Abstract
Neurodegenerative proteinopathies are a group of pathologically similar, progressive disorders of the nervous system, characterised by structural alterations within and toxic misfolding of susceptible proteins. Oligomerisation of Aβ, tau, α-synuclein and TDP-43 leads to a toxin gain- or loss-of-function contributing to the phenotype observed in Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis and frontotemporal dementia. Misfolded proteins can adversely affect mitochondria, and post-mitotic neurones are especially sensitive to metabolic dysfunction. Misfolded proteins impair mitochondrial dynamics (morphology and trafficking), preventing functional mitochondria reaching the synapse, the primary site of ATP utilisation. Furthermore, a direct association of misfolded proteins with mitochondria may precipitate or augment dysfunctional oxidative phosphorylation and mitochondrial quality control, causing redox dyshomeostasis observed in disease. As such, a significant interest lies in understanding mechanisms of mitochondrial toxicity in neurodegenerative disorders and in dissecting these mechanisms with a view of maintaining mitochondrial homeostasis in disease. Recent advances in understanding mitochondrially controlled cell death pathways and elucidating the mitochondrial permeability pore bioarchitecture are beginning to present new avenues to target neurodegeneration. Novel mitochondrial roles of deubiquitinating enzymes are coming to light and present an opportunity for a new class of proteins to target therapeutically with the aim of promoting mitophagy and the ubiquitin–proteasome system. The brain is enormously metabolically active, placing a large emphasis on maintaining ATP supply. Therefore, identifying mechanisms to sustain mitochondrial function may represent a common intervention point across all proteinopathies.
Keywords: amyloid, mitochondria, mitophagy, neurodegeneration, tau proteins, α-synuclein
Introduction
The energetic requirements of neuronal excitability, synaptic activity and plasticity are extensive and are almost exclusively fulfilled by mitochondrial oxidative phosphorylation (OXPHOS) [1]. Mitochondria are often trafficked long distances to meet spatiotemporal adenosine triphosphate (ATP) requirements and dynamic mechanisms determine mitochondrial localisation to best satisfy local demands of the neurone. It is also now clear that, during the lifetime of a neurone, mitochondria can become dysfunctional to the extent they cannot maintain a proton motive force sufficient for ATP generation. Cellular mechanisms engage to remove damaged mitochondria and replenish the mitochondrial pool. These cycles of mitochondrial fusion and fission can become defective in neurodegenerative disorders and consequent accumulation of damaged components within mitochondria can adversely affect mitochondrial function and cellular homeostasis.
Mitochondrial dysfunction in neurodegenerative proteinopathies
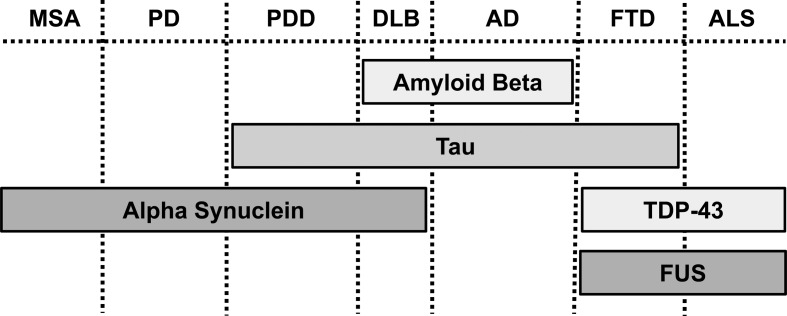
Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are pathologically similar, progressive neurodegenerative proteinopathies [2] (Figure 1). Proteinopathy refers to a disease-causing conformational change in a protein that normally has other roles in cell biology. Such proteins undergo pathogenic misfolding and oligomerisation into higher-order structures, revealing self-templating conformations, and have an ability to undergo prion-like spreading between cells, together resulting in toxin gain- or loss-of-function [2,3]. Misfolded proteins can adversely affect mitochondria, either through a direct association, damaging mitochondrial DNA, altering trafficking and dynamics, deregulating bioenergetics and quality control pathways or promoting mitochondria-dependent cell death pathways. Mitochondrial dysfunction as a cause or a consequence of neurodegenerative disease pathogenesis is still debated and a self-perpetuating feed-forward toxic cycle may exist (Figure 2).
Figure 1. Pathological overlap of proteinopathies in different neurodegenerative diseases.
Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; DLB, dementia with Lewy bodies; MSA, multiple systems atrophy; PD, Parkinson's disease; PDD, Parkinson's disease dementia; TDP-43, TAR DNA-binding protein 43; FUS, fused in sarcoma.
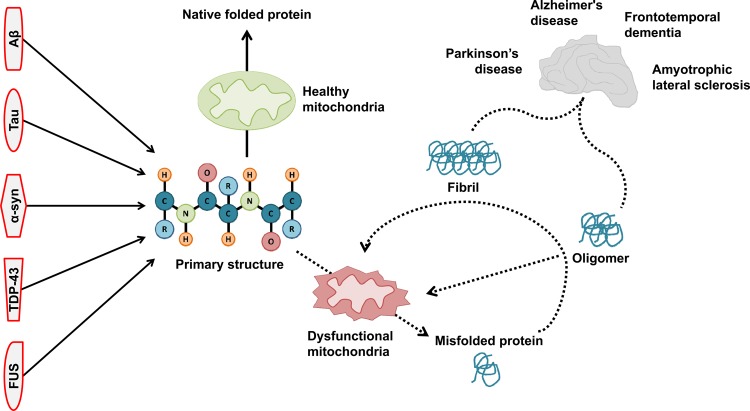
Figure 2. Reciprocal relationship between mitochondrial function and protein misfolding.
Mitochondrial function is adversely affected by toxic misfolded proteins. Additionally, mitochondrial function is necessary for correct protein folding and processing in neurodegenerative proteinopathies.
Mitochondrial relationship with amyloid β and tau
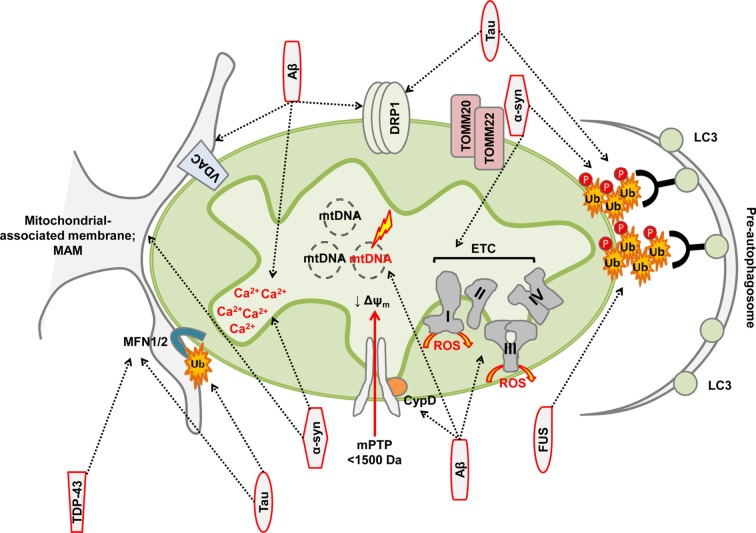
AD is the most prevalent proteinopathy, characterised by accumulation of extracellular plaques containing amyloid β (Aβ) and intracellular tangles of tau. Aβ and tau both have detrimental effects on mitochondria [4]. Disruptions in mitochondrial DNA maintenance, protein import, electron transport chain (ETC) activity and redox balance are all consequences of Aβ-induced toxicity [5–12] (Figure 3 and Table 1). Mitochondrial localisation of Aβ peptide has been observed, albeit the exact sub-mitochondrial topology remains less well defined [13,14] and, indeed, Aβ may be produced at the mitochondrial-associated membrane (MAM) [15], linking mitochondrial–ER contact sites, Ca2+ handling and bioenergetics to Aβ toxicity.
