Abstract
Changes in mucin-type O-linked glycosylation are seen in over 90% of breast cancers where increased sialylation is often observed and a change from branched glycans to linear glycans is often seen. There are many mechanisms involved including increased/altered expression of glycosyltransferases and relocalisation to the endoplasmic reticulum of the enzymes responsible for the addition of the first sugar, N-acetyl-d-galactosamine. It is now becoming clear that these changes can contribute to tumour growth and progression by modulating the micro-environment through glycan-sensing lectins expressed on immune cells, by modulating interactions with tumour surface receptors and by binding to selectins. The understanding of how changes in mucin-type O-linked glycosylation influence tumour growth and progression reveals new potential targets for therapeutic intervention in the treatment of breast cancer.
Keywords: breast cancer, glycosyltransferases, immunotherapy, lectins, O-linked mucin-type glycosylation
Introduction
While genomics and proteomics are producing unparalleled discoveries that are advancing the understanding of biological processes and cancer, this is often incomplete without a knowledge and understanding of post-translational modifications (PTMs) of proteins, which greatly increase the size, diversity and function of the proteome [1]. Glycosylation is the most abundant PTM and in eukaryotes the majority of proteins that are expressed on the cell membrane or are secreted carry glycans, one of the four fundamental macromolecular components of all cells, together with nucleic acid, proteins and lipids. Glycoproteins carry glycans covalently attached to the peptide backbone, usually via nitrogen (N-linked) or oxygen (O-linked) linkages [2].
N-linked glycans are attached to asparagine within the consensus sequon Asn-X-Ser/Thr, where X is any amino acid except proline. The linkage sugar in eukaryote N-glycans is N-acetyl-d-glucosamine (GlcNAc) and the sugars are added en bloc in the endoplasmic reticulum. During trafficking through the secretory pathway sugars can be trimmed and added to give the final glycan side chain [3].
In contrast with N-glycans, O-linked glycans are added to the peptide core individually and sequentially [4]. There are many different types of O-linked glycosylation in which different sugars are O-linked to serine or threonine, for example O-fucose, O-mannose, O-glucose, O-xylose [2]. Moreover, GlcNAc can be O-linked to serine or threonine on proteins found in the nucleus, cytoplasm and mitochondria [5]. However, this review will be confined to mucin-type O-linked glycosylation, where N-acetyl-d-galactosamine (GalNAc) is added in O-linkage to serine or threonine, and the changes that occur in this type of glycosylation in breast cancer. For a more extensive review on mucin-type glycosylation in cancer in general please see the reviews by Kudelka et al. [6] and Pinho and Reis [7].
Aberrant glycosylation occurs in essentially all types of human cancer and glycosyltransferase (GT) gene expression can be used to classify cancer subtypes [8]. Although aberrant glycosylation occurring in tumours has been recognised for over forty years [9], it is relatively recent that its critical role in tumour growth and progression has been recognised. Indeed glycan changes contribute to the malignant phenotype, metastasis and immune evasion (see below). Changes in O-linked mucin-type glycosylation are observed in over 90% of breast cancers, as demonstrated, for example, by the expression of the Tn antigen [10] and the loss of core 2 glycans [11]; see below. Such selectivity throughout cancer evolution suggests that mucin-type O-linked glycosylation may be a driver of tumour progression.
Mucin-type O-linked glycosylation
Mucin-type O-linked glycosylation is so-called as it was originally found abundantly on mucins, which carry many and often hundreds of glycans in this linkage [4]. However, this type of glycosylation is also found on many other types of cell membrane glycoproteins where only 1 or 2 sites may be present [12].
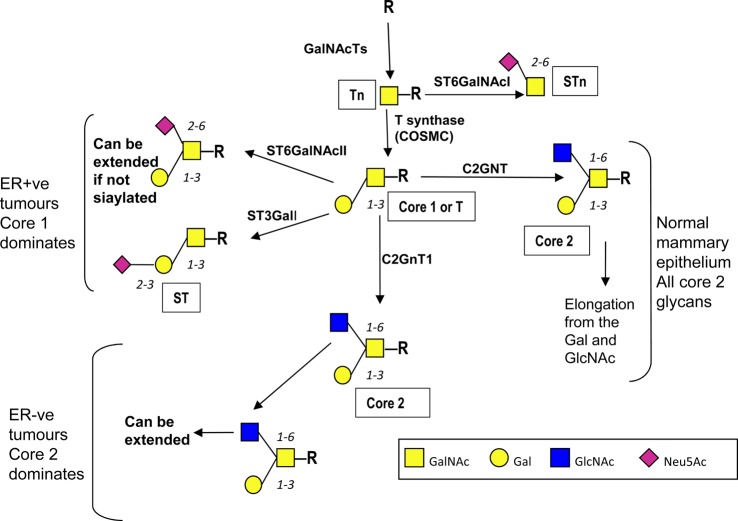
Mucin-type O-linked glycosylation (for clarity and brevity from now on referred to as O-GalNAc glycosylation) is characterised by the addition of GalNAc to serine and threonine, which is mediated by a large family of polypeptide N-acetylgalactosaminyltransferases (GalNAc-Ts), and, in contrast with N-linked glycosylation and most other types of O-linked glycosylation, is initiated in the Golgi apparatus [13]. After the addition of the first sugar many so-called core structures can be formed. In mammary gland epithelial cells the addition of galactose (Gal) results in the formation of core 1 which is converted into core 2 by the addition of GlcNAc to form the branched core 2 structure ([4,14]; see Figure 1). Extension of the glycan chains can then occur from the GlcNAc and/or the Gal; see Figure 1.
Figure 1. Pathways of O-GaalNAc glycosylation in the normal and malignant mammary gland.
