Abstract
IMPORTANCE
Cancer is the second leading cause of death worldwide. Current estimates on the burden of cancer are needed for cancer control planning.
OBJECTIVE
To estimate mortality, incidence, years lived with disability (YLDs), years of life lost (YLLs), and disability-adjusted life-years (DALYs) for 32 cancers in 195 countries and territories from 1990 to 2015.
EVIDENCE REVIEW
Cancer mortality was estimated using vital registration system data, cancer registry incidence data (transformed to mortality estimates using separately estimated mortality to incidence [MI] ratios), and verbal autopsy data. Cancer incidence was calculated by dividing mortality estimates through the modeled MI ratios. To calculate cancer prevalence, MI ratios were used to model survival. To calculate YLDs, prevalence estimates were multiplied by disability weights. The YLLs were estimated by multiplying age-specific cancer deaths by the reference life expectancy. DALYs were estimated as the sum of YLDs and YLLs. A sociodemographic index (SDI) was created for each location based on income per capita, educational attainment, and fertility. Countries were categorized by SDI quintiles to summarize results.
FINDINGS
In 2015, there were 17.5 million cancer cases worldwide and 8.7 million deaths. Between 2005 and 2015, cancer cases increased by 33%, with population aging contributing 16%, population growth 13%, and changes in age-specific rates contributing 4%. For men, the most common cancer globally was prostate cancer (1.6 million cases). Tracheal, bronchus, and lung cancer was the leading cause of cancer deaths and DALYs in men (1.2 million deaths and 25.9 million DALYs). For women, the most common cancer was breast cancer (2.4 million cases). Breast cancer was also the leading cause of cancer deaths and DALYs for women (523 000 deaths and 15.1 million DALYs). Overall, cancer caused 208.3 million DALYs worldwide in 2015 for both sexes combined. Between 2005 and 2015, age-standardized incidence rates for all cancers combined increased in 174 of 195 countries or territories. Age-standardized death rates (ASDRs) for all cancers combined decreased within that timeframe in 140 of 195 countries or territories. Countries with an increase in the ASDR due to all cancers were largely located on the African continent. Of all cancers, deaths between 2005 and 2015 decreased significantly for Hodgkin lymphoma (−6.1% [95% uncertainty interval (UI), −10.6% to −1.3%]). The number of deaths also decreased for esophageal cancer, stomach cancer, and chronic myeloid leukemia, although these results were not statistically significant.
CONCLUSION AND RELEVANCE
As part of the epidemiological transition, cancer incidence is expected to increase in the future, further straining limited health care resources. Appropriate allocation of resources for cancer prevention, early diagnosis, and curative and palliative care requires detailed knowledge of the local burden of cancer. The GBD 2015 study results demonstrate that progress is possible in the war against cancer. However, the major findings also highlight an unmet need for cancer prevention efforts, including tobacco control, vaccination, and the promotion of physical activity and a healthy diet.
In 2015, cancer caused over 8.7 million deaths globally and was the second leading cause of death behind cardiovascular diseases.1 Even though these impressive numbers are testimony that the “war on cancer” has not been won, recent developments in personalized medicine and novel treatment approaches like immunotherapy have raised hope of significantly improving cancer survival.2–4 These expectations for patients with cancer in high-income countries contrast with the challenge of making basic diagnostic and treatment options widely available in low-resource settings.5 Both the equity and affordability of cancer care from individual and societal perspectives are increasingly being questioned.6 Survival rates between and within high-income countries differ for reasons such as variation in education, access to specialized care, effective treatment, and insurance status.7–9 The full potential of cancer prevention for reducing incidence and mortality is far from being realized, and efforts are especially lagging in low-income countries.10 Awareness of this “cancer divide,” with substantially worse outcomes and a high burden in socioeconomically disadvantaged populations, has led to a focus on global oncology by the international health community.4,5,10 This is reflected in the third Sustainable Development Goal (SDG) to “by 2030, reduce by one-third premature mortality from non-communicable diseases through prevention and treatment and promote mental health andwell-being.”11 Estimates of the burden of cancer are produced annually as part of the Global Burden of Disease (GBD) study providing a unique means of tracking progress in closing this divide. Here, we present results of the GBD 2015 study for 32 cancer groups covering cancer incidence, mortality, years of life lost (YLLs), years lived with disability (YLDs), and disability-adjusted life years (DALYs) for 195 countries or territories from 1990 to 2015 for both sexes across age groups.
Methods
Differences Between GBD 2015 and GBD 2013
General methods for GBD 2015 and prior GBD studies have been described previously.1,12 Here, we present methods and results specific to the GBD 2015 cancer estimation. The general framework for the cancer estimation in GBD 2015 has remained similar to GBD 2013, exceptions are detailed below.13 The GBD 2015 study is compliant with the newly developed Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER).14 A chart detailing fulfillment of GATHER requirements is provided in eTable 1 in the Supplement; flowcharts and a detailed description for each estimation step are also available in the eAppendix and in the numerous eTables and eFigures in the Supplement. Box 1 includes a list of the figures and tables in this article. Further details about methods and data sources are provided in the eAppendix, eFigures, and eTables in the Supplement. Box 2 contains a list of the supplementary figures and tables. Additional information is available from the authors in Web Tables 1 through 3; the web addresses for these items are listed in Box 3. Hereinafter, citations to Web Tables are for those given in Box 3. Data sources for GBD 2015 are listed in eTable 2 in the Supplement, including which new sources were added compared with GBD 2013.
Box 1. List of Figures and Tables in the Article.
Figure 1. Age-Specific Global Contributions of Cancer Types to Total Cancer Incidence and Mortality for Both Sexes, 2015
Figure 2. Relative Changes in Age-Standardized Cancer Incidence Rates in Both Sexes for All Cancers in 195 Countries or Territories From 2005 to 2015
Figure 3. Relative Changes in Age-Standardized Cancer Mortality Rates in Both Sexes for All Cancers in 195 Countries or Territories From 2005 to 2015
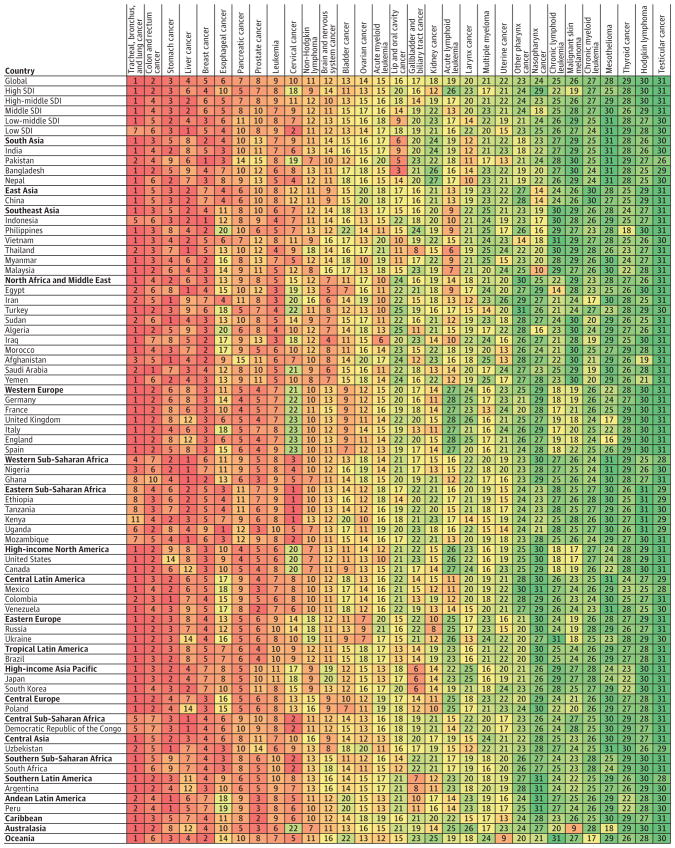
Figure 4. Cancers Ranked by Number of Incident Cases in Both Sexes, Global, by Region, by Sociodemographic Index, and in the 50 Most Populous Countries, 2015
Figure 5. Cancers Ranked by Number of Deaths in Both Sexes, Global, by Region, by Sociodemographic Index, and in the 50 Most Populous Countries, 2015
Figure 6. Cancers Ranked Globally and for Both Sexes by Absolute Years of Life Lost
Figure 7. Trends in Age-Standardized Incidence Rates for Breast Cancer, 1990–2015
Figure 8. Trends in Age-Standardized Incidence Rates for Tracheal, Bronchus, and Lung Cancer, 1990–2015
Figure 9. Trends in Age-Standardized Incidence Rates for Colon and Rectum Cancer, 1990–2015
Figure 10. Trends in Age-Standardized Incidence Rates for Prostate Cancer, 1990–2015
Figure 11. Trends in Age-Standardized Incidence Rates for Stomach Cancer, 1990–2015
Figure 12. Trends in Age-Standardized Incidence Rates for Liver Cancer, 1990–2015
Figure 13. Trends in Age-Standardized Incidence Rates for Non-Hodgkin Lymphoma, 1990–2015
Figure 14. Trends in Age-Standardized Incidence Rates for Leukemia, 1990–2015
Figure 15. Trends in Age-Standardized Incidence Rates for Bladder Cancer, 1990–2015
Figure 16. Trends in Age-Standardized Incidence Rates for Cervical Cancer, 1990–2015
Table 1. 2015 Global Incidence and Deaths for All Cancers and 32 Cancer Groups
Table 2. Decomposition Analysis of Cancer Trends in Global Incidence, Both Sexes, 2005 to 2015
Box 2. List of Supplementary Material, eAppendix, eTables, and eFigures.
