Abstract
Antibiotics are used to fight bacterial infections. However, a selective pressure gave rise to bacteria resistant to antibiotics. This leaves scientists worried about the danger to human and animal health. Some strategies can be borrowed to reduce the use of antibiotics in chicken farms. Much research has been carried out to look for natural agents with similar beneficial effects of growth promoters. The aim of these alternatives is to maintain a low mortality rate, a good level of animal yield while preserving environment and consumer health. Among these, the most popular are probiotics, prebiotics, enzymes, organic acids, immunostimulants, bacteriocins, bacteriophages, phytogenic feed additives, phytoncides, nanoparticles and essential oils.
Keywords: Broiler chicken, Antibiotic, Probiotic, Prebiotic, Enzyme, Essential oil
1. Introduction
The discovery of antibiotics was a success in controlling infectious pathologies and increasing feed efficiencies (Engberg et al., 2000). Antibiotics, either of natural or synthetic origin are used to both prevent proliferation and destroy bacteria. Antibiotics are produced by lower fungi or certain bacteria. They are routinely used to treat and prevent infections in humans and animals. However, scientific evidence suggests that the massive use of these compounds has led to increased problem of antibiotic resistance (Diarra et al., 2007, Forgetta et al., 2012, Furtula et al., 2010), and presence of antibiotics residues in feed and environment (Carvalho and Santos, 2016, Gonzalez Ronquillo et al., 2017), compromises human and animal health (Diarra et al., 2010). Hence, there is a growing need to find effective alternatives to control infectious diseases and limit the spread of resistant bacteria, but more importantly, keep antibiotics a useful tool for the future. This literature review synthesizes the current state of antibiotics use, as well as alternative strategies available in broiler chicken production.
2. Use of antibiotics in broiler chicken production
Over the past 50 years, the use of antibiotics combined with strict biosecurity and hygiene measures has helped the poultry industry to grow by preventing the negative impacts of many avian diseases (Bermudez, 2003). Even as biosecurity may be sufficient, vaccination can also be used as an additional measure. A vaccine provides assistance to the immune system by preparing it against certain pathogens such as viruses or bacteria to which it may be exposed in the future. Vaccination protocols and the type of vaccine used vary from country to country and from farm to farm. Many factors can influence the choice of vaccination method such as species, place, number of manpower, type of production, and production cycle. The choice of vaccination method also depends on general health status of poultry, maternal immunity, and vaccine costs (Rauw et al., 2009). Livestock vaccination against specific diseases is compulsory (e.g., Newcastle disease) in many countries (Belgium, Netherlands, Germany), while in other such as France only long-lived poultry (laying and breeding) are vaccinated (Rauw et al., 2009).
Antibiotics are not effective against fungal and viral pathogens. They only treat infectious diseases whose causative agents are bacteria. In general, antibiotics are used in phytosanitary treatments, fish farming, animal feed, and human or veterinary medicine where they can be used as a preventive or curative treatment. Antibiotics are classified according to their chemical family, mode of action and the species of bacteria on which they act. Bactericidal antibiotics kill bacteria and bacteriostatics weaken them by inhibiting their proliferation and facilitating their phagocytosis by the immune system. Thus, mortality rate decreases because animals become more resistant.
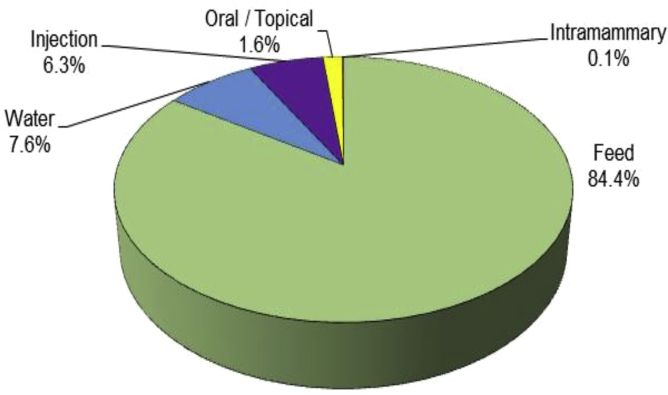
In intensive poultry farming, especially in North America, antibiotics such as tetracycline, bacitracin, tylosin, salinomycin, virginiamycin and bambermycin are often used (Diarra and Malouin, 2014). In the United States, tetracyclines represent more than two-thirds of antimicrobials administered to animals (Gonzalez Ronquillo and Angeles Hernandez, 2017), while in European Union (EU) they represent only 37% (Carvalho and Santos, 2016). In 2015, the overall sales of veterinary antimicrobial agent were 8,361 t in EU (ESVAC, 2017). This figure is calculated without counting growth promoters in animal production (Kummerer, 2009). The use of antibiotics as growth factors is not allowed in the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) participating countries (ESVAC, 2017). In 2014, 1.5 million kg of active antimicrobial ingredients were distributed for use in animals in Canada, up 5% from 2013. For antimicrobials distributed, 99% were for farm animals and less than 1% were for pets. In 2014, 81% of the antimicrobials used in Canada on broiler farms were for prevention purposes. In the feed, 84% of these antimicrobials were used (Fig. 1). They were primarily intended to prevent necrotic enteritis caused by Clostridium perfringens and coccidiosis (CSCRA, 2016).
Fig. 1.
Quantity of antimicrobials (% of total weight in kg) distributed for veterinary use by route of administration in Canada (CSCRA, 2016).
3. Antibiotic impacts
3.1. Impact on chicken growth, digestive tract and immune systems
The poultry industry uses antibiotics to improve meat production through increased feed conversion, growth rate promotion and disease prevention. Antibiotics can be used successfully at sub-therapeutic doses in poultry production to promote growth (Barceló, 2007, Chattopadhyay, 2014, Engberg et al., 2000, Harms et al., 1986, Khodambashi Emami, 2012, Rosen, 1996) and protect the health of birds by modifying the immune status of broiler chickens (Lee et al., 2012). This is mainly due to the control of gastrointestinal infections and microbiota modification in the intestine (Dibner and Richards, 2005, Singh et al., 2013, Torok et al., 2011). The mechanism remains unclear, but antibiotics are likely to act by remodeling microbial diversity and relative abundance in the intestine to provide an optimal microbiota for growth (Dibner and Richards, 2005). For example, meta-genome sequencing approaches have demonstrated that diets with salinomycin (60 ppm) has an impact on microbiome dynamics in chicken ceca (Fung et al., 2013). Similarly, the use of virginiamycin (100 ppm) as a growth promoter has been associated with an increased abundance of Lactobacillus species in broiler duodenal loop at proximal ileum. This indicates that virginiamycin alters the composition of chicken gut microbiota (Dumonceaux et al., 2006). In addition, populations of Lactobacillus spp. in the ileum of chickens receiving feed containing tylosin, a bacteriostatic, are significantly lower than those in chickens receiving no tylosin (Lin et al., 2013). This decrease in Lactobacilli species following the use of antibiotics has been demonstrated in other studies (Danzeisen et al., 2011, Lee et al., 2012, Zhou et al., 2007). For reminder, Lactobacillus are the primary commensal bacteria for the production of bile hydrolase salt. The decrease in the Lactobacillus population in antibiotic-treated animals probably reduces the intestinal activity of the bile hydrolase salts, which would increase the relative abundance of conjugated bile salts, thus promotes lipid metabolism and energy harvesting and increases animal weight gain (Lin et al., 2013).
A change in the intestinal microbiota of chickens can influence their immunity and their health. However, changes in the intestinal microbiota of chickens can be influenced by several factors. These factors include housing conditions, exposure to pathogens, diet composition and the presence of antibiotics in feed (Lee et al., 2012).