In the normal mammary gland epithelial cells O-GalNAc glycans are core 2-based and extension can occur from the Gal and the GlcNAc. COSMC is indicated under the T synthase as it acts as a private chaperone for this enzyme and hence is required for T synthase activity ([21], see text). In many breast cancers truncated mucin-type O-linked glycans are seen often terminating in sialic acid due to up-regulation of sialyltransferases [16]. However, in ER-ve breast cancer core 2-based glycans are expected to be present and may dominate due to the overexpression of the C1GALT1 and GCNT1 in ER-ve tumours compared with ER+ve breast cancers [20]. Symbols used are based on the nomenclature recommended in Varki et al. [79].
Changes in O-GalNAc glycosylation seen in breast cancer
In breast cancer there can be a change in the number of O-GalNAc glycans added to the peptide core of glycoproteins, as well as changes in the core structures, which often results in increased sialylation [7,15–17]. Overall there is change from core 2-based glycans to more simple glycans with many breast cancers showing exposure of the first sugar GalNAc (Tn) and the disaccharide which forms core 1 (T), as originally described by Springer in 1984 [18]. The sialylated derivatives of these glycans are also commonly observed, giving the sialylated Tn (STn) and sialylated core 1 (ST) glycans; see Figure 1. The readers are referred to Ju et al. [19] for a comprehensive review of the Tn and STn antigens in cancer. However, estrogen receptor-positive (ER+ve) and estrogen receptor-negative (ER-ve) breast cancers show different profiles of genes encoding GTs associated with O-linked glycosylation [20]. These data suggest that ER+ve breast cancer, which accounts for ∼75% of all breast cancers, carry mostly simple core 1-based glycans on their O-linked glycoproteins, whereas ER-ve cancers can also carry the branched elongated core 2 glycans associated with a normal glycophenotype [17,20].
Mechanisms responsible for aberrant O-GalNAc glycosylation
There are many mechanisms that have been shown to contribute to changes in O-GalNAc glycosylation in breast cancer including relocalisation of GalNAc-Ts, changes in expression of GTs as well as altered glycosidase activity. There is a single enzyme responsible for adding Gal to GalNAc to form the core 1 glycan (T antigen) and this is known as T synthase (core 1 β3-galactosyltransferase) encoded by the C1GALT1 gene (see Figure 1). A private chaperone is required for the correct folding and activity of T synthase and this is known as Cosmc. Although mutations in COSMC and methylation of its promoter play a role in the expression of Tn in cervical and pancreatic cancers, respectively [21,22], this does not appear to be the case for breast cancers [23–25].
Changes in expression of GTs: GalNAcTs
There are twenty GalNAcTs (GalNAcT1-T20) that are capable of initiating O-GalNAc glycosylation and each has distinct peptide substrate specificities although some overlap exists [13]. Each tissue can express a specific profile of GalNAcTs, and GalNAcT1 and GalNAcT2 (encoded by GALNT1 and GALNT2), which are often thought of as ‘house-keeping' GalNAcTs, are expressed in normal mammary epithelial cells [26,27]. However, in breast cancer increased expression of other GalNAcTs is seen and these include GalNAcT6 [27] and GalNAcT14 [28]. GalNAcT6 (encoded by GALNT6) is expressed in the majority of breast cancers [26,29,30] but no expression was seen in sections of normal breast tissue [26,30]. In breast cancer GalNAcT6 can glycosylate and stabilise the MUC1 mucin [30], resulting in increased proliferation and decreased cell adhesion [30]. Interestingly, GalNAcT6 is also expressed in colon adenocarcinoma but not normal colon, and its expression results in dysplastic growth [31]. By immunohistochemistry, GalNAcT14 is expressed in 84% of breast cancers, while only being found in 14.6% of non-malignant breast tissue [28]. Its expression is associated with grade, but the functional significance has yet to be elucidated.
Relocalisation of GalNAcTs results in the increased expression of Tn
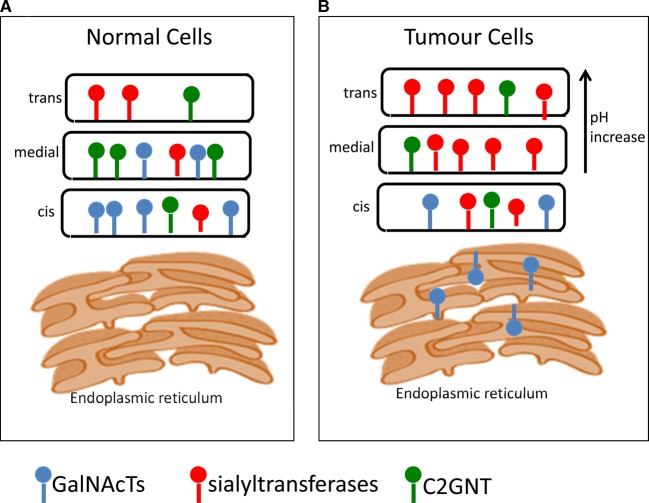
O-GalNAc glycosylation is initiated by the GalNAcTs localised within the Golgi apparatus [13]; see Figure 2A for a simplified diagram of the Golgi apparatus. However, recent data suggest that GalNAcTs can relocate to the endoplasmic reticulum in some cancer cells and that this is driven by Src kinase regulating the COPI transport machinery [24,32]; see Figure 2. This relocation results in the increased expression of the Tn antigen [24,32]. Staining of breast cancer tissue microarrays (TMAs) with the Vicia Villosa lectin (VVA), which recognises Tn, showed an endoplasmic reticulum localisation of Tn in 70% of the cancers [24], suggesting O-GalNAc glycosylation had been initiated in this organelle. Moreover, there was a marked effect on cell motility and invasiveness with endoplasmic reticulum localised GalNAcT2 [24]. Interestingly, in an in vivo murine model of liver cancer, endoplasmic reticulum localisation of GalNAcT1 increased tumour growth driven by increased glycosylation of MMP14 [33].
Figure 2. Mucin-type O-GalNAc glycosylation is initiated in the Golgi.