eAppendix. Definitions, data sources, mortality to incidence ratio estimation, modeling parameters, and other study methods
eTable 1. GATHER Guidelines Checklist
eTable 2. Sources for Cancer Incidence and MI Ratio Data by Country, Year, and Registry
eTable 3. Number of Site-years for Cancer Mortality Data by Type
eTable 4. List of International Classification of Diseases (ICD) Codes Mapped to the Global Burden of Disease Cause List for Cancer Incidence Data
eTable 5. List of International Classification of Diseases (ICD) Codes Mapped to the Global Burden of Disease Cause List for Cancer Mortality Data
eTable 6. Undefined Cancer Code Categories (ICD-10) and Respective Target Codes for Cancer Registry Dncidence Data
eTable 7. Final MI Ratio Model Selection
eTable 8. Sociodemographic Index Groupings by Geography, Based on 2015 Values
eTable 9. Covariates Selected for CODEm for Each GBD Cancer Group and Expected Direction of Covariate
eTable 10. Comparison of GBD 2013 and GBD 2015 Covariates Used and Level of Covariates
eTable 11. Results for CODEm Model Testing
eTable 12. Percent Change Before and After CoDCorrect by Cancer for All Ages, Both Sexes Combined, 2015
eTable 13. Duration of 4 Prevalence Phases by Cancer
eTable 14. Disability Weights
eTable 15. Decomposition of Trends in Incidence by SDI Quintile, Both Sexes, 2005 to 2015
eTable 16. Probability of Developing Cancer Within Selected Age Intervals, Global, and by SDI Quintile, by Sex, 2010–2015 in% (Odds)
eFigure 1. Flowchart GBD Cancer Mortality, YLL Estimation
eFigure 2. Flowchart GBD Cancer Incidence, Prevalence, YLD Estimation
eFigure 3. Flowchart of Algorithm Used to Adjust MI Ratios
eFigure 4. Sociodemographic Index Quintiles, 2015
eFigure 5. Percentage of Deaths Added to Original ICD Codes After Redistribution of Garbage Codes, 2010, Male
eFigure 6. Percentage of Deaths Added to Original ICD Codes After Redistribution of Garbage Codes, 2010, Female
eFigure 7. Cancer Ranking by Total Incidence Based on Global Level for Developing and Developed Regions and All Countries, Both Sexes, 2015
eFigure 8. Cancer Ranking by Total Mortality Based on Global Level for Developing and Developed Regions and All Countries, Both Sexes, 2015
eFigure 9. Top Ranked Cancers by Absolute Incident Cases for All Ages in Males, 2015
eFigure 10. Top Ranked Cancers by Absolute Incident Cases for All Ages in Females, 2015
eFigure 11. Top Ranked Cancers by Absolute Deaths for All Ages in Males, 2015
eFigure 12. Top Ranked Cancers by Absolute Deaths for All Ages in Females, 2015
eFigure 13. Contribution of YLDs and YLLs to DALYs by Cancer, Global, Both Sexes, 2015
eFigure 14. Trends in Age-Standardized Incidence Rates for Esophageal Cancer, 1990–2015
eFigure 15. Trends in Age-Standardized Incidence Rates for Uterine Cancer, 1990–2015
eFigure 16. Trends in Age-Standardized Incidence Rates for Pancreatic Cancer, 1990–2015
eFigure 17. Trends in Age-Standardized Incidence Rates for Kidney Cancer, 1990–2015
eFigure 18. Trends in Age-Standardized Incidence Rates for Lip and Oral Cavity Cancer, 1990–2015
eFigure 19. Trends in Age-Standardized Incidence Rates for Malignant Melanoma, 1990–2015
eFigure 20. Trends in Age-Standardized Incidence Rates for Thyroid Cancer, 1990–2015
eFigure 21. Trends in Age-Standardized Incidence Rates for Brain and Nervous System Cancer, 1990–2015
eFigure 22. Trends in Age-Standardized Incidence Rates for Ovarian Cancer, 1990–2015
eFigure 23. Trends in Age-Standardized Incidence Rates for Larynx Cancer, 1990–2015
eFigure 24. Trends in Age-Standardized Incidence Rates for Chronic Lymphoid Leukemia, 1990–2015
eFigure 25. Trends in Age-Standardized Incidence Rates for Acute Myeloid Leukemia, 1990–2015
eFigure 26. Trends in Age-Standardized Incidence Rates for Gallbladder and Biliary Tract Cancer, 1990–2015
eFigure 27. Trends in Age-Standardized Incidence Rates for Other Pharynx Cancer, 1990–2015
eFigure 28. Trends in Age-Standardized Incidence Rates for Acute Lymphoid Leukemia, 1990–2015
eFigure 29. Trends in Age-Standardized Incidence Rates for Multiple Myeloma, 1990–2015
eFigure 30. Trends in Age-Standardized Incidence Rates for Nasopharynx Cancer, 1990–2015
eFigure 31. Trends in Age-Standardized Incidence Rates for Hodgkin Lymphoma, 1990–2015
eFigure 32. Trends in Age-Standardized Incidence Rates for Testicular Cancer, 1990–2015
eFigure 33. Trends in Age-Standardized Incidence Rates for Chronic Myeloid Leukemia, 1990–2015
eFigure 34. Trends in Age-Standardized Incidence Rates for Mesothelioma, 1990–2015
eFigure 35. Trends in Age-Standardized Incidence Rates, Other Cancers, 1990–2015
Box 3. Web Table Addresses.
Web Table 1. Incidence (absolute numbers and rates) by sex, 2005, 2015 (ja.ma/healthdataorg_webtable1_incidence)
Web Table 2. Mortality (absolute numbers and rates) by sex, 2005, 2015 (ja.ma/healthdataorg_webtable2_mortality)
Web Table 3. DALYs (absolute numbers and rates) by sex, 2005, 2015 (ja.ma/healthdataorg_webtable3_DALYs)
Relevant changes in the estimation strategy since GBD 2013 include the addition of 7 territories (American Samoa, Bermuda, Greenland, Guam, Northern Mariana Islands, Puerto Rico, and the US Virgin Islands), which previously were only included in the GBD regional totals. Results for the United Kingdom are reported for Northern Ireland, Scotland, Wales, and England). Changes to the GBD causes include dividing “leukemia” into acute lymphoid leukemia, chronic lymphoid leukemia, acute myeloid leukemia, and chronic myeloid leukemia. Methodological updates were made to the mortality to incidence (MI) ratio estimation, which are described in detail in the eAppendix in the Supplement. Major updates for the MI ratio predictions were out-of-sample validation of multiple model types and selection of 1 model per cancer based on the out-of-sample root-mean-squared error.