3.2. Impact on meat quality and eggs
Campylobacter is a major cause of food-borne diarrheal diseases in humans. Campylobacter infections can be severe or fatal in immunocompromised or elderly people and very young children. Escherichia coli bacteria are very common and can also cause diseases. The most common type of E. coli infection that causes illness in people is called E. coli O157:H7. Salmonellosis is one of the most common and widespread food-borne illnesses in the world. Salmonella infections usually cause mild gastroenteritis. These 3 bacteria and others are monitored by specialized agencies around the world, for example, Public Health Agency of Canada in Canada, Food and Drug Administration (FDA) in USA, European Food Safety Authority (EFSA) in EU. Tens of millions of cases of these bacterial infections occur in humans every year worldwide. Each year in the United States there are approximately 76 million food-borne illnesses (Zhao et al., 2001). Approximately 325,000 cases result in hospitalization, and 5,000 cases are fatal, and 1.4 million cases are caused by nontyphoidal Salmonella serovars, Campylobacter spp. Nearly 2.4 million cases and 270,000 cases are caused by pathogenic E. coli, including E. coli O157:H7 (Mead et al., 1999, Zhao et al., 2001).
According to CSCRA (2016) report, chicken contamination rates for E. coli, Campylobacter and Salmonella spp. are respectively 96%, 25% and 34% in Canada. In addition, antibiogram test revealed multi-pharmacological resistance in Enterobacteriaceae isolates from eggs and broiler meat (Diarra et al., 2010, Singh et al., 2010, Yulistiani et al., 2017). Eggs are frequently implicated in Salmonella transmission (Singh et al., 2010). This contamination is due mainly to the proliferation of pathogens in the intestines. There are secondary contaminations along the production line by resistant bacteria in foods of animal origin. Schwaiger et al. (2012) reported that the prevalence of multi-resistant of Salmonella was higher in retail samples compared to slaughterhouse samples.
3.3. Impact on consumer health and the environment
In addition to bio-resistance, antibiotics abuse has resulted in drug residues in animal products (Gonzalez Ronquillo and Angeles Hernandez, 2017). Several antibiotics such as penicillin, tetracycline, macrolide, aminoglycoside and amphenicol have been detected in foods (Diarra and Malouin, 2014). Residues in livestock production can actually have adverse impact on human health; this is the case for tetracyclines, which interfere with teeth development in young children (Kummerer, 2009). This is also the case with beta-agonists, such as clenbuterol, leading sometimes to food poisoning and muscle tremors, palpitations and tachycardia (Chan, 1999). Clenbuterol is prohibited in EU. Some breeders use it to produce meat containing less fat and more protein. Further, chloramphenicol illustrates both potential problems (Gassner and Wuethrich, 1994). Gassner and Wuethrich (1994) have demonstrated the presence of chloramphenicol metabolites in meat products. These authors concluded a possibility link between the presence of these antibiotic residues in meat and the occurrence of aplastic anemia in humans.
The administration and restriction of the use of antibiotics in the EU are regulated by Directive 96/23/EC (European-Commission, 1996). This directive focuses on measures to monitor residues in animal products. Certain limits of antibiotic residues are imposed in food and animal products (meat and eggs). Limits on the quantities of antibiotic residues in eggs and chicken meat are reported in Table 1.
Table 1.
Maximum residue limits (MRL) for antimicrobials in poultry products applied in EU (adapted from Gonzalez Ronquillo and Angeles Hernandez, 2017).
| Substance | Chemical group | Matrix | MRL, mg/kg |
|---|---|---|---|
| Tetracycline | Tetracyclines | Muscle | 100 |
| Liver | 300 | ||
| Kidney | 600 | ||
| Eggs | 200 | ||
| Streptomycin | Aminoglycosides | Muscle | 600 |
| fat | 600 | ||
| Liver | 600 | ||
| Kidney | 1,000 | ||
| Tilmicosin | Macrolides | Muscle | 150 |
| Liver | 2,400 | ||
| Kidney | 600 | ||
| Skin and fat | 250 | ||
| Florfenicol | Amphenicols | Muscle | 100 |
| Skin and fat | 20 | ||
| Liver | 2,500 | ||
| Kidney | 750 | ||
| Tiamulin | Pleuromutilins | Muscle | 100 |
| Liver | 1,000 | ||
| Skin and fat | 100 | ||
| Eggs | 1,000 |
The global consumption of antibiotics in human and animal production is estimated between 1 × 105 and 2 × 105 t (Manzetti and Ghisi, 2014). Releasing thereby large quantities of antibiotics into the environment entertains the cycle of biotransformation and bioaccumulation of antibiotics in the environment. According to Manzetti and Ghisi (2014), the most vulnerable ecosystems to antibiotic contamination are confined aquatic ecosystems such as ponds and lakes and soils close to urban sites. Aquatic compartments, such as water and sediments, can thus play an important role in the transfer, evolution and ecology of antibiotic resistance genes (Marti et al., 2014). Large amounts of antibiotics administered to animals are excreted into the environment via urine and faeces (Carvalho and Santos, 2016). After metabolic changes in animals, 30% to up 90% of the dose consumed is found in the urine and feces as parent compounds and/or metabolite compounds (Carvalho and Santos, 2016). This makes sewage disposal systems one of the most important routes by which antibiotics can enter in the environment (Gonzalez Ronquillo and Angeles Hernandez, 2017) and contaminate even coastal waters (Chen et al., 2015).
Antibiotics' risks in the aquatic environment and sediments are important because they can influence aquatic life behavior (Kummerer, 2009). Antimicrobials have qualitative and quantitative effects on the microbial community residing in sediments, which in turn can affect the degradation of organic matter (Kummerer, 2009). Residues of antibiotics present in the water contribute strongly to the maintenance, emergence and dissemination of bacterial populations with a low level of resistance and are ready to evolve towards resistance (Corvaglia, 2006). According to Chen et al. (2015), the spatial distribution of antibiotics in the marine environment is significantly correlated with environmental variables such as chemical oxygen demand (COD) and nitrates (NO3—N).
The aquatic environment is considered as an important point for acquisition and spread of antibiotic resistance genes by bacteria (Devarajan et al., 2017). Studies (Caplin et al., 2008, Devarajan et al., 2017) demonstrated a widespread presence of antibiotic resistance determinants in aquatic sediment ecosystems. Devarajan et al. (2017) reported multi-resistance profiles in Pseudomonas spp. in aquatic sediment samples, which is potentially transferable to humans. Laroche et al. (2009) reported resistance genes in estuary samples, mainly carried by Enterococcus spp. and E. coli. In addition, a study Furtula et al. (2013) carried out on samples collected from 2 poultry farms showed that 58% of Enterococcus spp. isolates in surface waters and 100% of isolates in groundwater were resistant to more than an antibiotic. According to Carvalho and Santos (2016), the toxic effects of antibiotics in the aquatic environment increased when combined with other antibiotics.
In the soil, antibiotic's behavior differs according to their physicochemical properties, soil characteristics, as well as climate conditions. Acid rain accelerates the antibiotics accumulation in animal manure and soil surface while long-lasting rains foster antibiotics' migration in deeper parts of the soil (Pan and Chu, 2017). According to Pan and Chu (2017), antibiotics leaching is higher in sandy soils than in clay and silty soils. Norfloxacin and tetracycline tend to persist in the soil surface while sulfamethazine and erythromycin pose a higher risk for deeper soil layers and groundwater. The soil can be also contaminated by antibiotics in litter. Animal bedding contains residues of antimicrobial compounds. Residues of bacitracin, salinomycin, penicillin and virginiamycin were detected in chicken litter at concentrations ranging from 0.07 to 66 mg/L (Furtula et al., 2010). When this bedding material is used as nitrogen amendment, the resistant bacteria can live in the soil for several months (Merchant et al., 2012). According to De Liguoro et al. (2003), biotransformation and biodegradation of antibiotics on agricultural sites can take up to 150 days. In addition, antibiotic by-products in the environment remain bioactive and can be potentially more toxic, stable and mobile than their parent compounds (Carvalho and Santos, 2016). Bio-resistant bacteria (Staphylococcus xylosus) have also been reported in air in broiler farms (Vela et al., 2012). Liu et al. (2012a) have shown that airborne transmission causes the spread of epidemic diseases and also poses impend over public health.
