. Statistical analyses were performed with StatView software (Abacus Concepts). P values less than 0.05 were considered significant.
RESULTS
Altered gene pattern expression in podocytes cultured with IgG purified from the sera of SLE patients.
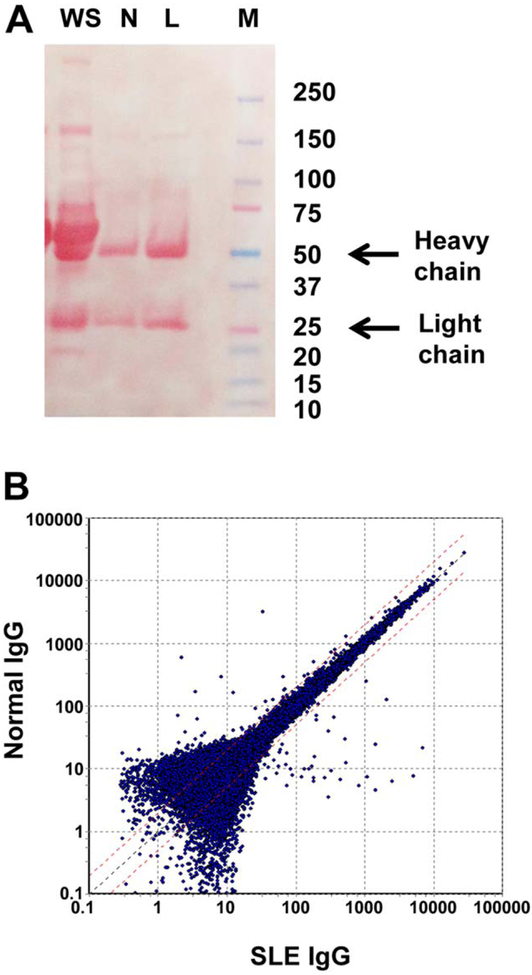
Purified IgG from the sera of SLE patients and normal individuals and whole serum were subjected to electrophoresis to document their purity (Figure 1A). Podocytes were cultured in the presence of SLE or normal IgG, and the extracted mRNA was subjected to microarray analysis as described in Patients and Methods. A scatterplot summary of the entire gene set expressed (P < 0.05) in podocytes, as determined by microarray analysis, is shown in Figure 1B. When an arbitrary difference of 1.5-fold (or greater) change was selected, 860 genes were identified as being up-regulated and 1,317 as down-regulated in podocytes exposed to SLE IgG as compared to normal IgG.
Figure 1.
A, Electrophoresis of whole serum (WS) or fractionated serum IgG derived from a systemic lupus erythematosus (SLE) patient (L) and a normal subject (N). Molecular weight marker (M) is shown at the right. B, Scatterplot of entire gene set considered by microarray analysis to be expressed in podocytes (P < 0.05). The position of each dot on the scatterplot corresponds to the normalized average signal intensity (log scale) of a single gene. The normalized average signal intensity after exposure to SLE IgG and normal IgG is shown on the x and y axes, respectively. Broken red lines represent normal IgG:SLE IgG ratios of 2.0 (top; 2-fold greater expression in normal IgG) and 0.5 (bottom; 2-fold greater expression in SLE IgG). Black broken line is the mean.
Altered expression of genes related to podocyte damage after exposure to IgG from LN patients.
To evaluate the effect of IgG from normal subjects and LN patients on podocytes, we identified candidate genes in gene ontology terms using the DNA microarray data. This analysis identified 20 specific ontologies that were up-regulated and had the highest Z scores. We present ontologies that were overrepresented relative to normal IgG when podocytes were exposed to IgG from LN patients. We focused on ontologies containing ≥9 genes and having a Z score that was >2.0 (Table 1). The ontologies that were affected by LN-derived IgG included those involved in the activation of immune cells or podocyte damage through myofibril rearrangement. We used the term “regulation of T cell activation” because the genes with the highest changes included HLA-DQB1, IL10, PTPN22, CAMK4, CD80, and CD86, all of which have also been known to be linked to the pathogenesis of lupus (Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39499/abstract) (1,22). We also identified 44 specific ontologies that were down-regulated and had the highest Z scores. Interestingly, processes such as regulation of cell proliferation and developmental growth involved in morphogenesis were down-regulated in cells exposed to LN-derived IgG (Supplementary Table 2).