For GBD 2015, a sociodemographic index (SDI) was developed, which is a summary indicator derived from measures of income per capita, educational attainment, and fertility. Detailed methods describing computation of the SDI are reported elsewhere.1 In brief, the SDI weighs each component, which is rescaled between 0 and 1, equally. The composite SDI index is the mean of the 3 rescaled components. An SDI of 1.0 can be interpreted as a location that has the highest observed educational attainment, the highest log income per capita, and the lowest fertility rate. For GBD 2015, SDI quintiles were used to group countries that are similar based on their development status. Locations were grouped into quintiles based on their SDI value in 2015. Quintile cutoffs were based on the distribution of geography-years from 1980 to 2015 with the exception of populations smaller than 1 million. eFigure 4 and eTable 8 in the Supplement show the SDI quintile for each country. As for every GBD study, the full time series estimated for each GBD cycle supersedes prior GBD studies. For GBD 2015, the full time series from 1990 to 2015 was estimated. W e focus here on changes over the last decade. Estimates before 2005 as well as additional results can be found online (https://vizhub.healthdata.org/gbd-compare/).
Estimation Framework
The initial process in the burden of cancer estimation is the modeling of cancer mortality. One of the GBD study’s principles is to identify, and ideally use, all available data.15 Data inputs for cancer mortality estimation therefore come from 2 major pathways: (1) mortality data and (2) cancer registry incidence data transformed to mortality estimates. Mortality data from vital registration systems, verbal autopsies, and other sources like disease surveillance records were processed and added to a cause-of-death database. Methods and data sources have been described in detail previously.1
To maximize data availability and take advantage of cancer registry data in countries with scarce mortality data, incidence data from cancer registries were transformed to mortality estimates through the use of separately estimated MI ratios. Modeling of the MI ratios is described in detail in the eAppendix in the Supplement. In brief, the estimation followed a 3-step approach, the creation of logit random effect models, spatiotemporal smoothing, and Gaussian process regression. A final model was selected based on out-of-sample validation. Updated cancer registry data for GBD 2015 was obtained from the GBD collaborator network or downloaded from publically available sources. All data sources used for MI ratio estimation, as well as those used for incidence data transformed to mortality estimates, are listed in the eAppendix and eTable 2 in the Supplement.
For cancer estimation, 333 513 site-years were used from vital registration systems, 785 site-years from verbal autopsy, 619 site years from surveillance data, and 69 013 site years from cancer registry data. The number of site-years used by source type and by cancer can be found in eTable 3 in the Supplement. All data sources were extracted at the most detailed cause- and age-specific level and mapped to the GBD cause list. Codes from the International Classification of Diseases, Ninth Revision (ICD-9), and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), for each GBD cancer group as well as a description of data processing steps can be found in the eAppendix in the Supplement. The 32 cancer groups, together referred to as the “all cancer” group, encompass all malignant neoplasms as defined in the ICD-10 except for nonmelanoma skin cancer (NMSC, ICD-10 code C44) and Kaposi sarcoma (ICD-10 code C46). Although NMSC is the most common cancer in many populations, most cancer registries do not include NMSC, which necessitates different estimation methods from the cancers presented here.16 Deaths due to Kaposi sarcoma are not separately included because these were attributed to human immunodeficiency virus/AIDS in the GBD study.
The combined data on individual causes of death were used as input for the Cause of Death Ensemble model (CODEm).17 Covariates used for each cancer are listed in the eAppendix in the Supplement. Individual cause mortality estimates from CODEm were constrained to fit independently modeled, all-cause mortality estimates using the tool CodCorrect.1 We calculated YLLs by multiplying each death with the life expectancy for that age taken from a normative life table; life expectancy at birth was 86.5 years, which is based on the lowest observed death rate in each 5-year age group in populations over 5 million.1
Final mortality estimates were transformed into incidence estimates using modeled MI ratios. Uncertainty from the mortality estimation and from the MI ratio estimation was propagated to the incidence estimates. Ten-year cancer prevalence was modeled by estimating cancer survival using an MI ratio–based scaling factor, which takes into account location, year, and sex (see the eAppendix in the Supplement for details). This factor was used to scale the incidence cohort between a theoretical best-case and a theoretical worst-case survival. The absolute survival estimates allowed calculation of 10-year prevalence for each incidence cohort.
Total prevalence was divided into 4 sequelae reflecting varying degrees of disability during the cancer continuum: (1) diagnosis/treatment, (2) remission, (3) metastatic/disseminated, and (4) terminal phase. Duration of the 4 prevalence phases by cancer can be found in eTable 13 in the Supplement. Since data sources including stage distribution and treatment approaches are not available for most countries, the simplifying assumption of a constant duration of the diagnosis and treatment, metastatic/disseminated, and terminal phase for all ages, over time, and all countries was made. After dividing total prevalence into these 3 sequelae, we attributed the remaining prevalence to the remission phase.
To calculate YLDs, the prevalence for each sequela was multiplied with a disability weight. Additional disability was estimated for procedures and procedure-related morbidities associated with the treatment of breast, larynx, colorectal, bladder, and prostate cancer (mastectomy, laryngectomy, stoma, urinary incontinence, and impotence) under the assumption that these are major disabling sequelae after cancer treatment. Disability weights used for the different sequelae as well as methods to determine disability prevalence for these cancer-related outcomes can be found in the eAppendix in the Supplement. The sum of the YLDs for each general sequela, as well as for procedure-related sequelae, represent the total YLDs for each cancer. DALYs are the sum of YLLs and YLDs. One DALY can be regarded as 1 lost year of “healthy life.”
We calculated 2 scenarios to analyze the contribution of population aging, population growth, and changes in the age-specific incidence rates on the absolute change of cancer incidence. In the first scenario, the age structure, sex structure, and the age-specific rates from 2005 were applied to the total population of the year 2015. The difference between the total number of cases in 2005 and the hypothetical scenario were attributed to population growth. In the second hypothetical scenario, the age-specific rates from 2005 were applied to the age structure, sex structure, and population size of 2015. Differences between the second hypothetical scenario and the first hypothetical scenario were attributed to population aging. Differences between the total number of cases in 2015 and the second hypothetical scenario were attributed to changes in the age-specific rates.
In this publication, all rates are reported per 100 000 person-years. The GBD world population standard was used for the calculation of age-standardized weights.18 We report 95% uncertainty intervals (UIs) for all estimates (listed in parentheses after point estimates).
Results
Global Incidence, Mortality, and DALYs
In 2015, there were 17.5 million incident cancer cases worldwide and 8.7 million cancer deaths, as detailed in Table 1. Cancer caused 208.3 million DALYs in 2015, of which 96% came from YLLs and 4% came from YLDs (Web Table 3). At the global level, the odds of developing cancer during a lifetime (age 0–79 years) differed between the sexes: they were 1 in 3 for men and 1 in 4 for women (eTable 16 in the Supplement). These odds differ substantially among SDI categories. In the lowest SDI quintile, the odds of developing cancer for men aged between 0 and 79 years were 1 in 6, whereas in the highest SDI quintile, 1 in 2 men developed cancer. For women, the odds of developing cancer was 1 in 5 in the lowest SDI quintile and 1 in 3 in the highest quintile.
Table 1.
