Abstract
The increasing pressure of abolishing and/or decreasing the use of antibiotics as antimicrobial growth promoters for livestock calls for alternative solutions to sustain the efficiency of current livestock production. Among the alternatives, essential oils have a great potential and are generally considered natural, less toxic, and free from residues. Essential oils have been proven in numerous in vitro studies to exert antimicrobial effects on various pathogens. The current review touched on the basics of essential oils, and the in vivo effects of essential oils on growth, intestinal microflora, anti-oxidation, immune functionality, meat qualities as well as the possible modes of action in poultry and pigs, and the future research areas were proposed.
Keywords: Antibiotics, Chickens, Essential oils, Pathogens, Pigs
1. Introduction
The recognition of microorganisms being responsible for a variety of diseases in the late half of the 19th century ushered in the discoveries of antibiotics, which saw its golden era between 1950s and 1970s. But since then, no new classes of antibiotics had been discovered (Aminov, 2010). Meanwhile, the improper use of antibiotics resulted in the selection of bacteria resistant to antibiotics. One of the solutions is to implement bans of using antibiotics as antimicrobial growth promoters (AGP) for farm animals, which makes it imperative to find effective alternatives to antibiotics to sustain the efficiency of current livestock production. Among the alternatives, essential oils have a great potential. The essential oils are generally considered natural, less toxic, and free from residues when compared with antibiotics (Gong et al., 2014).
Essential oils are complex mixtures of volatile compounds produced by living organisms and isolated by physical means only (pressing and distillation) from a whole plant or plant part of known taxonomic origin (Franz and Novak, 2009). The term “essential oils” emerged because “oils” were wishfully believed to be “essential” to life, and have a long history of being used by human for cosmetic and medicinal purposes. The development of essential oils, however, was delayed by the advent of antibiotics in the middle of the 19th century, and was renewed recently. It was estimated that, out of 3,000 known essential oils, 300 were recognized as commercially important and mainly used in the flavors and fragrances market (van de Braak and Leijten, 1994). The global essential oil market is expected to reach 11.67 billion USD by 2022.
The aim of the current review was to identify the well-recognized efficacy of essential oils for poultry and pigs as well as the conflicting research findings, whereupon more research efforts could be directed to the inconclusive area to facilitate a better understanding of essential oils.
2. Basics of essential oils
Essential oils are a sum of constituent volatiles, and thus the effects of essential oils should be a totality of effects of all components and their interactions. However, 2 or 3 components could account for up to 85% of the total mixture compared with the minors (Miguel, 2010), and thereby contribute to the primary property of the mixture. For example, the phenols (thymol and carvacrol) constitute about 80% of the essential oils of oregano, the most widespread species of Lamiaceae family, and are mainly responsible for its antibacterial and antioxidant activities. Besides thymol and carvacrol, ρ-cymene was found as another dominant component of oregano (Bouhaddouda et al., 2016). Although the ρ-cymene is not an effective antimicrobial agent by itself, it could facilitate the transport of carvacrol across the cytoplasmic membrane (Oke et al., 2009).
The composition of essential oils is primarily determined by the homogeneity of the starting materials, whose characteristics could be influenced by a plethora of factors. For example, the total content of monoterpene hydrocarbons (mostly γ-terpinene and ρ-cymene) and phenol terpenes (mostly thymol and carvacrol) ranges from 57.3% to 62.5% of the essential oils from a Thyme (Thymus pulegioides L.), relatively constant over different harvesting times, but the phenol content starts to increase at the beginning of the flowering and reaches its greatest value during the full flowering period of the plant (Senatore, 1996). The biological activities in in vivo trials largely depend on the chemical profile of essential oils.
Essential oils account for only a small proportion (usually less than 1%) of the wet weight of plant materials, which makes it imperative to improve the yield of essential oils by continuous developments in relevant fields such as genetic engineering and extraction methods. These developments presented challenges to the concept of essential oils as well as the knowledge of biological activities of essential oils. For example, the steam-distilled essential oils from Origanum vulgare showed a great antibacterial activity against reference strains with a moderate antioxidant activity, while the methanolic extract exhibits no antibacterial activity but a high antioxidant activity (Bouhaddouda et al., 2016), which suggests that the bioactivity of essential oils is indeed based on the method of extraction (Vigan, 2010). In addition, there is a growing part of chemically-synthesized essential oils used in feed industry.
Most constituents of essential oils are terpenoids and phenylpropanoids. Phenylpropanoids occur less frequently and less abundantly than terpenoids (Hammer and Carson, 2011). The well-known plant families for producing essential oils with medicinal and industrial values include Alliaceae, Apiaceae, Asteraceae, Lamiaceae, Myrtaceae, Poaceae, and Rutaceae (Raut and Karuppayil, 2014). Some representative essential oils include, but not limited to, anise (Apiaceae), oregano (Lamiaceae), cinnamon (Lauraceae), garlic (Liliaceae), thyme (Myrtaceae), black pepper (Piperaceae), and Turmeric (Zingiberaceae).
3. Essential oils for poultry
3.1. Growth performance
Essential oils are perceived as growth promoters in poultry diets (Zhang et al., 2014). Animal trial results, however, are considerably variable. Table 1 gives a summary of the factors which could influence the efficacy of essential oils for both poultry and pigs. These factors relate to the experimental essential oils, animals, diets, and environment.
Table 1.
Factors influencing the efficacy of essential oils.