Table 1.
High expression of gene ontologies in podocytes after exposure to IgG from systemic lupus erythematosus patients*
| Ontology | Term | No. of changed genes |
Total no. of genes |
Z score | P |
|---|---|---|---|---|---|
| Biologic process | Cellular component assembly involved in morphogenesis |
10 (0) | 257 (0) | 2.787 | 0.01313 |
| Biologic process | Regulation of T cell activation† | 12 (0) | 397 (8) | 2.112 | 0.04711 |
| Biologic process | Actin filament–based process | 25 (0) | 945 (1) | 2.353 | 0.02711 |
| Cellular component | Cell–substrate adherens junction | 10 (0) | 290 (12) | 2.383 | 0.03349 |
| Cellular component | Focal adhesion | 10 (10) | 282 (282) | 2.478 | 0.03051 |
| Cellular component | Axon part | 10 (2) | 281 (37) | 2.49 | 0.03018 |
| Cellular component | I band | 10 (3) | 185 (28) | 3.986 | 0.00146 |
| Cellular component | Cell–substrate junction | 11 (2) | 305 (12) | 2.66 | 0.02082 |
| Cellular component | Early endosome | 12 (10) | 362 (256) | 2.463 | 0.02293 |
| Cellular component | Contractile fiber part | 13 (0) | 290 (0) | 3.761 | 0.00156 |
| Cellular component | Sarcomere | 13 (1) | 260 (33) | 4.214 | 0.000606 |
| Cellular component | Contractile fiber | 14 (0) | 335 (11) | 3.608 | 0.00196 |
| Cellular component | Myofibril | 14 (1) | 318 (55) | 3.827 | 0.00123 |
| Cellular component | Endosome membrane | 16 (9) | 525 (282) | 2.487 | 0.02362 |
| Cellular component | Endosomal part | 17 (0) | 541 (0) | 2.697 | 0.0161 |
| Cellular component | Basolateral plasma membrane | 17 (5) | 585 (258) | 2.357 | 0.032 |
| Cellular component | Endosome | 27 (14) | 1,040 (587) | 2.361 | 0.02568 |
| Cellular component | Z disc | 9 (9) | 171 (171) | 3.687 | 0.00294 |
| Molecular function | Cytokine receptor binding | 10 (0) | 303 (11) | 2.234 | 0.03951 |
| Molecular function | Magnesium ion binding | 12 (12) | 368 (368) | 2.403 | 0.03678 |
Specific ontologies with the highest Z scores were selected. Criteria for inclusion in the table were an ontology containing ≥9 genes and having a Z score of >2.0. Numbers in parentheses are the numerical value considered in the hierarchical structure of each gene ontology term.
Term selected because the genes with the highest changes included HLA–DQB1, IL10, PTPN22, CAMK4, CD80, and CD86, all of which have also been known to be linked to the pathogenesis of lupus.
Entry of IgG into podocytes through the FcRn.
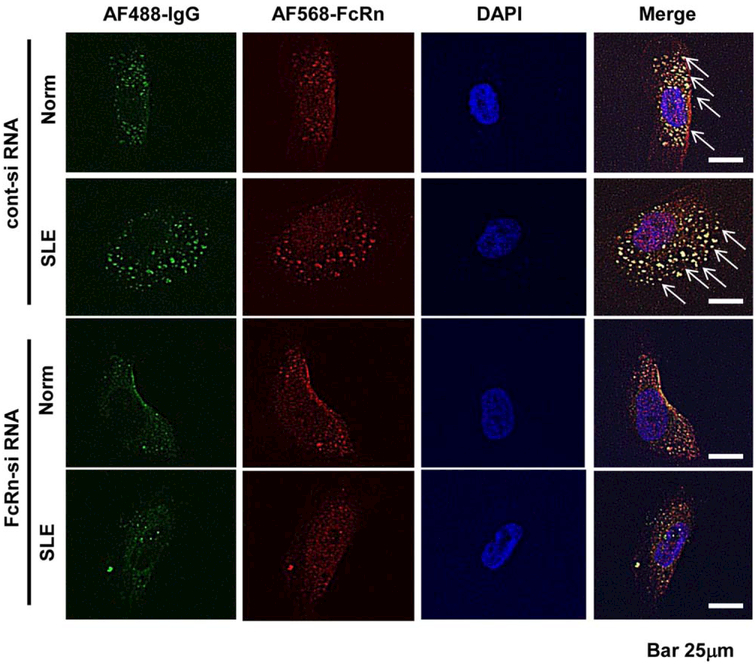
Next, we analyzed the localization of normal and LN-derived IgG (Alexa Fluor 488 labeled) in podocytes by immunofluorescent staining before or after silencing of the FcRn (Alexa Fluor 568 labeled), the receptor for IgG. As shown in Figure 2, exposure of podocytes to IgG from normal individuals and LN patients resulted in entry of the IgG into the cytoplasm. The amount of IgG that entered the podocytes was not significantly different between the normal individuals and the LN patients (Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39499/abstract). We found that IgG entered podocytes through the FcRn because when the FcRn was silenced with an siRNA FcRn, lesser amounts of IgG were found in the cytoplasm.