Figure 3. Mitochondrial toxicity of misfolded proteins.
Red outline depicts misfolded proteins and arrows indicate mitochondrial directed association or toxicity.
Table 1. Mitochondrial relationship and toxicities with AD associated pathogenic proteins.
| Pathogenic protein | Mitochondrial toxicity or association | Reference |
|---|---|---|
| Amyloid β | Mitochondrial association and protein import: | [6,13–15,37] |
| ||
| Mitochondrial DNA maintenance: | [5,11,12] | |
| ||
| ETC and bioenergetics: | [6–10] | |
| ||
| Oxidative stress and cell death pathways: | [4,6,8,38] | |
| ||
| Tau | Mitochondrial association and protein import: | [6,17,22,23] |
| ||
| Deregulated mitophagy: | [17,19,20] | |
| ||
| Mitochondrial trafficking and dynamics: | [17,18,21,24,25] | |
|
Tau is a highly soluble, natively unfolded protein [16]. Tau hyperphosphorylation impairs its ability to bind and stabilise microtubules, and tau aggregation and intraneuronal filaments are common in the pathology of tauopathies (Figure 1). The influence of tau on mitochondrial dynamics and quality control is well documented [17–21] (Figure 3). N-terminal tau fragments are associated with mitochondrial functional changes and defects in mitochondrial quality control, and the accumulation of tau fragments in human mitochondria isolated from synaptosomes correlates with synaptic changes observed in AD [17,22,23]. Changes in mitochondrial dynamics are also observed following tau overexpression in cultured cells or in in vivo models of tauopathy [18,24,25] (Table 1).
Some literature suggests that the accumulation of (phospho-)tau and Aβ is a direct consequence of mitochondrial dysfunction [26–30]. Perturbations to mitochondrial proteases and chaperones have demonstrated relationships with AD disease markers. Missense mutations in the mitochondrial matrix peptidase, pitrilysin metalloprotease 1 (PITRM1), are associated with Aβ-positive deposits and a slowly progressing neurodegenerative phenotype [31]. In addition, decreased activity in pre-sequence protease (PreP) has been observed in the temporal lobe region of AD patients [32] and has been linked to Aβ processing [33]. Overexpression of heat shock chaperone, mortalin, alleviates Aβ-induced toxicity, while pharmacological inhibition or siRNA down-regulation of mortalin induces DRP-1 (dynamin-related protein 1)-mediated mitochondrial fission and potentiates Aβ-induced mitochondrial and cellular toxicity [34,35]. With respect to pathogenic tau, antioxidant treatment of sod2 nullizygous neonatal mice reverses phospho–tau accumulation, placing mitochondrial oxidative stress upstream of tau pathology [26]. Finally, mitochondrial dysfunction induced by loss of either prohibitin 2 (PHB2), a mitochondrial membrane scaffold protein, or the m-AAA protease subunit, AFG3L2, both results in tau hyperphosphorylation [27,36], potentially linking mitochondrial dysfunction and the cytoskeleton. Taken together, these observations link mitochondrial dynamics, quality control and function to accumulation and toxicity of Aβ and tau.
Mitochondrial toxicity associated with synucleinopathies
PD is characterised by loss of dopaminergic neurones within the substantia nigra pars compacta and reduced dopamine innovation to the striatum. Mitochondrial dysfunction is a prominent pathological feature of both sporadic and familial diseases [39–45], and many PD-causing genes have overt mitochondrial phenotypes [44–53]. Accumulation of insoluble α-synuclein (α-syn) is a common feature of many clinical phenotypes, known collectively as synucleinopathies [54] (Figure 1).
Mitochondrial localisation of α-syn negatively affects mitochondrial function, morphology and dynamics [46,55–60] (Figure 3 and Table 2). Constitutive import of α-syn to mitochondria is transmembrane potential-dependent and is facilitated through a cryptic mitochondrial targeting sequence within the N-terminal region [57]. α-Syn associates with the mitochondrial inner membrane where a direct interaction and toxicity towards mitochondrial complex I has been observed [46,57]. Oligomeric and dopamine-modified α-syn-dependent reduction in protein import occurs via disruption of the association between translocase of the outer mitochondrial membrane (TOMM)-20 and its co-receptor, TOMM22 [61]. As a result, diminished protein import in nigrostriatal neurones impairs mitochondrial function, decreasing respiration and transmembrane potential, and increasing mitochondrial reactive oxygen species (ROS) [61]. Purification of crude mitochondrial preparations has led to the hypothesis that α-syn is, in fact, localised to the MAM [58] and α-syn point mutations reduce mitochondria–ER contacts, causing mitochondrial fragmentation [58]. Finally, α-syn has significant effects on mitochondrial quality control and its accumulation is becoming recognised as a consequence of deficient mitophagy [50,62,63]. α-Syn competes with LC3 for cardiolipin on the outer mitochondrial membrane. Cardiolipin facilitates refolding of α-syn oligomers; however, following prolonged exposure, LC3 is recruited. Mutated α-Syn is less able to compete with LC3, contributing to increased mitophagic flux observed in disease cells [64].
Table 2. Mitochondrial toxicities associated with synucleinopathies.
| Pathogenic protein | Mitochondrial toxicity or association | Reference |
|---|---|---|
| α-Synuclein | Mitochondrial association and protein import: | [46,57–59,61] |
| ||
| Mitochondrial dynamics and morphology: | [58–60,65] | |
| ||
| ETC and bioenergetics: | [46,56,57,66] | |
| ||
| Deregulated mitophagy: | ||
|
||
| Oxidative stress and cell death pathways: | [55,57,66,67] | |
|
Mitochondrial toxicity in ALS and FTD-linked proteinopathies
ALS and FTD share pathological and genetic similarities and potentially common neurodegenerative pathways [68]. Aggregated transactive response DNA-binding protein 43 kDa (TDP-43) and fused in sarcoma (FUS) are pathological hallmarks of both ALS and FTD (Figure 1). Both are ribonuclear proteins and contain prion-like domains, rich in glycine molecules, increasing their propensity for aggregation and cell-to-cell transmission. Dysfunction in OXPHOS, Ca2+ handling and ROS have all been proposed as key mitochondrially-associated determinants of ALS pathogenesis [69] (Table 3). Furthermore, mitochondrial trafficking defects are responsible for accumulation of defective mitochondria around cell bodies in motor neurones [69].
Table 3. Mitochondrial toxicities for ALS/FTD associated pathogenic proteins.
| Pathogenic protein | Mitochondrial toxicity or association | Reference |
|---|---|---|
| TDP-43 | Mitochondrial association and protein import: | [74] |
| ||
| Mitochondrial dynamics and morphology: | [73,75] | |
| ||
| Mitochondrial trafficking: | [72,73] | |
| ||
| Deregulated mitophagy: | [74] | |
| ||
| FUS | Mitochondrial dynamics and morphology: | [70,71] |
|
Ubiquitin-positive aggregates are observed in aged, mutant FUS-expressing transgenic animals and correlate with neuronal loss. Aggregates are also positive for mitochondrial cytochrome C oxidase (COX-IV), suggesting that defective mitochondria may be tagged for removal through the mitophagic machinery [70]. Similar pathology has been observed in a single post-mortem analysis of an FUS mutation carrier [71]. C- and N-terminal fragments of TDP-43 have been identified within mitochondria in Amyloid precursor protein (APP)/PS1 mice and mitochondrial dynamic changes, including trafficking and quality control defects, organelle redistribution and clustering within cytoplasmic inclusions, as well as morphological and ultrastructural alterations, are observed in animal models of TDP-43 pathology [72–74]. Taken together, these observations suggest a phenotype of dysfunctional, mislocalised and fragmented mitochondria in ALS and FTD (Figure 3).