(A) Normally O-GalNAc glycosylation is initiated in the Golgi. GalNAcTs are not confined to the cis-Golgi, and ST3GalI and C2GNT overlap to some degree [80]. (B) In the malignant cell some GalNAcTs can be relocated to the endoplasmic reticulum where normally unmodified serine/threonines in proteins can be modified with GalNAc by the ER-relocated GalNAcTs. This results in increasing the density of glycosylation and expression of Tn. This relocation process is termed the GALA pathway [24,32,33]. ST3GalI is overexpressed in 90% of breast cancers [16], and ST6GalNAcI is seen in 25% of breast cancers [34]. Changes in pH across the Golgi cisternae can also be observed in breast cancer [43,44]. For clarity only GalNAcTs, sialyltransferases and C2GNT are depicted.
Changes in expression of GTs: the core forming and extension GTs
Expression of ST6GalNAcI to form STn
The Tn antigen can be converted into STn by the action of ST6GalNAcI. This is due to the transcriptional activation of ST6GALNACI in ∼25% of breast carcinomas where there is a complete concordance between mRNA expression and the glycan being seen in the cancers [34]. However, in studies using only immunohistochemistry with different antibodies to define the presence of STn in breast cancers, there is little agreement on the percentage of breast cancers carrying this glycan [35]. Nevertheless, in vitro and in vivo experiments with breast cancer cell lines indicate that STn is associated with tumour progression and migration [36], and in primary breast cancers STn expression is associated with resistance to adjuvant chemotherapy in node-positive patients [37].
Increased expression of ST3GalI and ST6GalNAcII to form sialylated and disialylated core 1
The core 2 branch seen on O-linked glycoproteins expressed by the normal mammary epithelial cells is initiated by the action of the GT C2GNT (encoded by GCNT1; see Figure 1). This enzyme uses as a substrate Galβ1,3GalNAc known as core 1 or T, which is synthesised by the action of T synthase. Galβ1,3GalNAc (core 1) also forms the substrate for the competing sialyltransferases ST3GalI and ST6GalNAcII (Figure 1), which terminate chain extension. Thus, the relative expression of these GTs determines whether glycoproteins with O-GalNAc glycans carry core 2 structures that allow further extension, or sialylated core 1 glycans [38] such as ST. Although ST3GalI expression is elevated in 90% of breast cancers [16], the ST glycan is found in ∼50% of these cancers due to competition with C2GNT, which is only lost in a proportion of breast cancers [16]. In addition, the presence of T is often seen [23]. Moreover, in breast cancer subtypes, changes are seen in both the relative levels of core 1 versus core 2 structures and in the terminal units carried by these structures. Thus, as predicted from profiles of GTs expressed, ER+ve breast cancers show a dominance of core 1-based glycans [20] and sialylated cores (ST) due to an increased expression of ST3GalI. On the other hand, in ER-ve breast cancers, core 2-based structures appear to be dominant [20]. In these cancers the core 2 structures can be carrying the sialyl Lewis X (sLeX) glycan, which is not found on core 2 in the normal breast. Selectin interactions with glycans including sLeX are crucial to immune cell trafficking [39]. It therefore appears that breast cancers have hijacked this normal lectin/glycan interaction to aid in the metastatic process (see below).
Higher levels of expression of ST6GalNAcII, which forms disialylated core 1 (see Figure 1), are associated with ER+ve compared with ER-ve breast cancers. However, high levels of this sialyltransferase are associated with an increased survival time in ER-ve tumours [40]. This sialyltransferase has been identified as a metastasis suppressor gene as its knockdown results in an increase in metastasis in an in vivo model [40]. Evidence suggests that the addition of sialic acid in the α2,6-position to the core 1 structure inhibits the cells' ability to bind to galectin-3, a lectin associated with many processes involved in tumour progression and metastasis [40].
Changes in expression of glycosidases
α-l-fucosidase 1 (FUCA1) is found in lysosomes and catalyses the removal of terminal fucose. The mRNA encoding this enzyme is highly overexpressed in breast cancer compared with normal breast tissue but is associated with early-stage tumours [41]. This association with early-stage disease of the fucosidase may be due to fucose being part of LeX glycan, the sialylated version of which binds to selectins and may play a role in metastasis. Moreover, higher levels of FUCA1 predicted a better overall survival in patients with triple negative breast cancer [41], which by definition, is ER-ve.
Changes in pH of the Golgi apparatus and availability of sugar nucleotides
Regulation of pH within organelles is essential for their correct physiological function, and the pH of the Golgi apparatus is strictly controlled [42]. The presence of the unsubstituted T antigen (core 1) in most breast cancers [23] is difficult to explain by changes in expression of GTs. However, small increases in pH within the Golgi apparatus have been associated with an increased presence of the T antigen in cell lines [43], which may be the result of mislocalisation of certain GTs within the Golgi [44]; see Figure 2.
Another possible contributing factor to aberrant mucin-type glycosylation is that there may be a change in the availability of the nucleotide sugar donors, and elevated levels of UDP-GlcNAc and UDP-GalNAc have been found in breast cancer cell lines [45].
Role of changes in mucin-type O-GalNAc glycosylation in tumour growth and metastasis
The presence of tumour-associated carbohydrate antigens is generally associated with a poor prognosis and reduced overall survival [46]. In O-GalNAc glycosylation overexpression of ST3GalI leading to the increased expression of sialylated core 1 (ST) by tumour cells leads to increased tumour growth in transplantable [47] and spontaneous models of breast cancer [48]. Moreover, loss of core 1 O-glycans in spontaneous mammary cancer models delayed the onset and growth of the tumours and impaired the localisation of Muc1 [49]. Data are now emerging as to the mechanisms involved in this accelerated initiation and tumour growth by core 1 and sialylated core 1 glycans; see Figure 3.
Figure 3. Mucin-type O-GalNAc glycosylation changes seen in breast cancer lead to increase in tumour growth and progression through a number of mechanisms.
