Abstract
Recent biochemical characterization of Arsenic resistance protein 2 (Ars2) has established it as central to determining the fate of nascent RNA polymerase II (RNAPII) transcripts. Through interactions with the nuclear 5′-7-methylguanosine (7mG) cap binding complex (CBC), Ars2 promotes co-transcriptional processing coupled with nuclear export or degradation of several classes of RNAPII transcripts, allowing for gene expression programs that facilitate rapid and sustained proliferation of immortalized cells in culture. However, rapidly dividing cells in culture do not represent the physiological condition of the vast majority of cells in an adult mammal. To examine functions of Ars2 in a physiological setting we generated inducible Ars2 knockout mice and found that deletion of Ars2 from adult mice resulted in defective hematopoiesis in bone marrow and thymus. Importantly, only some of this defect could be explained by the requirement of Ars2 for rapid proliferation, which we found to be cell-type specific in vivo. Rather Ars2 was required for survival of developing thymocytes and for limiting differentiation of bone marrow resident long-term hematopoietic stem cells (LT-HSCs). As a result, Ars2 knockout led to rapid thymic involution and loss of the ability of mice to regenerate peripheral blood following myeloablation. These in vivo data demonstrate that Ars2 expression is important at several steps of hematopoiesis, likely because Ars2 acts on gene expression programs underlying essential cell fate decisions such as the decision to die, to proliferate, or to differentiate.
Introduction
Arsenic resistance protein 2 (Ars2) is an essential, highly conserved protein with mammals sharing more than 98% amino acid identity [1]. Ars2 was initially described as a modulator of arsenic sensitivity in a cDNA screen [2]; subsequent analysis determined that the ability of Ars2 to modulate arsenic sensitivity likely resulted from a dominant-negative effect of the partial cDNA sequence used in the screen [3]. Full length Ars2 did not impart arsenic resistance, but rather was found to be a component of the 5′-7-methylguanosine (7mG) cap binding complex (CBC) that coordinated RNA polymerase II (RNAPII) transcription with nuclear RNA processing and export [3–9]. Ars2 was required for rapid proliferation of cells in culture [2, 3] and, unlike other protein components of the CBC, Ars2 could be induced by mitogenic signals [3, 10]. The intimate relationship between Ars2 and cellular proliferation likely stems from its role in 3′ end processing of replication-dependent histone (RDH) mRNAs [4, 11, 12]. In addition to RDH mRNAs, Ars2 was shown to be involved in maturation and/or nuclear export of microRNAs (miRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), and select mRNAs [10, 11, 13–15]. As a balance to its anabolic effects on RNAPII transcripts, Ars2 supports transcription termination-coupled degradation of superfluous transcripts such as promoter upstream transcripts (PROMPTs) and enhancer RNAs (eRNAs) [5, 16, 17]. Overall, data indicate that the net effect of Ars2 is increased efficiency of productive RNAPII transcription.
In vivo characterization of Ars2 in mammals, while limited, demonstrated roles for Ars2 that do not stem from its requirement for rapid cellular proliferation. Wilson et al. demonstrated that embryos at the blastocyst stage rely on Ars2 to promote cell survival [1], while Gruber et al. found similar viability-promoting properties of Ars2 in adult peripheral immune organs [3]. Finally, Andreu-Agullo et al. demonstrated that Ars2 is essential for maintenance of neural stem cells in the absence of effects on their proliferation or viability [18]. Rather, Ars2 activated transcription of Sox2, a transcription factor that suppresses differentiation of neural stem cells.
In the current study we set out to identify additional physiologic roles for Ars2 in adult mammals. To this end, we generated inducible whole-body Ars2 knockout (Ars2 iKO) mice by crossing Ars2-floxed mice [3] with mice expressing the Cre-ERT2 transgene [19]. Initial characterization of Ars2 iKO mice following induced whole-body Ars2 deletion confirmed that developing blood cells are sensitive to Ars2 deletion, as originally observed by Gruber et al. following MxCre-mediated Ars2 deletion [3]. Adult mammals continually regenerate blood cells in a well characterized, step-wise process termed hematopoiesis. Hematopoiesis begins in the bone marrow with multipotent long-term hematopoietic stem cells (LT-HSC) that are capable of indefinite self-renewal and differentiation to generate all blood cells throughout the life of an organism [20, 21]. In adult mammals, the majority of LT-HSCs are quiescent to prevent exhaustion and malignant transformation [22, 23]. Upon activation by peripheral signaling, LT-HSCs enter cell cycle and undergo asymmetrical division to self-renew and differentiate into downstream lineage committed stem and progenitor cells that ultimately produce every mature blood cell [23–25].
While many blood cells mature prior to leaving the bone marrow, most T-cells require additional, antigen-dependent maturation that takes place in the thymus. Bone marrow derived T-cell progenitors enter the thymus and progress through a series of maturation and selection processes termed thymopoiesis that ultimately yields mature CD4+ helper T-cells and CD8+ cytotoxic T-cells [26–28]. Early thymic maturation during the CD4/CD8 double-negative (DN) stage of thymopoiesis is driven by recombination of T-cell receptor (TCR) genes to produce TCR diversity [29]. Following TCR recombination, thymopoiesis proceeds to the CD4/CD8 double-positive (DP) stage during which positive and negative selection eliminate non-MHC-restricted and autoreactive T-cell progenitors. The small fraction of T-cell progenitors that survive positive and negative selection down-regulate either CD4 or CD8 to become mature CD8+ or CD4+ T-cells, respectively [30].
The current study extends cursory observations regarding roles for Ars2 in normal mammalian hematopoiesis made by Gruber et al [3]. Specifically, we show that thymopoiesis is critically dependent on expression of Ars2, both in developing thymocytes and in thymic stroma. Additionally we report that hematopoiesis within the bone marrow of Ars2 deficient mice is compromised as the result of defective proliferation of progenitor cells combined with loss of self-renewal capacity of LT-HSCs. Taken together, data indicate that Ars2 is essential for multiple processes involved in production of mature blood cells and that Ars2 influences these processes through intrinsic effects on developing blood cells along with effects on stromal cells that support hematopoiesis.
Results
Deletion of Ars2 is lethal to adult mice
While growing evidence has established Ars2 as a vital factor for transcription regulation and nuclear RNA processing, little is known about the relevance of Ars2 to normal mammalian physiology. To begin to explore physiological relevance of Ars2 in mammals, we generated inducible Ars2 knockout (iKO) mice by cross breeding Ars2fl/fl mice [3] with mice expressing the tamoxifen-inducible Cre recombinase Cre-ERT2 [19]. In these mice exons 2–20 of the Srrt gene, which codes for Ars2, are deleted by daily intraperitoneal injection of tamoxifen for five consecutive days (Fig. 1A). Deletion of Srrt following tamoxifen injection was confirmed by endpoint PCR in multiple organs (Fig. S1A) and changes in Ars2 mRNA expression was determined by quantitative real-time polymerase chain reaction (qRT-PCR). Consistent with previous observations [1, 3], Ars2 mRNA expression was high in hematopoietic tissues of control mice. Reduced expression of Ars2 mRNA was observed in most organs eight days following first tamoxifen injection of Ars2 iKO mice (Fig. 1B). Several organs from Ars2 iKO mice failed to demonstrate reduction in Ars2 mRNA expression despite clear evidence of Srrt deletion (Fig. S1A), suggesting extended half-life of Ars2 mRNA in these organs. Importantly, tamoxifen treatment of Ars2 iKO mice led to rapid weight loss (Fig. 1C) and death (Fig. 1D) within two weeks, with no differences observed between male and female mice (Fig. S1B). In contrast, Ars2fl/fl mice injected with tamoxifen survived for the duration of each experiment (20 to 60 days) and showed no signs of distress (Fig. 1C, D). Ars2 iKO mice injected with vehicle control corn oil and Ars2fl/WT mice injected with tamoxifen also demonstrated normal survival (data not shown).