3.4. Antibiotic and bacterial resistance
Scientific evidence suggests that the use of antimicrobials in livestock production can promote bacterial resistance in treated animals (O'Brien, 2002). Antibiotic resistance is defined as the ability of microorganisms to proliferate in presence of an antibiotic that generally inhibits or kills microorganisms of the same species (RUMA, 2016). Resistance is by mutation or acquisition of genes carried by mobile genetic elements such as transposons, integrons, plasmids or phages (Kempf and Zeitouni, 2012). Chicken harbors large proportion of Enterobacteriaceae resistant to aminosides in its digestive tract and tetracycline in its meat (Guillot et al., 1977, Yulistiani et al., 2017). Bacterial resistance to antibiotics has been the subject of several studies in the recent years (Diarra et al., 2007, Forgetta et al., 2012, Furtula et al., 2010, Furtula et al., 2013, Johnson et al., 2012). In one study on Salmonella enterica isolates collected from poultry farms in British Columbia (Canada), Diarra et al. (2014) showed that more than 43% of the isolates were simultaneously resistant to ampicillin, amoxicillin-clavulanic acid, ceftiofur, cefoxitim and ceftriaxone. Another Canadian study (Diarra and Malouin, 2014) highlights the existence of different stereotypes of Salmonella, isolated from broiler farms, resistant and multi-resistant to antibiotics. In addition, antibiotic resistance in Enterococci (Silbergeld et al., 2008), Mycoplasma gallisepticum (Pakpinyo and Sasipreeyajan, 2007) and Salmonella spp. (Manning et al., 2015) isolated in broilers have been reported. A study in Germany (Schwaiger et al., 2012) showed that resistant and multi-resistant isolates are very common in chicken meat. Another study in Italy (Bacci et al., 2012) reported that 86% of S. enterica isolated from chicken carcasses were resistant to tetracycline, while 30% of isolates showed multipharmacological phenotypic resistance to ampicillin, sulfamethoxazole and tetracycline. In Ecuador, a study by Braykov et al. (2016) showed that tetracycline resistance was detected in 78% of production bird (broilers and laying hens). More than half of the isolates were resistant to sulfisoxazole and trimethoprim-sulfamethoxazole (69% and 63%, respectively).
Bacterial resistance to animal antibiotics is a public health issue. In Canada, for example, poultry meat may play a role in human infections (Diarra et al., 2010, Manges et al., 2007). In addition, Hur et al. (2011) founded that isolates of S. enterica from egg and chicken carcasses were resistant to penicillins, sulfisoxazole, treptomycin, tetracycline and quinolones. S. enterica isolates were resistant to at least 21 antibiotics used by the authors. Most isolates harbored genes associated with SPI-1 and SPI-2 and the spv operon, which are known to be associated with human infections. This represents a threat to human health. This situation is mainly due to the misuse of certain antibiotics such as penicillins, tetracyclines, macrolides and aminoglycosides (Diarra and Malouin, 2014). The abusive use of antibiotics and the associated selection pressure have led to decreased therapeutic efficacy and created populations of antibiotic-resistant microorganisms. Antibiotic resistance may spread over time despite the suspension of antibiotic use. Indeed, strains of E. coli resistant to trimethoprim and streptomycin have been shown to persist for several weeks in a chicken farm without using the antibiotics mentioned above (Chaslus-Dancla et al., 1987). On the other hand, antibiotic resistance is lower in organic farms (Hegde et al., 2016). Thus, it is imperative to determine the exact sources and ecology of resistant bacteria in order to develop strategies to stop their proliferation (Diarra and Malouin, 2014).
4. Alternatives to the use of antibiotics
Consumers' pressure and worries towards harmful effects of antibiotic use and the ban of antibiotics in EU have prompted researchers to think about alternatives to antibiotics (Diarra and Malouin, 2014). The aim of these alternatives is to maintain a low mortality rate, a good level of animal yield while preserving environment and consumer health. Much research has been carried out to look for natural agents with similar beneficial effects of growth promoters. There are indeed a number of non-therapeutic alternatives that can substitute antibiotics use. Among these, the most popular are probiotics, prebiotics, enzymes, organic acids, immunostimulants, bacteriocins, bacteriophages, phytogenic feed additives, phytocides, nanoparticles and essential oils.
4.1. Phytogenic feed additives
Phytogenic feed additives (PFA) derived from plants, herbs and spices are used to improve animal performance. They have been very successful because of their positive effects on growth, improved immune system and reduced stress response. Recent results showed that PFA were good alternatives to antibiotics (Frankic et al., 2009, Ghasemi et al., 2014, Toghyani et al., 2011, Windisch et al., 2008) and promoted broiler chicken growth (Ghasemi et al., 2014, Li et al., 2015, Toghyani et al., 2011). For example, inclusion of cinnamon 2 g/kg of the diet had a positive effect on growth performance at 28 days of age (974 vs. 850 g) and at 42 days of age (2,111 vs. 1,931 g) (Toghyani et al., 2011). Also, inclusion of Lippia javanica at 5 g/kg in broiler feed had beneficial effects on average daily gain (ADG) in the grower period (67 vs. 30 g ), slaughter weight (2,213 vs. 1,967 g) and fatty acid profiles of broiler chicken meat (Mpofu et al., 2016). According to Mpofu et al. (2016), phytogenic extracts in L. javanica leaf meal can stimulate glycolysis and increase utilization of energy production and ultimately growth. In addition, a mixture of garlic (5 g/kg) and black pepper (1 g/kg) powder had positive effects on weight gain and broiler chicken consumption index (Kirubakaran et al., 2016).
The study by Jarriyawattanachaikul et al. (2016) showed that certain herbs, especially Cratoxylum formosum, can have an antimicrobial activity against E. coli, C. jejuni and S. aureus isolated from chicken caecum. At a dose of 150 mg/kg for 39 days, PFA contained extracts from fennel (Foeniculum vulgarae var. dulcemil), Melissa balm (Melissa officinalis L.), peppermint (Mentha arvensis L.), anise (Pimpinella anisum L.), oak (Quercus cortex), clove (Syzygium aromaticum L.), and thyme (Thymusvulgaris L.) was as effective as bacitracin methylene disalicylate in controlling inoculated Clostridium, Salmonella and E. coli in broiler chicken (day 28) (Wati et al., 2015). Some medicinal plants have anti-microbial and antioxidant properties (El-Ghorab, 2006, Mahboubi et al., 2008). The use of pennyroyal (Mentha pulegium L.) in broiler chicken diets has shown positive effects. Goodarzi and Nanekarani (2014) showed that the use of 2% of pennyroyal in broiler feed has positive effects on their ADG (49 g compared with the negative control [43 g] and Virginimacin control [49 g]) and carcass traits.
A positive influence of PFA has been reported on the immune system. Indeed, supplementation with 7 g/kg of neem (Azadirachta indica) induced favorable influences on the immune responses of broiler chickens and ADG compared with chicken supplemented with flavophospholipol (Landy et al., 2011). Similarly, supplementation with 10 to 20 g/kg of black seed (Nigella sativa L.) improved plasma lipid profile (triglyceride and low density lipoprotein) and antibody-mediated immunity (Ghasemi et al., 2014). In a comparative study, between the use of lincomycin and licorice extract (Glycyrrhiza glabra) (0.1%, 0.2% or 0.3% in drinking water) in broiler chickens, Khamisabadi et al. (2015) concluded that licorice extract can reduce abdominal fat and serum levels of low density lipoprotein cholesterol and total cholesterol without any adverse effects on broilers performance and immune status.
It has been also shown that the use of spices in the feeding of broiler chickens could have a lipotropic effect. This effect is achieved by affecting lipid metabolism by the transport of fatty acids (Cross et al., 2007). Some aspects of the effects of PFA remain unclear (Wati et al., 2015). However the reason for using these plants is the many chemical components with antibacterial, antioxidant and conservative activity they possess (Mabona et al., 2013). According to Platel and Srinivasan (2004), it is likely that essential oils contained in PFA promote intestinal functions by stimulating bile secretion, digestive enzymes and mucus. Biologically active constituents of the plants are mainly terpenoids, phenolics, glycosides and alkaloids. Phytogenic feed additives promote growth by reducing the incidence and severity of subclinical infections, improving nutrient uptake (Huyghebaert et al., 2011). The phytogenic efficacy of broiler chickens depends on inclusion level in feed and the physiological period of the chicken (Mountzouris et al., 2011). Most beneficial effects of additives are observed in the growth and finishing period.