Mitochondrially targeted strategies as disease-modifiers in neurodegenerative proteinopathies
Despite evidence of mitochondrial dysfunction in the pathology of proteinopathies and exacerbation of neurodegenerative disorders, the exact biochemical, neurotoxic mechanisms of misfolded, aggregated proteins remain poorly understood. Protecting mitochondrial function therefore may be one plausible drug discovery strategy for neuroprotective or disease-modifying end-points.
Inhibition of mitochondrial permeability transition pore opening
Mitochondrial permeability transition pore (mPTP) opening has been implicated as a major cell death pathway in multiple neurodegenerative diseases [76–78]. A shift in the mitochondrial redox balance towards oxidative stress, coupled with Ca2+ overload, triggers opening of the mPTP leading to osmotic swelling, uncoupling of electron transport and metabolic collapse [79–86]. The mitochondrial matrix enzyme, cyclophilin D (CypD), is a known positive regulator of mPTP opening [86]. Genetic ablation or pharmacological inhibition of CypD desensitises the pore to Ca2+, restricting pore opening [85,86]. Direct binding between Aβ and CypD links amyloid toxicity to mPTP opening in AD [38,87] and CypD deficiency corrects mitochondrial trafficking defects observed in AD models [88]. Previous literature supports a role for the F1F0-ATP synthase in pore formation [89,90], suggesting that the oligomycin-sensitivity conferring protein (OSCP) serves as a docking site for CypD [90]. Interestingly, OSCP is decreased during AD progression and may directly interact with Aβ [37]. Given the relationship between CypD, OSCP, Aβ and the propensity for mPTP opening, it is plausible that targeting these processes may have clinical benefits. Indeed, recently, phenotypic screening approaches have identified mPTP inhibitors and CypD-binding compounds in a model of Aβ-induced mPTP opening [91–94]. Overexpression of an N-terminal region of α-syn has also been observed to regulate mitochondrial membrane permeability [67], linking mPTP to synucleinopathies. Moreover, following overexpression, α-syn associates with the adenine nucleotide translocase (ANT), another putative pore component [95,96]. Interestingly, pharmacological inhibition of ANT partially reverses the associated α-syn-induced mitochondrial toxicity [97].
Homology within the cyclophilin isoenzyme family makes selective targeting of CypD therapeutically challenging [98]. Moreover, since CypD does not constitute a principal pore component, and effects are indirect, mitochondria remain capable of permeability transition given enough stimuli [86,99]. CypD confers sensitivity to the mPTP inhibitor, cyclosporin A (CsA) [85,100], and number of CsA analogues have been developed [101,102]. CsA and its derivatives are large molecular mass natural products and penetrate the blood–brain barrier poorly, limiting efficacy in neurodegenerative disease. Many groups have developed CypD-independent inhibitors [103–107], but to date, none, as far as we are aware, have been tested in models of neurodegenerative disease.
Activating mitophagy to improve mitochondrial function in neurodegenerative proteinopathies
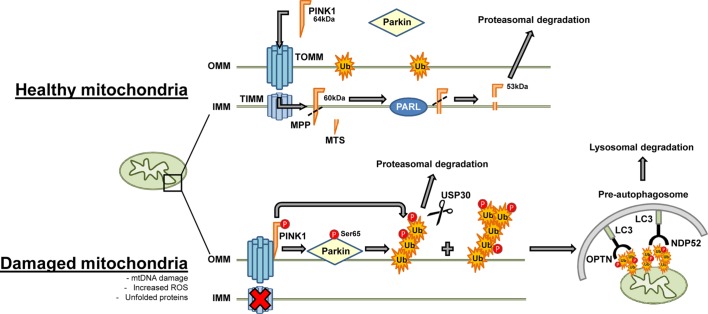
Dysfunctional lysosomal and proteasomal degradation pathways have been implicated in neurodegenerative diseases. A selective form of macroautophagy, termed mitophagy, is responsible for the clearance of defective mitochondria from cells [44,45,108–110]. PTEN-induced putative kinase 1 (PINK1) and parkin are regulators of mitophagy and are integral to a mechanism that identifies and tags defective mitochondria for removal [110,111] (Figure 4). The association between mutations in these proteins and dysfunction in the mitophagy pathway has direct implications in both familial and sporadic PD [43,51,112].
Figure 4. Defective mitochondria are removed from the cell by mitophagy.
In healthy mitochondria, mitophagy proceeds at a slow rate due to the low abundance of ubiquitinated mitochondrial proteins, PINK1 import and degradation. PINK1 is stabilised on the OMM following mitochondrial depolarisation and phosphorylates both parkin and ubiquitin. Parkin is activated and translocates to mitochondria. Parkin ubiquitinates outer membrane proteins which then serve as targets for autophagic adaptor proteins and mitochondria are then cleared through the autophagic machinery. Abbreviations: IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane; TIMM, translocase of the inner mitochondrial membrane; MPP, mitochondrial processing peptidase; MTS, mitochondrial targeting sequence; TOMM, translocase of the outer mitochondrial membrane; Ub, ubiquitin.
PINK1 and parkin function may be necessary for α-syn clearance. Preceding neurodegeneration, α-syn A53T transgenic mice accumulate neuronal inclusions containing mitochondrial remnants and autophagic markers which increase in size and number with PINK1 or parkin knockout [62]. PINK1 loss-of-function potentiates the A53T phenotype, decreasing lifespan and enhancing movement deficits and protein aggregation [63]. Similarly, iPSCs from mutant PINK1/parkin carriers accumulate cytoplasmic inclusions and insoluble α-syn, a phenotype which can be partially corrected following PINK1 re-expression [50]. Interestingly, following mitochondrial uncoupling, autophagic α-syn removal is reduced and the likelihood of aggregate formation in oligodendrocytes is enhanced, suggesting that mitochondrial damage over time may play a role in α-syn accumulation [48]. Finally, a novel mechanism of PINK1 protection in α-syn models has been proposed as being mediated through protein phosphatase 2A activity [113].
Mitophagy has been linked to both Aβ and tau in AD. PINK1 is down-regulated in AD patients and in transgenic AD models [114]. Furthermore, the absence of PINK1 augments the mutant APP phenotype and stereotaxic injection of rAAV2–PINK1 into the hippocampus of mutant APP mice significantly reduced Aβ compared with control, improving synaptic function and memory [114]. An association between deficient mitophagy and abnormal tau accumulation has been found in AD patient brain homogenate and transgenic mice. This deficit can be rescued by up-regulating parkin expression [20]. Exogenous parkin has also been found to decrease Aβ levels in vitro and Aβ-induced plaque formation in transgenic mice [115–117].
Parkin activation may be a promising strategy to enhance mitophagy in disease models. Nilotinib, originally discovered as a tyrosine kinase inhibitor, increases parkin abundance and ubiquitination, potentially increasing parkin recycling via the proteasome [118]. Nilotinib-mediated c-ABL inhibition also prevents parkin tyrosine phosphorylation, resulting in release of parkin auto-inhibition and demonstrating protection in PD models [119]. Additionally, nilotinib has been demonstrated to increase the parkin–beclin 1 interaction and increase clearance of Aβ in transgenic APP mice following chronic treatment [120]. Finally, with respect to ALS and FTD, motor and cognitive deficits measured in TDP-43 transgenic mice have also been reversed using nilotinib [121].