Figure 1. Induced whole-body Ars2 knockout leads to rapid death of adult mice.
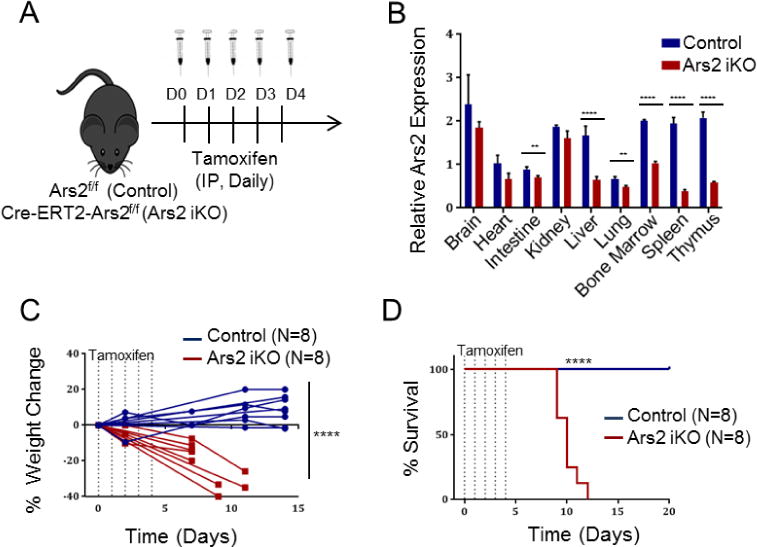
A) Schematic of whole-body inducible Ars2 knockout model. Littermate mice with either Ars2fl/fl (Control) or Cre-ERT2+, Ars2fl/fl (Ars2 iKO) genotype were injected intraperitoneally with tamoxifen (75 mg/kg) for five consecutive days and then monitored for up to 60 days. B) qRT-PCR was performed on RNA isolated from several organs removed from Ars2 iKO or control mice eight days following initial tamoxifen injection. Relative Ars2 expression was calculated using the ΔCT method with TBP as endogenous control. Bars represent mean ± SD of technical quadruplicates. C) Rapid weight loss of Ars2 iKO following Ars2 deletion. Lines represent change in weight of individual mice relative to initiation of tamoxifen injection. D) Survival of Ars2 iKO mice following tamoxifen injection (red line) was reduced relative to littermate controls (blue line). **P ≤ 0.01, ****P ≤ 0.0001.
Ars2 is required for normal thymopoiesis
Necropsy of tamoxifen-treated Ars2 iKO mice revealed no obvious cause of death, but a marked reduction in the size of Ars2 deleted thymi when compared to littermate control mice (Fig. 2A). Reduction in size was reflected in significant reduction in cellularity and mass (Fig. 2B) of thymi from tamoxifen-treated Ars2 iKO mice when compared to littermate controls. In contrast, other parenchymal and immune organs such as kidney, liver and spleen appeared normal on gross examination (Fig. S2A, B).
Figure 2. Ars2 supports survival of immature thymocytes.
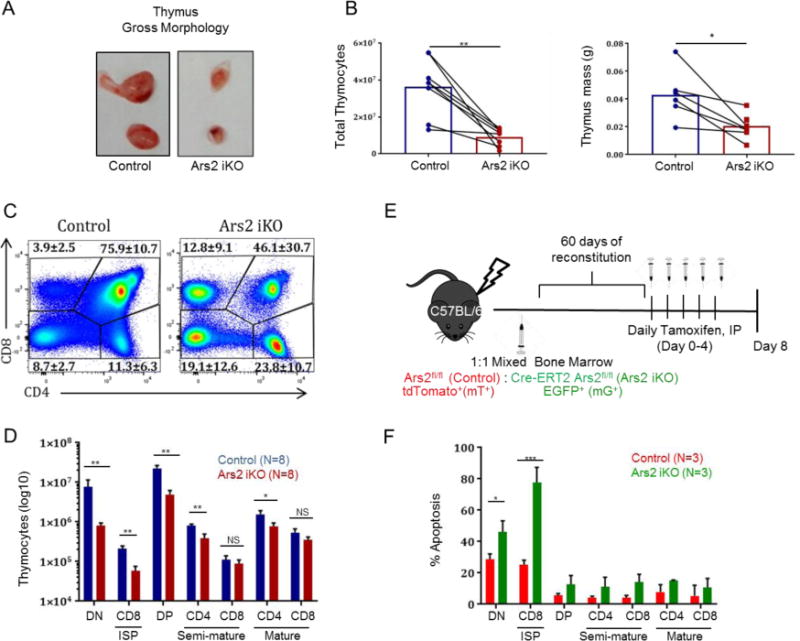
A) Gross anatomy of thymi showing smaller size following tamoxifen-induced Ars2 depletion. B) (left) Cellularity and (right) mass of thymi from 6 to 8 weeks old Ars2 iKO (n=8) mice was reduced compared to littermate controls (n=8) seven to ten days following initial Tamoxifen injection (**p = 0.001 and *p=0.01, respectively). Dots represent individual mice with lines connecting littermates. C) Representative dot plots showing distribution of lineage-negative (B220−, CD11b−, Ly-6G−, NK1.1−) thymocytes between four major developmental stages based on CD4 and CD8 staining. Numbers represent mean ± standard error for each of four thymocyte populations, CD4/CD8 double-negative (DN, p = 0.002), CD4/CD8 double-positive (DP, p = 0.007), CD4 single-positive (p = 0.003) and CD8 single-positive (p = 0.04) comparing Ars2 iKO (n=8) versus control (n=8) mice 7 to 8 days following initial tamoxifen injection. D) Average number ± standard deviation of DN, immature single positive CD8 (CD8ISP, TCRβ−, CD24+), DP, semi-mature (TCRβ+, CD24+) CD4 and CD8 single positive and mature (TCRβ+, CD24−) CD4 and CD8 single-positive thymocytes isolated from Ars2 iKO (n=8) versus control (n=8) mice 7 to 8 days following initial tamoxifen injection (NS: P > 0.05, *: P ≤ 0.05, **: P ≤ 0.01). E) Schematics of mixed mT/mG chimera model. F) Percent apoptosis (Annexin V+) ± standard deviation of immature, semi-mature and mature thymocytes from mixed mT/mG chimeric mice (n=4, *P = 0.01, ***p <0.0008)
To assess whether thymocyte populations were altered by Ars2 depletion, multicolor flow cytometry was performed [31]. Significant decrease in the percent of CD4/CD8 double positive (DP) thymocytes (p = 0.007) with concomitant increase in the percent of CD8 single positive (CD8SP, p = 0.04), CD4 single positive (CD4SP, p = 0.003), and CD4/CD8 double negative (DN, p = 0.002) thymocytes was observed in thymi of Ars2 iKO mice one week following initial tamoxifen injection (Fig. 2C). These changes likely reflect rapid loss of immature Ars2 knockout thymocytes, as the number of mature thymocytes remained relatively stable (Fig. 2D, S2C).
To further explore effects of Ars2 deletion on thymic T-cell development, Ars2fl/fl mice were crossed with mice expressing Cre recombinase driven by the Lck promoter to induce deletion of Ars2 in thymocytes [32]. Lck-cre expressing Ars2fl/fl mice were viable (Fig. S3A) yet exhibited significant reduction in thymic cellularity and mass (Fig. S3B), potentially as a result of increased thymocyte apoptosis (Fig. S3C). Comparison of immature thymocyte populations from mice in which Ars2 deletion was driven by Cre-ERT2 versus Lck-cre demonstrated similar decrease in the number of DP thymocytes in both models, suggesting that thymocyte-intrinsic Ars2 is required for maturation beyond the DN stage (Fig. S3D). In contrast, the number of DN thymocytes decreased when Ars2 deletion was driven by Cre-ERT2 but not when Ars2 deletion was driven by Lck-cre (Fig. S3D).