2015 Global Incidence and Deaths for All Cancers and 32 Cancer Groupsa
| Cancerb | Incident Cases, Thousandsc
|
ASIRc
|
Deaths, Thousandsc
|
ASDRc
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Male | Female | Total | Male | Female | Male | Female | |
| All cancers | 17 481 | 9269 | 8212 | 304.6 | 229.2 | 8713 | 5046 | 3667 | 170.7 | 103.5 |
|
| ||||||||||
| (16 847–18 177) | (8768–9947) | (7904–8570) | (288.5–326.9) | (220.6–239.0) | (8539–8894) | (4907–5196) | (3576–3756) | (166.1–175.8) | (101.0–106.0) | |
|
| ||||||||||
| Lip and oral cavity cancer | 410 (388–435) | 263 (244–283) | 148 (136–160) | 8.1 (7.6–8.7) | 4.1 (3.8–4.5) | 146 (142–151) | 98 (94–101) | 48 (46–51) | 3.1 (3.0–3.3) | 1.4 (1.3–1.4) |
|
| ||||||||||
| Nasopharynx cancer | 123 (99–144) | 88 (65–108) | 34 (26–45) | 2.6 (1.9–3.1) | 0.9 (0.7–1.2) | 63 (51–67) | 46 (34–49) | 17 (16–18) | 1.4 (1.0–1.5) | 0.5 (0.5–0.5) |
|
| ||||||||||
| Other pharynx cancer | 161 (152–172) | 118 (111–128) | 43 (39–48) | 3.6 (3.4–3.9) | 1.2 (1.1–1.3) | 64 (62–67) | 47 (45–49) | 17 (16–19) | 1.5 (1.4–1.5) | 0.5 (0.5–0.5) |
|
| ||||||||||
| Esophageal cancer | 483 (437–549) | 352 (312–416) | 130 (116–150) | 11.6 (10.3–13.7) | 3.7 (3.3–4.3) | 439 (423–457) | 318 (302–335) | 121 (115–128) | 10.5 (10.0–11.1) | 3.5 (3.3–3.7) |
|
| ||||||||||
| Stomach cancer | 1313 (1238–1404) | 872 (806–957) | 440 (413–471) | 29.2 (27.0–31.8) | 12.5 (11.7–13.4) | 819 (795–844) | 535 (516–556) | 284 (274–294) | 18.3 (17.7–19.0) | 8.1 (7.8–8.3) |
|
| ||||||||||
| Colon and rectum cancer | 1653 (1601–1714) | 920 (878–965) | 733 (702–767) | 30.9 (29.6–32.3) | 20.8 (19.9–21.7) | 832 (812–855) | 456 (442–468) | 376 (363–391) | 15.9 (15.4–16.3) | 10.7 (10.3–11.1) |
|
| ||||||||||
| Liver cancer | 854 (768–961) | 591 (517–691) | 264 (227–314) | 18.6 (16.3–21.6) | 7.5 (6.4–8.9) | 810 (750–863) | 577 (524–622) | 234 (204–255) | 18.2 (16.6–19.6) | 6.6 (5.8–7.2) |
|
| ||||||||||
| Gallbladder and biliary tract cancer | 188 (175–199) | 81 (76–87) | 107 (96–117) | 2.8 (2.6–3.0) | 3.0 (2.7–3.3) | 140 (131–147) | 60 (56–62) | 81 (73–87) | 2.1 (2.0–2.2) | 2.3 (2.1–2.5) |
|
| ||||||||||
| Pancreatic cancer | 426 (412–439) | 220 (210–230) | 206 (198–216) | 7.4 (7.1–7.7) | 5.9 (5.6–6.2) | 412 (404–421) | 215 (210–220) | 197 (191–203) | 7.3 (7.1–7.5) | 5.6 (5.4–5.8) |
|
| ||||||||||
| Larynx cancer | 238 (226–253) | 190 (178–205) | 48 (45–52) | 6.0 (5.6–6.4) | 1.3 (1.3–1.4) | 106 (103–109) | 86 (83–90) | 19 (19–20) | 2.8 (2.7–2.9) | 0.6 (0.5–0.6) |
|
| ||||||||||
| Tracheal, bronchus, and lung cancer | 2019 (1906–2149) | 1379 (1281–1499) | 640 (602–690) | 46.1 (42.9–49.6) | 18.2 (17.1–19.6) | 1722 (1674–1773) | 1206 (1165–1252) | 517 (497–538) | 41.0 (39.6–42.5) | 14.7 (14.2–15.3) |
|
| ||||||||||
| Malignant skin melanoma | 352 (282–445) | 190 (124–273) | 162 (142–175) | 6.0 (3.8–8.5) | 4.5 (4.0–4.9) | 60 (48–73) | 32 (21–45) | 27 (24–29) | 1.1 (0.7–1.5) | 0.8 (0.7–0.8) |
|
| ||||||||||
| Breast cancer | 2422 (2280–2541) | 44 (40–49) | 2378 (2236–2497) | 1.4 (1.2–1.5) | 65.5 (61.7–68.8) | 534 (502–553) | 10 (9–11) | 523 (492–543) | 0.3 (0.3–0.4) | 14.6 (13.7–15.1) |
|
| ||||||||||
| Cervical cancer | 526 (483–571) | NA | 526 (483–571) | NA | 14.3 (13.2–15.6) | 239 (225–252) | NA | 239 (225–252) | NA | 6.6 (6.2–7.0) |
|
| ||||||||||
| Uterine cancer | 455 (409–507) | NA | 455 (409–507) | NA | 12.6 (11.4–14.0) | 90 (86–94) | NA | 90 (86–94) | NA | 2.5 (2.4–2.7) |
|
| ||||||||||
| Ovarian cancer | 251 (239–266) | NA | 251 (239–266) | NA | 6.9 (6.6–7.3) | 161 (157–167) | NA | 161 (157–167) | NA | 4.5 (4.4–4.7) |
|
| ||||||||||
| Prostate cancer | 1618 (1321–2222) | 1618 (1321–2222) | NA | 56.7 (45.9–78.4) | NA | 366 (303–460) | 366 (303–460) | NA | 14.2 (11.8–17.9) | NA |
|
| ||||||||||
| Testicular cancer | 72 (67–77) | 72 (67–77) | NA | 1.9 (1.8–2.1) | NA | 9 (9–10) | 9 (9–10) | NA | 0.3 (0.3–0.3) | NA |
|
| ||||||||||
| Kidney cancer | 425 (406–447) | 268 (253–286) | 157 (146–172) | 8.6 (8.1–9.2) | 4.4 (4.1–4.9) | 137 (133–141) | 89 (86–93) | 48 (46–49) | 3.0 (2.9–3.1) | 1.4 (1.3–1.4) |
|
| ||||||||||
| Bladder cancer | 541 (517–567) | 412 (390–437) | 129 (121–137) | 14.1 (13.4–15.0) | 3.6 (3.4–3.9) | 188 (183–193) | 137 (133–141) | 51 (49–53) | 5.1 (4.9–5.2) | 1.5 (1.4–1.5) |
|
| ||||||||||
| Brain and nervous system cancer | 321 (293–348) | 175 (150–198) | 146 (134–160) | 5.2 (4.4–5.8) | 4.1 (3.7–4.4) | 229 (210–245) | 127 (108–141) | 102 (96–106) | 3.9 (3.3–4.3) | 2.8 (2.7–3.0) |
|
| ||||||||||
| Thyroid cancer | 334 (310–353) | 141 (123–153) | 194 (181–210) | 4.3 (3.7–4.7) | 5.4 (5.1–5.9) | 32 (29–33) | 13 (11–14) | 18 (17–20) | 0.5 (0.4–0.5) | 0.5 (0.5–0.6) |
|
| ||||||||||
| Mesothelioma | 37 (35–39) | 27 (25–29) | 10 (9–11) | 0.9 (0.9–1.0) | 0.3 (0.3–0.3) | 32 (31–33) | 23 (22–24) | 9 (9–10) | 0.8 (0.8–0.8) | 0.3 (0.3–0.3) |
|
| ||||||||||