Figure 2.
IgG entry into podocytes via the neonatal Fc receptor (FcRn). Localization of IgG derived from a normal subject (Norm) and a patient with systemic lupus erythematosus (SLE) nephritis (Alexa Fluor [AF] 488 stained) in podocytes was analyzed by immunofluorescence staining, with or without silencing of FcRn (Alexa Fluor 568 stained), the receptor of IgG, using FcRn small interfering RNA (siRNA) or control siRNA. Arrows indicate entry of IgG into the cytoplasm, which did not occur when FcRn was silenced.
LN-derived IgG induction of CaMKIV and CD86 expression in podocytes.
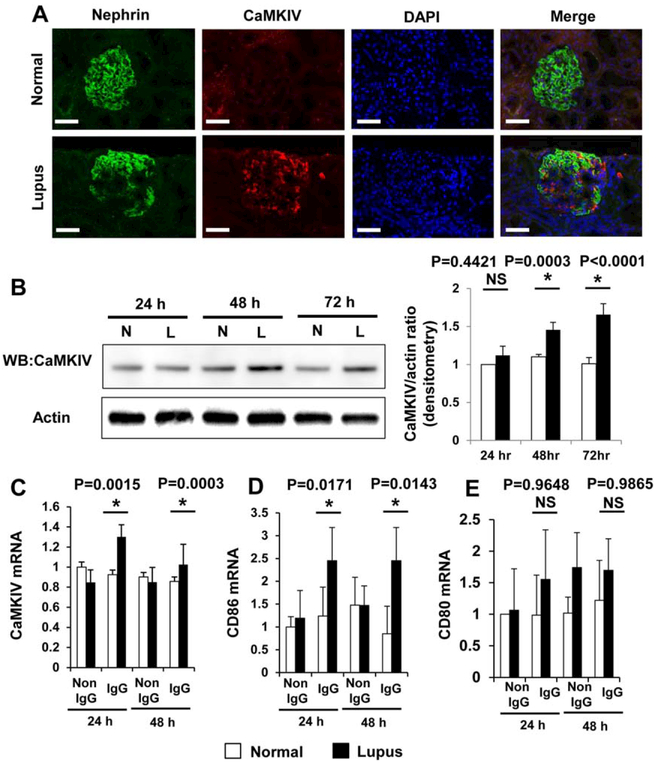
We examined the coexpression of nephrin, a specific podocyte protein, and CaMKIV in glomeruli from healthy individuals and patients with LN. As shown in Figure 3A, CaMKIV expression was found to be increased in podocytes from kidney biopsy specimens obtained from LN patients. Increased expression of CaMKIV seems to be limited to the renal glomeruli of LN patients because we did not detect increased expression of CaMKIV in renal glomeruli obtained from patients with IgA nephropathy (data not shown).
Figure 3.
A, Coexpression of nephrin and calcium/calmodulin-dependent protein kinase IV (CaMKIV) in the glomeruli of normal subjects and patients with lupus nephritis. Bars = 50 μm. B–E, Lupus serum induction of the expression of CaMKIV protein (B), CaMKIV mRNA (C), CD86 mRNA (D), and CD80 mRNA (E) after incubation for the indicated times. Podocytes (cell line) were stimulated with IgG derived from sera obtained from normal controls (N) and lupus nephritis patients (L). Protein expression of CaMKIV was determined by Western blotting (WB) (B). Actin was included as a loading control. The Western blotting results were quantified by densitometry and are shown at the right. Values are the mean ± SD of 4–8 subjects per group. * = P < 0.05 versus normal controls. NS = not significant.