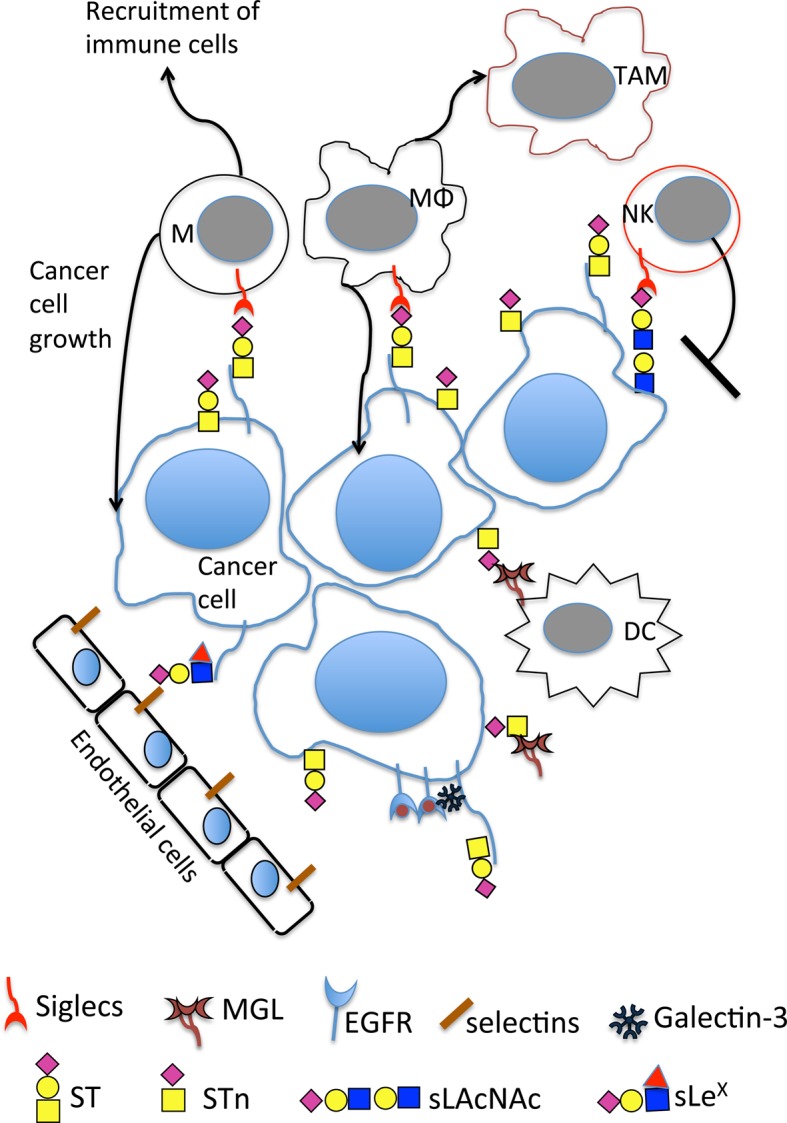
O-GalNAc changes lead to novel interactions with lectins on immune cells including the binding of sialylated glycans to siglecs on monocytes, macrophages and NK cells. This includes the binding of MUC1-ST to siglec-9 on monocytes and macrophages and the binding of sialylated LacNAc (which can be carried on core 1 and core 2 branches) to siglec-7 on NK. Binding of Tn and STn to MGL on dendritic cells and macrophages is also observed. Expression of sLeX can lead to binding to selectins on endothelial cells, and different core glycans can dictate how cancer cells respond to EGF binding. M, monocytes; MΦ, macrophages; TAM, tumour-associated macrophages; NK, natural killer cells; DC, dendritic cells; MGL, macrophage galactose-specific lectin; EGFR, epidermal growth factor receptor. Symbols for the glycans are as in Figure 1 with the addition of the red triangle for fucose.
Modification of the immune micro-environment
The dramatic changes seen in O-GalNAc glycosylation in breast cancers leads to new interactions with carbohydrate-binding lectins expressed by immune cells [50]. This allows the immune compartment to respond to changes in glycosylation, which often leads to activating inhibitory pathways.
As described above, increased sialylation in breast and many other cancers is a common change seen in malignancy. Importantly, both the Tn and STn glycans can bind to the macrophage galactose-specific lectin (MGL) expressed by macrophages and dendritic cells [23], inducing inhibitory signals resulting in effector T-cell apoptosis [51].
Although Tn is highly expressed by most breast cancers, it is mainly observed inside the cells [23,24]. STn, on the other hand, is found at the cell surface in ∼25% of breast cancers and hence can interact with MGL on dendritic cells and macrophages [23,34].
Sialic acid patterns on the cell surface can be recognised as ‘self' by the immune system, and the term SAMPs (self-associated molecular patterns) has been coined to describe these glycans [52]. Siglecs (sialic acid-binding immunoglobulin-like lectins) are a family of sialic acid-binding lectins mainly expressed on cells of the immune system that have evolved to recognise these SAMPs and deliver signals that negatively regulate the immune system [53]. Like the immune checkpoint receptor PD1 (programmed cell death 1), many siglecs contain ITIMs (immunoreceptor tyrosine-based inhibition motif) within their cytoplasmic tails [53]. It has recently become apparent that siglecs play a role in cancer immune suppression, the hypersialylation seen in cancers inducing binding to these lectins [54–58].
Siglec-7 is expressed by NK cells, and increased sialylation on tumour cells inhibits NK activation through binding to this siglec [56]. Interestingly, the use of trastuzumab, an antibody to HER-2 used in the clinic for the treatment of HER-2 positive breast cancers, to deliver sialidase to tumour cells reduced binding to siglec-7 and enhanced NK killing [57].
MUC1 is a mucin that is highly glycosylated with O-GalNAc glycans and is overexpressed and aberrantly glycosylated in greater than 90% of breast cancers [11]. A tumour-associated glycoform of MUC1, MUC1-ST can interact with siglec-9 expressed by monocytes and macrophages, resulting in the secretion of many factors that are involved in tumour progression and immune recruitment [58]. The binding of MUC1-ST to siglec-9 on macrophages results in the expression of a tumour-associated macrophage (TAM) type phenotype [58], which is associated with poor prognosis in breast cancer [59]. These TAMS show increased expression of the programmed death ligand 1 that binds to the immune checkpoint receptor PD1 expressed by T cells. Antibody blocking of this immune checkpoint is now in clinical use with some extremely encouraging results particularly in melanoma and lung cancer [60]. The binding of siglec-9 to MUC1 has also been reported to increase the growth of the tumour cell via recruitment of β-catenin [61]. Thus the siglec glyco-immune checkpoint is a prime target for the development of novel immunotherapies [50,62,63].