| Factors | Essential oils | Results or speculations | Species | Reference |
|---|---|---|---|---|
| Dietary form | Menthol, cinnamaldehyde | A pelleting temperature of 58 °C led a recovery of 17% to 56% of the indicator substances | Pigs | Maenner et al., 2011 |
| Dietary nutrient density | Buckwheat, thyme, curcuma, black pepper, ginger | Benefits of essential oils are more dramatic with high nutrient density diets | Pigs | Yan et al., 2010 |
| Dietary composition | Thymol, cinnamaldehyde, CRINA Poultry | Highly digestible diet may diminish the efficacy of essential oils | Broilers | Lee et al., 2003 |
| Essential oils composition | Menthol, cinnamaldehyde | The essential oil mixture with menthol, not cinnamaldehyde, as the primary component improved gain to feed | Pigs | Maenner et al., 2011 |
| Essential oils composition | Caraway, fennel | Caraway oil, not fennel oil, at 100 mg/kg feed, tended to decrease feed intake | Pigs | Schöne et al., 2006 |
| Essential oils composition and quality | Thyme, oregano, marjoram, rosemary, yarrow | Various herbs and oils have different effects, which may be primarily related to differences in their terpene composition | Broilers | Cross et al., 2007 |
| Dosage | Cinnamon, thyme, oregano | The absence of benefit could be due to improper doses | Pigs | Namkung et al., 2004 |
| Dosage | Oregano | Feed intake and weight gain responded to the supplementation of oregano quadratically | Broilers | Abdel-Wareth et al., 2012 |
| Environment | Menthol, cinnamaldehyde | Feeding trials under simulated research station or commercial farm conditions gave similar results | Pigs | Maenner et al., 2011 |
| Environment | Anis, citrus, oregano, flavors | The supplementation of phytobiotics was not beneficial in research facility without sufficient disease challenges | Pigs | Kommera et al., 2006 |
| Environment | Caraway, fennel | A positive effect of fennel and caraway oil seems to occur only during gastrointestinal disorders | Pigs | Schöne et al., 2006 |
| Environment | Thymol, cinnamaldehyde, CRINA Poultry | A clean environment led to diminished efficacy | Broilers | Lee et al., 2003 |
| Age of animals | Thymol, cinnamaldehyde, CRINA Poultry | The effect on endogenous enzyme activities decreased with increasing age | Broilers | Lee et al., 2003 |
| Growth performance level | Oregano | Little or no response to oregano oil can be expected at high performance levels, but at low poor performance levels the response may increase | Broilers | Botsoglou et al., 2002 |
3.1.1. Feed intake
Recently-published reviews (Brenes and Roura, 2010, Bozkurt et al., 2014, Franz et al., 2010, Hashemi and Davoodi, 2010, Hippenstiel et al., 2011) reported that feed intake in chicks was unchanged or slightly reduced by dietary inclusion of essential oils. For the decreased feed consumption, one possible explanation is that essential oils possess an irritating smell, which renders the palatability of diet disagreeable to birds. Amad et al. (2011) and Halle et al. (2004) reported that daily feed intake of broilers was numerically decreased by increasing the dietary level of a blend of thyme, star anise, and origanum leaves, and its associated essential oils compared with control. Similarly, Cabuk et al. (2006) noted a significantly reduced feed intake of broilers from young breeders by graded inclusion of a cocktail of essential oils (oregano oil, laurel leaf oil, sage leaf oil, myrtle leaf oil, fennel seed oil, and citrus peel oil). In contrast to pigs, information of poultry concerning feed preference was scarce. Moran (1982) reported that poultry might not be sensitive to flavor as pigs, and Roura et al. (2008) reported that birds are more tolerant to exposure of moderate levels of essential oils than pigs.
3.1.2. Feed utilization
Unlike feed intake, improvements in weight gain and feed conversion ratio dominate the observations. Two well-accepted mechanisms are the stimulation of digestive enzyme secretion and the stabilization of ecosystem of gut microflora, leading to improved feed utilization and less exposure to growth-depressing disorders associated with digestion and metabolism (Bento et al., 2013, Franz et al., 2010, Kurekci et al., 2014, Lee et al., 2003, O'Bryan et al., 2015, Williams and Losa, 2001). The positive effects of essential oils on digestive enzyme secretion from pancreas and intestinal mucosal have been reported in many broiler studies (Basmacioğlu Malayoğlu et al., 2010, Jamroz et al., 2006, Jang et al., 2007). These effects were confirmed by the increased digestibility of nutrients, but did not translate into improvement in growth performance (Amad et al., 2011, Botsoglou et al., 2004, Garcia et al., 2007, Hernández et al., 2004, Lee et al., 2003). It is noteworthy that there is an inadequate description of the environmental conditions under which these trials were conducted, and poor hygienic conditions might be instrumental for essential oils to favorably affect the growth performance of broilers.
3.2. Antimicrobial and anticoccidial activity
The antimicrobial activity of essential oils has been explored in many in vitro assays which showed that thymol, eugenol and carvacrol have high antimicrobial activity against pathogenic bacteria such as Escherichia coli and Salmonella typhimurium, both of which are potential risk factors of enteric infections (Bassolé and Juliani, 2012, Franz et al., 2010, Hippenstiel et al., 2011). Thymol, eugenol and carvacrol are structurally similar, and have been proved to exert synergistic or additive antimicrobial effects when combined at lower concentrations (Bassolé and Juliani, 2012). Therefore, it is necessary to unravel the synergistic mechanism to optimize their formulation. Different in vitro methods as well as different pathogens exist for ranking the antimicrobial capacity of essential oil components, which could vary dramatically as shown in Table 2. In in vivo studies, essential oils used either individually or in combination have shown clear growth inhibition of Clostridium perfringens and E. coli in the hindgut and ameliorated intestinal lesions and weight loss than the challenged control birds (Jamroz et al., 2006, Jerzsele et al., 2012, Mitsch et al., 2004). One well-known mechanism of antibacterial activity is linked to their hydrophobicity, which disrupts the permeability of cell membranes and cell homeostasis with the consequence of loss of cellular components, influx of other substances, or even cell death (Brenes and Roura, 2010, Solórzano-Santos and Miranda-Novales, 2012, Windisch et al., 2008, O'Bryan et al., 2015). It is of note that Gram-negative bacteria are more tolerant to the actions of essential oil than Gram-positive bacteria due to their hydrophilic constituents in the outer membrane (Brenes and Roura, 2010, Giannenas et al., 2013, Seow et al., 2014).
Table 2.