Expression of Cre recombinase under the control of the Lck promoter was found to increase basal apoptosis of immature thymocytes [33], making interpretation of increased apoptosis observed when Ars2 deletion was driven by Lck-Cre difficult. To circumvent this difficulty and to determine if effects of Ars2 on thymocytes apoptosis are cell-intrinsic, we generated mixed chimeras in which Ars2 iKO thymocytes could be distinguished from Cre negative Ars2-floxed thymocytes by expression of fluorescent reporter proteins. In this model, 1:1 ratio of bone marrow from Cre-ERT2-positive (Ars2 iKO) and Cre-ERT2-negative (control) Ars2fl/fl mice with transgenic expression of the mT/mG Cre reporter, which expresses membrane-targeted tandem dimer Tomato (mT) prior to Cre-mediated excision and membrane-targeted green fluorescent protein (mG) after excision [34], was transplanted into lethally irradiated C57BL/6J mice (Fig. 2E). Decreased expression of Ars2 in mG+ thymocytes was confirmed by qRT-PCR (Fig. S3E). After two months of reconstitution, the gene coding Ars2 was deleted by five daily tamoxifen injections and apoptosis of thymocytes populations was analyzed by Annexin V staining. High rates of apoptosis were found in mG+, Ars2 iKO thymocytes within the two most immature thymocytes populations analyzed, CD4/CD8 double negative (DN) and CD8 immature single positive (CD8 ISP) (Fig. 2F, P = 0.01 and <0.0008, respectively). These data conclusively demonstrate a cell-intrinsic role for Ars2 in limiting apoptosis of immature thymocytes.
Ars2 is required for maintenance of long-term hematopoietic stem cells (LT-HSCs)
During characterization of mice in which Ars2 was deleted by Mx-Cre, Gruber et al. found decreased bone marrow cellularity and attributed this finding to the role of Ars2 in promoting cellular proliferation [3]. To begin to assess bone marrow function in our Ars2 iKO mice, we performed serial complete blood counts (CBCs) prior to and following tamoxifen injection. Ars2 iKO mice showed progressive reduction in peripheral white blood cells (WBC) and red blood cells (RBC) following tamoxifen injection, indicating underlying bone marrow dysfunction (Fig. 3A, S4A, B). Consistent with this observation, bone marrow cellularity was significantly reduced in tamoxifen-treated Ars2 iKO mice compared to littermate controls (Fig. 3B, p = 0.002).
Figure 3. Ars2 sustains proliferation of bone marrow progenitor cells.
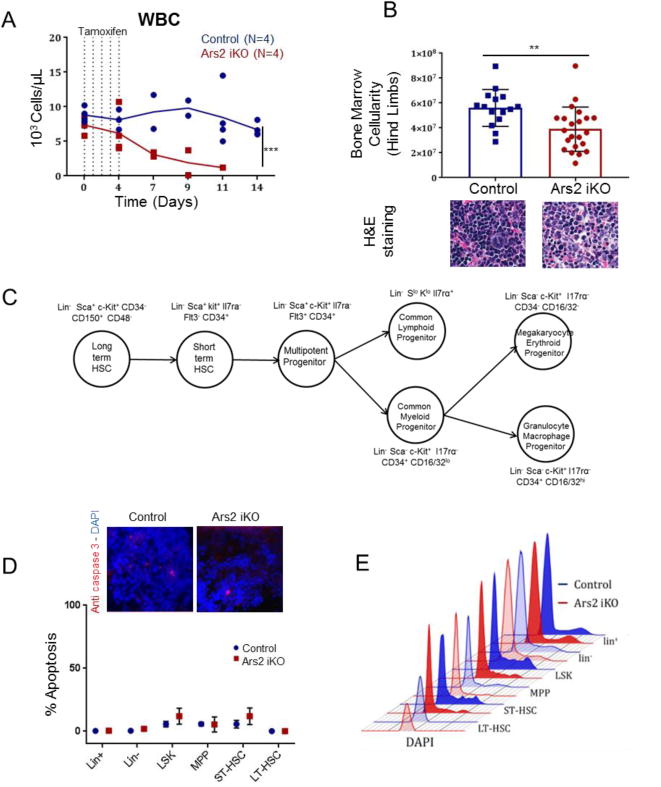
A) Serial counting of white blood cells (WBC) from Ars2 iKO and control mice prior to and following five daily tamoxifen injections. Solid lines represent mean values. Dotted lines represent tamoxifen injections. Dots represent individual mice (***P ≤ 0.001). B) (Top) Total number of bone marrow cells obtained from hind limbs of Ars2 iKO (39.01×106±17.8×106) or littermate control (56.09×106±14.7×106) mice 7-10 days following initial tamoxifen injection (**p = 0.002). Dots represent individual mice. (Bottom) H&E staining of paraffin-embedded femurs from control and Ars2 iKO mice 10 days following initial tamoxifen injection. C) Hematopoiesis hierarchy demonstrating various populations and their corresponding surface markers used in this study. D) (Top) Immunohistochemistry staining for anti-caspase 3 maker on bone marrow of Ars2 iKO and control mice 8 days post first tamoxifen injection. (Bottom) Percent apoptosis (Annexin V+,DAPI+) mean ± standard deviation among bone marrow mature and immature populations in control (n=2) and Ars2 iKO (n=2) mice 8 days post first tamoxifen injection. E) Bone marrow cells were stained for surface markers and then fixed and processed according to DAPI staining protocol for cell cycle analysis. Representative cell cycle histograms are presented for each population from control (blue) and Ars2 iKO (red) mice one week following initial tamoxifen injection.
To explore the underlying cause of bone marrow dysfunction in Ars2 iKO mice, cell cycle and apoptosis of mature bone marrow resident cells (lin+), immature bone marrow resident cells (lin−), and several lin− sub-populations (Fig. 3C) were measured by flow cytometry three, five and eight days after initiation of tamoxifen injections. In contrast to thymocytes, Ars2 knockout bone marrow cells exhibited very little apoptosis (<10%) as assessed by flow cytometry (Fig. 3D, S5A, B) and immunofluorescence staining for active caspase-3 (Fig. 3D). Furthermore, when comparing Ars2 knockout to littermate control mice no significant changes in apoptosis were observed in bone marrow sub-populations examined (Fig. 3D, S5C). These data indicate that reduced bone marrow cellularity in Ars2 iKO mice did not result from increased apoptosis of bone marrow resident cells following Ars2 deletion.
When cell cycle of bone marrow sub-populations was examined, no change in bulk lin+ or lin− populations was observed in tamoxifen-treated Ars2 iKO mice compared to littermate controls (Fig. 3E). In contrast, hematopoietic progenitor sub-populations from tamoxifen-treated Ars2 iKO mice, including Lin−, Sca+, c-Kit+ (LSK) [35], multipotetnt progenitors (MPP: lin−, Sca+, c-Kit+, CD34+, Il7ra−, Flt3+) and short-term hematopoietic stem cells (ST-HSC: lin−, Sca+, c-Kit+, CD34+, Il7ra−, Flt3−) [36], exhibited decreased frequency of cells in S and G2/M phase of the cell cycle (Fig. 3E). These cell types normally express high levels of Ars2 (Fig. S6A) and like immortalized cell lines in culture [3] appear to rely on Ars2 to maintain continued proliferation.