Role of aberrant mucin O-GalNAc glycosylation in tumour growth and progression of breast cancer
Glycoproteins that carry large amounts of O-GalNAc glycans, such as MUC1 are often overexpressed in cancer and contribute to the bulk of the glycocalyx facilitating integrin clustering that enhances interactions with ligands [64]. Thus, a bulky glycocalyx may contribute to tumour growth by enhancing integrin-mediated cell growth and survival [64].
Galectins are a family of carbohydrate-binding proteins that display a plethora of functions. Galectin-3 is elevated in the sera of breast and other cancer patients [65] and it has been shown to interact with MUC1 carrying the core 1 glycan (T glycan), enhancing tumour cell aggregation [66,67] and promoting adhesion to endothelial cells by inducing polarisation of MUC1, and thus exposing adhesion molecules [66]. EGF binding to EGFR expressed by breast cancer cells carrying core1- or core2-based glycans induces different patterns of gene expression (Tajadura-Ortega et al. submitted for publication). The mechanism responsible involves a MUC1/EGFR complex formation with galectin-3. Moreover, it has recently been shown that MUC1–galectin-3 interaction enhances the MUC1 interaction with EGFR, leading to increased dimerisation of EGFR and subsequent signalling [68].
The glycan sLeX can be carried on core 1 and core 2 branches, as well as N-linked glycans. In breast cancers, its expression has been associated with a high risk of metastasis [46], although others have found no association with disease survival [20]. The combination of sLeX and one of its carrier glycoproteins, (BST-2) is associated with a higher risk of brain and liver metastasis, and a 3-fold decrease in disease survival in ER-ve breast cancer patients [69]. However, although sLeX is found on a higher number of ER-ve breast cancers, when high levels are expressed by ER+ve breast cancers, there is an association with metastasis to the bone [20]. Importantly, bone microvasculature cells are known to express E-selectin, one of the receptors for sLeX [70], and selectin binding blocking strategies are now actively being explored as a therapeutic option in cancer [71]. These data indicate that it is the context in which sLeX is found rather than the glycan itself that dictates metastatic tropism.
Summary
Changes in O-linked glycosylation are a common feature of many cancers, including breast cancer, and result from many mechanisms. There is now convincing evidence that this is not just a passenger effect but can contribute to driving the malignant phenotype and cancer progression ([72] and see above). Understanding the mechanisms involved has allowed the development of novel therapeutics such as blocking glyco-immune checkpoints as described above. In addition, other types of immunotherapy such as the development of chimeric antigen receptors (CARs) specific for tumour-associated O-GalNAc glycoforms are being developed [73,74]; see reviews by Taylor-Papadimitriou et al. [75] and Steentoft et al. [76].
As in many fields, it is becoming clear that a reductionist approach will not adequately explain the complex interactions between glycans and glycan-binding proteins, glycan:glycan interactions and cell:cell interactions mediated by glycans. Within the breast cancer micro-environment cross-talk occurs between cancer cells, immune cells and stromal cells [77], often mediated by glycan-binding proteins [72]; therefore complex co-culture methods will be needed to really investigate and dissect the role of aberrant mucin-type glycosylation in breast cancer. Moreover, the recent developments in mass spectrometry allowing the collection of molecular information including glycans from formalin-fixed paraffin-embedded tissue and displaying the data as intensity maps should prove a great technological advance to the analysis of the glycome [78]. As our understanding of how changes in glycosylation can affect tumour growth and progression increases, the potential to develop further therapeutic approaches for the treatment of breast cancer will increase.
Abbreviations
- CARs
chimeric antigen receptors
- ER
estrogen receptor
- FUCA1
α-l-fucosidase 1
- GalNAc
N-acetyl-d-galactosamine
- GalNAc-Ts
N-acetylgalactosaminyltransferases
- GlcNAc
N-acetyl-d-glucosamine
- GTs
glycosyltransferases
- MGL
macrophage galactose-specific lectin
- NK
natural killer cells
- PD1
programmed cell death 1
- PTMs
post-translational modifications
- SAMPs
self-associated molecular patterns
- sLeX
sialyl Lewis X
- STn
sialylated Tn
- TAM
tumour-associated macrophage
Author Contribution
J.M.B. prepared the manuscript and wrote the first draft. All the authors contributed to the final draft.
Funding
R.G. was funded by a grant from the KHP R&D Challenge Fund. V.T.-O. and R.B. were funded by grants from the Medical Research Council [MR/J007196/1 and MR/R000026/1].
Competing Interests
J.M.B. is a consultant for Palleon Pharmaceuticals.