Rankings of in vitro antimicrobial capacity of some essential oil components.
| Reference | Test methods | Pathogens | Rankings |
|---|---|---|---|
| Kim et al., 19951 | Disk diffusion method | E. coli | Citronellal > perillaldehyde > citral > geraniol > linalool > eugenol > terpineol > carvacrol |
| Kim et al., 19951 | Disk diffusion method | S. typhimurium | Citronellal > citral > geraniol > perillaldehyde > linalool > eugenol > terpineol > carvacrol |
| Ait-Ouazzou et al., 2011 | Disk diffusion method | S. enteritidis | Carvacrol > terpineol > linalool |
| Ait-Ouazzou et al., 2011 | Disk diffusion method | E. coli O157:H7 | Carvacrol > terpineol > linalool |
| Frideman et al., 2002 | Microdilution + agar culture | E. coli | Carvacrol, cinnamaldehyde > thymol > eugenol > geraniol |
| Frideman et al., 2002 | Microdilution + agar culture | S. enterica | Cinnamaldehyde > thymol > carvacrol > eugenol > geraniol |
| Frideman et al., 2002 | Microdilution + agar culture | C. jejuni | Cinnamaldehyde > carvacrol > eugenol > thymol > geraniol |
| Si et al., 20062 | Microdilution + optical density | E. coli K88 | Thymol, carvacrol > cinnamon oil > clove oil > eugenol |
| Si et al., 20062 | Microdilution + optical density | E. coli O157:H7 | Cinnamon oil > thymol > geraniol, clove oil, carvacrol > eugenol |
| Si et al., 20062 | Microdilution + optical density | S. typhimurium DT 104 | Cinnamon oil > carvacrol > thymol > clove oil |
| Van Zyl., 20063 | Microdilution + p-iodonitrotetrazolium violet | S. aureus ATCC 25923 | Carvacrol > geraniol > linalool > citronellal > eugenol |
| Van Zyl., 20063 | Microdilution + p-iodonitrotetrazolium violet | B. cereus ATCC 11778 | Eugenol > carvacrol > geraniol > linalool > citronellal |
| Van Zyl., 20063 | Microdilution + p-iodonitrotetrazolium violet | E. coli ATCC 11775 | Eugenol > carvacrol > geraniol > linalool > citronellal |
| Michiels et al., 20094 | Simulated stomach | Total anaerobic bacteria | Carvacrol > thymol > eugenol > trans-cinnamaldehyde |
| Michiels et al., 2009 | Simulated jejunum | Coliform bacteria | Trans-cinnamaldehyde > carvacrol > thymol > eugenol |
| Michiels et al., 2009 | Simulated jejunum | E. coli | Trans-cinnamaldehyde > carvacrol > thymol > eugenol |
The ranking was based on 5% concentration.
The ranking was based on minimum bactericidal concentrations.
The ranking was based on the concentration that resulted in complete growth inhibition of 107 cfu/mL.
The ranking was based on the concentration that gives a reduction of 0.5 log10 cfu/mL compared to control.
Coccidiosis, a common parasitosis disease caused by protozoa of the genus Eimeria, leads to malnutrition and performance depression in poultry. There is an increasing interest in using essential oils against coccidiosis infection. The supplementation of essential oils led to a significant reduction of coccidian oocyst excretion and an alleviation of intestinal lesions in chicks (Alp et al., 2012, Bozkurt et al., 2014, Barbour et al., 2015). However, the underlying mechanisms still need to be elucidated.
3.3. Anti-oxidative activity and carcass hygiene
Chicken body antioxidative stability could be improved by essential oils. Karadas et al. (2014) fed a blend of carvacrol, cinnamaldehyde, and capsicum oleoresin to Ross 308 broilers, and found a significant increase in the hepatic concentration of carotenoids and coenzyme Q10 at d 21 of age. Habibi et al. (2014) and Placha et al. (2014) observed that malondialdehyde concentration in liver, duodenal mucosa, and kidney was significantly decreased by supplementing ginger power and thyme oil to broiler diet.
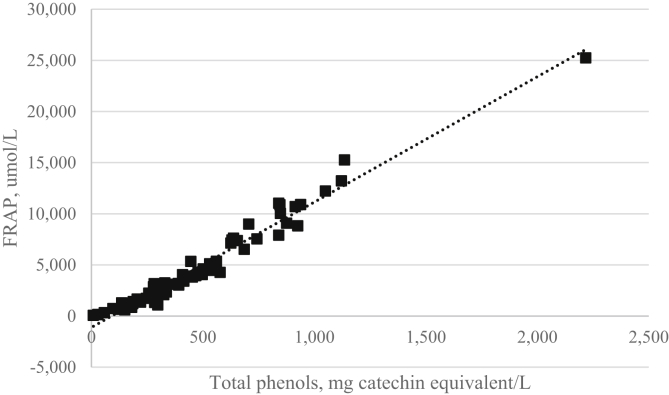
Poultry products are particularly prone to oxidative deterioration due to their high concentrations of polyunsaturated fatty acids. The reviews by Lee et al. (2003) and Khan et al. (2012) showed that thyme oil is effective in retarding oxidant degradation in poultry-derived products, such as meat and eggs. The possible reason might be the antioxidant activity derived from the phenolic OH group which acts as a donor of hydrogen interacting with peroxy radicals during the initial process in lipid oxidation and thereby inhibiting or retarding the hydroxyl peroxide formation (Lee et al., 2003). There was a linear relationship between the amount of total phenols and antioxidant capacity of medicinal plants (Fig. 1). Rosemary, oregano, and sage of the Labiate family were also reported for their effective antioxidant activities in broiler meat (Brenes and Roura, 2010, Franz et al., 2010, Windisch et al., 2008). Regarding the metabolic fate of essential oils in poultry, the research is still lacking. Kohlert et al. (2000) reported that the metabolic fates of essential oils are chemical structure depended; essential oil components are quickly eliminated; and accumulation is unlikely due to the high clearance and short half-lives in human, which lends support to no residue of essential oils in animal products.
Fig. 1.
Linear relationship between the amount of total phenols and antioxidant capacity, ferric reducing/antioxidant power (FRAP) of infusates of 70 medicinal plants, r = 0.9825. (Source: Katalinic et al., 2006; reprinted with permission from authors).
Carcass hygiene could be improved by essential oils, which should be attributed to their reducing the load of pathogens. Alali et al. (2013) reported that a blend of carvacrol, thymol, eucalyptol, and lemon might reduce the Salmonella heidelberg-positive crops and subsequently reduce the cross-contamination in carcass processing. Venkitanarayanan et al. (2013) reported that a blend of caprylic acid and essential oils (trans-cinnamaldehyde, eugenol, carvacrol, and thymol) reduced Salmonella Enteritidis and Campylobacter Jejuni in cecal contents of birds, which indicates a less likelihood of microbial contamination of poultry meat and eggs. An interesting study by Witkowska and Sowińska (2013) showed improvement in hygiene conditions in poultry house via air disinfectants using thyme and peppermint oils individually as primary components.