Deubiquitinating (deubiquitinase; DUB) enzymes in neurodegenerative proteinopathies
Down-regulation of the ubiquitin–proteasome system (UPS) is common across neurodegenerative diseases and promoting UPS activity is an emerging strategy for the treatment of proteinopathies. Deubiquitinases (DUBs) hydrolyse isopeptide bonds covalently binding ubiquitin to proteins, regulating degradation, localisation or activity. Multiple DUBs regulate mitochondrial function [122–126]. Ubiquitin-specific protease 15 (USP15) and USP30 both antagonise parkin-mediated mitophagy [122,125]. USP30 is the only DUB exclusively localised to mitochondria [127], tethered to the outer mitochondrial membrane. USP30 deubiquitinates parkin substrates, including TOMM20 and MIRO1 [122], and inhibition has been proposed to enhance parkin-mediated mitophagy [128,129] (Figure 4). In one study, USP35 was also found to oppose parkin-mediated mitophagy, with both a distinct mechanism to USP30 and lack of influence on the translocation of parkin [124]. USP8 enhances mitophagy by removing lysine-6-linked ubiquitin from parkin, promoting its turnover [126]. However, confusingly, USP8 knockout also limits toxicity in an α-syn model in Drosophila melanogaster [130]. Other DUBs localise to mitochondria, albeit not exclusively; Ataxin-3 [131,132] and the X-chromosome-linked deubiquitinase, USP9x [132] have demonstrated mitochondrial localisation under specific conditions. USP9x deubiquitinates α-syn and silencing increases the abundance of mono-ubiquitinated α-syn, enhancing its propensity for aggregation [133].
Eliminating ROS in neurodegenerative proteinopathies
Mitochondria are a principal source of cellular ROS. ROS are generated as a by-product of OXPHOS and their abundance presents a fine balance between signalling and toxicity. Much interest has focussed around limiting oxidative stress in neurodegenerative disease. Exogenous expression of a mitochondrially targeted catalase decreases monomeric and oligomeric Aβ and Aβ plaques in mice carrying the APP KM670/671NL (Swedish) mutation [134]. Synthetic analogues of mitochondrial coenzyme Q10 prevent Aβ oligomer-induced changes in mitochondrial mRNA transcript expression, protecting cells against oligomeric Aβ damage [135]. Although perturbation of ROS in preclinical models has so far proved beneficial, to date translation to human disease has been challenging and yielded multiple clinical failures across multiple neurodegenerative diseases [136]. Interestingly, the antioxidant MitoQ has been assessed in many models of ageing and neurodegenerative disease. MitoQ is a redox active ubiquinone, targeted to mitochondria [137]. MitoQ has demonstrated positive effects in an SOD1G93A ALS mouse model [138], a triple transgenic AD mouse [139] and in models of AD in Caenorhabditis elegans [140], together linking mitochondrial ROS and proteinopathy-related neuropathologies. MitoQ is currently in clinical trials testing the efficacy for improving vascular, motor and cognitive function in middle-aged and older adults (NCT02597023).
Conclusion and prospects
Compelling evidence suggests that mitochondrial dysfunction plays a significant role in neurodegenerative proteinopathies. Neuronal ATP is provided almost exclusively through mitochondrial OXPHOS, and complicated processes controlling mitochondrial dynamics, redox equilibrium, protein import and mitochondrial quality control work in concert to meet spatiotemporal bioenergetic demands. Aberrant misfolded proteins disrupt these processes, triggering mitochondrial dysfunction and having wider effects on cellular homeostasis.
Numerous disease-modifying strategies targeting mitochondria are currently under investigation. Further development of mPTP inhibitors is warranted due to the emerging evidence of the involvement of Ca2+ homeostasis, ROS and mPTP opening in multiple neurodegenerative diseases. Accelerating removal of damaged mitochondria has been proposed as a novel disease-modifying strategy not only in PD, but in many proteinopathies. Identification of a mechanism to enhance mitophagy may demand increased understanding of DUB biology and the substrate diversity and selectivity of these enzymes. Clinical trials of mitochondrially targeted antioxidants will provide proof-of-concept concerning ROS manipulation. Taken together, thoroughly understanding the mitochondrial relationship with neurodegenerative proteinopathies is likely to pave the way for the development of targeted therapies, potentially modifying the disease course of these progressive degenerative disorders.
Acknowledgements
The authors thank Jim Staddon for helpful comments and advice in the course of manuscript preparation.
Abbreviations
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- ANT
adenine nucleotide translocase
- APP
amyloid precursor protein
- ATP
adenosine triphosphate
- Aβ
amyloid β
- COX
cytochrome C oxidase
- CsA
cyclosporin A
- CypD
cyclophilin D
- DRP-1
dynamin-related protein 1
- DUB
deubiquitinase
- ETC
electron transport chain
- fis1
mitochondrial fission 1
- FTD
frontotemporal dementia
- FUS
fused in sarcoma
- MAM
mitochondrial-associated membrane
- mPTP
mitochondrial permeability transition pore
- OPA-1
optic atrophy 1
- OSCP
oligomycin-sensitivity conferring protein
- OXPHOS
oxidative phosphorylation
- PD
Parkinson's disease
- PINK1
PTEN-induced putative kinase 1
- ROS
reactive oxygen species
- TDP-43
transactive response DNA-binding protein 43 kDa
- TOMM
translocase of the outer mitochondrial membrane
- UPS
ubiquitin–proteasome system
- USP
ubiquitin-specific protease
- α-syn
α-synuclein
Author Contribution
T.B. and A.R.H. wrote the manuscript.
Competing Interests
T.B. and A.R.H. are employees of Eisai Ltd.