Despite observed reductions in proliferating cells within the LSK, MPP and ST-HSC bone marrow populations, the frequency of these cells within bone marrow of tamoxifen-treated Ars2 iKO mice was similar to littermate controls one week after initiation of tamoxifen treatment (Fig. 4A, B). Frequencies of additional downstream bone marrow progenitor populations, including common lymphoid progenitors (CLPs: lin−, Scalo, c-Kitlo, Il7ra+), common myeloid progenitors (CMPs: lin−, Sca−, c-Kit+, Il7ra−, CD34+, CD16/32lo), granulocyte-monocyte progenitors (GMPs: lin−, Sca−, c-Kit+, Il7ra−, CD34+, CD16/32hi), and megakaryocyte-erythrocyte progenitors (MEPs: lin−, Sca−, c-Kit+, Il7ra−, CD34−, CD16/32−) were also similar between tamoxifen-treated Ars2 iKO mice and littermate controls (Fig. S6B, C). Strikingly, only long-term hematopoietic stem cells (LT-HSCs: lin−, Sca+, c-Kit+, CD34−, CD48−, CD150+) [36] were significantly reduced in tamoxifen-treated Ars2 iKO mice when compared to littermate controls (Fig. 4A,B, p = 0.008). This reduction was rapid, as 3 of 5 Ars2 iKO mice examined displayed reduced LT-HSC frequency relative to littermate control mice five days following initiation of tamoxifen injections (Fig. S5D). Addition of Flt3 to our multicolor flow cytometry panel [23] had no effect on observed loss of LT-HSC from bone marrow of Ars2 deficient mice (Fig. S7A), while elimination of CD34 as a marker of HSC [36] resulted in loss of the phenotype (Fig. S7B). Since CD34 staining was essential to discriminate the LT-HSC population sensitive to Ars2 deletion, we used a second CD34 antibody to stain Ars2 iKO and littermate control bone marrow and confirmed that lin−, Sca+, c-Kit+, CD34−, CD48−, CD150+ LT-HSCs were reduced from Ars2 iKO bone marrow (Fig. S7C, D).
Figure 4. Ars2 is required for maintenance of long-term hematopoietic stem cells (LT-HSCs).
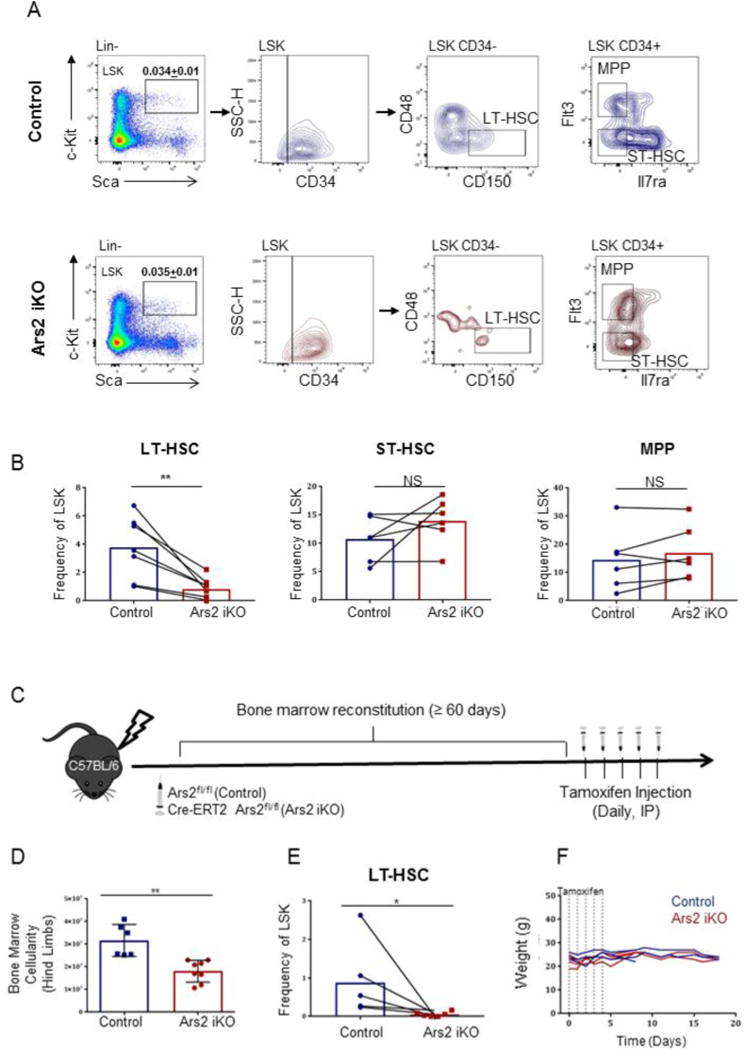
A) Representative flow cytometry plots and gating strategy for lin− (CD3−, CD11b−, B220−, Ly6-G−, TER119−) Sca+, c-Kit+ (LSK), long-term hematopoietic stem cell (LT-HSC), short-term hematopoietic stem cell (ST-HSC) and multipotent progenitor (MPPs) populations. B) Frequency of LT-HSCs, ST-HSCs and MPPs within the LSK population comparing Ars2 iKO (n=6) mice to littermate controls (n=6) 7-8 days following initial tamoxifen injection. Dots represent individual mice with lines connecting littermates. **p = 0.008, NS: p > 0.05. C) Schematic depicting experiments performed using bone marrow chimera mice. Donor bone marrow obtained from either Ars2fl/fl (control) or Cre-ERT2-Ars2fl/fl (Ars2 iKO) mice was transplanted into lethally irradiated wild-type C57BL/6J mice. After 2 months of bone marrow reconstitution, mice were injected with five daily doses of tamoxifen (75 mg/kg IP) and sacrificed 8 days after first tamoxifen injection or monitored for survival. D) Bone marrow cellularity was significantly reduced in Ars2 iKO bone marrow chimeras (18.54 ×106 ± 4.98 ×106) compared to control chimeras (28.47 ×106 ± 6.02 ×106, **p = 0.01). E) LT-HSC frequency of LSK was significantly reduced in Ars2 iKO chimeras (0.05 ± 0.02) compared to littermate control chimeras (0.88 ± 0.36, *p= 0.04). Dots represent individual mice with lines connecting littermates. E) Bone marrow chimeras were able to survive after Ars2 depletion with no significant changes in weight (Ars2fl/fl donor into wildtype host, N=3, Cre-ERT2-Ars2fl/fl donor into wildtype host, N=4).
Effects of Ars2 deletion on bone marrow are stroma-independent
Bone marrow stroma has myriad effects on hematopoietic progenitor cells, including LT-HSCs [37]. To determine if Ars2 deletion from stromal cells was the cause of phenotypes observed in tamoxifen-treated Ars2 iKO mice, lethally irradiated wild-type C57BL/6 mice were reconstituted with bone marrow from either Ars2 iKO mice or Ars2fl/fl mice as controls (Fig. 4C). Similar to induced whole-body Ars2 deletion, thymic involution occurred rapidly (data not shown) and examination of Ars2-deleted bone marrow from chimeric animals a week to ten days after initial tamoxifen injection (a similar timeframe as whole-body knockouts) revealed significant reduction in bone marrow cellularity (Fig. 4D, p = 0.01) and the LT-HSC population (Fig. 4E, p = 0.04) relative to control chimeras. However, unlike whole-body Ars2 knockout, induced deletion of Ars2 from bone marrow (and bone marrow derived cells) did not result in death and weight loss of mice (Fig. 4F).
Ars2 is required for hematopoiesis within bone marrow following myeloablation
Maintenance of LT-HSC population within the bone marrow relies on a balance between differentiation, self-renewal, and cell death, all of which can be affected by signals from bone marrow stroma cells [25]. Since data indicate that deletion of Ars2 does not alter viability of LT-HSCs and that wild-type stroma cannot rescue reduced numbers of Ars2 knockout LT-HSCs (Fig. 3D, 4E), we examined the self-renewal capacity of Ars2 deficient LT-HSCs by performing stressed hematopoiesis experiments.