The use of PFA contributes to the maintenance of regular digestive function and chicken microflora (Mountzouris et al., 2011). According to Wati et al. (2015), supplementation with PFA in broiler chicken diets can improve nitrogen (N) retention and raw fiber digestibility, and may induce decreased digestive transit time compared to negative (without antibiotics) or positive control (supplemented with bacitracin methylene disalicylate [BMD]). The use of PFA does not alter carcass or meat quality. For example, the use of garlic and cinnamon (2 to 4 g/kg for both) in broiler feed had no effect on olfactory meat parameters (Toghyani et al., 2011). Li et al. (2015) concluded that phytoncide (0.5 to 1 g/kg) extracted from korean pine could be considered as an alternative to tylosin. Inclusion of phytocides, molecules excreted by trees in the air, has been able to improve performance and to reduce excreted gas emissions in broiler chickens (Li et al., 2015). Excreta ammonia, total mercaptans, acetic acid, and hydrogen sulfide all linearly decreased (P = 0.02, 0.04, 0.05 and 0.02, respectively) with increasing levels of phytoncide (Li et al., 2015).
Some research on chicken feed additives has focused on nanoparticles. Certain nanoparticles such as silver (Ag) and copper (Cu) as well as certain oxidized metals (Al2O3, Fe3O4, CeO2, ZrO2) may also have antimicrobial action and can be beneficial when used in feeds (Gangadoo et al., 2016).
4.2. Essential oils
Essential oils are the hydrophobic liquid of odoriferous and volatile aromatic compounds of a plant. Essential oils can be natural (vegetable origin) or synthetic. Only a few essential oils have useful antibacterial properties. The most used are thymol, trans-cinnamaldhyde, carvacrol and eugenol. Their modes of action lie in their interference with the enzymatic system of the bacteria and the modulation of immune responses and inflammation. Some studies (Khattak et al., 2014, Peng and Li, 2016, Pirgozliev et al., 2015) showed that essential oils are promising alternatives to growth promoter antibiotics (e.g., avilamycin) in improving chicken production. Essential oils can also play a preventive and curative role in necrotic enteritis in broilers (Jerzsele et al., 2012). The use of essential oils has a positive effect on growth, meat and carcass quality as well as chicken health. Peng et al. (2016) reported that adding oregano essential oil (Origanum genus) at 300 and 600 mg/kg in broiler chicken feed increased ADG. According to the authors, this result may be related to increased villus height and decreased crypt depth in the jejunum of broiler chickens. In addition, the administration of 600 mg/kg of feed of oregano essential oil improved the percentage of thigh muscle and decreased abdominal fat percentage in broiler chickens. Nevertheless, peppermint (Mentha piperita) was a good alternative to virginiamycin in broiler chickens (Khodambashi Emami et al., 2012).
4.3. Probiotics
Probiotics are defined as “live micro-organisms, when administered in adequate amounts, confer a health benefit to the host” (WHO, 2001). Probiotic feed supplementation improves growth, feed efficiency and intestinal health (Ghasemi et al., 2014, Giannenas et al., 2012, Samli et al., 2007). This improvement is achieved by reducing intestinal pH, intestinal bacteria composition and digestive activity. Mechanisms of action of probiotics include stimulation of endogenous enzymes, reduction of metabolic reactions that produce toxic substances, and production of vitamins or antimicrobial substances (Hassanein and Soliman, 2010). Probiotic bacteria produce molecules with antimicrobial activities such as bacteriocins which inhibits toxins' production and pathogens' adhesion (Pan and Yu, 2014). On the other hand, probiotics stimulate the immune response and increase resistance to colonization of bacteria (Hassanein and Soliman, 2010).
Administration of Enterococcus faecium in chicken feed had an antibacterial effect on bacterial microflora in the small intestine (Levkut et al., 2012). Similar results were reported with Streptomyces sp. (Latha et al., 2016) and Bacillus subtilis (Zhang et al., 2013). In a study (Zhang et al., 2013), comparing B. subtilis with enramycin, widely used as a feed additive for chickens to prevent necrotic enteritis, administration of 105 cfu of B. subtilis UBT-MO2/kg in broiler feed increased body weight by 4.4% and relative weight of the thymus. In addition, the treatment reduced NH3 and H2S concentrations in chicken excretions leading to less odor emissions.
Probiotics have positive effects on poultry meat quality (Hassanein and Soliman, 2010, Popova, 2017). They improve pH, color, fatty acid profile, chemical composition, water retention capacity and oxidation stability (Popova, 2017). The probiotics affect the protein and fat contents of meat and thus the meat quality. Abdurrahman et al. (2016) reported that lipid oxidation is one of the main causes of deterioration in feed quality. This hypothesis can be confirmed by other studies that showing the inclusion of Aspergillus awamori and Saccharomyces cerevisiae in chicken feed reduced blood saturated fatty acids and increased the polyunsaturated (Saleh et al., 2012). Another similar study of Liu et al. (2012b) showed that treatment with Bacillus licheniformis significantly increased the protein content and the respective essential and aromatic amino acids (Liu et al., 2012b). Feed containing B. licheniformis improves meat color, juiceness and flavor of broiler chickens (Liu et al., 2012b). These factors are very important in terms of consumer appreciation especially the color.
Probiotics may also have anticoccidial role. Results of Giannenas et al. (2012) suggest that treatment with probiotics may mitigate the impact of parasitic infection on chickens in the absence of anticoccidial infections. The use of probiotics exerted coccidiostatic effect against Eimeria tenella. This can help to minimize the risk and spread of coccidiosis and maintain intestinal health.
4.4. Organic acids
Organic acids are conservation agents used to protect feed from microbial and fungal proliferation (Kum et al., 2010). These acids are mainly carboxylic acids carrying a hydroxyl group on alpha carbon such as malic, lactic and tartaric acids. The organic acids can also be simple monocarboxylic acids such as acetic, formic, butyric and propionic acids. The antimicrobial action of organic acids is due to the fact that non-dissociated acids can diffuse through lipophilic bacteria membrane and disrupt enzymatic reactions and transport system (Cherrington et al., 1991). Some studies (Hassan et al., 2010, Nava et al., 2009) showed that organic acids addition to broiler feed promotes growth, feed conversion rate and feed utilization. Adding organic acids in drinking water gives young chicks a protective efficacy against Campylobacter infection (Chaveerach et al., 2004). These acids also have a protective action against E. coli (Izat et al., 1990). Thus, it has been shown (Mohammadagheri et al., 2016) that supplementation with citric acid (2%) can improve cell proliferation epithelial and villi height of gastrointestinal tract. Organic acid blend, formic and propionic acid supplementation (0.0525% in drinking water) generates more homogeneous and distinct populations in the intestinal microbiota and increases the colonization of Lactobacillus spp. in ileum of chicken (Nava et al., 2009). These changes in the intestinal microbiota and the increase in Lactobacillus populations show that organic acid can be used as an alternative to antibiotics (bacitracin in this study) to reduce pathogenic bacteria in the gastrointestinal tract (Nava et al., 2009).
Some organic acids would play a role in digestion. Indeed, a diet with low digestible protein in chicken leads to more protein reaching the gut, resulting in an increase in protein fermentation. Protein fermentation produces ammonia, branched-chain fatty acids, volatile fatty acids and intermediate products such as lactate and succinate as well as gases (hydrogen, carbon dioxide and methane). Some of these compounds may have adverse effects on growth performance (Bikker et al., 2007). Organic acids, such as butyric acid, added as a feed additive can be used to improve the digestibility of ileal proteins from poorly digestible protein sources (Adil et al., 2010).
Butyric acid is a saturated carboxylic acid produced in the cecum and colon of animals via the fermentation of carbohydrates such as dietary fiber and unabsorbed starch (Hu and Guo, 2007). Butyric acid is a readily available source of energy for intestinal epithelial cells and stimulates their multiplication and differentiation, as a result improves the feed efficiency in chickens (Adil et al., 2010, Józefiak et al., 2004). Indeed, Hu and Guo (2007) showed that body weight gain in chickens increased linearly during the period from 0 to 21 days as the dietary supplementation of butyrate increased. Further, according to Hu and Guo (2007) dietary supplementation of butyrate influenced feed conversion ratio in a positive quadratic fashion during the period from 0 to 42 days. Qaisrani et al. (2015) reported that diet supplemented with butyric acid improved the growth performance of chickens fed proteins of low digestible sources.