3.4. Future research for poultry
Environmental conditions play a key role in poultry husbandry. The interaction between environment and the effects of essentials oils should be more researched. Detailed description of the hygienic conditions is necessary for better interpretation of the experimental results and variations. The anticoccidial and immunomodulatory properties of essential oils in poultry have gained interest and require more in-depth research. The metabolic fates of essential oils in poultry should be studied, and the corresponding analytical methods should be established to track the active compounds and their metabolites. Research on the interactions among individual essential oils and with other categories of feed additives should be explored for identifying practical applications.
4. Essential oils for swine
4.1. Growth performance
4.1.1. Feed intake
Voluntary feed intake could be influenced by many factors in association with housing and social environments, and dietary characteristics (Nyachoti et al., 2004), one of which could become the predominant determinant over the other factors under certain conditions. Essential oils usually possess a pungent smell, which might make the feed appealing and thereby arouse the interest of pigs to explore in a larger degree and then consume more frequently and/or a larger amount at each meal before another factor such as the gut fill seizes the dominance and weakens the feeding drive of pigs, or, on the contrary, simply deter pigs from feeding due to the aversive smell.
The effects of essential oils on feed intake of pigs were ambiguous. Feed intake change relative to control due to dietary supplementation of essential oils ranged from −9% to 12% in the review of Franz et al. (2010), and a recent review by Zeng et al. (2015b) reported a range of −3% to 19%. These numerical changes derived mainly from growth performance trials might not qualify as evidence for the animal's preference or aversion to essential oils-supplemented feed because of the latent assumption that more feed should be consumed if it is desirable. The observed improvement in feed intake might be only affiliated to the improved growth rate of animals as commonly seen with most growth-promoting additives (Windisch et al., 2008). In feeding-preference studies, fennel oil significantly decreased the feed intake of piglets (Schöne et al., 2006), and piglets showed no preference for feed supplemented with 125 and 500 mg/kg thymol, while the supplemental level of 2,000 mg/kg almost caused a complete refusal of feed (Michiels et al., 2009). The effects of essential oils on feed intake of pigs are not consistent in the feeding-preference and growth performance trials conducted by Michiels et al. (2009). Therefore, it is advisable to take cautions to judge the feeding preference from different types of animal trials, and more in-depth investigations are warranted to reconcile the incongruent results.
4.1.2. Feed utilization
The literature has registered several studies showing the improved digestibility of energy and nutrients with the supplementation of essential oils. Cinnamaldehyde and thymol (250 mg/kg) significantly improved apparent total tract digestibility (ATTD) of dry matter, crude protein, and energy in piglets (Li et al., 2012, Zeng et al., 2015a). A cocktail of essential oils (250 mg/kg) significantly improved ATTD of crude protein and energy in grower-finisher pigs (Yan et al., 2010). The apparent ileal digestibility of crude protein and most amino acids were improved by the cocktail of essential oils (300 mg/kg) with menthol as the primary component, but not by the cocktail with cinnamaldehyde (Maenner et al., 2011).
The improved apparent digestibility of fat and nutrients could be attributable to the enhanced secretion of bile and enzymes, which was suggested as the primary mode for the digestive stimulant action of spices by Platel and Srinivasan (2004), and in some degree, to the decreased endogenous losses of nutrients (Maenner et al., 2011).
Essential oils have also been shown to regulate the relaxation and contraction of the gut, and thereby to influence the transit of digesta and the resultant interaction between feed and the endogenous enzymes in the gut. The essential oils (cineol, methyl-eugenol and terpineol) of Croton nepetaefolius were myorelaxant and antispasmodic, and were capable of stimulating intestinal transit possibly due to decreased luminal resistance to propulsion of intestinal digesta (Magalhães et al., 1998). Both Manuka and Kanuka oils (tea-tree oils) were shown to have spasmolytic action, but Kanuka had a strong initial spasmogenic action, which might be related to the difference in their chemical compositions (sesquiterpenens vs. monoterpenens; Lis-Balchin and Hart, 1998). The backstage mechanisms might relate to terpenes being well recognized as novel compounds targeting voltage-gated ion channels (de Araújo et al., 2011). The essential oils from Ferula heuffelii inhibited spontaneous contraction of isolated rat ileum dose-dependently possibly by blocking voltage Ca2+ channels or by opening K+ channels (Pavlović et al., 2012). Blocking calcium channels seemed to be of higher significance for Agathosma betulina essential oils than Agathosma crenulata regarding their initial spasmogenic action followed by a spasmolytic action on the guinea-pig ileum (Lis-Balchin et al., 2001).
4.2. Health
4.2.1. Antimicrobial
In piglets, carvacrol, thymol, eugenol, and cinnamaldehyde were found to be absorbed nearly completely in the stomach and the proximal small intestine within 2 h after oral administration (Michiels et al., 2008). Therefore, to deliver the essential oils and their antimicrobial activities to the hindgut to favorably modify the microflora ecosystem, essential oils need to be protected to circumvent the absorption in the foregut (de Lange et al., 2010).
Some essential oils could influence the gut microflora selectively. A cocktail of carvacrol, cinnamaldehyde, and capsicum oleoresin increased the population of Lactobacilli and the ratio of Lactobacilli to Enterobacteria in the jejunum (Manzanilla et al., 2006) and cecum (Castillo et al., 2006) of early-weaned piglets using qPCR method. This is in agreement with the in vitro study results that cinnamaldehyde was highly inhibitory for coliform bacteria and E. coli while it hardly inhibited the growth of Lactobacilli (Michiels et al., 2008). The antimicrobial activity of essential oils could also be exploited as a green preservative to prevent food from contamination of pathogens. Tuscan sausages treated with bay leaf essential oils showed reduced population of total coliforms (da Silveira et al., 2014). To realize the same in vitro antimicrobial effects, however, it is necessary to use 10 to 100-fold higher concentrations of an essential oil in foods (Burt, 2004).
4.2.2. Immunity
Essential oils could change the lymphocyte distribution in the gut. Since a cross talk between gut microbiota and the mucosal immune system is beneficial for a mutual growth and survival (Purchiaroni et al., 2013), it is understandable to surmise that essential oils could leverage the development and function of the gut immune system via modifying the gut microflora, and/or the essential oils might be recognized as foreign stimulants and directly attacked by the immune system. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin decreased the population of intraepithelial lymphocyte (IEL) in jejunum and ileum, but increased lymphocyte in the lamina propria of early-weaned pigs (Manzanilla et al., 2006). Capsaicin and cinnamaldehyde might be responsible for the reduced IEL population in jejunum because of their inhibition of the activation or proliferation of T cells (Nofrarias et al., 2006). It is difficult to conclude whether these changes could be construed as beneficial for the gut immune system, because a healthy gut immune system calls for a delicate balance between recognizing threats such as pathogens followed by initiation of immune defense attacks and tolerating the non-threating foreign substances.