To confirm the validity of the microarray data, we analyzed several of the most strongly expressed genes known to be involved in the regulation of T cell activation using real-time quantitative reverse transcription-PCR (qRT-PCR) (Supplementary Table 1 and Supplementary Figure 2, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39499/abstract). We evaluated the expression levels of CaMKIV protein (Figure 3B) and mRNA (Figure 3C) in cultured podocytes and found that culture of podocytes in the presence of LN-derived IgG led to a 1.4-fold increase in the expression of CaMKIV protein and mRNA. We also found that the levels of mRNA for CD86, but not CD80, were elevated 2-fold in podocytes exposed to LN-derived IgG (Figures 3D and E). This observation documents the relevance of our in vitro data to events occurring in patients with LN. Apparently, podocytes in LN patients are exposed to circulating IgG, and this exposure results in up-regulation of CaMKIV.
CAMK4 regulation of CD86 expression.
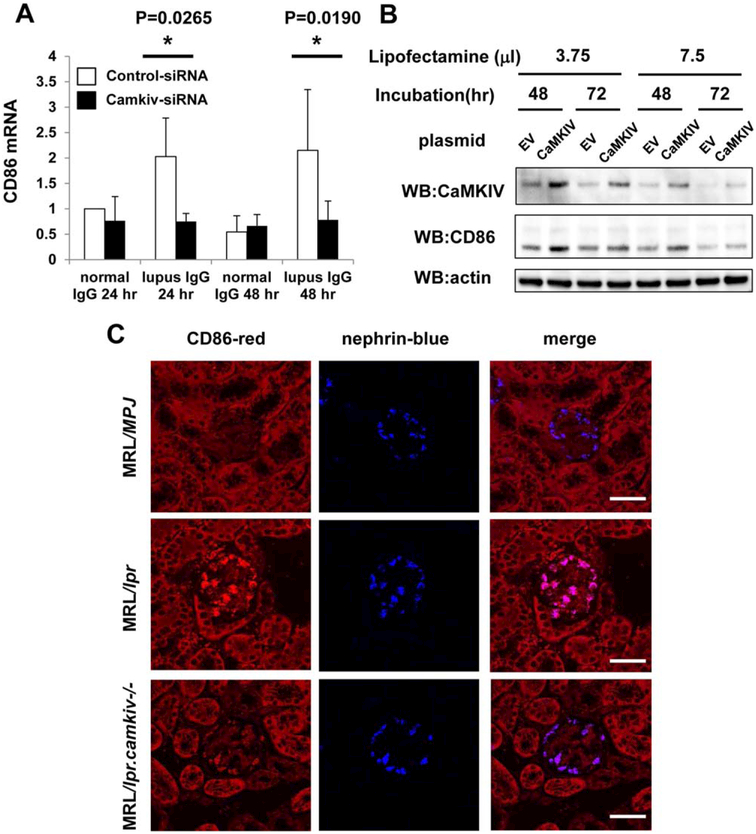
To evaluate the regulation of CD86 expression in podocytes, we cultured human podocytes with normal and LN-derived IgG for 24 or 48 hours and then transfected them with CAMK4 siRNA. The expression of mRNA for CD86 was down-regulated in cultured human podocytes after silencing of CAMK4 (Figure 4A). Additionally, we found that forced expression of CAMK4 in podocytes increased CD86 expression (Figure 4B and Supplementary Figure 3, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39499/abstract).
Figure 4.
A, Suppression of CD86 expression by silencing of calcium/calmodulin-dependent protein kinase IV (CaMKIV) in human podocytes exposed to IgG derived from normal subjects and from patients with lupus nephritis. Human podocytes were exposed to IgG for 24 or 48 hours and were then transfected with CAMK4 or control scrambled small interfering RNA (siRNA). Values are the mean ± SD of 4 subjects per group. * = P < 0.05 versus control siRNA. B, Expression of mRNA for CD86 in human podocytes following forced expression of CAMK4. Cultured human podocytes were transfected with either empty vector (EV) or pCMV-sport6 CaMKIV. After 48 or 72 hours, cell lysates were collected, and the expression of CaMKIV, CD86, and actin (loading control) protein was examined by Western blotting (WB). C, Reduced expression of CD86 in the glomeruli of lupus-prone mice lacking CaMKIV. The coexpression of CD86 mRNA and nephrin mRNA in the glomeruli of MRL/MpJ, MRL/lpr, and MRL/lpr.camkiv−/− mice was analyzed by in situ hybridization. Bars = 50 μm.