4.5. Prebiotics
Prebiotics are non-digestible feed components that are potentially beneficial to host health because of their fermentable properties that stimulate bacteria growth and/or activity in the ileum and caecum (Gibson and Roberfroid, 1995). It generally consists of short chain polysaccharides and oligosaccharides. Several prebiotics are generated from yeast cell walls and fermentation products. Prebiotics are not digestible by the host but commensal intestinal bacteria can metabolize them to produce short chain fatty acids like propionate, acetate and butyrate (Józefiak et al., 2008). These prebiotic components have positive effects on poultry productivity and contribute to a healthy intestinal tract and can be a good alternative to antibiotics (Morales-Lopez et al., 2009, Zhang et al., 2005). When ingested, the prebiotics alter the caecal microbial composition resulting in changes in the proteobacteria and changes in the genus and family of bacteria which causes change in growth (Park et al., 2016).
The addition of a product rich in mannose and mannoproteins in chicken feed significantly increased the number of intestinal villus cells (Baurhoo et al., 2007). Further, administration of mannanoligosaccharide (0.2%) in the chicken diet conferred intestinal health benefits over antibiotics. These advantages are expressed by a reduction of pathogenic bacteria, a morphological development (height of the villus and number of goblet cells) and an increased colonization by beneficial bacteria (Baurhoo et al., 2009). Another way of administration reported by Bednarczyk et al. (2016) is the ingestion of in ovo prebiotics in the chicken embryo. It is an effective practice that can replace antibiotic supplementation in water after hatching. The doses of prebiotics used in ovo are 10 times lower than after hatching.
4.6. Amino acids and enzymes
The feed additive enzymes are produced through fungi and bacteria fermentations. They are used to maximize feed conversion. Enzymes facilitate components degradation such as proteins, phytates and glucans. For example, endo-b-1-4-xylanases and b-1-3,1-4-glucanases have been used in wheat and barley diets of broilers to improve their digestion (Cowieson et al., 2006). Also, phytase enzyme can increase villus width and decrease crypt depth which can improve ADG (Mohammadagheri et al., 2016). Lysins are bacteriophage endolysins representing an innovative alternative therapeutic option of antibacterial. Lysins are phage-encoded peptidoglycan hydrolases which bring about the bacterial cell lysis when applied exogenously to Gram-positive bacteria (Fenton et al., 2010, Rios et al., 2016). According to Volozhantsev et al. (2011), administration of a combination of a group of lysins containing peptidases, amidases and lysozymes produces an antimicrobial effect against C. perfringens in poultry. For example, Ply3626 lysine is an enzyme which has been shown lytic activity against several strains of C. perfringens, which is an important cause of food poisoning and leads to economic losses in poultry production (Fenton et al., 2010, Zimmer et al., 2002).
4.7. Other studies
Other trials aiming at identifying compounds with positive effects on chicken health and productivity have focused on the use of natural products such as milk and propolis, resinous mixture produced by honey-bees. An immunodulatory effect was observed in broilers fed a feed containing propolis (Daneshmand et al., 2015). Broilers fed this had heavier lymphatic organs and a higher concentration of antibodies against Newcastle virus. Inclusion of milk kefir (2%) in chicken drinking water had a significant effect on body mass and chicken consumption index between 28 and 42 days of breeding (Toghyani et al., 2015).
5. Conclusion
Over the years, antibiotics have played an important role in fighting infectious diseases and stimulating poultry growth. Scientific evidence suggests that their large-scale use has led to antibiotic resistance and residues in the food and environment, particularly aquatic ecosystem, which can lead to public health problems. Many trials of potential alternatives to antibiotics have shown very relevant results. These alternatives give equal or better effects to antibiotics (namely good livestock performance), reduce mortality rates and protect environment and consumer health. Application of the results generated by these studies in feed industries, as well as livestock breeders and veterinary practice is very appealing. Some studies show that antibiotic use can be dropped or reduced. It should also be noted that some trials have shown the efficacy of vaccination of broiler chickens as a prophylactic treatment against necrotic enteritis induced by C. perfringens (Caly et al., 2015). Another way of research in the future is to test the interactive effect of using combinations of these alternatives. The aim will be to maintain a high level of viability and optimum productivity in poultry antibiotic free farms.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This work was done under the funding of MITACS, 2017.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Youcef Mehdi, Email: youcef.mehdi.1@ulaval.ca.
Stéphane Godbout, Email: stephane.godbout@irda.qc.ca.
References
- Abdurrahman Z.H., Pramono Y.B., Suthama N. Meat characteristic of crossbred local chicken fed inulin of dahlia tuber and lactobacillus sp. Media Peternakan. 2016;39:112–118. [Google Scholar]
- Adil S., Banday T., Bhat G.A., Mir M.S., Rehman M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet Med Int. 2010;2010:479485. doi: 10.4061/2010/479485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci C., Boni E., Alpigiani I., Lanzoni E., Bonardi S., Brindani F. Phenotypic and genotypic features of antibiotic resistance in salmonella enterica isolated from chicken meat and chicken and quail carcasses. Int J Food Microbiol. 2012;160:16–23. doi: 10.1016/j.ijfoodmicro.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Barceló D. Pharmaceutical-residue analysis. Trends Anal Chem. 2007;26:454–455. [Google Scholar]
- Baurhoo B., Ferket P.R., Zhao X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult Sci. 2009;88:2262–2272. doi: 10.3382/ps.2008-00562. [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Phillip L., Ruiz-Feria C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult Sci. 2007;86:1070–1078. doi: 10.1093/ps/86.6.1070. [DOI] [PubMed] [Google Scholar]
- Bednarczyk M., Stadnicka K., Kozlowska I., Abiuso C., Tavaniello S., Dankowiakowska A. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal. 2016;10:1271–1279. doi: 10.1017/S1751731116000173. [DOI] [PubMed] [Google Scholar]
- Bermudez A.J. Principles of disease prevention: diagnosis and control. In: Saif Y.M., editor. Diseases of poultry. Iowas State University Press; Ames, Ia, USA: 2003. pp. 3–60. [Google Scholar]
- Bikker P., Dirkzwager A., Fledderus J., Trevisi P., le Huërou-Luron I., Lallès J. Dietary protein and fermentable carbohydrates contents influence growth performance and intestinal characteristics in newly weaned pigs. Livest Sci. 2007;108:194–197. doi: 10.2527/jas.2006-076. [DOI] [PubMed] [Google Scholar]
- Braykov N.P., Eisenberg J.N., Grossman M., Zhang L., Vasco K., Cevallos W. Antibiotic resistance in animal and environmental samples associated with small-scale poultry farming in northwestern ecuador. mSphere. 2016;1 doi: 10.1128/mSphere.00021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly D.L., D'Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist's perspective. Front Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplin J.L., Hanlon G.W., Taylor H.D. Presence of vancomycin and ampicillin-resistant enterococcus faecium of epidemic clonal complex-17 in wastewaters from the south coast of England. Environ Microbiol. 2008;10:885–892. doi: 10.1111/j.1462-2920.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- Carvalho I.T., Santos L. Antibiotics in the aquatic environments: a review of the European scenario. Environ Int. 2016;94:736–757. doi: 10.1016/j.envint.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Chan T.Y. Health hazards due to clenbuterol residues in food. J Toxicol Clin Toxicol. 1999;37:517–519. doi: 10.1081/clt-100102525. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Gerbaud G., Lagorce M., Lafont J.P., Courvalin P. Persistence of an antibiotic resistance plasmid in intestinal Escherichia Coli of chickens in the absence of selective pressure. Antimicrob Agents Chemother. 1987;31:784–788. doi: 10.1128/aac.31.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M.K. Use of antibiotics as feed additives: a burning question. Front Microbiol. 2014;5:334. doi: 10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaveerach P., Keuzenkamp D.A., Lipman L.J., van Knapen F. Effect of organic acids in drinking water for young broilers on campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poult Sci. 2004;83:330–334. doi: 10.1093/ps/83.3.330. [DOI] [PubMed] [Google Scholar]