Essential oils might potentiate the immune responses. Supplementing essential oils improved serum lymphocyte proliferation rate, phagocytosis rate, immunoglobulin (Ig) G, IgA, IgM, C3 and C4 levels in piglets (Li et al., 2012). An essential oil cocktail (250 mg/kg) consisting of oregano, clove, and cinnamon induced more lymphocytes against the non-specific mitogens, but not to the specific mitogen (Halas et al., 2011). Halas et al. (2011) concluded that both the non-specific cellular and humoral immune responses were enhanced with the supplementation of essential oils to the weaned piglets. On the contrary, the essential oil failed to increase the serum IgG level in piglets (Cho et al., 2006), and there was no significant effect of the essential oils on serum IgG level in grower-finisher pigs (Yan et al., 2010). Ariza-Nieto et al. (2011) proved that adding 250 mg/kg oregano to the diet of sows during gestation and lactation did not improve the immune responses in suckling piglets. But Tan et al. (2015) proved that adding 15 mg/kg oregano to the diet of sows improved the performance of their piglets by counterbalancing the oxidative stress experienced by sows during the late gestation and early lactation.
4.3. Meat quality
Using essential oils to improve the meat quality is promising due to its easier acceptance by customers than synthetic preservatives. Meat quality could be influenced via the dietary supplementation of essential oils by being integrated in meat to modify the fatty acid profile of meat or to change the oxidative stability of meat and meat products (Wenk, 2003), or by being directly applied to meat products. Meanwhile, essential oils as antioxidants could be cytotoxic at certain concentrations because, after penetrating cells, the antioxidants are oxidized and react as pro-oxidant damaging DNA and protein (Bakkali et al., 2008).
Dietary supplementation of essential oils or oleoresins of rosemary, garlic, oregano, or ginger (500 mg/kg) failed to change the pork quality attributes and fatty acid profile, but the oregano-fed pork showed a tendency for less lipid oxidation (Janz et al., 2007). A feed additive of oregano essential oil and sweet chestnut wood extract increased anti-oxidant species in the blood, induced higher levels of glutathione peroxidase and glutathione reductase, and prevented lipid oxidation of pork, but did not affect the cooking loss, drip loss, Warner-Bratzler shear force values, and chemical composition of the pork (Ranucci et al., 2015).
It is generally accepted that lipid oxidation is mainly responsible for meat quality deterioration during storage. Oxygen availability is the most critical factor in the development of lipid oxidation (Jo et al., 1999). Meanwhile, protein oxidation increases meat product toughness, which is characterized by the oxidation of protein thiol and the resultant formation of myosin heavy chain disulfide cross-links (Nieto et al., 2013). The rosemary essential oil (150 mg/kg) significantly reduced the generation of thiobarbituric acid reactive substances (TBARS) and hexanal, indicators of lipid oxidation, and carbonyl, an indicator of protein oxidation in White pigs, while higher levels of 300 and 600 mg/kg had no effect on lipid oxidation, but significantly enhanced the oxidation of proteins; for Iberian pigs, the inhibitory effects of rosemary essential oil were stronger at higher supplementary concentrations (Estévez and Cava, 2006). Nieto et al. (2013) also showed essential oils of oregano and rosemary can protect the thiols in pork patties and reduce the cross-links of the myosin heavy chains, while essential oils of garlic exhibited the opposite effects.
Meat could contract the desirable or distasteful flavor of essential oils, which depends on a myriad of factors such as the application method and the dosage of the selected essential oils, the flavor preference of the customers of different geographical locations, and others. The sensory panelists did not differentiate the pork of pigs fed diets supplemented with essential oils (Janz et al., 2007). But the overall liking was higher in all the tests for the meat of pigs supplemented with a combination of oregano essential oil and sweet chestnut extract than the control (Ranucci et al., 2015). In addition, using bay leaf essential oils to pork sausages significantly impacted all the sensory evaluation attributes including appearance, odor, firmness, flavor, and overall acceptability (da Silveira et al., 2014).
4.4. Future research for pigs
Research on the olfactory effects of essentials oils is still scarce. The direct physiological effects of essentials oils on the relaxation, contraction, resistance, and peristalsis of gut could be a significant factor behind the improved digestibility of energy and nutrients. The absorbable attribute of essentials oils makes it essential to explore different techniques to deliver their release at the targeted sites in the gastro-intestinal tract. Different challenge models should be employed to ascertain the specific modulatory effects of essential oils on the immune system.
5. Summary
The success of essential oils depends on whether our knowledge of their actions is based on a solid scientific ground. This review shows that the positive effects of essential oils on the growth performance of poultry and pigs exist both abundantly and controversially, which makes the speculated underlying mechanisms further questionable. Firstly, more attention should be paid to dietary characteristics and experimental environments, and the description of the tested essential oils should be stricter. The description of the starting materials and extraction methods cannot guarantee the stable chemical composition of essential oils in animal trials, which should be further disclosed by analytical methods. These analytical results are essential for congregating the animal trial results and reconciling the conclusions. Secondly, the metabolism of essential oils should be better understood, which could cast light on the chemical forms and the locales in the animal body for the essential oils per se or their derivative metabolites to present themselves, and consequently to connect to their declared benefits. Lastly, the evidence for the link between essential oils and animal health is not robust yet. Anecdotal evidence and in vitro studies could not warrant the in vivo effects of essential oils. The direct effects on gut microflora and the indirect effects via the gut-associated immune system should be further explored.