To further confirm the validity of this in vitro observation, we analyzed the expression of CD86 in MRL/lpr lupus-prone mice in which CAMK4 had been genetically eliminated. To this end, we analyzed the coexpression of mRNA for CD86 and nephrin in the glomeruli of MRL/MpJ, MRL/lpr, and MRL/lpr.camkiv−/− mice by in situ hybridization. The expression of CD86 in podocytes was reduced in MRL/lpr.camkiv−/− mice as compared to MRL/lpr mice (Figure 4C and Supplementary Figure 4, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39499/abstract). Thus, LN-derived IgG up-regulates CaMKIV in podocytes and, through this, the expression of CD86.
DISCUSSION
We present novel evidence which sheds light on the biochemical events that precede podocyte injury and proteinuria in lupus nephritis. Specifically, IgG derived from patients with LN enters podocytes by way of the FcRn, where it causes an increase in CaMKIV and an alteration of the expression of genes that are known to be involved in podocyte damage and, as we unexpectedly found, in T cell activation.
Kidney podocytes have been known as the filtration barrier in the kidney since it prevents urinary protein loss (2). It has been shown that progressive worsening of histologic nephritis and proteinuria correlate with increased foot process effacement and reduced glomerular expression of nephrin and podocin in (NZB × NZW)F1 mice and in patients with LN (23). Autoantibodies have been shown to deposit in the kidneys of patients and mice with LN and are believed to play a key role in causing renal inflammation and dysfunction. However, in a subset of lupus patients with proteinuria, there is no evidence of the typical immune complexes, but instead, there appears to be extensive podocyte effacement (3). In addition, the molecular mechanisms whereby autoantibodies and/or immune complexes cause podocyte injury are not understood.
Our experiments were designed to investigate mechanisms whereby IgG from patients with LN affects podocyte function and mediates injury. Exposure of cultured podocytes to IgG induces early phenotype changes consisting of cytoskeleton F-actin rearrangement with a marked decrease in synaptopodin, an actin-associated protein, which is linked to the formation of foot processes, a hallmark of the differentiated podocyte phenotype (24–26). Dexamethasone, which is frequently used to treat kidney disease linked to podocyte malfunction, promotes maturation and process formation of cultured human podocytes in vitro (27). Our gene ontologies analysis (Table 1) showed high expression of sarcomere (P < 0.000606), which is the repeating unit of a myofibril, myofibril (P < 0.00123), which is the contractile element of skeletal and cardiac muscle, and actin filament–based process (P < 0.02711) in podocytes treated with IgG from LN patients. Therefore, exposure of podocytes to LN-derived IgG results in altered expression of proteins linked to contractile structure.
Previous studies have demonstrated that immune complexes can bind directly to renal parenchymal cells. It is conceivable that in vivo, podocytes use the classic Fcγ receptors and megalin (28) to internalize protein. Additionally, it has been reported that the IgG transport receptor, FcRn, is expressed by podocytes (29). Podocytes express FcRn and may transcytose IgG and albumin to the glomerular filtrate to prevent clogging of the filter. Since kidney damage is commonly caused by deposition of IgG and immune complexes at the glomerular barrier, accumulation of IgG in GBM spaces may lead to proteinuria in LN. This provides indirect evidence for the involvement of the receptor in the pathogenesis of glomerulopathies. Our results showed that IgG enters podocytes, in part, by using the FcRn, because when podocytes were treated with FCRN siRNA, less IgG was found in their cytoplasm (Figure 2). Also, we found that the quantity of IgG which entered podocytes was not significantly different between normal and LN-derived IgG (Supplementary Figure 1), but obviously, LN-derived IgG alters gene expression patterns to enable podocyte damage (Figure 1B). The microarray data and real-time qRT-PCR analysis showed that gene expression of CAMK4 and CD86 were significantly elevated in podocytes treated with LN-derived IgG (Figure 3C and Supplementary Table 1).