We generated mT/mG chimeric mice by transplanting bone marrow from Cre-ERT2-positive (Ars2 iKO) or Cre-ERT2-negative (control) Ars2fl/fl mice expressing mT/mG Cre reporter into lethally irradiated C57BL/6J mice. After a two month reconstitution period, mT/mG chimeras were injected with tamoxifen to induce Ars2 deletion from bone marrow and bone marrow-derived cells, followed by weekly injections of a sub-lethal dose of 5-fluorouricil (5-FU, 120 mg/kg IP, Fig. 5A). Depletion of Ars2 from mG+ cells was confirmed by qRT-PCR in bone marrow and peripheral immune organs (Fig. S3E). Tamoxifen-treated Ars2 iKO chimeric mice lacked the ability to reconstitute peripheral white and red blood cells (WBC, RBC) following initial 5-FU injection (Fig. S8A). Additionally, rapid weight loss (Fig. 5B) and death (Fig. 5C) of tamoxifen-treated Ars2 iKO chimeric mice occurred following a second (n = 7) or third (n = 3) weekly 5-FU injections. In contrast, control chimeras demonstrated more robust reconstitution of peripheral WBC following each of three weekly 5-FU injections (Fig. S8A), stabilized their weight over the course of the experiment (Fig. 5B), and did not die until after third (n=1) or fourth (n=5) weekly 5-FU injections (Fig. 5C).
Figure 5. Ars2 is required for hematopoiesis within bone marrow following myeloablation.
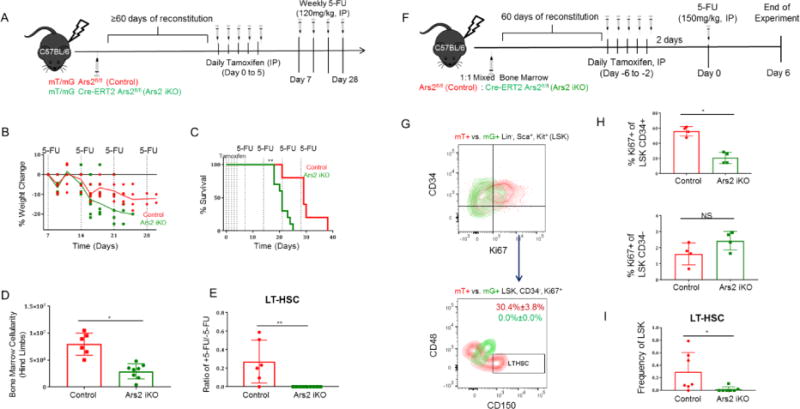
A) Schematic of stress hematopoiesis experiments performed using Cre-reporter (mT/mG) bone marrow chimeric mice. B) Change in weight of control (n=6, red line) and Ars2 iKO (n=10, green line) bone marrow chimera mice following Ars2 deletion and subsequent weekly 5-FU injections (120 mg/kg IP). Solid lines represent mean values. Each dot represents a mouse. C) Survival of control (n=6, red line) and Ars2 iKO (n=10, green line) bone marrow chimera mice prior to and following Ars2 deletion and subsequent weekly 5-FU injections (120 mg/kg IP). **p= 0.001. D) Total number of bone marrow cells obtained from hind limbs of control (7.95 ×106 ± 0.83 ×106, red bar) and Ars2 iKO (2.94×106 ± 0.438×106, green bar) chimeras following repeated 5-FU exposure. *p = 0.03. E) Change in frequency of LT-HSCs obtained from bone marrow of control (red bar) and Ars2 iKO (green bar) chimeras following repeated 5-FU exposure. ** p=0.003. F) Schematic depicting generation and use of mixed chimera mice. G) Proliferation of mT+ LSK cells overlaid with mG+ LSK cells and subdivided by CD34 and Ki67 markers from mixed chimera mice six days following injection of 5-FU (150 mg/kg IP). Proliferating (Ki67+) CD34− LSK cells were further examined for expression of LT-HSC markers (CD48−, CD150+). Quiescent (Ki67−) CD34− LSK cells showed a similar lack of mG+, CD48−, CD150+ LT-HSCs (data not shown). H) (Top) Percent of Ki67+ cells among mG+ CD34+ LSK cells was significantly reduced (*p= 0.02) compared to mT+ CD34+ LSK cells while (bottom) Ki67 positivity was unaffected in CD34− compartment of LSK cells (n =4). I) Ars2 knockout, mG+ LT-HSC frequency was significantly reduced compared to wild-type, mT+ LT-HSC frequency in bone marrow from mixed chimeras exposed to single dose of 5-FU (n=7, *p=0.03). (See Table 1). (D, E, H, I) Each dot represents a mouse.
Upon necropsy, tamoxifen-treated Ars2 iKO chimeras had significantly reduced bone marrow cellularity relative to control chimeras (Fig. 5D, p= 0.03). Flow cytometry revealed that repeated exposure of control chimeras to 5-FU (four weekly doses) resulted in approximately 70% reduction in mT+, Ars2-expressing LT-HSCs relative to control chimeras that never received 5-FU. Strikingly, mG+, Ars2 knockout LT-HSCs were reduced to an undetectable level in all mice examined following 2 to 3 weekly doses of 5-FU (Fig. 5E, p= 0.003).
Self-renewal of LT-HSCs is accomplished by asymmetric division to produce one daughter cell identical to its parent and one daughter cell that can expand and differentiate into all blood lineage cells [25]. Complete lack of identifiable LT-HSCs in tamoxifen-treated Ars2 iKO chimeras following multiple doses of 5-FU precluded analysis of their proliferation and/or differentiation in response to hematopoietic stress. Additionally, severe defects in peripheral blood reconstitution in 5-FU treated Ars2 knockout chimeras could multiply hematopoietic stress signals, potentially exacerbating depletion of LT-HSCs. To mitigate these possibilities we generated mixed chimeras by transplanting a 1:1 mix of bone marrow cells from mT/mG-expressing Ars2 iKO and control mice into lethally irradiated C57BL/6J mice. Following reconstitution, mixed mT/mG chimeras were given five daily injections of tamoxifen (75 mg/kg IP) followed by a single dose of 5-FU (150, mg/kg IP) (Fig. 5F).
As expected 5-FU treatment strongly induced proliferation of wild-type, mT+ LSK cells measured by Ki-67 staining (57.7% ± 6.1% Ki-67+). In contrast, proliferation was only moderately induced in Ars2 knockout mG+ LSK cells (23.3% ± 7.7% Ki-67+, Fig. 5G, S8B, p=0.02). Delineation of LSK sub-populations using CD34, a marker whose expression is unchanged by 5-FU treatment [38], revealed that the proliferation defect observed in Ars2 knockout mG+ cells was limited to CD34+ cells (Fig. 5H, p = 0.02), while CD34−, mT+ wild-type and CD34−,mG+ Ars2 knockout LSK cells demonstrated similar Ki-67 positivity (Fig. 5H, p = 0.3). When CD34− LSK cells were further subdivided using markers of LT-HSC (CD48−, CD150+), the vast majority of wild-type mT+ LT-HSCs were found to be Ki-67+ whereas Ars2 knockout mG+ LT-HSCs were not detected in Ki-67 stained bone marrow (p= 0.02, Fig. 5G,I). In fact, mG+ Ars2 knockout LT-HSCs were detected in only 2 of 7 mixed chimeras exposed to a single dose of 5-FU while Ars2 wild-type mT+ LT-HSCs were observed in 6 of 7 (Table 1).
Table 1.