Conflict of interest
The authors declare there is no conflict of interests.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdel-Wareth A.A.A., Kehraus S., Hippenstiel F., Südekum K.H. Effects of thyme and oregano on growth performance of broilers from 4 to 42 days of age and on microbial counts in crop, small intestine and caecum of 42-day-old broilers. Anim Feed Sci Technol. 2012;178:198–202. [Google Scholar]
- Ait-Ouazzou A., Cherrat L., Espina L., Lorán S., Rota C., Pagán R. The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innovat Food Sci Emerg Technol. 2011;12:320–329. [Google Scholar]
- Alali W., Hofacre C., Mathis G., Faltys G. Effect of essential oil compound on shedding and colonization of Salmonella enterica serovar Heidelberg in broilers. Poult Sci. 2013;92:836–841. doi: 10.3382/ps.2012-02783. [DOI] [PubMed] [Google Scholar]
- Alp M., Midilli M., Kocabağlı N., Yılmaz H., Turan N., Gargılı A. The effects of dietary oregano essential oil on live performance, carcass yield, serum immunoglobulin G level, and oocyst count in broilers. J Appl Poult Res. 2012;21:630–636. [Google Scholar]
- Amad A., Männer K., Wendler K., Neumann K., Zentek J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult Sci. 2011;90:2811–2816. doi: 10.3382/ps.2011-01515. [DOI] [PubMed] [Google Scholar]
- Aminov R.I. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010;1:1–7. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza-Nieto C., Bandrick M., Baidoo S.K., Anil L., Molitor T.W., Hathaway M.R. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J Anim Sci. 2011;89:1079–1089. doi: 10.2527/jas.2010-3514. [DOI] [PubMed] [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Barbour E.K., Bragg R.R., Karrouf G., Iyer A., Azhar E., Harakeh S. Control of eight predominant Eimeria spp. involved in economic coccidiosis of broiler chicken by a chemically characterized essential oil. J Appl Microbiol. 2015;118:583–591. doi: 10.1111/jam.12731. [DOI] [PubMed] [Google Scholar]
- Basmacioğlu Malayoğlu H., Baysal Ş., Misirlioğlu Z., Polat M., Yilmaz H., Turan N. Effects of oregano essential oil with or without feed enzymes on growth performance, digestive enzyme, nutrient digestibility, lipid metabolism and immune response of broilers fed on wheat-soybean meal diets. Br Poult Sci. 2010;51:67–80. doi: 10.1080/00071660903573702. [DOI] [PubMed] [Google Scholar]
- Bassolé I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento M., Ouwehand A., Tiihonen K., Lahtinen S., Nurminen P., Saarinen M. Essential oils and their use in animal feeds for monogastric animals-Effects on feed quality, gut microbiota, growth performance and food safety: a review. Vet Med. 2013;58:449–458. [Google Scholar]
- Botsoglou N.A., Florou-Paneri P., Christaki E., Fletouris D.J., Spais A.B. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br Poul Sci. 2002;43:223–230. doi: 10.1080/00071660120121436. [DOI] [PubMed] [Google Scholar]
- Botsoglou N., Christaki E., Florou-Paneri P., Giannenas I., Papageorgiou G., Spais A. The effect of a mixture of herbal essential oils or á-tocopheryl acetate on performance parameters and oxidation of body lipid in broilers. South Afr J Anim Sci. 2004;34:52–61. [Google Scholar]
- Bouhaddouda N., Aouadi S., Labiod R. Evaluation of chemical composition and biological activities of essential oil and methanolic extract of Origanum vulgare L. ssp. glandulosum (Desf.) Ietswaart from Algeria. Int J Pharmacognosy Phytochem Res. 2016;8:104–112. [Google Scholar]
- Bozkurt M., Aysul N., Küçükyilmaz K., Aypak S., Ege G., Çatli A. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poult Sci. 2014;93:389–399. doi: 10.3382/ps.2013-03368. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim Feed Sci Technol. 2010;158:1–14. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Cabuk M., Bozkurt M., Alcicek A., Akbas Y., Kucukyilmaz K. Effect of a herbal essential oil mixture on growth and internal organ weight of broilers from young and old breeder flocks. South Afr J Anim Sci. 2006;36:135–141. [Google Scholar]
- Castillo M., Martín-Orúe S., Roca M., Manzanilla E., Badiola I., Perez J. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. J Anim Sci. 2006;84:2725–2734. doi: 10.2527/jas.2004-556. [DOI] [PubMed] [Google Scholar]
- Cho J., Chen Y., Min B., Kim H., Kwon O., Shon K. Effects of essential oils supplementation on growth performance, IgG concentration and fecal noxious gas concentration of weaned pigs. Asian-Australas J Anim Sci. 2006;19:80–85. [Google Scholar]
- Cross D.E., McDevitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br Poult Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- da Silveira S.M., Luciano F.B., Fronza N., Cunha A., Scheuermann G.N., Vieira C.R.W. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7°C. LWT-Food Sci and Technol. 2014;59:86–93. [Google Scholar]
- de Araújo D.A.M., Freitas C., Cruz J.S. Essential oils components as a new path to understand ion channel molecular pharmacology. Life Sci. 2011;89:540–544. doi: 10.1016/j.lfs.2011.04.020. [DOI] [PubMed] [Google Scholar]
- de Lange C., Pluske J., Gong J., Nyachoti C. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci. 2010;134:124–134. [Google Scholar]
- Estévez M., Cava R. Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: contradictory effects in different types of frankfurters. Meat Sci. 2006;72:348–355. doi: 10.1016/j.meatsci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Franz C., Baser K., Windisch W. Essential oils and aromatic plants in animal feeding-a European perspective. A review. Flavour Fragr J. 2010;25:327–340. [Google Scholar]
- Franz C., Novak J. Sources of essential oils. In: Baser K.H.C., Buchbauer G., editors. Handbook of essential oils: science, technology, and applications. CRC Press/Taylor & Francis Group; Boca Raton: 2009. pp. 39–82. [Google Scholar]
- Friedman M., Henika P.R., Mandrell R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. 2002;65:1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- Garcia V., Catala-Gregori P., Hernández F., Megias M., Madrid J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J Appl Poult Res. 2007;16:555–562. [Google Scholar]
- Giannenas I., Bonos E., Christaki E., Florou-Paneri P. Essential oils and their applications in animal nutrition. Med Aromat Plants. 2013;2:1–12. [Google Scholar]