References
- 1.Silver I. and Erecińska M. (1998) Oxygen and ion concentrations in normoxic and hypoxic brain cells In Oxygen Transport to Tissue XX (Hudetz A.G., Bruley D.F., eds), pp. 7–16. Springer, Boston, MA: [DOI] [PubMed] [Google Scholar]
- 2.Bayer T.A. (2015) Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur. Neuropsychopharmacol. 25, 713–724 10.1016/j.euroneuro.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 3.Ugalde C.L., Finkelstein D.I., Lawson V.A. and Hill A.F. (2016) Pathogenic mechanisms of prion protein, amyloid-β and α-synuclein misfolding: the prion concept and neurotoxicity of protein oligomers. J. Neurochem. 139, 162–180 10.1111/jnc.13772 [DOI] [PubMed] [Google Scholar]
- 4.Cardoso S.M., Swerdlow R.H. and Oliveira C.R. (2002) Induction of cytochrome c-mediated apoptosis by amyloid β 25–35 requires functional mitochondria. Brain Res. 931, 117–125 10.1016/S0006-8993(02)02256-4 [DOI] [PubMed] [Google Scholar]
- 5.Manczak M., Park B.S., Jung Y. and Reddy P.H. (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer's disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 5, 147–162 10.1385/NMM:5:2:147 [DOI] [PubMed] [Google Scholar]
- 6.Manczak M. and Reddy P.H. (2012) Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease. Hum. Mol. Genet. 21, 5131–5146 10.1093/hmg/dds360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhein V., Baysang G., Rao S., Meier F., Bonert A., Müller-Spahn F. et al. (2009) Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell. Mol. Neurobiol. 29, 1063–1071 10.1007/s10571-009-9398-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhein V., Song X., Wiesner A., Ittner L.M., Baysang G., Meier F. et al. (2009) Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc. Natl Acad. Sci. U.S.A. 106, 20057–20062 10.1073/pnas.0905529106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker W.D. Jr, Filley C.M. and Parks J.K. (1990) Cytochrome oxidase deficiency in Alzheimer's disease. Neurology 40, 1302–1303 10.1212/WNL.40.8.1302 [DOI] [PubMed] [Google Scholar]
- 10.Parker W.D., Ba, J.P., Filley C.M. and Kleinschmidt-DeMasters B.K. (1994) Electron transport chain defects in Alzheimer's disease brain. Neurology 44, 1090–1091 10.1212/WNL.44.6.1090 [DOI] [PubMed] [Google Scholar]
- 11.Lin M.T., Simon D.K., Ahn C.H., Kim L.M. and Beal M.F. (2002) High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer's disease brain. Hum. Mol. Genet. 11, 133–145 10.1093/hmg/11.2.133 [DOI] [PubMed] [Google Scholar]
- 12.Coskun P.E., Beal M.F. and Wallace D.C. (2004) Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc. Natl Acad. Sci. U.S.A. 101, 10726–10731 10.1073/pnas.0403649101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cenini G., Rub C., Bruderek M. and Voos W. (2016) Amyloid β-peptides interfere with mitochondrial preprotein import competence by a coaggregation process. Mol. Biol. Cell 27, 3257–3272 10.1091/mbc.e16-05-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson Petersen C.A., Alikhani N., Behbahani H., Wiehager B., Pavlov P.F., Alafuzoff I. et al. (2008) The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl Acad. Sci. U.S.A. 105, 13145–13150 10.1073/pnas.0806192105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiner B., Hedskog L., Wiehager B. and Ankarcrona M. (2015) Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers Dis. 43, 369–374 10.3233/JAD-132543 [DOI] [PubMed] [Google Scholar]
- 16.Duan A.R., Jonasson E.M., Alberico E.O., Li C., Scripture J.P., Miller R.A. et al. (2017) Interactions between tau and different conformations of tubulin: implications for tau function and mechanism. J. Mol. Biol. 429, 1424–1438 10.1016/j.jmb.2017.03.018 [DOI] [PubMed] [Google Scholar]
- 17.Amadoro G., Corsetti V., Florenzano F., Atlante A., Ciotti M.T., Mongiardi M.P. et al. (2014) AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol. Dis. 62, 489–507 10.1016/j.nbd.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 18.DuBoff B., Götz J. and Feany M.B. (2012) Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron 75, 618–632 10.1016/j.neuron.2012.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corsetti V., Florenzano F., Atlante A., Bobba A., Ciotti M.T., Natale F. et al. (2015) NH2-truncated human tau induces deregulated mitophagy in neurons by aberrant recruitment of Parkin and UCHL-1: implications in Alzheimer's disease. Hum. Mol. Genet. 24, 3058–3081 10.1093/hmg/ddv059 [DOI] [PubMed] [Google Scholar]
- 20.Hu Y., Li X.C., Wang Z.H., Luo Y., Zhang X., Liu X.P. et al. (2016) Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin. Oncotarget 7, 17356–17368 10.18632/oncotarget.7861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X.-C., Hu Y., Wang Z.-h., Luo Y., Zhang Y., Liu X.-P. et al. (2016) Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci. Rep. 6, 24756 10.1038/srep24756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amadoro G., Corsetti V., Stringaro A., Colone M., D'Aguanno S., Meli G. et al. (2010) A NH2 tau fragment targets neuronal mitochondria at AD synapses: possible implications for neurodegeneration. J. Alzheimers Dis. 21, 445–470 10.3233/JAD-2010-100120 [DOI] [PubMed] [Google Scholar]
- 23.Amadoro G., Corsetti V., Atlante A., Florenzano F., Capsoni S., Bussani R. et al. (2012) Interaction between NH-tau fragment and Aβ in Alzheimer's disease mitochondria contributes to the synaptic deterioration. Neurobiol. Aging 33, 833.e1–833.e25 10.1016/j.neurobiolaging.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 24.Kopeikina K.J., Carlson G.A., Pitstick R., Ludvigson A.E., Peters A., Luebke J.I. et al. (2011) Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer's disease brain. Am. J. Pathol. 179, 2071–2082 10.1016/j.ajpath.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoothoff W., Jones P.B., Spires-Jones T.L., Joyner D., Chhabra E., Bercury K. et al. (2009) Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J. Neurochem. 111, 417–427 10.1111/j.1471-4159.2009.06316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melov S., Adlard P.A., Morten K., Johnson F., Golden T.R., Hinerfeld D. et al. (2007) Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE 2, e536 10.1371/journal.pone.0000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merkwirth C., Martinelli P., Korwitz A., Morbin M., Bronneke H.S., Jordan S.D. et al. (2012) Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet. 8, e1003021 10.1371/journal.pgen.1003021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva D.F., Esteves A.R., Oliveira C.R. and Cardoso S.M. (2011) Mitochondria: the common upstream driver of amyloid-β and tau pathology in Alzheimer's disease. Curr. Alzheimer Res. 8, 563–572 10.2174/156720511796391872 [DOI] [PubMed] [Google Scholar]
- 29.Iijima-Ando K., Sekiya M., Maruko-Otake A., Ohtake Y., Suzuki E., Lu B. et al. (2012) Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer's disease-related tau phosphorylation via PAR-1. PLoS Genet. 8, e1002918 10.1371/journal.pgen.1002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joh Y. and Choi W.-S. (2017) Mitochondrial complex I inhibition accelerates amyloid toxicity. Dev. Reprod. 21, 417–424 10.12717/DR.2017.21.4.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunetti D., Torsvik J., Dallabona C., Teixeira P., Sztromwasser P., Fernandez-Vizarra E. et al. (2016) Defective PITRM1 mitochondrial peptidase is associated with Aβ amyloidotic neurodegeneration. EMBO Mol. Med. 8, 176–190 10.15252/emmm.201505894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alikhani N., Guo L., Yan S., Du H., Pinho C.M., Chen J.X. et al. (2011) Decreased proteolytic activity of the mitochondrial amyloid-β degrading enzyme, PreP peptidasome, in Alzheimer's disease brain mitochondria. J. Alzheimers Dis. 27, 75–87 10.3233/JAD-2011-101716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falkevall A., Alikhani N., Bhushan S., Pavlov P.F., Busch K., Johnson K.A. et al. (2006) Degradation of the amyloid β-protein by the novel mitochondrial peptidasome, PreP. J. Biol. Chem. 281, 29096–29104 10.1074/jbc.M602532200 [DOI] [PubMed] [Google Scholar]