Mixed mT/mG chimera bone marrow analysis, single dose of 5-FU (150mg/kg, IP).
| Frequency of LSK
| ||||||
|---|---|---|---|---|---|---|
| Mouse
|
LT-HSC
|
ST-HSC
|
MPP
|
|||
| mT+ | mG+ | mT+ | mG+ | mT+ | mG+ | |
|
|
|
|
||||
| 1 | 0.082 | 0 | 0.71 | 0.066 | 5.9 | 3.75 |
| 2 | 0.48 | 0.11 | 0.032 | 0 | 8.6 | 3.82 |
| 3 | 0.063 | 0.021 | 0.079 | 0.021 | 6.6 | 3.1 |
| 4 | 0.096 | 0 | N/A | N/A | N/A | N/A |
| 5 | 0.56 | 0 | N/A | N/A | N/A | N/A |
| 6 | 0.79 | 0 | N/A | N/A | N/A | N/A |
| 7 | 0 | 0 | N/A | N/A | N/A | N/A |
|
|
|
|
|
|||
| Average | 0.29 | 0.018 | 0.27 | 0.029 | 7.03 | 3.55 |
Self-renewal defect in Ars2 knockout LT-HSCs is cell-intrinsic
Near complete loss of Ars2-deficient LT-HSCs, but not Ars2-expressing LT-HSCs, from 5-FU treated mixed chimeras strongly suggests that Ars2 is necessary for self-renewal of LT-HSCs following in vivo stress. Alternatively, rapid loss of LT-HSCs following 5-FU exposure could have resulted from increased sensitivity of Ars2 knockout LT-HSCs to death induced by 5-FU. Examination of tamoxifen-treated mixed chimera mice in the absence of 5-FU treatment (Fig. 6A) revealed a profound decrease in mG+, Ars2 knockout LT-HSCs relative to mT+, wild-type LT-HSCs one week following initiation of tamoxifen treatment (p= 0.002, Fig. 6B,C). Importantly, mixed chimera mice displayed no signs of bone marrow failure and had normal bone marrow cellularity upon necropsy (86.6×106 ± 15.2×106 bone marrow cells). Consistent with lack of self-renewal potential of Ars2 knockout LT-HSCs, singly sorted LT-HSCs from Ars2 knockout chimeras failed to produce large, differentiated colonies in single cell colony formation assays (Fig. 6D,E) [39]. As expected control LT-HSCs formed large colonies consisting mainly of morphologically mature blood cells. In contrast, LT-HSCs from tamoxifen-treated Ars2 iKO chimeras produced few small colonies consisting of uniform, morphologically undifferentiated cells (p = 0.007, Fig. 6F).
Figure 6. Defect in Ars2 knockout LT-HSCs is cell-intrinsic.
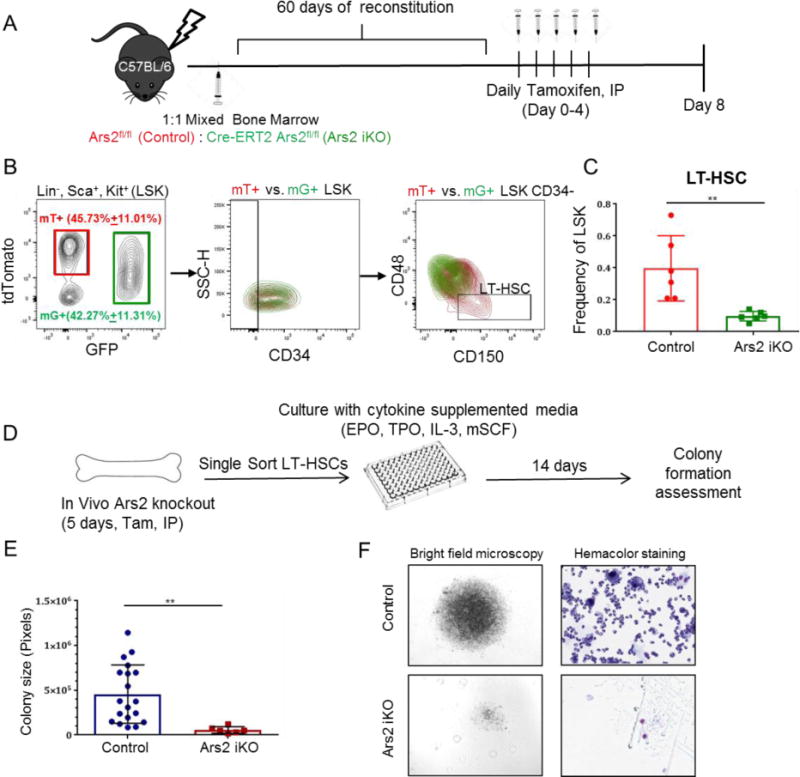
A) Schematic of mixed mT/mG chimera generation. Two months after reconstitution with 1:1 bone marrow cells from both Cre+ mT/mG and Cre- mT/mG mice, mice were injected with daily tamoxifen and bone marrow was analyzed at day 8. B) Representative density plots with mT+, wild-type (red) and mG+, Ars2 knockout (green) cells isolated from a single mixed chimera overlaid to demonstrate gating strategy and intrinsic differences between cell populations. C) Frequency of Ars2 knockout, mG+ LT-HSCs (green bar) was reduced compared to wild-type, mT+ LT-HSCs (red bar) in mixed chimeras eight days following initial tamoxifen injection. n=6, **p=0.002. D) Schematic of single cell colony formation assay. Single LT-HSCs from tamoxifen-treated control or Ars2 iKO chimeras were FACS sorted into 96 well plates and cultured for 14 days in cytokine supplemented media. E) Size of colonies formed from Ars2 iKO LT-HSCs was significantly less than size of colonies formed from control LT-HSCs (**p = 0.007). Each dot represents a colony. F) Comparison of representative colony size (left) and differentiation (right) obtained from a singly sorted LT-HSC from a tamoxifen-treated control chimera (top) versus a tamoxifen-treated Ars2 iKO chimera (bottom).
Discussion
Several recent studies have established Ars2 as a critical mediator of transcription and co-transcriptional RNA processing [4, 6, 11, 12]. However, little is known about physiological roles for Ars2 in mammals, as its germline deletion led to early embryonic lethality [1]. Here we describe generation and characterization of whole-body inducible Ars2 knockout mice. Using these mice we provide mechanistic insight into the requirement for Ars2 in hematopoiesis, both in the bone marrow and during thymic T-cell maturation.
Our data indicate that the in vivo ability of long-term hematopoietic stem cells (LT-HSCs) to self-renew is critically dependent on expression of Ars2. Additionally, proliferation of several bone marrow progenitor populations decreased upon Ars2 deletion. These data likely explain reduced bone marrow cellularity initially observed when Ars2 was inducibly deleted from bone marrow cells using MxCre [3] and again when inducibly deleted from the whole body (Fig. 3,4) or from bone marrow chimeras (Fig. 4) using Cre-ERT2. In addition to confirming and extending the bone marrow phenotype of Ars2 knockout mice reported by Gruber et al. [3], we found that Ars2 supports T-cell development in the thymus (Fig. 2). Interestingly, reported phenotypes required deletion of both alleles of Ars2, as heterozygous mice or cells from heterozygous mice displayed no phenotype (data not shown).
Self-renewal of stem cells is accomplished through cell division in the absence of differentiation of one daughter cell [21, 22]. Determining whether Ars2 influences self-renewal of LT-HSCs through effects on proliferation versus differentiation has proven difficult, although data point to a role for Ars2 in limiting LT-HSC differentiation. Depletion of the Ars2fl/fl Cre-reporter (mG+) LT-HSC pool from mixed chimera mice within one week of tamoxifen injection (Fig. 6) provides the best evidence for spontaneous differentiation of LT-HSCs following Ars2 knockout. In these mice downstream bone marrow and peripheral blood populations were maintained by wild-type progenitors co-occupying the bone marrow, thus limiting signals that activate LT-HSCs to enter cell cycle. Moreover, LT-HSCs from tamoxifen-treated Ars2 iKO mice displayed a cell cycle profile containing predominantly 1N, non-proliferating cells (Fig. 3E) suggesting that Ars2 knockout alone is insufficient to stimulate LT-HSCs to proliferate. Rather, data point to a scenario in which LT-HSC differentiation is suppressed by a gene expression program that relies on continued expression of Ars2. Mechanistic studies similar to those performed by Andreu-Agullo et al. in neural stem cells [18] are required to conclusively establish that Ars2 suppresses differentiation of LT-HSCs. However, these studies are beyond the scope of the current manuscript.