- Gong J., Yin F., Hou R., Yin Y.L. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: potential and challenges in application. Can J Anim Sci. 2014;94:223–241. [Google Scholar]
- Habibi R., Sadeghi G., Karimi A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br Poult Sci. 2014;55:228–237. doi: 10.1080/00071668.2014.887830. [DOI] [PubMed] [Google Scholar]
- Halas V., Nochta I., Pásti Z., Szabó C., Tóthi R., Tossenberger J. Cellular immune response of weaned pigs fed diet supplemented with an essential oil. Agric Conspec Sci. 2011;76:279–282. [Google Scholar]
- Halle I., Thomann R., Bauermann U., Henning M., Köhler P. Effects of a graded supplementation of herbs and essential oils in broiler feed on growth and carcass traits. Landbauforshung Volkenrod. 2004;54:219–229. [Google Scholar]
- Hammer K.A., Carson C.F. Antibacterial and antifungal activities of essential oils. In: Thormar H., editor. Lipids and essential oils as antimicrobial agents. Wiley; Iceland: 2011. pp. 255–306. [Google Scholar]
- Hashemi S., Davoodi H. Phytogenics as new class of feed additive in poultry industry. J Ani Vet Adv. 2010;9:2295–2304. [Google Scholar]
- Hernández F., Madrid J., Garcia V., Orengo J., Megias M. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Hippenstiel F., Abdel-Wareth A., Kehraus S., Südekum K. Effects of selected herbs and essential oils, and their active components on feed intake and performance of broilers-a review. Arch Geflügelk. 2011;75:226–234. [Google Scholar]
- Jamroz D., Wertelecki T., Houszka M., Kamel C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J Anim Physiol Anim Nutr. 2006;90:255–268. doi: 10.1111/j.1439-0396.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- Jang I., Ko Y., Kang S., Lee C. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim Feed Sci Technol. 2007;134:304–315. [Google Scholar]
- Janz J., Morel P., Wilkinson B., Purchas R. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. 2007;75:350–355. doi: 10.1016/j.meatsci.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Jo C., Lee J., Ahn D. Lipid oxidation, color changes and volatiles production in irradiated pork sausage with different fat content and packaging during storage. Meat Sci. 1999;51:355–361. doi: 10.1016/s0309-1740(98)00134-x. [DOI] [PubMed] [Google Scholar]
- Karadas F., Pirgozliev V., Rose S., Dimitrov D., Oduguwa O., Bravo D. Dietary essential oils improve the hepatic antioxidative status of broiler chickens. Br Poult Sci. 2014;55:329–334. doi: 10.1080/00071668.2014.891098. [DOI] [PubMed] [Google Scholar]
- Katalinic V., Milos M., Kulisic T., Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. [Google Scholar]
- Khan R., Naz S., Nikousefat Z., Tufarelli V., Laudadio V. Thymus vulgaris: alternative to antibiotics in poultry feed. World Poult Sci J. 2012;68:401–408. [Google Scholar]
- Kim J., Marshall M.R., Wei C. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem. 1995;43:2839–2845. [Google Scholar]
- Kohlert C., Van Rensen I., März R., Schindler G., Graefe E., Veit M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000;66:495–505. doi: 10.1055/s-2000-8616. [DOI] [PubMed] [Google Scholar]
- Kommera S.K., Mateo R.D., Neher F.J., Kim S.W. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian-Australas J Anim Sci. 2006;19:1784–1789. [Google Scholar]
- Kurekci C., Al Jassim R., Hassan E., Bishop-Hurley S.L., Padmanabha J., McSweeney C.S. Effects of feeding plant-derived agents on the colonization of Campylobacter jejuni in broiler chickens. Poult Sci. 2014;93:1–10. doi: 10.3382/ps.2014-03950. [DOI] [PubMed] [Google Scholar]
- Lee K.-W., Everts H., Kappert H., Frehner M., Losa R., Beynen A. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br Poult Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Li P., Piao X., Ru Y., Han X., Xue L., Zhang H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas J Anim Sci. 2012;25:1617–1626. doi: 10.5713/ajas.2012.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis-Balchin M., Hart S.L. An investigation of the actions of the essential oils of Manuka (Leptospermum scoparium) and Kanuka (Kunzea ericoides), Myrtaceae on Guinea-pig smooth muscle. J Pharm Pharmacol. 1998;50:809–811. doi: 10.1111/j.2042-7158.1998.tb07144.x. [DOI] [PubMed] [Google Scholar]
- Lis-Balchin M., Hart S., Simpson E. Buchu (Agathosma betulina and A. crenulata, Rutaceae) essential oils: their pharmacological action on Guinea-pig ileum and antimicrobial activity on microorganisms. J Pharm Pharmacol. 2001;53:579–582. doi: 10.1211/0022357011775703. [DOI] [PubMed] [Google Scholar]
- Maenner K., Vahjen W., Simon O. Studies on the effects of essential-oil-based feed additives on performance, ileal nutrient digestibility, and selected bacterial groups in the gastrointestinal tract of piglets. J Anim Sci. 2011;89:2106–2112. doi: 10.2527/jas.2010-2950. [DOI] [PubMed] [Google Scholar]
- Magalhães P.J., Criddle D.N., Tavares R.A., Melo E.M., Mota T.L., Leal-Cardoso J.H. Intestinal myorelaxant and antispasmodic effects of the essential oil of Croton nepetaefolius and its constituents cineole, methyl-eugenol and terpineol. Phytother Res. 1998;12:172–177. [Google Scholar]
- Manzanilla E., Nofrarias M., Anguita M., Castillo M., Perez J., Martin-Orue S. Effects of butyrate, avilamycin, and a plant extract combination on the intestinal equilibrium of early-weaned pigs. J Anim Sci. 2006;84:2743–2751. doi: 10.2527/jas.2005-509. [DOI] [PubMed] [Google Scholar]
- Michiels J., Missotten J., Dierick N., Fremaut D., Maene P., De Smet S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J Sci Food Agric. 2008;88:2371–2381. [Google Scholar]
- Michiels J., Missotten J.A.M., Fremaut D., De Smet S., Dierick N.A. In vitro characterisation of the antimicrobial activity of selected essential oil components and binary combinations against the pig gut flora. Anim Feed Sci Technol. 2009;151:111–127. [Google Scholar]
- Miguel M. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr J. 2010;25:291–312. [Google Scholar]
- Mitsch P., Zitterl-Eglseer K., Köhler B., Gabler C., Losa R., Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult Sci. 2004;83:669–675. doi: 10.1093/ps/83.4.669. [DOI] [PubMed] [Google Scholar]
- Moran E.T. University of Guelph; 1982. Comparative nutrition of fowl and swine. The gastrointestinal system. [Google Scholar]