- 34.Park S.J., Shin J.H., Jeong J.I., Song J.H., Jo Y.K., Kim E.S. et al. (2014) Down-regulation of Mortalin exacerbates Aβ-mediated mitochondrial fragmentation and dysfunction. J. Biol. Chem. 289, 2195–2204 10.1074/jbc.M113.492587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu M., Zhou Z., Xu S., Chen C., Yu Z. and Wang D. (2011) Mortalin overexpression attenuates beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Brain Res. 1368, 336–345 10.1016/j.brainres.2010.10.068 [DOI] [PubMed] [Google Scholar]
- 36.Kondadi A.K., Wang S., Montagner S., Kladt N., Korwitz A., Martinelli P. et al. (2014) Loss of the m-AAA protease subunit AFG3L2 causes mitochondrial transport defects and tau hyperphosphorylation. EMBO J. 33, 1011–1026 10.1002/embj.201387009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck S.J., Guo L., Phensy A., Tian J., Wang L., Tandon N. et al. (2016) Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer's disease. Nat. Commun. 7, 11483 10.1038/ncomms11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du H., Guo L., Zhang W., Rydzewska M. and Yan S. (2011) Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging 32, 398–406 10.1016/j.neurobiolaging.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose A. and Beal M.F. (2016) Mitochondrial dysfunction in Parkinson's disease. J. Neurochem. 139(Suppl 1), 216–231 10.1111/jnc.13731 [DOI] [PubMed] [Google Scholar]
- 40.Luo Y., Hoffer A., Hoffer B. and Qi X. (2015) Mitochondria: a therapeutic target for Parkinson's disease? Int. J. Mol. Sci. 16, 20704–20730 10.3390/ijms160920704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osellame L.D., Rahim A.A., Hargreaves I.P., Gegg M.E., Richard-Londt A., Brandner S. et al. (2013) Mitochondria and quality control defects in a mouse model of Gaucher disease—links to Parkinson's disease. Cell Metab. 17, 941–953 10.1016/j.cmet.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickrell A.M. and Youle R.J. (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257–273 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valente E.M., Abou-Sleiman P.M., Caputo V., Muqit M.M.K., Harvey K., Gispert S. et al. (2004) Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 10.1126/science.1096284 [DOI] [PubMed] [Google Scholar]
- 44.Clark I.E. Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H. et al. (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162–1166 10.1038/nature04779 [DOI] [PubMed] [Google Scholar]
- 45.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S. et al. (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 10.1038/nature04788 [DOI] [PubMed] [Google Scholar]
- 46.Chinta S.J., Mallajosyula J.K., Rane A. and Andersen J.K. (2010) Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci. Lett. 486, 235–239 10.1016/j.neulet.2010.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindström V., Gustafsson G., Sanders L.H., Howlett E.H., Sigvardson J., Kasrayan A. et al. (2017) Extensive uptake of α-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol. Cell Neurosci. 82, 143–156 10.1016/j.mcn.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 48.Pukaß K., Goldbaum O. and Richter-Landsberg C. (2015) Mitochondrial impairment and oxidative stress compromise autophagosomal degradation of α-synuclein in oligodendroglial cells. J. Neurochem. 135, 194–205 10.1111/jnc.13256 [DOI] [PubMed] [Google Scholar]
- 49.Sherer T.B., Betarbet R., Stout A.K., Lund S., Baptista M., Panov A.V. et al. (2002) An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered α-synuclein metabolism and oxidative damage. J. Neurosci. 22, 7006–7015 10.1523/JNEUROSCI.22-16-07006.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung S.Y., Kishinevsky S., Mazzulli J.R., Graziotto J., Mrejeru A., Mosharov E.V. et al. (2016) Parkin and PINK1 patient iPSC-derived midbrain dopamine neurons exhibit mitochondrial dysfunction and α-synuclein accumulation. Stem Cell Rep. 7, 664–677 10.1016/j.stemcr.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsieh C.-H., Shaltouki A., Gonzalez A.E., Bettencourt da Cruz A., Burbulla L.F., St Lawrence E. et al. (2016) Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson's disease. Cell Stem Cell 19, 709–724 10.1016/j.stem.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burchell V.S., Nelson D.E., Sanchez-Martinez A., Delgado-Camprubi M., Ivatt R.M., Pogson J.H. et al. (2013) The Parkinson's disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat. Neurosci. 16, 1257–1265 10.1038/nn.3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Z.D., Xie S.P., Sathiyamoorthy S., Saw W.T., Sing T.Y., Ng S.H. et al. (2015) F-box protein 7 mutations promote protein aggregation in mitochondria and inhibit mitophagy. Hum. Mol. Genet. 24, 6314–6330 10.1093/hmg/ddv340 [DOI] [PubMed] [Google Scholar]
- 54.Jellinger K.A. (2010) Synucleinopathies. Encyclopedia of Movement Disorders, pp. 203–207. Academic Press, Oxford [Google Scholar]
- 55.Hsu L.J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M. et al. (2000) α-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 157, 401–410 10.1016/S0002-9440(10)64553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin L.J., Pan Y., Price A.C., Sterling W., Copeland N.G., Jenkins N.A. et al. (2006) Parkinson's disease α-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26, 41–50 10.1523/JNEUROSCI.4308-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devi L., Raghavendran V., Prabhu B.M., Avadhani N.G. and Anandatheerthavarada H.K. (2008) Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 283, 9089–9100 10.1074/jbc.M710012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guardia-Laguarta C., Area-Gomez E., Rub C., Liu Y., Magrane J., Becker D. et al. (2014) α-synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 34, 249–259 10.1523/JNEUROSCI.2507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pozo Devoto V.M., Dimopoulos N., Alloatti M., Pardi M.B., Saez T.M., Otero M.G. et al. (2017) αsynuclein control of mitochondrial homeostasis in human-derived neurons is disrupted by mutations associated with Parkinson's disease. Sci. Rep. 7, 5042. 10.1038/s41598-017-05334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakamura K., Nemani V.M., Azarbal F., Skibinski G., Levy J.M., Egami K. et al. (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α-synuclein. J. Biol. Chem. 286, 20710–20726 10.1074/jbc.M110.213538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Maio R., Barrett P.J., Hoffman E.K., Barrett C.W., Zharikov A., Borah A. et al. (2016) α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson's disease. Sci. Transl. Med. 8, 342ra78 10.1126/scitranslmed.aaf3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L., Xie Z., Turkson S. and Zhuang X. (2015) A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration. J. Neurosci. 35, 890–905 10.1523/JNEUROSCI.0089-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gispert S., Brehm N., Weil J., Seidel K., Rüb U., Kern B. et al. (2015) Potentiation of neurotoxicity in double-mutant mice with Pink1 ablation and A53T-SNCA overexpression. Hum. Mol. Genet. 24, 1061–1076 10.1093/hmg/ddu520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryan T., Bamm V.V., Stykel M.G., Coackley C.L., Humphries K.M., Jamieson-Williams R. et al. (2018) Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat. Commun. 9, 817 10.1038/s41467-018-03241-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ordonez D.G., Lee M.K. and Feany M.B. (2018) α-synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97, 108–24.e6 10.1016/j.neuron.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keeney P.M., Xie J., Capaldi R.A. and Bennett J.P. (2006) Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 26, 5256–5264 10.1523/JNEUROSCI.0984-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen J., Du T., Wang X., Duan C., Gao G., Zhang J. et al. (2014) α-Synuclein amino terminus regulates mitochondrial membrane permeability. Brain Res. 1591, 14–26 10.1016/j.brainres.2014.09.046 [DOI] [PubMed] [Google Scholar]