| Hodgkin lymphoma | 78 (70–91) | 49 (43–61) | 28 (24–36) | 1.4 (1.2–1.7) | 0.8 (0.6–1.0) | 24 (22–29) | 15 (13–19) | 9 (7–12) | 0.5 (0.4–0.6) | 0.2 (0.2–0.3) |
|
| ||||||||||
| Non-Hodgkin lymphoma | 666 (584–710) | 379 (319–415) | 287 (249–313) | 11.7 (9.7–12.8) | 8.1 (7.0–8.8) | 231 (196–244) | 133 (109–143) | 98 (82–104) | 4.4 (3.5–4.7) | 2.8 (2.3–2.9) |
|
| ||||||||||
| Multiple myeloma | 154 (145–162) | 82 (77–87) | 72 (66–78) | 2.7 (2.5–2.9) | 2.0 (1.9–2.2) | 101 (98–104) | 52 (51–54) | 49 (46–51) | 1.8 (1.7–1.9) | 1.4 (1.3–1.5) |
|
| ||||||||||
| Leukemia | 606 (573–643) | 352 (325–385) | 254 (235–275) | 10.8 (10.1–11.7) | 7.1 (6.6–7.7) | 353 (345–363) | 204 (197–212) | 149 (144–154) | 6.6 (6.3–6.8) | 4.2 (4.0–4.3) |
|
| ||||||||||
| Acute lymphoid leukemia | 161 (141–184) | 95 (79–114) | 66 (57–78) | 2.7 (2.3–3.2) | 1.8 (1.6–2.2) | 110 (101–118) | 65 (57–72) | 45 (43–49) | 1.9 (1.7–2.1) | 1.3 (1.2–1.4) |
|
| ||||||||||
| Chronic lymphoid leukemia | 191 (179–204) | 106 (97–116) | 85 (78–93) | 3.4 (3.2–3.7) | 2.4 (2.2–2.6) | 61 (58–65) | 34 (32–38) | 27 (25–28) | 1.2 (1.2–1.4) | 0.8 (0.7–0.8) |
|
| ||||||||||
| Acute myeloid leukemia | 190 (175–209) | 113 (98–131) | 78 (71–85) | 3.5 (3.0–4.0) | 2.2 (2.0–2.4) | 147 (137–157) | 85 (76–95) | 62 (59–64) | 2.7 (2.5–3.0) | 1.7 (1.7–1.8) |
|
| ||||||||||
| Chronic myeloid leukemia | 64 (60–68) | 39 (35–43) | 25 (23–27) | 1.2 (1.1–1.4) | 0.7 (0.6–0.8) | 35 (33–38) | 20 (19–23) | 15 (14–16) | 0.7 (0.6–0.8) | 0.4 (0.4–0.4) |
|
| ||||||||||
| Other neoplasms | 756 (680–809) | 386 (329–429) | 370 (335–399) | 12.0 (10.2–13.3) | 10.3 (9.3–11.1) | 372 (336–392) | 191 (160–206) | 181 (162–192) | 6.1 (5.1–6.5) | 5.1 (4.6–5.4) |
Abbreviations: ASDR, age-standardized death rate per 100 000 person-years; ASIR, age-standardized incidence rate per 100 000 person-years; NA, not applicable.
All data reported as number or rate (95% UI).
Cancer groups are defined based on International Classification of Diseases, Ninth Revision (ICD-9), and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), codes and include all codes pertaining to neoplasms (ICD-9 140–208; ICD-10 C00–C96) except for nonmelanoma skin cancer (C44) and Kaposi sarcoma (C46). eTables 4 and 5 in the Supplement detail how the original ICD codes were mapped to the standardized Global Burden of Disease cause list.
Detailed results for incidence, mortality, and disability-adjusted life-years for the global level, by sociodemographic index quintile, region, and country are reported in Web Tables 1 through 3.
In 2015, prostate cancer, TBL (tracheal, bronchus, and lung) cancer, and colorectal cancer were the most common incident (95% UI) cancers in men—accounting for 42% of all cancer cases among men—with 1.6 million (1.3–2.2 million), 1.4 million (1.3–1.5 million), and 920 000 (878 000–965 000) cases, respectively (Table 1). The most common causes of cancer deaths for men were TBL, liver, and stomach cancer with 1.21 (1.16–1.25) million, 577 000 (524 000–622 000), and 535 000 (516 000–556 000) deaths, respectively. The leading causes for cancer DALYs in 2015 for men were TBL, liver, and stomach cancer, with 25.9 million (25.0–27.0 million), 15.4 million (14.0–16.7 million), and 11.7 million (11.2–12.2 million), respectively. For women in 2015, the most common incident cancers were breast, colorectal, and TBL cancer, with 2.4 million (2.2–2.5 million), 733 000 (702 000–767 000), and 640 000 (602 000–690 000), respectively. These cancers were responsible for 46% of all incident cases among women. The leading causes of cancer deaths were breast, TBL, and colorectal cancer, 523 000 (492 000–543 000), 517 000 (497 000–538 000), and 376 000 (363 000–391 000) deaths, respectively. Breast, TBL, and colorectal cancer were also the leading causes for female cancer DALYs in 2015, with 15.1 million (14.2–15.9 million), 10.5 million (10.1–11.0 million), and 7.2 million (7.0–7.5 million), respectively.
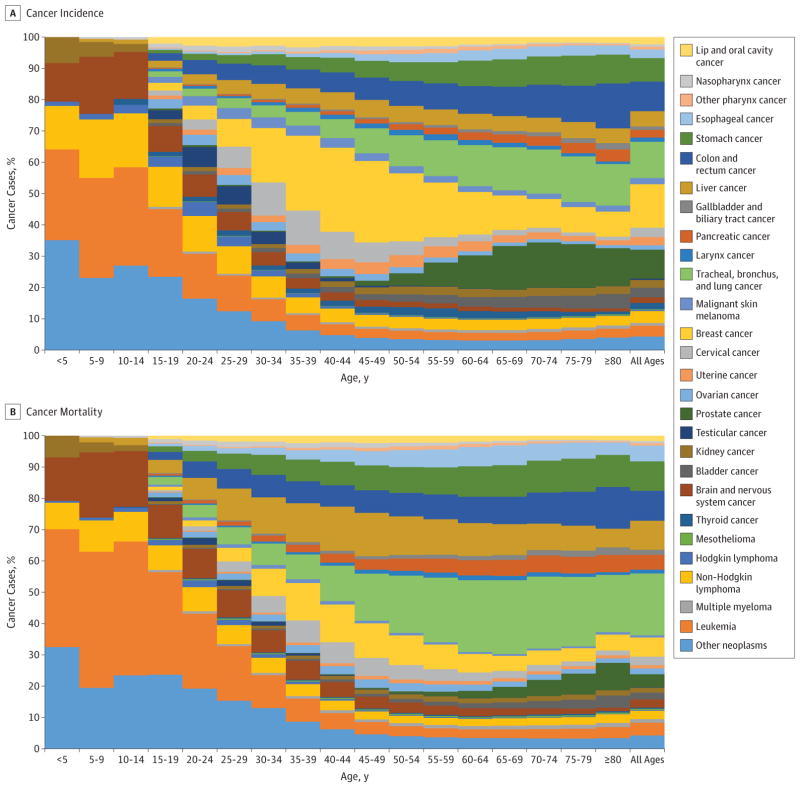
Figure 1 shows the pattern of cancer incidence and mortality by age group. For childhood cancers (age 0–14 years), the most common were leukemia, other neoplasms (see eTables 4 and 5 in the Supplement for ICD codes included under “other neoplasms”), non-Hodgkin lymphoma (NHL), and brain and nervous system cancers (Figure 1A). Leukemia, other neoplasms, and brain and nervous system cancers were also the leading contributors to childhood cancer deaths (Figure 1B). For adolescents and young adults (age 15–39 years) the most common cancers at the global level were breast cancer, cervical cancer, and other neoplasms. The main causes of cancer deaths for this age group were leukemia, other neoplasms, and liver cancer. For the population older than 39 years, the cancers contributing the most incident cases were TBL, breast, prostate, and colorectal cancer, while the main contributors to cancer deaths in this age group were TBL, stomach, and colorectal cancer.
Figure 1.