References
- 1.Schjoldager K.T.-B.G. and Clausen H. (2012) Site-specific protein O-glycosylation modulates proprotein processing — deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim. Biophys. Acta, Gen. Subj. 1820, 2079–2094 10.1016/j.bbagen.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 2.Haltiwanger R.S., Wells L., Freeze H.H. and Stanley P. (2015–2017) Other Classes of Eukaryotic Glycans In Essentials of Glycobiology, 3rd edn (Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M. et al., eds), Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY) Chapter 13 [Google Scholar]
- 3.Stanley P., Taniguchi N. and Aebi M. (2015–2017) N-Glycans In Essentials of Glycobiology, 3rd edn (Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M. et al., eds), Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY), Chapter 9 [Google Scholar]
- 4.Brockhausen I. (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 7, 599–604 10.1038/sj.embor.7400705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zachara N., Akimoto Y. and Hart G.W. (2015–2017) The O-GlcNAc modification In Essentials of Glycobiology, 3rd edn (Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M. et al., eds), Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY), Chapter 19 [Google Scholar]
- 6.Kudelka M.R., Ju T., Heimburg-Molinaro J. and Cummings R.D. (2015) Simple sugars to complex disease-mucin-type O-glycans in cancer. Adv. Cancer Res. 126, 53–135 10.1016/bs.acr.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho S.S. and Reis C.A. (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- 8.Ashkani J. and Naidoo K.J. (2016) Glycosyltransferase gene expression profiles classify cancer types and propose prognostic subtypes. Sci. Rep. 6, 26451 10.1038/srep26451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahmberg C.G., Kiehn D. and Hakomori S.-I. (1974) Changes in a surface-labelled galactoprotein and in glycolipid concentrations in cells transformed by a temperature-sensitive polyoma virus mutant. Nature 248, 413–415 10.1038/248413a0 [DOI] [PubMed] [Google Scholar]
- 10.Au G.H.T., Mejias L., Swami V.K., Brooks A.D., Shih W.Y. and Shih W.-H. (2014) Quantitative assessment of Tn antigen in breast tissue micro-arrays using CdSe aqueous quantum dots. Biomaterials 35, 2971–2980 10.1016/j.biomaterials.2013.12.034 [DOI] [PubMed] [Google Scholar]
- 11.Girling A., Bartkova J., Burchell J., Gendler S., Gillett C. and Taylor-Papadimitriou J. (1989) A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int. J. Cancer 43, 1072–1076 10.1002/ijc.2910430620 [DOI] [PubMed] [Google Scholar]
- 12.Steentoft C., Vakhrushev S.Y., Vester-Christensen M.B., Schjoldager K.T.-B.G., Kong Y., Bennett E.P. et al. (2011) Mining the O-glycoproteome using zinc-finger nuclease–glycoengineered SimpleCell lines. Nat. Methods 8, 977–982 10.1038/nmeth.1731 [DOI] [PubMed] [Google Scholar]
- 13.Bennett E.P., Mandel U., Clausen H., Gerken T.A., Fritz T.A. and Tabak L.A. (2012) Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 10.1093/glycob/cwr182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burchell J.M., Mungul A. and Taylor-Papadimitriou J. (2001) O-linked glycosylation in the mammary gland: changes that occur during malignancy. J. Mammary Gland Biol. Neoplasia 6, 355–364 10.1023/A:1011331809881 [DOI] [PubMed] [Google Scholar]
- 15.Müller S., Alving K., Peter-Katalinic J., Zachara N., Gooley A.A. and Hanisch F.-G. (1999) High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. J. Biol. Chem. 274, 18165–18172 10.1074/jbc.274.26.18165 [DOI] [PubMed] [Google Scholar]
- 16.Burchell J., Poulsom R., Hanby A., Whitehouse C., Cooper L., Clausen H. et al. (1999) An α2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 9, 1307–1311 10.1093/glycob/9.12.1307 [DOI] [PubMed] [Google Scholar]
- 17.Lloyd K.O., Burchell J., Kudryashov V., Yin B.W.T. and Taylor-Papadimitriou J. (1996) Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J. Biol. Chem. 271, 33325–33334 10.1074/jbc.271.52.33325 [DOI] [PubMed] [Google Scholar]
- 18.Springer G.F. (1984) T and Tn, general carcinoma autoantigens. Science 224, 1198–1206 10.1126/science.6729450 [DOI] [PubMed] [Google Scholar]
- 19.Ju T., Wang Y., Aryal R.P., Lehoux S.D., Ding X., Kudelka M.R. et al. (2013) Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics Clin. Appl. 7, 618–631 10.1002/prca.201300024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julien S., Ivetic A., Grigoriadis A., QiZe D., Burford B., Sproviero D. et al. (2011) Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 71, 7683–7693 10.1158/0008-5472.CAN-11-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju T., Lanneau G.S., Gautam T., Wang Y., Xia B., Stowell S.R. et al. (2008) Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 68, 1636–1646 10.1158/0008-5472.CAN-07-2345 [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan P., Dabelsteen S., Madsen F.B., Francavilla C., Kopp K.L., Steentoft C. et al. (2014) Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl Acad. Sci. U.S.A. 111, E4066–E4075 10.1073/pnas.1406619111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beatson R., Maurstad G., Picco G., Arulappu A., Coleman J., Wandell H.H. et al. (2015) The breast cancer-associated glycoforms of MUC1, MUC1-Tn and sialyl-Tn, are expressed in COSMC wild-type cells and bind the C-type lectin MGL. PLoS ONE 10, e0125994 10.1371/journal.pone.0125994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill D.J., Tham K.M., Chia J., Wang S.C., Steentoft C., Clausen H. et al. (2013) Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl Acad. Sci. U.S.A. 110, E3152–E3161 10.1073/pnas.1305269110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo N.J., Kim M.S. and Lee S.H. (2008) Absence of COSMC gene mutations in breast and colorectal carcinomas. APMIS 116, 154–155 10.1111/j.1600-0463.2008.00965.x [DOI] [PubMed] [Google Scholar]
- 26.Brooks S.A., Carter T.M., Bennett E.P., Clausen H. and Mandel U. (2007) Immunolocalisation of members of the polypeptide N-acetylgalactosaminyl transferase (ppGalNAc-T) family is consistent with biologically relevant altered cell surface glycosylation in breast cancer. Acta Histochem. 109, 273–284 10.1016/j.acthis.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Freire T., Berois N., Sóñora C., Varangot M., Barrios E. and Osinaga E. (2006) UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int. J. Cancer 119, 1383–1388 10.1002/ijc.21959 [DOI] [PubMed] [Google Scholar]