- 68.Ferrari R., Kapogiannis D., Huey E.D. and Momeni P. (2011) FTD and ALS: a tale of two diseases. Curr. Alzheimer Res. 8, 273–294 10.2174/156720511795563700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muyderman H. and Chen T. (2014) Mitochondrial dysfunction in amyotrophic lateral sclerosis — a valid pharmacological target? Br. J. Pharmacol. 171, 2191–2205 10.1111/bph.12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang C., Zhou H., Tong J., Chen H., Liu Y.-J., Wang D. et al. (2011) FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet. 7, e1002011 10.1371/journal.pgen.1002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang E.J., Zhang J., Geser F., Trojanowski J.Q., Strober J.B., Dickson D.W. et al. (2010) Extensive FUS-immunoreactive pathology in juvenile amyotrophic lateral sclerosis with basophilic inclusions. Brain Pathol. 20, 1069–1076 10.1111/j.1750-3639.2010.00413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shan X., Chiang P.-M., Price D.L. and Wong P.C. (2010) Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc. Natl Acad. Sci. U.S.A. 107, 16325–16330 10.1073/pnas.1003459107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y.-F., Gendron T.F., Zhang Y.-J., Lin W.-L., D'Alton S., Sheng H. et al. (2010) Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J. Neurosci. 30, 10851–10859 10.1523/JNEUROSCI.1630-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davis S.A., Itaman S., Khalid-Janney C.M., Sherard J.A., Dowell J.A., Cairns N.J. et al. (2018) TDP-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics. Neurosci. Lett. 678, 8–15 10.1016/j.neulet.2018.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W., Li L., Lin W.-L., Dickson D.W., Petrucelli L., Zhang T. et al. (2013) The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 22, 4706–4719 10.1093/hmg/ddt319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Supnet C. and Bezprozvanny I. (2010) Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer's disease. J. Alzheimers Dis. 20(Suppl 2), S487–S498 10.3233/JAD-2010-100306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Supnet C. and Bezprozvanny I. (2010) The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 47, 183–189 10.1016/j.ceca.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pivovarova N.B. and Andrews S.B. (2010) Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 277, 3622–3636 10.1111/j.1742-4658.2010.07754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haworth R.A. and Hunter D.R. (1979) The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 195, 460–467 10.1016/0003-9861(79)90372-2 [DOI] [PubMed] [Google Scholar]
- 80.Szabó I., Bernardi P. and Zoratti M. (1992) Modulation of the mitochondrial megachannel by divalent cations and protons. J. Biol. Chem. 267, 2940–2946 PMID: [PubMed] [Google Scholar]
- 81.Bernardi P., Vassanelli S., Veronese P., Colonna R., Szabó I. and Zoratti M. (1992) Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J. Biol. Chem. 267, 2934–2939 PMID: [PubMed] [Google Scholar]
- 82.Scorrano L., Petronilli V. and Bernardi P. (1997) On the voltage dependence of the mitochondrial permeability transition pore. A critical appraisal. J. Biol. Chem. 272, 12295–12299 10.1074/jbc.272.19.12295 [DOI] [PubMed] [Google Scholar]
- 83.Crompton M. (1999) The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 341, 233–249 10.1042/bj3410233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Briston T., Roberts M., Lewis S., Powney B., Staddon J.M, Szabadkai G. et al. (2017) Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci. Rep. 7, 10492 10.1038/s41598-017-10673-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crompton M., Ellinger H. and Costi A. (1988) Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 255, 357–360 PMID: [PMC free article] [PubMed] [Google Scholar]
- 86.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A. et al. (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 10.1038/nature03434 [DOI] [PubMed] [Google Scholar]
- 87.Du H., Guo L., Fang F., Chen D., Sosunov A.A., McKhann G.M. et al. (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat. Med. 14, 1097–1105 10.1038/nm.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo L., Du H., Yan S., Wu X., McKhann G.M., Chen J.X. et al. (2013) Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons. PLoS ONE 8, e54914 10.1371/journal.pone.0054914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Giorgio V., Bisetto E., Soriano M.E., Dabbeni-Sala F., Basso E., Petronilli V. et al. (2009) Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J. Biol. Chem. 284, 33982–33988 10.1074/jbc.M109.020115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M. et al. (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl Acad. Sci. U.S.A. 110, 5887–5892 10.1073/pnas.1217823110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elkamhawy A., Park J.-e., Hassan A.H.E., Pae A.N., Lee J., Park B.-G. et al. (2018) Synthesis and evaluation of 2-(3-arylureido)pyridines and 2-(3-arylureido)pyrazines as potential modulators of Aβ-induced mitochondrial dysfunction in Alzheimer's disease. Eur. J. Med. Chem. 144, 529–543 10.1016/j.ejmech.2017.12.045 [DOI] [PubMed] [Google Scholar]
- 92.Park J.-e., Elkamhawy A., Hassan A.H.E., Pae A.N., Lee J., Paik S. et al. (2017) Synthesis and evaluation of new pyridyl/pyrazinyl thiourea derivatives: neuroprotection against amyloid-β-induced toxicity. Eur. J. Med. Chem. 141, 322–334 10.1016/j.ejmech.2017.09.043 [DOI] [PubMed] [Google Scholar]
- 93.Valasani K.R., Sun Q., Fang D., Zhang Z., Yu Q., Guo Y. et al. (2016) Identification of a small molecule cyclophilin D inhibitor for rescuing Aβ-mediated mitochondrial dysfunction. ACS Med. Chem. Lett. 7, 294–299 10.1021/acsmedchemlett.5b00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim Y.S., Jung S.h., Park B.-G., Ko M.K., Jang H.-S., Choi K. et al. (2013) Synthesis and evaluation of oxime derivatives as modulators for amyloid beta-induced mitochondrial dysfunction. Eur. J. Med. Chem. 62, 71–83 10.1016/j.ejmech.2012.12.033 [DOI] [PubMed] [Google Scholar]
- 95.Woodfield K., Rück A., Brdiczka D. and Halestrap A.P. (1998) Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 336(Pt 2), 287–290 10.1042/bj3360287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richardson A.P. and Halestrap A.P. (2016) Quantification of active mitochondrial permeability transition pores using GNX-4975 inhibitor titrations provides insights into molecular identity. Biochem. J. 473, 1129–1140 10.1042/BCJ20160070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu Y., Duan C., Lü L., Gao H., Zhao C., Yu S. et al. (2011) α-Synuclein overexpression impairs mitochondrial function by associating with adenylate translocator. Int. J. Biochem. Cell Biol. 43, 732–741 10.1016/j.biocel.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 98.Wang P. and Heitman J. (2005) The cyclophilins. Genome Biol. 6, 226 10.1186/gb-2005-6-7-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Basso E., Fante L., Fowlkes J., Petronilli V., Forte M.A. and Bernardi P. (2005) Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 280, 18558–18561 10.1074/jbc.C500089200 [DOI] [PubMed] [Google Scholar]
- 100.Halestrap A.P. and Davidson A.M. (1990) Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 268, 153–160 10.1042/bj2680153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hansson M.J., Mattiasson G., Månsson R., Karlsson J., Keep M.F., Waldmeier P. et al. (2004) The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J. Bioenerg. Biomembr. 36, 407–413 10.1023/B:JOBB.0000041776.31885.45 [DOI] [PubMed] [Google Scholar]
- 102.Tiepolo T., Angelin A., Palma E., Sabatelli P., Merlini L., Nicolosi L. et al. (2009) The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1−/− myopathic mice. Br. J. Pharmacol. 157, 1045–1052 10.1111/j.1476-5381.2009.00316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schaller S., Paradis S., Ngoh G.A., Assaly R., Buisson B., Drouot C. et al. (2010) TRO40303, a new cardioprotective compound, inhibits mitochondrial permeability transition. J. Pharmacol. Exp. Ther. 333, 696–706 10.1124/jpet.110.167486 [DOI] [PubMed] [Google Scholar]
- 104.Roy S., Šileikyte˙ J., Neuenswander B., Hedrick M.P., Chung T.D.Y., Aubé J. et al. (2016) N-Phenylbenzamides as potent inhibitors of the mitochondrial permeability transition pore. ChemMedChem 11, 283–288 10.1002/cmdc.201500545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fancelli D., Abate A., Amici R., Bernardi P., Ballarini M., Cappa A. et al. (2014) Cinnamic anilides as new mitochondrial permeability transition pore inhibitors endowed with ischemia-reperfusion injury protective effect in vivo. J. Med. Chem. 57, 5333–5347 10.1021/jm500547c [DOI] [PubMed] [Google Scholar]