We have previously shown that a small-molecule inhibitor of CaMKIV, KN-93, and CAMK4 deletion mitigate disease development in lupus-prone mice by suppressing cytokine production (6–8) and costimulatory molecule CD86 and CD80 expression (9) in lymphocytes. Moreover, the proliferation of mesangial cells in lupus nephritis was inhibited by deletion of CAMK4 through suppression of AP-1 binding to the IL-6 promoter (19). Our results indicate that CaMKIV represents a kinase involved in the pathogenesis of kidney dysfunction and proteinuria in lupus nephritis. Similar results have been reported in doxorubicin-induced nephrotic syndrome (30), where inhibition of CaMKIV signaling was effective in protecting renal function even during the proteinuric state. However, the pathogenic role of CaMKIV in podocyte functioning is still unknown. We show that the expression of CaMKIV increased in LN podocytes (Figures 3A–C) and that CaMKIV expression was increased in biopsy specimens from patients with classes IV and V LN, but not class II LN (Supplementary Figure 5, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39499/abstract). In in vitro experiments, we found that culture of podocytes in the presence of IgG from LN sera led to a 1.4-fold increase in the expression of CaMKIV protein and mRNA.
CD80 and CD86 are usually found on B cells and are involved in T cell costimulation. Induced expression of CD80 on podocytes was previously considered to be involved in the development of proteinuria by rearranging actin cytoskeleton, and CD80 deficiency is known to protect mice from proteinuria because of alterations in glomerular permeability with actin rearrangement (4,10).
Our gene array analysis of podocytes exposed to IgG from LN patients revealed that CD86, another molecule involved T cell costimulation which shares the same ligand with CD80, is also induced. We also found that CD86 expression increased in podocytes from the glomeruli of MRL/lpr mice (Figure 4C). CaMKIV was previously shown to be involved in the up-regulation of CD86 in immune cells (9,31). Our experiments showed that expression of mRNA for CD86 was down-regulated in cultured human podocytes after CaMKIV was silenced and that forced expression of CaMKIV in podocytes increased CD86 expression (Figures 4A and B and Supplementary Figure 3). In vitro experiments supported these results, indicating that CD86 expression is regulated by CaMKIV in LN podocytes (Figure 4C and Supplementary Figure 4). Although at this point, we do not know whether CD86 expression can lead to podocyte injury, it is quite possible that it is involved in the regulation of migration, integrin activation, and slit diaphragm rearrangement/foot process effacement and, ultimately, podocyte damage in a manner similar to that of CD80 (10,32,33). Therefore, the podocyte CD86 and CD80 pathway seems to play an important role in certain patients with nephrotic syndrome, including those with LN. We hypothesize that CD86 and CD80 act together to advance podocyte injury and that blocking them may protect from podocyte damage.
In conclusion, we present evidence that LN-derived IgG enters podocytes by way of the Ig transporter FcRn and induces the expression of CaMKIV in podocytes, which leads to cell damage and proteinuria. Thus, inhibition of CaMKIV in patients with LN should restore podocyte function and mitigate proteinuria.
Supplementary Material
Acknowledgments
Supported by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research 24790999 to Dr. Ichinose) and the NIH (grant R01-AR-064350 to Dr. Tsokos).
REFERENCES
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011; 365:2110–21. [DOI] [PubMed] [Google Scholar]
- 2.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol 2012;74:299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraft SW, Schwartz MM, Korbet SM, Lewis EJ. Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol 2005;16:175–9. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi S, Zeier M, Reiser J. Role of podocytes in lupus nephritis. Nephrol Dial Transplant 2009;24:3607–12. [DOI] [PubMed] [Google Scholar]