- 28.Wu C., Guo X., Wang W., Wang Y., Shan Y., Zhang B. et al. (2010) N-Acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer 10, 123 10.1186/1471-2407-10-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J.H., Katagiri T., Chung S., Kijima K. and Nakamura Y. (2011) Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin. Neoplasia 13, 320–326 10.1593/neo.101440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J.-H., Nishidate T., Kijima K., Ohashi T., Takegawa K., Fujikane T. et al. (2010) Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 70, 2759–2769 10.1158/0008-5472.CAN-09-3911 [DOI] [PubMed] [Google Scholar]
- 31.Lavrsen K., Dabelsteen S., Vakhrushev S.Y., Levann A.M.R., Haue A.D., Dylander A. et al. (2018) De novo expression of human polypeptide N-acetylgalactosaminyltransferase 6 (GalNAc-T6) in colon adenocarcinoma inhibits the differentiation of colonic epithelium. J. Biol. Chem. 293, 1298–1314 10.1074/jbc.M117.812826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill D.J., Chia J., Senewiratne J. and Bard F. (2010) Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J. Cell Biol. 189, 843–858 10.1083/jcb.201003055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen A.T., Chia J., Ros M., Hui K.M., Saltel F. and Bard F. (2017) Organelle specific O-glycosylation drives MMP14 activation, tumor growth, and metastasis. Cancer Cell 32, 639–653.e6 10.1016/j.ccell.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 34.Sewell R., Bäckström M., Dalziel M., Gschmeissner S., Karlsson H., Noll T. et al. (2006) The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 281, 3586–3594 10.1074/jbc.M511826200 [DOI] [PubMed] [Google Scholar]
- 35.Julien S., Videira P.A. and Delannoy P. (2012) Sialyl-tn in cancer: (how) did we miss the target? Biomolecules 2, 435–466 10.3390/biom2040435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Julien S., Adriaenssens E., Ottenberg K., Furlan A., Courtand G., Vercoutter-Edouart A.S. et al. (2006) ST6GalNAc i expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 16, 54–64 10.1093/glycob/cwj033 [DOI] [PubMed] [Google Scholar]
- 37.Miles D.W., Happerfield L.C., Smith P., Gillibrand R., Bobrow L.G., Gregory W.M. et al. (1994) Expression of sialyl-Tn predicts the effect of adjuvant chemotherapy in node-positive breast cancer. Br. J. Cancer 70, 1272–1275 10.1038/bjc.1994.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalziel M., Whitehouse C., McFarlane I., Brockhausen I., Gschmeissner S., Schwientek T. et al. (2001) The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem. 276, 11007–11015 10.1074/jbc.M006523200 [DOI] [PubMed] [Google Scholar]
- 39.Marth J.D. and Grewal P.K. (2008) Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 10.1038/nri2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murugaesu N., Iravani M., van Weverwijk A., Ivetic A., Johnson D.A., Antonopoulos A. et al. (2014) An in vivo functional screen identifies ST6GalNAc2 sialyltransferase as a breast cancer metastasis suppressor. Cancer Discov. 4, 304–317 10.1158/2159-8290.CD-13-0287 [DOI] [PubMed] [Google Scholar]
- 41.Cheng T.C., Tu S.H., Chen L.C., Chen M.Y., Chen W.Y., Lin Y.K. et al. (2015) Down-regulation of α-L-fucosidase 1 expression confers inferior survival for triple-negative breast cancer patients by modulating the glycosylation status of the tumor cell surface. Oncotarget 6, 21283–21300 10.18632/oncotarget.4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivinoja A., Pujol F.M., Hassinen A. and Kellokumpu S. (2012) Golgi pH, its regulation and roles in human disease. Ann. Med. 44, 542–554 10.3109/07853890.2011.579150 [DOI] [PubMed] [Google Scholar]
- 43.Rivinoja A., Kokkonen N., Kellokumpu I. and Kellokumpu S. (2006) Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell. Physiol. 208, 167–174 10.1002/jcp.20653 [DOI] [PubMed] [Google Scholar]
- 44.Rivinoja A., Hassinen A., Kokkonen N., Kauppila A. and Kellokumpu S. (2009) Elevated Golgi pH impairs terminal N-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J. Cell. Physiol. 220, 144–154 10.1002/jcp.21744 [DOI] [PubMed] [Google Scholar]
- 45.Nakajima K., Kitazume S., Angata T., Fujinawa R., Ohtsubo K., Miyoshi E. et al. (2010) Simultaneous determination of nucleotide sugars with ion-pair reversed-phase HPLC. Glycobiology 20, 865–871 10.1093/glycob/cwq044 [DOI] [PubMed] [Google Scholar]
- 46.Cazet A., Julien S., Bobowski M., Burchell J. and Delannoy P. (2010) Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 12, 204 10.1186/bcr2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mungul A., Cooper L., Brockhausen I., Ryder K., Mandel U., Clausen H. et al. (2004) Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int. J. Oncol. 25, 937–943 PMID: [PubMed] [Google Scholar]
- 48.Picco G., Julien S., Brockhausen I., Beatson R., Antonopoulos A., Haslam S. et al. (2010) Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 20, 1241–1250 10.1093/glycob/cwq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song K., Herzog B.H., Fu J., Sheng M., Bergstrom K., McDaniel J.M. et al. (2015) Loss of core 1-derived O-glycans decreases breast cancer development in mice. J. Biol. Chem. 290, 20159–20166 10.1074/jbc.M115.654483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.RodrÍguez E., Schetters S.T.T. and van Kooyk Y. (2018) The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 18, 204–211 10.1038/nri.2018.3 [DOI] [PubMed] [Google Scholar]
- 51.van Vliet S.J., Gringhuis S.I., Geijtenbeek T.B.H. and van Kooyk Y. (2006) Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat. Immunol. 7, 1200–1208 10.1038/ni1390 [DOI] [PubMed] [Google Scholar]