- 106.Briston T., Lewis S., Koglin M., Mistry K., Shen Y., Hartopp N. et al. (2016) Identification of ER-000444793, a cyclophilin D-independent inhibitor of mitochondrial permeability transition, using a high-throughput screen in cryopreserved mitochondria. Sci. Rep. 6, 37798 10.1038/srep37798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin L.J., Fancelli D., Wong M., Niedzwiecki M., Ballarini M., Plyte S. et al. (2014) GNX-4728, a novel small molecule drug inhibitor of mitochondrial permeability transition, is therapeutic in a mouse model of amyotrophic lateral sclerosis. Front. Cell. Neurosci. 8, 433 10.3389/fncel.2014.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang Y., Gehrke S., Imai Y., Huang Z., Ouyang Y., Wang J.-W. et al. (2006) Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl Acad. Sci. U.S.A. 103, 10793–10798 10.1073/pnas.0602493103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lemasters J.J. (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5 10.1089/rej.2005.8.3 [DOI] [PubMed] [Google Scholar]
- 110.Narendra D., Tanaka A., Suen D.-F. and Youle R.J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lazarou M., Jin S.M., Kane L.A. and Youle R.J. (2012) Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell 22, 320–333 10.1016/j.devcel.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S. et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 10.1038/33416 [DOI] [PubMed] [Google Scholar]
- 113.Yang W., Wang X., Liu J., Duan C., Gao G., Lu L. et al. (2018) PINK1 suppresses α-synuclein-induced neuronal injury: a novel mechanism in protein phosphatase 2A activation. Oncotarget 9, 37–53 10.18632/oncotarget.21554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Du F., Yu Q., Yan S., Hu G., Lue L.-F., Walker D.G. et al. (2017) PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease. Brain 140, 3233–3251 10.1093/brain/awx258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hong X., Liu J., Zhu G., Zhuang Y., Suo H., Wang P. et al. (2014) Parkin overexpression ameliorates hippocampal long-term potentiation and β-amyloid load in an Alzheimer's disease mouse model. Hum. Mol. Genet. 23, 1056–1072 10.1093/hmg/ddt501 [DOI] [PubMed] [Google Scholar]
- 116.Burns M.P., Zhang L., Rebeck G.W., Querfurth H.W. and Moussa C.E.-H. (2009) Parkin promotes intracellular Aβ1–42 clearance. Hum. Mol. Genet. 18, 3206–3216 10.1093/hmg/ddp258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khandelwal P.J., Herman A.M., Hoe H.-S., Rebeck G.W. and Moussa C.E.-H. (2011) Parkin mediates beclin-dependent autophagic clearance of defective mitochondria and ubiquitinated Aβ in AD models. Hum. Mol. Genet. 20, 2091–2102 10.1093/hmg/ddr091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lonskaya I., Hebron M.L., Desforges N.M., Schachter J.B. and Moussa C.E.-H. (2014) Nilotinib-induced autophagic changes increase endogenous parkin level and ubiquitination, leading to amyloid clearance. J. Mol. Med. 92, 373–386 10.1007/s00109-013-1112-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ko H.S., Lee Y., Shin J.-H., Karuppagounder S.S., Gadad B.S., Koleske A.J. et al. (2010) Phosphorylation by the c-Abl protein tyrosine kinase inhibits Parkin's ubiquitination and protective function. Proc. Natl Acad. Sci. U.S.A. 107, 16691–16696 10.1073/pnas.1006083107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lonskaya I., Hebron M.L., Desforges N.M., Franjie A. and Moussa C.E.H. (2013) Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol. Med. 5, 1247–1262 10.1002/emmm.201302771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wenqiang C., Lonskaya I., Hebron M.L., Ibrahim Z., Olszewski R.T., Neale J.H. et al. (2014) Parkin-mediated reduction of nuclear and soluble TDP-43 reverses behavioral decline in symptomatic mice. Hum. Mol. Genet. 23, 4960–4969 10.1093/hmg/ddu211 [DOI] [PubMed] [Google Scholar]
- 122.Bingol B., Tea J.S., Phu L., Reichelt M., Bakalarski C.E., Song Q. et al. (2014) The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510, 370–375 10.1038/nature13418 [DOI] [PubMed] [Google Scholar]
- 123.Cunningham C.N., Baughman J.M., Phu L., Tea J.S., Yu C., Coons M. et al. (2015) USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria. Nat. Cell Biol. 17, 160–169 10.1038/ncb3097 [DOI] [PubMed] [Google Scholar]
- 124.Wang Y., Serricchio M., Jauregui M., Shanbhag R., Stoltz T., Di Paolo C.T. et al. (2015) Deubiquitinating enzymes regulate PARK2-mediated mitophagy. Autophagy 11, 595–606 10.1080/15548627.2015.1034408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cornelissen T., Haddad D., Wauters F., Van Humbeeck C., Mandemakers W., Koentjoro B. et al. (2014) The deubiquitinase USP15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum. Mol. Genet. 23, 5227–5242 10.1093/hmg/ddu244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Durcan T.M., Tang M.Y., Pérusse J.R., Dashti E.A., Aguileta M.A., McLelland G.L. et al. (2014) USP8 regulates mitophagy by removing K6-linked ubiquitin conjugates from parkin. EMBO J. 33, 2473–2491 10.15252/embj.201489729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakamura N. and Hirose S. (2008) Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol. Biol. Cell 19, 1903–1911 10.1091/mbc.e07-11-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thobois S. (2015) USP30: a new promising target for Parkinson's disease? Mov. Disord. 30, 340 10.1002/mds.26185 [DOI] [PubMed] [Google Scholar]
- 129.Yue W., Chen Z., Liu H., Yan C., Chen M., Feng D. et al. (2014) A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 24, 482–496 10.1038/cr.2014.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alexopoulou Z., Lang J., Perrett R.M., Elschami M., Hurry M.E.D., Kim H.T. et al. (2016) Deubiquitinase Usp8 regulates α-synuclein clearance and modifies its toxicity in Lewy body disease. Proc. Natl Acad. Sci. U.S.A. 113, E4688–E4697 10.1073/pnas.1523597113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pozzi C., Valtorta M., Tedeschi G., Galbusera E., Pastori V., Bigi A. et al. (2008) Study of subcellular localization and proteolysis of ataxin-3. Neurobiol. Dis. 30, 190–200 10.1016/j.nbd.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 132.Schwickart M., Huang X., Lill J.R., Liu J., Ferrando R., French D.M. et al. (2010) Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463, 103–107 10.1038/nature08646 [DOI] [PubMed] [Google Scholar]
- 133.Rott R., Szargel R., Haskin J., Bandopadhyay R., Lees A.J., Shani V. et al. (2011) α-Synuclein fate is determined by USP9X-regulated monoubiquitination. Proc. Natl Acad. Sci. U.S.A. 108, 18666–18671 10.1073/pnas.1105725108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mao P., Manczak M., Calkins M.J., Truong Q., Reddy T.P., Reddy A.P. et al. (2012) Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 21, 2973–2990 10.1093/hmg/dds128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mastroeni D., Nolz J., Khdour O.M., Sekar S., Delvaux E., Cuyugan L. et al. (2018) Oligomeric amyloid β preferentially targets neuronal and not glial mitochondrial-encoded mRNAs. Alzheimers Dement. https://www.sciencedirect.com/science/article/pii/S1552526017338761 10.1016/j.jalz.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 136.Kamat C.D., Gadal S., Mhatre M., Williamson K.S., Pye Q.N. and Hensley K. (2008) Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J. Alzheimers Dis. 15, 473–493 10.3233/JAD-2008-15314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelso G.F., Porteous C.M., Coulter C.V., Hughes G., Porteous W.K., Ledgerwood E.C. et al. (2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 276, 4588–4596 10.1074/jbc.M009093200 [DOI] [PubMed] [Google Scholar]
- 138.Miquel E., Cassina A., Martínez-Palma L., Souza J.M., Bolatto C., Rodríguez-Bottero S. et al. (2014) Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic. Biol. Med. 70, 204–213 10.1016/j.freeradbiomed.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 139.McManus M.J., Murphy M.P. and Franklin J.L. (2011) The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 31, 15703–15715 10.1523/JNEUROSCI.0552-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ng L.F., Gruber J., Cheah I.K., Goo C.K., Cheong W.F., Shui G. et al. (2014) The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 71, 390–401 10.1016/j.freeradbiomed.2014.03.003 [DOI] [PubMed] [Google Scholar]