- 5.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest 2005;115:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga T, Ichinose K, Mizui M, Crispin JC, Tsokos GC. Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. J Immunol 2012;189:3490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga T, Hedrich CM, Mizui M, Yoshida N, Otomo K, Lieberman LA, et al. CaMK4-dependent activation of AKT/mTOR and CREM-α underlies autoimmunity-associated Th17 imbalance. J Clin Invest 2014;124:2234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koga T, Mizui M, Yoshida N, Otomo K, Lieberman LA, Crispin JC, et al. KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3+ regulatory T cells in MRL/lpr mice. Autoimmunity 2014;47:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichinose K, Juang YT, Crispin JC, Kis-Toth K, Tsokos GC. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis Rheum 2011;63:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 2004;113:1390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan TM, Leung JK, Ho SK, Yung S. Mesangial cell-binding anti-DNA antibodies in patients with systemic lupus erythematosus. J Am Soc Nephrol 2002;13:1219–29. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Leung JC, Chan LY, Chan TM, Lai KN. The pathogenetic role of immunoglobulin G from patients with systemic lupus erythematosus in the development of lupus pleuritis. Rheumatology (Oxford) 2004;43:286–93. [DOI] [PubMed] [Google Scholar]
- 13.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 2008;105:967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 15.Weening JJ, D’Agati VD, Schwartz MM, Seshan Sv, Alpers CE, Appel GB, et al. , on behalf of the International Society of Nephrology and Renal Pathology Society Working Group on the Classification of Lupus Nephritis. The classification of glomerulonephritis in systemic lupus erythematosus revisited [published erratum appears in J Am Soc Nephrol 2004;15:835–6]. J Am Soc Nephrol 2004;15:241–50. [DOI] [PubMed] [Google Scholar]
- 16.Saleem MA, O’Hare mJ, Reiser J, Coward RJ, Inward CD, Farren T, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 2002;13:630–8. [DOI] [PubMed] [Google Scholar]
- 17.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. Nat Genet 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett T, Troup DB, Wilhite SE, Ledoux P, Evangelista C, Kim IF, et al. NCBI GEO: archive for functional genomics data sets—10 years on. Nucleic Acids Res 2011;39:D1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichinose K, Rauen T, Juang YT, Kis-Toth K, Mizui M, Koga T, et al. Calcium/calmodulin-dependent protein kinase type IV is essential for mesangial cell proliferation and lupus nephritis. J Immunol 2011;187:5500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichinose K, Maeshima Y, Yamamoto Y, Kitayama H, Takazawa Y, Hirokoshi K, et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 2005;54:2891–903. [DOI] [PubMed] [Google Scholar]
- 21.Ichinose K, Maeshima Y, Yamamoto Y, Kinomura M, Hirokoshi K, Kitayama H, et al. 2-(8-hydroxy-6-methoxy-1-oxo-1h-2-benzopyran-3-yl) propionic acid, an inhibitor of angiogenesis, ameliorates renal alterations in obese type 2 diabetic mice. Diabetes 2006;55:1232–42. [PubMed] [Google Scholar]
- 22.Cui Y, Sheng Y, Zhang X. Genetic susceptibility to SLE: recent progress from GWAS. J Autoimmun 2013;41:25–33. [DOI] [PubMed] [Google Scholar]
- 23.Perysinaki GS, Moysiadis DK, Bertsias G, Giannopoulou I, Kyriacou K, Nakopoulou L, et al. Podocyte main slit diaphragm proteins, nephrin and podocin, are affected at early stages of lupus nephritis and correlate with disease histology. Lupus 2011;20:781–91. [DOI] [PubMed] [Google Scholar]
- 24.Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, et al. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol 2005;166:1309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barisoni L, Kopp JB. Update in podocyte biology: putting one’s best foot forward. Curr Opin Nephrol Hypertens 2003;12:251–8. [DOI] [PubMed] [Google Scholar]
- 26.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997;236:248–58. [DOI] [PubMed] [Google Scholar]
- 27.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int 2006;70:1038–45. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki H, Saito A, Ooi H, Kobayashi N, Mundel P, Gejyo F. Differentiation-induced cultured podocytes express endocytically active megalin, a Heymann nephritis antigen. Nephron Exp Nephrol 2004;96:e52–8. [DOI] [PubMed] [Google Scholar]
- 29.Haymann JP, Levraud JP, Bouet S, Kappes V, Hagege J, Nguyen G, et al. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 2000;11:632–9. [DOI] [PubMed] [Google Scholar]
- 30.Ao Q, Cheng Q, Ma Q, Wang X, Liu S. Inhibition of calcium2+/calmodulin-dependent protein kinase type IV ameliorates experimental nephrotic syndrome. Intern Med 2013;52:1035–41. [DOI] [PubMed] [Google Scholar]
- 31.Illario M, Giardino-Torchia ML, Sankar U, Ribar TJ, Galgani M, Vitiello L, et al. Calmodulin-dependent kinase IV links Toll-like receptor 4 signaling with survival pathway of activated dendritic cells. Blood 2008;111:723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 2013;369:2416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiser J, Alachkar N. Proteinuria: abate or applaud abatacept in proteinuric kidney disease? Nat Rev Nephrol 2014;10:128–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.