- 52.Varki A. (2011) Since there are PAMPs and DAMPs, there must be SAMPs? Glycan ‘self-associated molecular patterns’ dampen innate immunity, but pathogens can mimic them. Glycobiology 21, 1121–1124 10.1093/glycob/cwr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macauley M.S., Crocker P.R. and Paulson J.C. (2014) Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 10.1038/nri3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jandus C., Boligan K.F., Chijioke O., Liu H., Dahlhaus M., Démoulins T. et al. (2014) Interactions between Siglec-7/9 receptors and ligands influence NK cell–dependent tumor immunosurveillance. J. Clin. Invest. 124, 1810–1820 10.1172/JCI65899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Läubli H., Pearce O.M.T., Schwarz F., Siddiqui S.S., Deng L., Stanczak M.A. et al. (2014) Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl Acad. Sci. U.S.A. 111, 14211–14216 10.1073/pnas.1409580111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudak J.E., Canham S.M. and Bertozzi C.R. (2014) Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 10, 69–75 10.1038/nchembio.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao H., Woods E.C., Vukojicic P. and Bertozzi C.R. (2016) Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl Acad. Sci. U.S.A. 113, 10304–10309 10.1073/pnas.1608069113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beatson R., Tajadura-Ortega V., Achkova D., Picco G., Tsourouktsoglou T.-D., Klausing S. et al. (2016) The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 17, 1273–1281 10.1038/ni.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiainen S., Tumelius R., Rilla K., Hämäläinen K., Tammi M., Tammi R. et al. (2015) High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 66, 873–883 10.1111/his.12607 [DOI] [PubMed] [Google Scholar]
- 60.Ascierto P.A. and McArthur G.A. (2017) Checkpoint inhibitors in melanoma and early phase development in solid tumors: what's the future? J. Transl. Med. 15, 173 10.1186/s12967-017-1278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanida S., Akita K., Ishida A., Mori Y., Toda M., Inoue M. et al. (2013) Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth. J. Biol. Chem. 288, 31842–31852 10.1074/jbc.M113.471318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams O.J., Stanczak M.A., von Gunten S. and Läubli H. (2017) Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology PMID: 10.1093/glycob/cwx108 [DOI] [PubMed] [Google Scholar]
- 63.Tomioka Y., Morimatsu M., Nishijima K.-i., Usui T., Yamamoto S., Suyama H. et al. (2014) A soluble form of Siglec-9 provides an antitumor benefit against mammary tumor cells expressing MUC1 in transgenic mice. Biochem. Biophys. Res. Commun. 450, 532–537 10.1016/j.bbrc.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 64.Paszek M.J., DuFort C.C., Rossier O., Bainer R., Mouw J.K., Godula K. et al. (2014) The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325 10.1038/nature13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iurisci I., Tinari N., Natoli C., Angelucci D., Cianchetti E. and Iacobelli S. (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 6, 1389–1393 PMID: [PubMed] [Google Scholar]
- 66.Zhao Q., Guo X., Nash G.B., Stone P.C., Hilkens J., Rhodes J.-M. et al. (2009) Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 69, 6799–6806 10.1158/0008-5472.CAN-09-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Q., Barclay M., Hilkens J., Guo X., Barrow H., Rhodes J.M. et al. (2010) Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 9, 154 10.1186/1476-4598-9-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piyush T., Chacko A.R., Sindrewicz P., Hilkens J., Rhodes J.M. and Yu L.-G. (2017) Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. 24, 1937–1947 10.1038/cdd.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodman N., Pinder S.E., Tajadura V., Le Bourhis X., Gillett C., Delannoy P. et al. (2016) Two E-selectin ligands, BST-2 and LGALS3BP, predict metastasis and poor survival of ER-negative breast cancer. Int. J. Oncol. 49, 265–275 10.3892/ijo.2016.3521 [DOI] [PubMed] [Google Scholar]
- 70.Schweitzer K.M., Dräger A.M., van der Valk P., Thijsen S.F., Zevenbergen A., Theijsmeijer A.P. et al. (1996) Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am. J. Pathol. 148, 165–175 PMID: [PMC free article] [PubMed] [Google Scholar]
- 71.Natoni A., Macauley M.S. and O'Dwyer M.E. (2016) Targeting selectins and their ligands in cancer. Front. Oncol. 6, 93 10.3389/fonc.2016.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodrigues J.G., Balmaña M., Macedo J.A., Poças J., Fernandes Â., de-Freitas-Junior J.C.M. et al. (2018) Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell Immunol. PMID: 10.1016/j.cellimm.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 73.Wilkie S., Picco G., Foster J., Davies D.M., Julien S., Cooper L. et al. (2008) Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J. Immunol. 180, 4901–4909 10.4049/jimmunol.180.7.4901 [DOI] [PubMed] [Google Scholar]
- 74.Posey A.D. Jr, Schwab R.D., Boesteanu A.C., Steentoft C., Mandel U., Engels B. et al. (2016) Engineered CAR T cells targeting the cancer-associated Tn-Glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 10.1016/j.immuni.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor-Papadimitriou J., Burchell J.M., Graham R. and Beatson R. (2018) Latest developments in MUC1 immunotherapy. Biochem. Soc. Res. Trans., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steentoft C., Migliorini D., King T.R., Mandel U., June C.H. and Posey A.D. Jr (2018) Glycan directed CAR-T cells. Glycobiology PMID: 10.1093/glycob/cwy008 [DOI] [PubMed] [Google Scholar]
- 77.Criscitiello C., Esposito A. and Curigliano G. (2014) Tumor–stroma crosstalk: targeting stroma in breast cancer. Curr. Opin. Oncol. 26, 551–555 10.1097/CCO.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 78.Hinneburg H., Korać P., Schirmeister F., Gasparov S., Seeberger P.H., Zoldoš V. et al. (2017) Unlocking cancer glycomes from histopathological formalin-fixed and paraffin-embedded (FFPE) tissue microdissections. Mol. Cell. Proteomics 16, 524–536 10.1074/mcp.M116.062414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varki A., Cummings R.D., Aebi M., Packer N.H., Seeberger P.H., Esko J.D. et al. (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324 10.1093/glycob/cwv091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitehouse C., Burchell J., Gschmeissner S., Brockhausen I., Lloyd K.O. and Taylor-Papadimitriou J. (1997) A transfected sialyltransferase that is elevated in breast cancer and localizes to the medial/trans-Golgi apparatus inhibits the development of core-2–based O-glycans. J. Cell Biol. 137, 1229–1241 10.1083/jcb.137.6.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]