Previous studies focusing on cellular context and consequences of Ars2 expression have demonstrated that high levels are necessary for cells to rapidly proliferate in culture [3, 10]. In contrast, Ars2 has been found to maintain viability [1, 3] or cell identity [18] when examined in the context of normal mammalian physiology. Studies of Ars2 expression in blood cancers further suggest that Ars2 may be less important for in vivo proliferation, as low expression of Ars2 was found to correlate with aggressive pediatric acute lymphocytic leukemia [40] and the region of human chromosome 7 containing SRRT is commonly deleted in acute myeloid leukemia [41]. Our whole-body inducible Ars2 knockout mice reveal that in vivo effects of Ars2 are context-dependent, with thymocytes relying on Ars2 for survival (Fig. 2) [3], bone marrow progenitor cells relying on Ars2 for cell cycle progression (Fig. 3), and long-term hematopoietic stem cells relying on Ars2 to limit differentiation (Figs. 4–6). These findings are not surprising in light of the molecular role of Ars2 in coordinating transcriptional and co-transcriptional events underlying gene expression programs. Whether context-dependent effects of Ars2 reflect cell type-specific molecular functions of Ars2 (transcriptional regulation of Sox2 to maintain neural stem cells[18] vs. 3′ end processing during replication-dependent histone mRNA biogenesis to support proliferation of cultured cells [4, 11]) or universal molecular functions of Ars2 resulting in distinct phenotypic outcomes due to underlying context-dependent gene expression programs remains an open question.
A striking consequence of whole-body Ars2 deletion was rapid demise of 100% of Ars2 deficient animals accompanied by clear signs of bone marrow failure (rapid weight loss, decreased peripheral blood and bone marrow cellularity). Surprisingly, deletion of Ars2 from bone marrow derived cells using two distinct models (inducible knockout in chimeras in this study and MxCre driven knockout in the Gruber et al. study[4]) did not kill mice even though reduced bone marrow cellularity was observed. This raises several possibilities, 1) Ars2 deletion from LT-HSCs was incomplete, 2) Ars2 expressed in bone marrow stroma cells is needed to support hematopoiesis downstream of LT-HSCs, or 3) death following Ars2 deletion did not result from bone marrow failure. An organ site whose failure following Ars2 deletion could explain rapid weight loss and death is the intestine. Microscopic examination of intestines from Ars2 knockout mice showed marked reduction in crypt depth and organization (data not shown). Similar to bone marrow, cells of the intestine are continuously replenished through differentiation of tissue-specific stem cells[42]. If, as data presented here and in Andreu-Agullo et al. study [18] suggest, Ars2 is broadly required for maintenance of stem cell populations, its deletion from intestinal stem cells could lead to rapid loss of intestinal epithelium and result in malabsorption, weight loss and death of Ars2 knockout animals.
Although further efforts are required to fully appreciate the importance of Ars2 in normal mammalian physiology, this study provides insight into roles for Ars2 in hematopoiesis. Data demonstrate that Ars2 is required for adult mice to maintain a pool of undifferentiated hematopoietic stem cells, likely by suppressing their differentiation, and that Ars2 is essential for survival of thymocytes during T cell maturation. While molecular studies will provide mechanistic details of how Ars2 affects LT-HSCs and thymocytes, the current study identifies a second type of stem cell that requires Ars2 to maintain its population in vivo, suggesting that Ars2 may play an especially important role in the biology of stem cells.
Material and methods
Mice
Mice were maintained in Laboratory Animal Shared Resource (LASR) at Roswell Park Cancer Institute and all procedures were approved by the institutional animal care (IACUC). Four different strains of mice were used in this study: C57BL/6J (B6), Stock No: 000664, B6.Cg-Tg(UBC-cre/ERT2)1Ejb/1J (Cre-ERT2), Stock No: 007001 and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (mT/mG), Stock No:007576 were purchased from Jackson Laboratory. Ars2 floxed (Ars2 fl/fl) mice were obtained from Thompson Lab[3]. Cre-ERT2 Ars2 fl/fl, mT/mG Ars2 fl/fl and mT/mG Cre-ERT2-Ars2 fl/fl were generated by crossbreeding of Cre-ERT2 and mT/mG with Ars2 fl/fl mice. To delete Ars2 gene from its floxed sites, Cre-ERT2 was activated by intra-peritoneal injection of 75μgr Tamoxifen (Sigma, 10540-29-1) per gram of 6-8 weeks old mouse for 5 days. Chimera mice were generated by tail vein injection of 2.5-5 million bone marrow cells from Ars2 fl/fl or Cre-ERT2 Ars2 fl/fl or mT/mG Ars2 fl/fl or mT/mG Cre-ERT2-Ars2 fl/fl into lethally irradiated (600 Rad) B6 mice. Mixed chimeras were generated by tail vein injection of 1:1 ratio of bone marrow cells from both Ars2 fl/fl and Cre-ERT2-Ars2 fl/fl or mT/mG Ars2 fl/fl and mT/mG Cre-ERT2-Ars2 fl/fl into lethally irradiated C57BL/6J mice.
Flow cytometry
To identify the stem/progenitor cell populations, bone marrow was harvested by flushing the hind limb bones using FACS buffer (1% bovine serum albumin (BSA) and 0.5% sodium azide in 1X PBS) and filtered through 40μM cell strainer. Red blood cells were lysed using ACK lysing buffer (Thermofisher scientific). Cells were stained in a two-step manner with CD34 as the first step and the rest of the antibodies as the second step. Data was collected and analyzed by BD LSR II A flow cytometer and flowjo V10 software, respectively. For cell cycle analysis, cells were stained with surface markers, fixed, permeabilized (Intrasure Kit) and DAPI stained. To analyze T cells, harvested thymus was meshed through a 40μM cell strainer and stained for flow cytometry. Markers used in this study are listed in supplementary Table-1.
Immunohistochemistry
Femur bones were formalin fixed, decalcified and 10μm sectioned by pathology core facility of Roswell Park Cancer Institute. Sections were deparaffinized by xylene and ethanol method [43], antigen retrieved (heat induced antigen retrieval using Reveal Decloaker, Biocare), permeabilized by Triton 0.5% and blocked by 5% normal goat serum in PBS. Slides were incubated with primary antibody (anti caspase 3 (Rb), 1:200) overnight. Next day, slides were washed (1XPBS) and incubated with secondary antibody (Goat anti rabit alexa fluor 488, 1:2000) for 2 hours. Lastly, slides were washed (1XPBS) and DAPI mounted. Images were taken by fluorescence microscope. Antibodies are listed in supplementary Table-1.
Self-renewal (5-FU) assays
At day 7 post tamoxifen injection, 5-fluorouracil (5-FU) was administered intraperitoneally at either four weekly 120mg/kg doses or single 150mg/kg dose. Blood was drawn from sub-mandibular vein and collected in 50 μM EDTA tubes. CBC was performed using HemaTrue. Moribund mice (identified by ruffled fur, hunched back, more than 20% reduction in weight) were euthanized according to IACUC protocol and bone marrow was harvested for flow cytometry.
Colony formation assay (CFA)
Single cell colony formation assay was performed according to Ema et al. protocol [39] from single sorted Lin−Sca+kit+CD34−CD150+CD48− LT-HSCs. After 14 days of culture, colonies were imaged and analyzed by ImageJ. Colonies were cytospined and stained for hemacolor according to its protocol (EMD Millipore).
Quantitative Real Time PCR (qRT-PCR)
RNA from harvested organs was extracted using trizol and miRNeasy kit (Qiagen). cDNA was generated using super script IV (Thermofisher Scientific) and qPCR with SYBR green master mix (Thermofisher Scientific) was performed using Quant studio 6 flex device. Primers are listed in supplementary Table-2. Expression of Ars2 was normalized by TBP using ΔCT method. (Expression level= 2 (CT TBP −CT Ars2).