- Chen H., Liu S., Xu X.R., Zhou G.J., Liu S.S., Yue W.Z. Antibiotics in the coastal environment of the hailing bay region, south China sea: spatial distribution, source analysis and ecological risks. Mar Pollut Bull. 2015;95:365–373. doi: 10.1016/j.marpolbul.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Cherrington C.A., Hinton M., Mead G.C., Chopra I. Organic acids: chemistry, antibacterial activity and practical applications. Adv Microb Physiol. 1991;32:87–108. doi: 10.1016/s0065-2911(08)60006-5. [DOI] [PubMed] [Google Scholar]
- Corvaglia A.R. Rôle des résidus D'antibiotiques dans L'environnement hydrique sur La sélection et La diffusion De bactéries résistantes des genres “aeromonas”, “acinetobacter” et “Legionella”. Thèse De Doctorat :. Univ Genève. 2006 no Sc. 3796. [Google Scholar]
- Cowieson A.J., Hruby M., Pierson E.E. Evolving enzyme technology: impact on commercial poultry nutrition. Nutr Res Rev. 2006;19:90–103. doi: 10.1079/NRR2006121. [DOI] [PubMed] [Google Scholar]
- Cross D.E., McDevitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br Poult Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- CSCRA . Gouvernement du Canada; 2016. Système canadien de surveillance de la résistance aux antimicrobiens – rapport De 2016. [Google Scholar]
- Daneshmand A., Sadeghi G.H., Karimi A., Vaziry A., Ibrahim S.A. Evaluating complementary effects of ethanol extract of propolis with the probiotic on growth performance, immune response and serum metabolites in male broiler chickens. Livest Sci. 2015;178:195–201. [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Liguoro M., Cibin V., Capolongo F., Halling-Sorensen B., Montesissa C. Use of oxytetracycline and tylosin in intensive calf farming: evaluation of transfer to manure and soil. Chemosphere. 2003;52:203–212. doi: 10.1016/S0045-6535(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Devarajan N., Kohler T., Sivalingam P., van Delden C., Mulaji C.K., Mpiana P.T. Antibiotic resistant Pseudomonas spp. In the aquatic environment: a prevalence study under tropical and temperate climate conditions. Water Res. 2017;115:256–265. doi: 10.1016/j.watres.2017.02.058. [DOI] [PubMed] [Google Scholar]
- Diarra M.S., Malouin F. Antibiotics in canadian poultry productions and anticipated alternatives. Front Microbiol. 2014;5:282. doi: 10.3389/fmicb.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra M.S., Rempel H., Champagne J., Masson L., Pritchard J., Topp E. Distribution of antimicrobial resistance and virulence genes in enterococcus spp. and characterization of isolates from broiler chickens. Appl Environ Microbiol. 2010;76:8033–8043. doi: 10.1128/AEM.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra M.S., Silversides F.G., Diarrassouba F., Pritchard J., Masson L., Brousseau R. Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, clostridium perfringens and enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia Coli isolates. Appl Environ Microbiol. 2007;73:6566–6576. doi: 10.1128/AEM.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dumonceaux T.J., Hill J.E., Hemmingsen S.M., van Kessel A.G. Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl Environ Microbiol. 2006;72:2815–2823. doi: 10.1128/AEM.72.4.2815-2823.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghorab A.H. The chemical composition of the mentha pulegium L. Essential oil from Egypt and its antioxidant activity. J Essent Oil Bearing Plants. 2006;9:183–195. [Google Scholar]
- Engberg R.M., Hedemann M.S., Leser T.D., Jensen B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult Sci. 2000;79:1311–1319. doi: 10.1093/ps/79.9.1311. [DOI] [PubMed] [Google Scholar]
- ESVAC . 2017. Sales of veterinary antimicrobial agents in 30 european countries in 2015. Trends from 2010 to 2015. Seventh Esvac Report. Ema/184855/2017. [Google Scholar]
- European-Commission Council directive 96/23/Ec of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing directives 85/358/Eec and 86/469/Eec and decision 89/187/Eec and 91/664/Eec. Offic J Eur Union. 1996;L125:10. [Google Scholar]
- Fenton M., Ross P., McAuliffe O., O'Mahony J., Coffey A. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs. 2010;1:9–16. doi: 10.4161/bbug.1.1.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgetta V., Rempel H., Malouin F., Vaillancourt R., Jr., Topp E., Dewar K. Pathogenic and multidrug-resistant Escherichia fergusonii from broiler chicken. Poult Sci. 2012;91:512–525. doi: 10.3382/ps.2011-01738. [DOI] [PubMed] [Google Scholar]
- Frankic T., Voljč M., Salobir J., Rezar V. Use of herbs and spices and their extracts in animal nutrition. Acta Agric Slov. 2009;94:95–102. [Google Scholar]
- Fung S., Rempel H., Forgetta V., Dewar E.T.K., Diarra M.S. 2013. “Ceca microbiome of mature broiler chickens fed with or without salinomycin,” in the gut microbiome: the effector/regulatory immune network conference (B3). Keystone symposia onmolecular and cellular biology (Taos) [Google Scholar]
- Furtula V., Farrell E.G., Diarrassouba F., Rempel H., Pritchard J., Diarra M.S. Veterinary pharmaceuticals and antibiotic resistance of Escherichia Coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult Sci. 2010;89:180–188. doi: 10.3382/ps.2009-00198. [DOI] [PubMed] [Google Scholar]
- Furtula V., Jackson C.R., Farrell E.G., Barrett J.B., Hiott L.M., Chambers P.A. Antimicrobial resistance in Enterococcus spp. isolated from environmental samples in an area of intensive poultry production. Int J Environ Res Publ Health. 2013;10:1020–1036. doi: 10.3390/ijerph10031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadoo S., Stanley D., Hughes R.J., Moore R.J., Chapman J. Nanoparticles in feed: progress and prospects in poultry research. Trends Food Sci Technol. 2016;58:115–126. [Google Scholar]
- Gassner B., Wuethrich A. Pharmacokinetic and toxicological aspects of the medication of beef-type calves with an Oral formulation of chloramphenicol palmitate. J Vet Pharmacol Therapeut. 1994;17:279–283. doi: 10.1111/j.1365-2885.1994.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Ghasemi H.A., Kasani N., Taherpour K. Effects of black cumin seed (nigella sativa L.), a probiotic, a prebiotic and a synbiotic on growth performance, immune response and blood characteristics of male broilers. Livest Sci. 2014;164:128–134. [Google Scholar]
- Giannenas I., Papadopoulos E., Tsalie E., Triantafillou E., Henikl S., Teichmann K. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet Parasitol. 2012;188:31–40. doi: 10.1016/j.vetpar.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gonzalez Ronquillo M., Angeles Hernandez J.C. Antibiotic and synthetic growth promoters in animal diets: review of impact and analytical methods. Food Contr. 2017;72:255–267. Part B. [Google Scholar]
- Goodarzi M., Nanekarani S. Effects of feeding mentha pulegium L. As an alternative to antibiotics on performance of broilers. APCBEE Procedia. 2014;8:53–58. [Google Scholar]
- Guillot J.F., Coudray M.C., Chaslus-Dancla E.T., Lafont J.P. Phénotypes D'escherichia Coli D'origine aviaire vis-À-vis des ominosides. Med Maladies Infect. 1977;7:449–455. [Google Scholar]
- Harms R.H., Ruiz N., Miles R.D. Influence of virginiamycin on broilers fed four levels of energy. Poult Sci. 1986;65:1984–1986. doi: 10.3382/ps.0651984. [DOI] [PubMed] [Google Scholar]
- Hassan H.M.A., Mohamed M.A., Youssef A.W., Hassan E.R. Effect of using organic acids to substitute antibiotic growth promoters on performance and intestinal microflora of broilers. Asian-Australas J Anim Sci. 2010;23:1348–1353. [Google Scholar]