Age-Specific Global Contributions of Cancer Types to Total Cancer Incidence and Mortality For Both Sexes, 2015
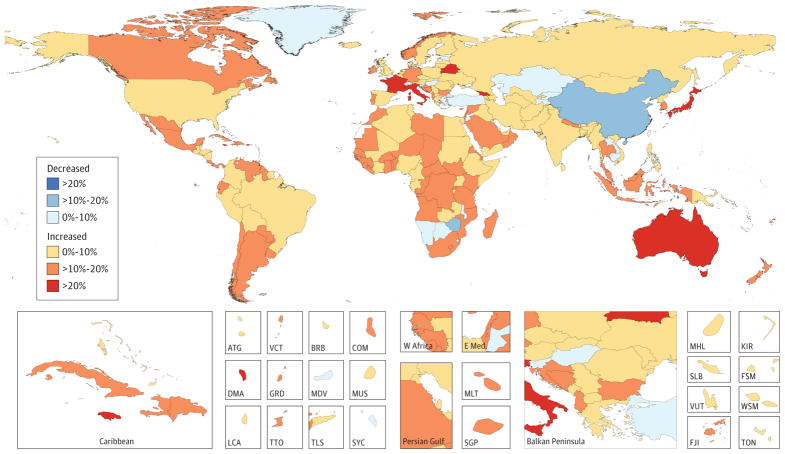
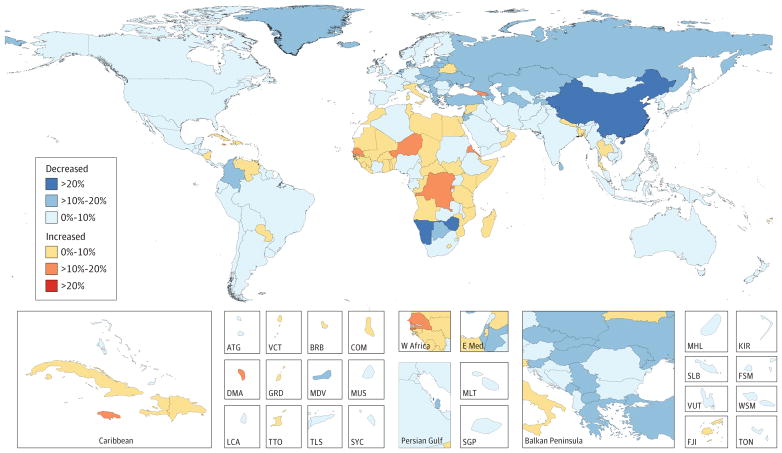
Between 2005 and 2015, age-standardized incidence rates (ASIRs) for all cancers combined increased in 174 of 195 countries or territories (Figure 2). China was a notable exception, with a 12% decrease in cancer incidence. In contrast, age-standardized death rates (ASDR) for all cancers combined decreased within that timeframe in 140 of 195 countries or territories, as shown in Figure 3, which also shows that countries with an increase in ASDR were largely located on the African continent.
Figure 2. Relative Changes in Age-Standardized Cancer Incidence Rates in Both Sexes for All Cancers in 195 Countries or Territories From 2005 to 2015.
Data reflect both sexes for all cancers excluding nonmelanoma skin cancer in 195 countries or territories from 2005 to 2015. The 95% UIs are reported in Web Table 1. ATG indicates Antigua and Barbuda; BRB, Barbados; COM, Comoros; DMA, Dominica; E Med: Eastern Mediterranean; FJI, Fiji; FSM, Federated States of Micronesia; GRD, Grenada; KIR, Kiribati; KS, Kaposi sarcoma; LCA, Saint Lucia; MDV, Maldives; MLT, Malta; MUS, Mauritius; MHL, Marshall Islands; NMSC, nonmelanoma skin cancer; SGP, Singapore; SLB, Solomon Islands; SYC, Seychelles; TLS, Timor-Leste; TON, Tonga; TTO, Trinidad and Tobago; VCT, Saint Vincent and the Grenadines; VUT, Vanuatu; W Africa, West Africa; WSM, Samoa.
Figure 3. Relative Changes in Age-Standardized Cancer Mortality Rates in Both Sexes for All Cancers in 195 Countries or Territories From 2005 to 2015.
Data reflect both sexes for all cancers excluding nonmelanoma skin cancer in 195 countries or territories from 2005 to 2015. The 95% UIs are reported in Web Table 2. ATG indicates Antigua and Barbuda; BRB, Barbados; COM, Comoros; DMA, Dominica; E Med: Eastern Mediterranean; FJI, Fiji; FSM, Federated States of Micronesia; GRD, Grenada; KIR, Kiribati; KS, Kaposi sarcoma; LCA, Saint Lucia; MDV, Maldives; MLT, Malta; MUS, Mauritius; MHL, Marshall Islands; NMSC, nonmelanoma skin cancer; SGP, Singapore; SLB, Solomon Islands; SYC, Seychelles; TLS, Timor-Leste; TON, Tonga; TTO, Trinidad and Tobago; VCT, Saint Vincent and the Grenadines; VUT, Vanuatu; W Africa, West Africa; WSM, Samoa.
The number (95% UI) of incident cases increased in all SDI quintiles between 2005 and 2015 for nearly all cancers; exceptions were esophageal cancer in middle and high-middle SDI countries, where incidence fell by 9% (−24.3% to 8.3%) and 4% (−17.7% to 14.0%), respectively, and cervical cancer in middle, high-middle, and high SDI countries, with a 5% (−19.6% to 12.3%), 5% (−14.3 to 6.2), and 2% (−7.4% to 2.9%) decrease, respectively (Web Table 1). However, these decreases were not statistically significant. The largest increase in cancer incident cases between 2005 and 2015 occurred in low SDI countries, with a 50% increase, of which population growth contributed 33%, changing age-specific incidence rates 13%, and changing age structure 4% (eTable 15 in the Supplement). The second largest increase occurred in the low-middle SDI quintile, with a 40% increase, followed by high SDI countries, with a 36% increase, high-middle SDI countries, with a 28% increase, and middle SDI countries, with a 27% increase (eTable 15 in the Supplement).
Global Top 10 Cancers in 2015
The top 10 cancers were ranked highest (top) number of incident cases (Figure 4).
Figure 4.
Cancers Ranked by Number of Incident Cases in Both Sexes, Global, by Region, by Sociodemographic Index (SDI), and in the 50 Most Populous Countries, 2015
1. Breast Cancer
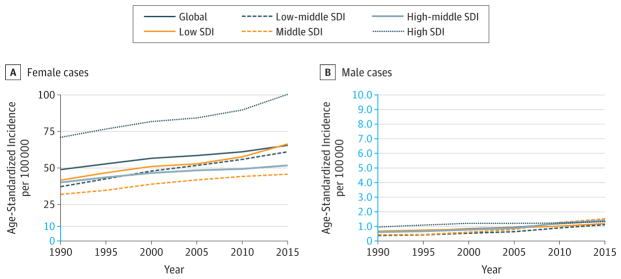
Breast cancer was the most common cancer overall, with an estimated 2.4 million (95% UI, 2.3–2.5 million) incident cases in 2015. The vast majority occurred in women, with 2.4 million (95% UI, 2.2–2.5 million) cases vs 44 000 (95% UI, 40 000–49 000) cases in men (Table 1). Breast cancer was the leading cause of cancer in all SDI quintiles except for the high and high-middle SDI quintiles where it was the second most common cancer (Figure 4). It was the cause of death for 523 000 (95% UI, 492 000–543 000) women and 10 000 (95% UI, 9000–11 000) men, making it the fifth leading cause of cancer deaths for both sexes in 2015 at the global and the low SDI countries, the fourth leading cause in high SDI countries, the sixth leading cause in high-middle and middle SDI countries, and the third leading cause in the low-middle SDI quintile (Figure 5). For women, breast cancer was the leading cause of death in 2015 (Table 1). Breast cancer caused 15.4 million (95% UI, 14.4–16.2 million) DALYs for both sexes, of which 88% came from YLLs, and 12% from YLDs (eFigure 13 in the Supplement). One in 14 women and 1 in 603 men developed breast cancer between birth and age 79 years (eTable 16 in the Supplement) at the global level. For women, the odds of developing breast cancer were the highest in high SDI countries, with 1 in 9 women developing breast cancer, compared with the lowest odds of 1 in 20 women in middle SDI countries developing breast cancer between age 0 and 79 years.
Figure 5.