Statistical analysis
All values are expressed in mean + SD/SEM. Unpaired student t-test was used to compare two experimental groups using GraphPad Prism 7 software. P value less than 0.05 was considered significant.
Supplementary Material
Highlights.
In vivo maintenance of LT-HSCs pool relies on RNA-binding protein Ars2.
Ars2 supports the proliferation of early hematopoietic progenitors.
Deletion of Ars2 results in thymocyte apoptosis.
Physiological effects of Ars2 are context-dependent.
Acknowledgments
This work was supported by National Cancer Institute (NCI) grants R00CA175189 and P30CA016056 involving the use of Roswell Park Comprehensive Cancer Center Flow and Image Cytometry, Pathology Network, Genomics and Laboratory Animal Shared Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson MD, et al. ARS2 is a conserved eukaryotic gene essential for early mammalian development. Molecular and cellular biology. 2008;28(5):1503–1514. doi: 10.1128/MCB.01565-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossman TG, Wang Z. Expression cloning for arsenite-resistance resulted in isolation of tumor-suppressor fau cDNA: possible involvement of the ubiquitin system in arsenic carcinogenesis. Carcinogenesis. 1999;20(2):311–316. doi: 10.1093/carcin/20.2.311. [DOI] [PubMed] [Google Scholar]
- 3.Gruber JJ, et al. Ars2 links the nuclear cap-binding complex to RNA interference and cell proliferation. Cell. 2009;138(2):328–339. doi: 10.1016/j.cell.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber JJ, et al. Ars2 promotes proper replication-dependent histone mRNA 3′ end formation. Molecular cell. 2012;45(1):87–98. doi: 10.1016/j.molcel.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen PR, et al. The human cap-binding complex is functionally connected to the nuclear RNA exosome. Nature structural & molecular biology. 2013;20(12):1367–1376. doi: 10.1038/nsmb.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabin LR, et al. Ars2 regulates both miRNA-and siRNA-dependent silencing and suppresses RNA virus infection in Drosophila. Cell. 2009;138(2):340–351. doi: 10.1016/j.cell.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visa N, et al. A nuclear cap-binding complex binds Balbiani ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. The Journal of cell biology. 1996;133(1):5–14. doi: 10.1083/jcb.133.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giacometti S, et al. Mutually exclusive CBC-containing complexes contribute to RNA fate. Cell reports. 2017;18(11):2635–2650. doi: 10.1016/j.celrep.2017.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze WM, Cusack S. Structural basis for mutually exclusive co-transcriptional nuclear cap-binding complexes with either NELF-E or ARS2. Nature Communications. 2017;8(1):1302. doi: 10.1038/s41467-017-01402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olejniczak SH, et al. Long-lived microRNA–Argonaute complexes in quiescent cells can be activated to regulate mitogenic responses. Proceedings of the National Academy of Sciences. 2013;110(1):157–162. doi: 10.1073/pnas.1219958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Sullivan C, et al. Mutagenesis of ARS2 Domains To Assess Possible Roles in Cell Cycle Progression and MicroRNA and Replication-Dependent Histone mRNA Biogenesis. Molecular and cellular biology. 2015;35(21):3753–3767. doi: 10.1128/MCB.00272-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallais M, et al. CBC–ARS2 stimulates 3′-end maturation of multiple RNA families and favors cap-proximal processing. Nature structural & molecular biology. 2013;20(12):1358–1366. doi: 10.1038/nsmb.2720. [DOI] [PubMed] [Google Scholar]
- 13.Laubinger S, et al. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proceedings of the National Academy of Sciences. 2008;105(25):8795–8800. doi: 10.1073/pnas.0802493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty SM, et al. Participation of the nuclear cap binding complex in pre-mRNA 3′ processing. Proceedings of the National Academy of Sciences. 1997;94(22):11893–11898. doi: 10.1073/pnas.94.22.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iasillo C, et al. ARS2 is a general suppressor of pervasive transcription. Nucleic acids research. 2017;45(17):10229–10241. doi: 10.1093/nar/gkx647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubas M, et al. Interaction profiling identifies the human nuclear exosome targeting complex. Molecular cell. 2011;43(4):624–637. doi: 10.1016/j.molcel.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Andreu-Agullo C, et al. Ars2 maintains neural stem-cell identity through direct transcriptional activation of Sox2. Nature. 2012;481(7380):195–198. doi: 10.1038/nature10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feil S, Valtcheva N, Feil R. Inducible cre mice. Gene Knockout Protocols: Second Edition. 2009:343–363. doi: 10.1007/978-1-59745-471-1_18. [DOI] [PubMed] [Google Scholar]
- 20.Babovic S, Eaves CJ. Hierarchical organization of fetal and adult hematopoietic stem cells. Experimental cell research. 2014;329(2):185–191. doi: 10.1016/j.yexcr.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Seita J, I, Weissman L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2010;2(6):640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheshier SH, et al. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proceedings of the National Academy of Sciences. 1999;96(6):3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Cabezas-Wallscheid N, et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell. 2017;169(5):807–823. e19. doi: 10.1016/j.cell.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453(7193):306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- 26.Krueger A, Ziętara N, Łyszkiewicz M. T Cell Development by the Numbers. Trends in immunology. 2016 doi: 10.1016/j.it.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Witt CM, Robey EA. Seminars in immunology. Elsevier; 2005. Thymopoiesis in 4 dimensions. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz BA, Bhandoola A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunological reviews. 2006;209(1):47–57. doi: 10.1111/j.0105-2896.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 29.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of γδ lineage during thymopoiesis. The Journal of Immunology. 2009;182(9):5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annual review of immunology. 2003;21(1):139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 31.Hu Q, et al. Examination of thymic positive and negative selection by flow cytometry. Journal of visualized experiments: JoVE. 201268 doi: 10.3791/4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennet T, et al. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci U S A. 1995;92(26):12070–4. doi: 10.1073/pnas.92.26.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carow B, et al. Lck-driven Cre expression alters T cell development in the thymus and the frequencies and functions of peripheral T cell subsets. The Journal of Immunology. 2016;197(6):2261–2268. doi: 10.4049/jimmunol.1600827. [DOI] [PubMed] [Google Scholar]
- 34.Muzumdar MD, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 35.Okada S, et al. In vivo and in vitro stem cell function of c-kit-and Sca-1-positive murine hematopoietic cells. Blood. 1992;80(12):3044–3050. [PubMed] [Google Scholar]
- 36.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell stem cell. 2013;13(1):102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood. 2015;125(17):2621–2629. doi: 10.1182/blood-2014-09-570192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishio N, et al. Changes in markers, receptors and adhesion molecules expressed on murine hemopoietic stem cells after a single injection of 5-fluorouracil. Stem Cells. 1996;14(5):584–91. doi: 10.1002/stem.140584. [DOI] [PubMed] [Google Scholar]
- 39.Ema H, et al. Adult mouse hematopoietic stem cells: purification and single-cell assays. Nature protocols. 2006;1(6):2979–2987. doi: 10.1038/nprot.2006.447. [DOI] [PubMed] [Google Scholar]
- 40.Cui L, et al. Low expressions of ARS2 and CASP8AP2 predict relapse and poor prognosis in pediatric acute lymphoblastic leukemia patients treated on China CCLG-ALL 2008 protocol. Leuk Res. 2015;39(2):115–23. doi: 10.1016/j.leukres.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Wilson MD, et al. Comparative analysis of the gene-dense ACHE/TFR2 region on human chromosome 7q22 with the orthologous region on mouse chromosome 5. Nucleic Acids Res. 2001;29(6):1352–65. doi: 10.1093/nar/29.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature reviews Molecular cell biology. 2014;15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G, et al. Deparaffinization compositions and methods for their use. 2003 Google Patents. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