- Namkung H., Li M., Gong J., Yu H., Cottrill M., de Lange C.F.M. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can J Anim Sci. 2004;84:697–704. [Google Scholar]
- Nieto G., Jongberg S., Andersen M.L., Skibsted L.H. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Sci. 2013;95:177–184. doi: 10.1016/j.meatsci.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Nofrarias M., Manzanilla E., Pujols J., Gibert X., Majo N., Segalés J. Effects of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J Anim Sci. 2006;84:2735–2742. doi: 10.2527/jas.2005-414. [DOI] [PubMed] [Google Scholar]
- Nyachoti C., Zijlstra R., de Lange C., Patience J. Voluntary feed intake in growing-finishing pigs: a review of the main determining factors and potential approaches for accurate predictions. Can J Anim Sci. 2004;84:549–566. [Google Scholar]
- O'Bryan C.A., Pendleton S.J., Crandall P.G., Ricke S.C. Potential of plant essential oils and their components in animal agriculture-in vitro studies on antibacterial mode of action. Front Vet Sci. 2015;2:1–8. doi: 10.3389/fvets.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke F., Aslim B., Ozturk S., Altundag S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia ten. Food Chem. 2009;112:874–879. [Google Scholar]
- Pavlović I., Petrović S., Radenković M., Milenković M., Couladis M., Branković S. Composition, antimicrobial, antiradical and spasmolytic activity of Ferula heuffelii Griseb. ex Heuffel (Apiaceae) essential oil. Food Chem. 2012;130:310–315. [Google Scholar]
- Placha I., Takacova J., Ryzner M., Cobanova K., Laukova A., Strompfova V. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br Poult Sci. 2014;55:105–114. doi: 10.1080/00071668.2013.873772. [DOI] [PubMed] [Google Scholar]
- Platel K., Srinivasan K. Digestive stimulant action of spices: a myth or reality? Indian J Med Res. 2004;119:167–179. [PubMed] [Google Scholar]
- Purchiaroni F., Tortora A., Gabrielli M., Bertucci F., Gigante G., Ianiro G. The role of intestinal microbiota and the immune system. Eur J Rev Med Pharmacol Sci. 2013;17:323–333. [PubMed] [Google Scholar]
- Ranucci D., Beghelli D., Trabalza-Marinucci M., Branciari R., Forte C., Olivieri O. Dietary effects of a mix derived from oregano (Origanum vulgare L.) essential oil and sweet chestnut (Castanea sativa Mill.) wood extract on pig performance, oxidative status and pork quality traits. Meat Sci. 2015;100:319–326. doi: 10.1016/j.meatsci.2014.09.149. [DOI] [PubMed] [Google Scholar]
- Raut J.S., Karuppayil S.M. A status review on the medicinal properties of essential oils. Ind Crop Prod. 2014;62:250–264. [Google Scholar]
- Roura E., Humphrey B., Tedó GIpharraguerre I. Unfolding the codes of short-term feed appetence in farm and companion animals. A comparative oronasal nutrient sensing biology review. Can J Anim Sci. 2008;88:535–558. [Google Scholar]
- Schöne F., Vetter A., Hartung H., Bergmann H., Biertümpfel A., Richter G. Effects of essential oils from fennel (Foeniculi aetheroleum) and caraway (Carvi aetheroleum) in pigs. J Anim Physiol Anim Nutr. 2006;90:500–510. doi: 10.1111/j.1439-0396.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- Senatore F. Influence of harvesting time on yield and composition of the essential oil of a thyme (Thymus pulegioides L.) growing wild in Campania (Southern Italy) J Agric Food Chem. 1996;44:1327–1332. [Google Scholar]
- Seow Y.X., Yeo C.R., Chung H.L., Yuk H.G. Plant essential oils as active antimicrobial agents. Crit Rev Food Sci Nutr. 2014;54:625–644. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- Si W., Gong J., Tsao R., Zhou T., Yu H., Poppe C. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J Appl Microbiol. 2006;100:296–305. doi: 10.1111/j.1365-2672.2005.02789.x. [DOI] [PubMed] [Google Scholar]
- Solórzano-Santos F., Miranda-Novales M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol. 2012;23:136–141. doi: 10.1016/j.copbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Tan C., Wei H., Sun H., Ao J., Long G., Jiang S. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. BioMed Res Int. 2015;2015:1–9. doi: 10.1155/2015/525218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Braak S., Leijten G. CBI, Centre for the Promotion of Imports from Developing Countries; Rotterdam: 1994. Essential oils and oleoresins: a survey in The Netherlands and other major markets in the European Union; p. 116. [Google Scholar]
- Van Zyl R.L., Seatlholo S.T., Van Vuuren S.F., Viljoen A.M. The biological activities of 20 nature identical essential oil constituents. J Essent Oil Res. 2006;18:129–133. [Google Scholar]
- Venkitanarayanan K., Kollanoor-Johny A., Darre M.J., Donoghue A.M., Donoghue D.J. Use of plant-derived antimicrobials for improving the safety of poultry products. Poult Sci. 2013;92:493–501. doi: 10.3382/ps.2012-02764. [DOI] [PubMed] [Google Scholar]
- Vigan M. Essential oils: renewal of interest and toxicity. Eur J Dermatol. 2010;20:685–692. doi: 10.1684/ejd.2010.1066. [DOI] [PubMed] [Google Scholar]
- Wenk C. Herbs and botanicals as feed additives in monogastric animals. Asian-Australas J Anim Sci. 2003;16:282–289. [Google Scholar]
- Williams P., Losa R. The use of essential oils and their compounds in poultry nutrition. World Poult Sci J. 2001;17:14–15. [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 2008;86:140–148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Witkowska D., Sowińska J. The effectiveness of peppermint and thyme essential oil mist in reducing bacterial contamination in broiler houses. Poult Sci. 2013;92:2834–2843. doi: 10.3382/ps.2013-03147. [DOI] [PubMed] [Google Scholar]
- Yan L., Wang J., Kim H., Meng Q., Ao X., Hong S. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and fecal noxious gas content in grower-finisher pigs. Livest Sci. 2010;128:115–122. [Google Scholar]
- Zhang Y., Gong J., Yu H., Guo Q., Defelice C., Hernandez M. Alginate-whey protein dry powder optimized for target delivery of essential oils to the intestine of chickens. Poult Sci. 2014;93:2514–2525. doi: 10.3382/ps.2013-03843. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Xu X., Zhang Q., Li P., Zhao P., Li Q. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim Sci J. 2015;86:279–285. doi: 10.1111/asj.12277. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J Anim Sci Biotechnol. 2015;6:1–10. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]