Cancers Ranked by Number of Deaths in Both Sexes, Global, by Region, by Sociodemographic Index (SDI), and in the 50 Most Populous Countries, 2015
For women (per 100 000 person-years) in 2015, ASIRs (95% UIs) and ASDRs (95% UIs) were the lowest in East Asia: ASIR 35.8 (27.5–45.4), ASDR 8.2 (6.9–9.3); South Asia: ASIR 44.4 (37.1–52.3), ASDR 11.9 (10.6–12.9); and Andean Latin America: ASIR 47.2 (39.6–54.6), ASDR 10.5 (9.1–12) (Web Tables 1 and 2). They were the highest in high-income North America: ASIR 124.8 (115.9–145.4), ASDR 19.9 (18.9–23.2);Western Europe: ASIR 124.7 (116.3–138.3), ASDR 21.8 (20.5–23.6);and Australasia: ASIR 123.7 (112.5–137.9), ASDR 19.8 (18.3–21.4).
Breast cancer was the most common cancer for women in 183 countries or territories and the most common cause of cancer deaths in women in 115 countries or territories (eFigures 10 and 12 in the Supplement).
Between 2005 and 2015, breast cancer remained the fifth leading cause of global cancer YLLs, as shown in Figure 6. If global population size and age structure had remained stable between 2005 and 2015, the change in age-specific incidence rates between 2005 and 2015 would have resulted in a 15% increase in incident cases (Table 2). Overall incident cases increased by 43% because of population growth (contributing an additional 13%) and aging (contributing 15%). The ASIR (95% UI) for women (per 100 000 person-years) between 2005 and 2015 increased by 12% (95% UI, 4.3%–19.5%) at the global level from 58.5 (55.7–61.9) to 65.5 (61.7–68.8). The largest increase occurred in low SDI countries, with a 26% increase, from 52.8 (43.8–70.2) to 66.4 (51.3–88.2). ASIR at the global level and for all SDI quintiles increased since 1990 (Figure 7). Age-standardized DALY rates (95% UI) for women between 2005 and 2015 decreased by 6% (−12.1% to −1.0%) at the global level, with the largest decrease of 10% (−17.9% to −3.3%) in high-middle SDI countries and the largest increase in low SDI countries of 10% (−12.5% to 38.5%), which was not statistically significant (Web Table 3).
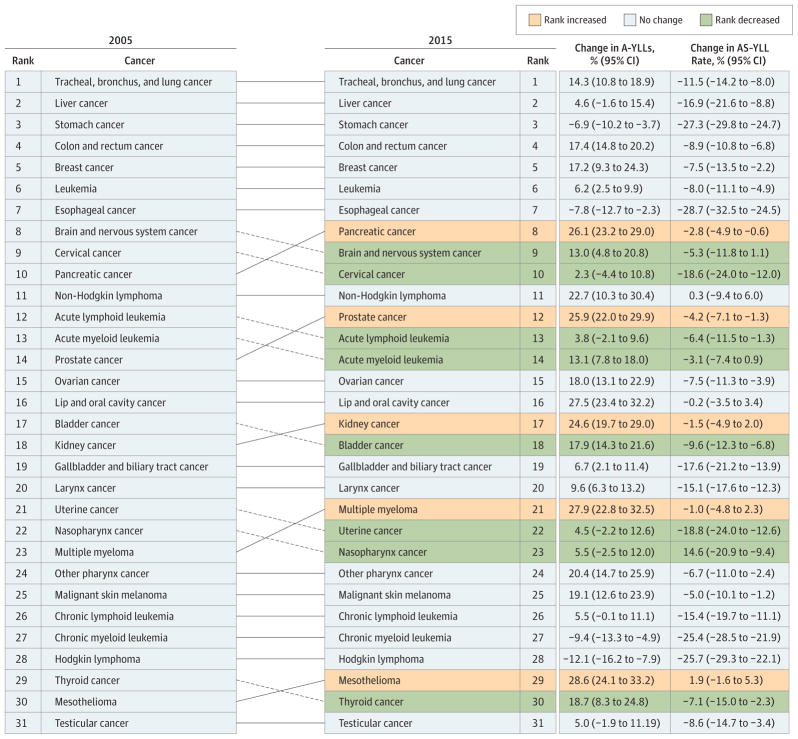
Figure 6. Cancers Ranked Globally and for Both Sexes by Absolute Years of Life Lost (YLLs).
Illustrated data include the percentage change in absolute YLLs (A-YLLs) and the percentage change in the age-standardized YLL (AS-YLL) rate between 2005 and 2015;. The “other cancers” group is not included in these data because it contains multiple different types of cancers. Solid lines connecting the 2005 and 2015 charts indicate increased or unchanged rank for the connected cancers; dotted lines indicate decreased rank.
Table 2.
Decomposition Analysis of Cancer Trends in Global Incidence, Both Sexes, 2005 to 2015
| Cancer | Incident Cases, No. | Expected Incident Cases, 2015, No. | Change in Incident Cases, 2005 to 2015, % | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Year 2005 | Year 2015 | Given Population Growth Alone | Given Population Growth and Aging | Due to Population Growth | Due to Change in Age Structure | Due to Change in Incidence Rates | Overall Change, % | |
| All cancers | 13 139 155 | 17 481 408 | 14 794 895 | 16 946 677 | 12.6 | 16.4 | 4.1 | 33.0 |
|
| ||||||||
| Lip and oral cavity cancer | 300 615 | 410 304 | 338 497 | 388 610 | 12.6 | 16.7 | 7.2 | 36.5 |
|
| ||||||||
| Nasopharynx cancer | 105 367 | 122 733 | 118 644 | 132 486 | 12.6 | 13.1 | −9.3 | 16.5 |
|
| ||||||||
| Other pharynx cancer | 124 247 | 161 427 | 139 904 | 162 449 | 12.6 | 18.1 | −0.8 | 29.9 |
|
| ||||||||
| Esophageal cancer | 459 299 | 482 578 | 517 178 | 601 758 | 12.6 | 18.4 | −25.9 | 5.1 |
|
| ||||||||
| Stomach cancer | 1 195 229 | 1 312 553 | 1 345 846 | 1 561 152 | 12.6 | 18.0 | −20.8 | 9.8 |
|
| ||||||||
| Colon and rectum cancer | 1 211 619 | 1 653 476 | 1 364 302 | 1 590 531 | 12.6 | 18.7 | 5.2 | 36.5 |
|
| ||||||||
| Liver cancer | 708 536 | 854 260 | 797 822 | 912 015 | 12.6 | 16.1 | −8.2 | 20.6 |
|
| ||||||||
| Gallbladder and biliary tract cancer | 158 742 | 188 233 | 178 746 | 210 027 | 12.6 | 19.7 | −13.7 | 18.6 |
|
| ||||||||
| Pancreatic cancer | 310 791 | 425 667 | 349 956 | 410 362 | 12.6 | 19.4 | 4.9 | 37.0 |
|
| ||||||||
| Larynx cancer | 193 477 | 238 150 | 217 859 | 251 416 | 12.6 | 17.3 | −6.9 | 23.1 |
|
| ||||||||
| Tracheal, bronchus, and lung cancer | 1 567 203 | 2 018 622 | 1 764 695 | 2 050 860 | 12.6 | 18.3 | −2.1 | 28.8 |
|
| ||||||||
| Malignant skin melanoma | 225 344 | 351 880 | 253 741 | 287 816 | 12.6 | 15.1 | 28.4 | 56.2 |
|
| ||||||||
| Breast cancer | 1 693 867 | 2 421 698 | 1 907 321 | 2 169 390 | 12.6 | 15.5 | 14.9 | 43.0 |
|
| ||||||||
| Cervical cancer | 532 132 | 525 907 | 599 189 | 663 070 | 12.6 | 12.0 | −25.8 | −1.2 |
|
| ||||||||
| Uterine cancer | 331 391 | 454 538 | 373 151 | 428 044 | 12.6 | 16.6 | 8.0 | 37.2 |
|
| ||||||||
| Ovarian cancer | 200 321 | 251 404 | 225 564 | 255 660 | 12.6 | 15.0 | −2.1 | 25.5 |
|
| ||||||||
| Prostate cancer | 974 188 | 1 618 087 | 1 096 951 | 1 289 311 | 12.6 | 19.7 | 33.7 | 66.1 |
|
| ||||||||
| Testicular cancer | 51 706 | 72 403 | 58 222 | 59 787 | 12.6 | 3.0 | 24.4 | 40.0 |
|
| ||||||||
| Kidney cancer | 278 569 | 425 111 | 313 673 | 360 896 | 12.6 | 17.0 | 23.1 | 52.6 |
|
| ||||||||
| Bladder cancer | 412 936 | 540 885 | 464 973 | 542 579 | 12.6 | 18.8 | −0.4 | 31.0 |
|
| ||||||||
| Brain and nervous system cancer | 257 203 | 320 907 | 289 615 | 314 329 | 12.6 | 9.6 | 2.6 | 24.8 |