- Hassanein S.M., Soliman N.K. Effect of probiotic (Saccharomyces Cerevisiae) adding to diets on intestinal microflora and performance of hy-line layers hens. J Am Sci. 2010;6 [Google Scholar]
- Hegde N.V., Kariyawasam S., DebRoy C. Comparison of antimicrobial resistant genes in chicken gut microbiome grown on Organic and conventional diet. Vet Anim Sci. 2016;1–2:9–14. doi: 10.1016/j.vas.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Guo Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim Feed Sci Technol. 2007;132:240–249. [Google Scholar]
- Hur J., Kim J.H., Park J.H., Lee Y.J., Lee J.H. Molecular and virulence characteristics of multi-drug resistant salmonella enteritidis strains isolated from poultry. Vet J. 2011;189:306–311. doi: 10.1016/j.tvjl.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Izat A.L., Tidwell N.M., Thomas R.A., Reiber M.A., Adams M.H., Colberg M. Effects of a buffered propionic acid in diets on the performance of broiler chickens and on microflora of the intestine and carcass. Poult Sci. 1990;69:818–826. doi: 10.3382/ps.0690818. [DOI] [PubMed] [Google Scholar]
- Jarriyawattanachaikul W., Chaveerach P., Chokesajjawatee N. Antimicrobial activity of Thai-Herbal plants against food-borne pathogens E. Coli, S. Aureus and C. Jejuni. Agri and Agri Sci Procedia. 2016;11:20–24. [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J.J. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Johnson T.J., Logue C.M., Johnson J.R., Kuskowski M.A., Sherwood J.S., Barnes H.J. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia Coli from humans and poultry. Foodb Pathog Dis. 2012;9:37–46. doi: 10.1089/fpd.2011.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefiak D., Kaczmarek S., Rutkowski A. A note on the effects of selected prebiotics on the performance and ileal microbiota of broiler chickens. J Anim Feed Sci. 2008;17:392–397. [Google Scholar]
- Józefiak D., Rutkowski A., Martin S. Carbohydrate fermentation in the avian ceca: a review. Anim Feed Sci Technol. 2004;113:1–15. [Google Scholar]
- Kempf I., Zeitouni S. Coût biologique De La résistance aux antibiotiques : analyse et conséquences. Pathol Biol. 2012;60:e9–e14. doi: 10.1016/j.patbio.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Khamisabadi H., Pourhesabi G., Chaharaein B., Naseri H.R. Comparison of the effects of licorice extract (Glycyrrhiza glabra) and lincomycine on abdominal fat biochemical blood parameter and immunity of broiler chickens. Anim Sci J. 2015;105:229–244. [Google Scholar]
- Khattak F., Ronchi A., Castelli P., Sparks N. Effects of natural blend of essential Oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult Sci. 2014;93:132–137. doi: 10.3382/ps.2013-03387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodambashi Emami N., Samie A., Rahmani H.R., Ruiz-Feria C.A. The effect of peppermint essential Oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim Feed Sci Technol. 2012;175:57–64. [Google Scholar]
- Kirubakaran A., Moorthy M., Chitra R., Prabakar G. Influence of combinations of fenugreek, garlic, and black pepper powder on production traits of the broilers. Vet World. 2016;9:470–474. doi: 10.14202/vetworld.2016.470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kum S., Eren U., Onol A., Sandikci M. Effects of dietary organic acid supplementation on the intestinal mucosa in broilers. Rev Med Vet. 2010;10:463–468. [Google Scholar]
- Kummerer K. Antibiotics in the aquatic environment–a review–part I. Chemosphere. 2009;75:417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- Landy N., Ghalamkari G., Toghyani M. Performance, carcass characteristics, and immunity in broiler chickens fed dietary neem (Azadirachta Indica) as alternative for an antibiotic growth promoter. Livest Sci. 2011;142:305–309. [Google Scholar]
- Laroche E., Pawlak B., Berthe T., Skurnik D., Petit F. Occurrence of antibiotic resistance and class 1, 2 and 3 integrons in Escherichia Coli isolated from a densely populated estuary (Seine, France) FEMS Microbiol Ecol. 2009;68:118–130. doi: 10.1111/j.1574-6941.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- Latha S., Vinothini G., John dickson calvin D., dhanasekaran D. In vitro probiotic profile based selection of indigenous actinobacterial probiont Streptomyces sp. Jd9 for enhanced broiler production. J Biosci Bioeng. 2016;121:124–131. doi: 10.1016/j.jbiosc.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Ho Hong Y., Lee S.H., Jang S.I., Park M.S., Bautista D.A. Effects of anticoccidial and antibiotic growth promoter programs on broiler performance and immune status. Res Vet Sci. 2012;93:721–728. doi: 10.1016/j.rvsc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Levkut M., Revajova V., Laukova A., Sevcikova Z., Spisakova V., Faixova Z. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium Ef55 and challenged with salmonella enteritidis. Res Vet Sci. 2012;93:195–201. doi: 10.1016/j.rvsc.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Li H.L., Zhao P.Y., Lei Y., Hossain M.M., Kim I.H. Phytoncide, phytogenic feed additive as an alternative to conventional antibiotics, improved growth performance and decreased excreta gas emission without adverse effect on meat quality in broiler chickens. Livest Sci. 2015;181:1–6. [Google Scholar]
- Lin J., Hunkapiller A.A., Layton A.C., Chang Y.J., Robbins K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodb Pathog Dis. 2013;10:331–337. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- Liu D., Chai T., Xia X., Gao Y., Cai Y., Li X. formation and transmission of Staphylococcus Aureus (including mrsa) aerosols carrying antibiotic-resistant genes in a poultry farming environment. Sci Total Environ. 2012;426:139–145. doi: 10.1016/j.scitotenv.2012.03.060. [DOI] [PubMed] [Google Scholar]
- Liu X., Yan H., Lv L., Xu Q., Yin C., Zhang K. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas J Anim Sci. 2012;25:682–689. doi: 10.5713/ajas.2011.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabona U., Viljoen A., Shikanga E., Marston A., van Vuuren S. Antimicrobial activity of southern african medicinal plants with dermatological relevance: from an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J Ethnopharmacol. 2013;148:45–55. doi: 10.1016/j.jep.2013.03.056. [DOI] [PubMed] [Google Scholar]
- Mahboubi M., Haghi G. Antimicrobial activity and chemical composition of mentha pulegium L. Essential oil. J Ethnopharmacol. 2008;119:325–327. doi: 10.1016/j.jep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Manges A.R., Smith S.P., Lau B.J., Nuval C.J., Eisenberg J.N., Dietrich P.S. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia Coli causing urinary tract infections: a case-control study. Foodb Pathog Dis. 2007;4:419–431. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- Manning J., Gole V., Chousalkar K. Screening for salmonella in backyard chickens. Prev Vet Med. 2015;120:241–245. doi: 10.1016/j.prevetmed.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Manzetti S., Ghisi R. The environmental release and fate of antibiotics. Mar Pollut Bull. 2014;79:7–15. doi: 10.1016/j.marpolbul.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Marti E., Variatza E., Balcazar J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014;22:36–41. doi: 10.1016/j.tim.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Mead P.S., Slutsker L., Dietz V., McCaig L.F., Bresee J.S., Shapiro C. Food-related illness and death in the United States. Emerg infec diseases. 1999;5:607. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant L.E., Rempel H., Forge T., Kannangara T., Bittman S., Delaquis P. Characterization of antibiotic-resistant and potentially pathogenic Escherichia Coli from soil fertilized with litter of broiler chickens fed antimicrobial-supplemented diets. Can J Microbiol. 2012;58:1084–1098. doi: 10.1139/w2012-082. [DOI] [PubMed] [Google Scholar]
- Mohammadagheri N., Najafi R., Najafi G. Effects of dietary supplementation of organic acids and phytase on performance and intestinal histomorphology of broilers. Vet Res Forum. 2016;7:189–195. [PMC free article] [PubMed] [Google Scholar]
- Morales-Lopez R., Auclair E., Garcia F., Esteve-Garcia E., Brufau J. Use of yeast cell walls; Beta-1, 3/1, 6-glucans; and mannoproteins in broiler chicken diets. Poult Sci. 2009;88:601–607. doi: 10.3382/ps.2008-00298. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Paraskevas V., Tsirtsikos P., Palamidi I., Steiner T., Schatzmayr G. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim Feed Sci Technol. 2011;168:223–231. [Google Scholar]
- Mpofu D.A., Marume U., Mlambo V., Hugo A. The effects of Lippia javanica dietary inclusion on growth performance, carcass characteristics and fatty acid profiles of broiler chickens. Anim Nutri. 2016;2:160–167. doi: 10.1016/j.aninu.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava G.M., Attene-Ramos M.S., Gaskins H.R., Richards J.D. Molecular analysis of microbial community structure in the chicken ileum following organic acid supplementation. Vet Microbiol. 2009;137:345–353. doi: 10.1016/j.vetmic.2009.01.037. [DOI] [PubMed] [Google Scholar]
- O'Brien T.F. Emergence, spread, and environmental effect of antimicrobial resistance: how use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin Infect Dis. 2002;34(Suppl. 3):S78–S84. doi: 10.1086/340244. [DOI] [PubMed] [Google Scholar]
- Pakpinyo S., Sasipreeyajan J. Molecular characterization and determination of antimicrobial resistance of mycoplasma gallisepticum isolated from chickens. Vet Microbiol. 2007;125:59–65. doi: 10.1016/j.vetmic.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M., Chu L.M. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci Total Environ. 2017;579:466–473. doi: 10.1016/j.scitotenv.2016.11.072. [DOI] [PubMed] [Google Scholar]
- Park S.H., Lee S.I., Ricke S.C. Microbial populations in naked neck chicken ceca raised on pasture flock fed with commercial yeast cell wall prebiotics via an illumina miseq platform. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q.Y., Li J.D., Li Z., Duan Z.Y., Wu Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim Feed Sci Technol. 2016;214:148–153. [Google Scholar]
- Pirgozliev V., Bravo D., Mirza M.W., Rose S.P. Growth performance and endogenous losses of broilers fed wheat-based diets with and without essential oils and xylanase supplementation. Poult Sci. 2015;94:1227–1232. doi: 10.3382/ps/peu017. [DOI] [PubMed] [Google Scholar]
- Platel K., Srinivasan K. Digestive stimulant action of spices: a myth or reality? Indian J Med Res. 2004;119:167–179. [PubMed] [Google Scholar]
- Popova T. Effect of probiotics in poultry for improving meat quality. Curr Opin Food Sci. 2017;14:72–77. [Google Scholar]
- Qaisrani S., Van Krimpen M., Kwakkel R., Verstegen M., Hendriks W. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poultry sci. 2015;94:2152–2164. doi: 10.3382/ps/pev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw F., Gardin Y., van den Berg T., Lambrecht B. La vaccination contre la maladie de newcastle chez le poulet (Gallus Gallus) Biotechnologie, Agronomie, Société et Environnement. 2009;13:587. [Google Scholar]
- Rios A.C., Moutinho C.G., Pinto F.C., Del Fiol F.S., Jozala A., Chaud M.V. Alternatives to overcoming bacterial resistances: state-of-the-art. Microbiol Res. 2016;191:51–80. doi: 10.1016/j.micres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Rosen G.D. Pronutrient antibiotic replacement standards discussed. Feedstuffs. 1996;75:11–13. [Google Scholar]
- RUMA . 2016. Responsible use of medicines in agriculture alliance (Ruma) information on antibiotic resistance: Ruma.org.UK/about/position-papers/ruma-information-note-antibiotics-responsible-use-antibiotics-farm-animals/ [Google Scholar]
- Saleh A.A., Eid Y.Z., Ebeid T.A., Ohtsuka A., Hioki K., Yamamoto M. The modification of the muscle fatty acid profile by dietary supplementation with aspergillus awamori in broiler chickens. Br J Nutr. 2012;108:1596–1602. doi: 10.1017/S0007114511007069. [DOI] [PubMed] [Google Scholar]
- Samli H.E., Senkoylu N., Koc F., Kanter M., Agma A. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch Anim Nutr. 2007;61:42–49. doi: 10.1080/17450390601106655. [DOI] [PubMed] [Google Scholar]
- Schwaiger K., Huther S., Holzel C., Kampf P., Bauer J. Prevalence of antibiotic-resistant Enterobacteriaceae isolated from chicken and pork meat purchased at the slaughterhouse and at retail in bavaria, Germany. Int J Food Microbiol. 2012;154:206–211. doi: 10.1016/j.ijfoodmicro.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Silbergeld E.K., Graham J., Price L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Publ Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- Singh P., Karimi A., Devendra K., Waldroup P.W., Cho K.K., Kwon Y.M. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult Sci. 2013;92:272–276. doi: 10.3382/ps.2012-02603. [DOI] [PubMed] [Google Scholar]
- Singh S., Yadav A.S., Singh S.M., Bharti P. Prevalence of salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res Int. 2010;43:2027–2030. [Google Scholar]
- Toghyani M., Mosavi Sk, Modaresi M., Landy N. Evaluation of kefir as a potential probiotic on growth performance, serum biochemistry and immune responses in broiler chicks. Animal Nutr. 2015;1:305–309. doi: 10.1016/j.aninu.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toghyani M., Toghyani M., Gheisari A., Ghalamkari G., Eghbalsaied S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest Sci. 2011;138:167–173. [Google Scholar]
- Torok V.A., Allison G.E., Percy N.J., Ophel-Keller K., Hughes R.J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl Environ Microbiol. 2011;77:3380–3390. doi: 10.1128/AEM.02300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela J., Hildebrandt K., Metcalfe A., Rempel H., Bittman S., Topp E. Characterization of Staphylococcus xylosus isolated from broiler chicken barn bioaerosol. Poult Sci. 2012;91:3003–3012. doi: 10.3382/ps.2012-02302. [DOI] [PubMed] [Google Scholar]
- Volozhantsev N.V., Verevkin V.V., Bannov V.A., Krasilnikova V.M., Myakinina V.P., Zhilenkov E.L. The genome sequence and proteome of bacteriophage Phicpv1 virulent for clostridium perfringens. Virus Res. 2011;155:433–439. doi: 10.1016/j.virusres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Wati T., Ghosh T.K., Syed B., Haldar S. Comparative efficacy of a phytogenic feed additive and an antibiotic growth promoter on production performance, caecal microbial population and humoral immune response of broiler chickens inoculated with enteric pathogens. Anim Nutr. 2015;1:213–219. doi: 10.1016/j.aninu.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2001. Food and agriculture organization of the united nations/world health organization (Fao/Who). Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. [Internet] Report of a Joint Fao/Who Expert Consultation. 2001. [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Yulistiani R., Praseptiangga D., Raharjo D., Shirakawa T. vol. 193. IOP Publishing; 2017. p. 012007. (2017. Prevalence of antibiotic-resistance enterobacteriaceae strains isolated from chicken meat at traditional markets in Surabaya, Indonesia, IOP conference series: materials science and Engineering). [Google Scholar]
- Zhang A.W., Lee B.D., Lee S.K., Lee K.W., An G.H., Song K.B. Effects of yeast (Saccharomyces Cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult Sci. 2005;84:1015–1021. doi: 10.1093/ps/84.7.1015. [DOI] [PubMed] [Google Scholar]
- Zhang Z.F., Cho J.H., Kim I.H. Effects of Bacillus subtilis Ubt-Mo2 on growth performance, relative immune Organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livest Sci. 2013;155:343–347. [Google Scholar]
- Zhao C., Ge B., De Villena J., Sudler R., Yeh E., Zhao S. Prevalence of Campylobacter spp., Escherichia Coli, and Salmonella serovars in retail chicken, Turkey, pork, and beef from the greater Washington, dc, area. Appl Environ Microbiol. 2001;67:5431–5436. doi: 10.1128/AEM.67.12.5431-5436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Gong J., Brisbin J.T., Yu H., Sanei B., Sabour P. Appropriate chicken sample size for identifying the composition of broiler intestinal microbiota affected by dietary antibiotics, using the polymerase chain reaction-denaturing gradient gel electrophoresis technique. Poult Sci. 2007;86:2541–2549. doi: 10.3382/ps.2007-00267. [DOI] [PubMed] [Google Scholar]
- Zimmer M., Vukov N., Scherer S., Loessner M.J. The murein hydrolase of the bacteriophage Phi3626 dual lysis system is active against all tested clostridium perfringens strains. Appl Environ Microbiol. 2002;68:5311–5317. doi: 10.1128/AEM.68.11.5311-5317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]