|
| ||||||||
| Thyroid cancer | 168 107 | 334 468 | 189 291 | 215 624 | 12.6 | 15.7 | 70.7 | 99.0 |
|
| ||||||||
| Mesothelioma | 26 376 | 36 925 | 29 700 | 34 468 | 12.6 | 18.1 | 9.3 | 40.0 |
|
| ||||||||
| Hodgkin lymphoma | 68 830 | 77 728 | 77 504 | 81 911 | 12.6 | 6.4 | −6.1 | 12.9 |
|
| ||||||||
| Non-Hodgkin lymphoma | 430 197 | 666 130 | 484 408 | 541 281 | 12.6 | 13.2 | 29.0 | 54.8 |
|
| ||||||||
| Multiple myeloma | 107 965 | 153 589 | 121 570 | 141 270 | 12.6 | 18.2 | 11.4 | 42.3 |
|
| ||||||||
| Leukemia | 481 088 | 606 025 | 541 712 | 590 363 | 12.6 | 10.1 | 3.3 | 26.0 |
|
| ||||||||
| Acute lymphoid leukemia | 130 912 | 160 885 | 147 409 | 151 484 | 12.6 | 3.1 | 7.2 | 22.9 |
|
| ||||||||
| Chronic lymphoid leukemia | 151 954 | 190 860 | 171 102 | 192 517 | 12.6 | 14.1 | −1.1 | 25.6 |
|
| ||||||||
| Acute myeloid leukemia | 141 772 | 190 194 | 159 638 | 174 768 | 12.6 | 10.7 | 10.9 | 34.2 |
|
| ||||||||
| Chronic myeloid leukemia | 56 450 | 64 087 | 63 564 | 71 595 | 12.6 | 14.2 | −13.3 | 13.5 |
|
| ||||||||
| Other neoplasms | 563 810 | 755 719 | 634 859 | 699 211 | 12.6 | 11.4 | 10.0 | 34.0 |
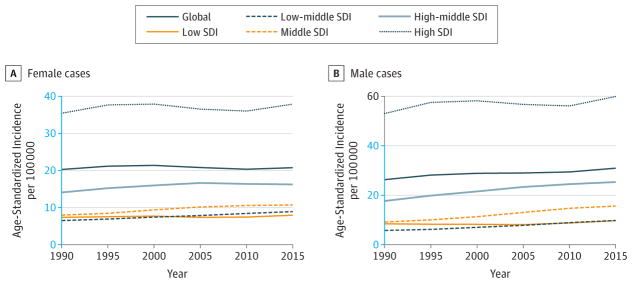
Figure 7. Trends in Age-Standardized Incidence Rates for Breast Cancer, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
2. Tracheal, Bronchus, and Lung Cancer
In 2015, there were 2 million (95% UI, 1.9–2.1 million) incident cases of TBL cancer and 1.7 million (95% UI, 1.67–1.77 million) deaths. Tracheal, bronchus, and lung cancer caused 36.4 million (95% UI, 35.4–37.6 million) DALYs in 2015, of which 99% came from YLLs and 1% from YLDs (eFigure 13 in the Supplement). Men were more likely to develop TBL cancer than women, with 1 in 18 men and 1 in 45 women developing TBL cancer between birth and age 79 years (eTable 16 in the Supplement). The odds were the highest in high SDI countries, with 1 in 13 men and 1 in 27 women developing TBL cancer. In low SDI countries the odds were substantially lower, with 1 in 70 men and 1 in 199 women developing TBL cancer between birth and age 79 years. Overall, TBL cancer had the second highest absolute incidence globally as well as in middle and low-middle SDI countries; TBL was the leading cause of cancer in high-middle SDI countries and ranked fourth in high SDI countries and tenth in low SDI countries (Figure 4). It was the most common cause of cancer deaths by absolute cases globally as well as in all SDI quintiles except for countries in the low SDI group, where TBL cancer ranked seventh (Figure 5).
ASIRs and ASDRs (95% UI) (per 100 000 person-years) for men were the lowest in Eastern Sub-Saharan Africa: ASIR 8.6 (6.9–10.7), ASDR 10.3 (8.1–13.0); Central Sub-Saharan Africa: ASIR 11.7 (7.8–17.0), ASDR 14.2 (9.3–20.9);and Western Sub-Saharan Africa: ASIR 12.8 (10.7–16.5), ASDR 13.9 (11.4–17.4). They were the highest in men in high-income North America: ASIR 70.9 (66.3–75.7), ASDR 50.3 (48.3–52.3); Central Europe: ASIR 70.5 (66.5–75.0), ASDR 61 (58.6–63.1); and high-income Asia Pacific: ASIR 67.5 (62.8–72.4), ASDR 42.1 (40.5–43.8). For women in 2015, incidence rates were the lowest in Eastern Sub-Saharan Africa: ASIR 2.7 (2.0–3.6), ASDR 3.2 (2.3–4.2); Western Sub-Saharan Africa: ASIR 5.2 (3.9–7.5), ASDR 5.6 (4.3–8.1); and South Asia: ASIR 5.3 (4.5–6.3), ASDR 5.5 (5.1–5.9). Incidence rates were the highest in high-income North America: ASIR 51.7 (48.2–55.5), ASDR 32.9 (31.6–34.1); Australasia: ASIR 28.9 (25.7–32.4), ASDR 19.3 (18.0–20.8); and high-income Asia Pacific: ASIR 25.4 (23.5–27.5), ASDR 12.6 (12.0–13.2). (Web Tables 1 and 2). Tracheal, bronchus, and lung cancer was the cause of the most incident cases for men in 38 countries and the most common cause for cancer deaths in 113 countries or territories (eFigures 9 and 11 in the Supplement). For women, TBL cancer was the most common cause of cancer deaths in 20 countries and territories (eFigure 12 in the Supplement).
Between 2005 and 2015, TBL cancer cases increased by 29% (95% UI, 21.5%–37.0%) (Web Table 1). Population growth alone contributed 13%. Aging of the population contributed 18% of the total increase. This increase was partially offset by a decrease in age-specific rates, which would have led to a 2% decrease in incidence if the age structure and population size had remained constant between 2005 and 2015. Figure 8 shows slightly decreasing ASIR at the global level for men and increasing trends for women between 1990 and 2015. This trend was much more pronounced for the high SDI quintile.
Figure 8. Trends in Age-Standardized Incidence Rates for Tracheal, Bronchus, and Lung Cancer, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
3. Colon and Rectum Cancer
In 2015, there were 1.7 million (95% UI, 1.6–1.7 million) incident cases of colon and rectum cancer, and it caused 832 000 (95% UI, 812 000–855 000) deaths (Table 1). Colon and rectum cancer caused 17 million (95% UI, 16.6–17.5 million) DALYs in 2015 of which 96% came from YLLs and 4% came from YLDs (eFigure 13 in the Supplement). The odds of developing colon and rectum cancer before age 79 years at the global level was higher for men than for women (1 in 28 men, 1 in 43 women, eTable 16 in the Supplement). The highest odds were in the high SDI quintile, with 1 in 14 men and 1 in 23 women developing colorectal cancer compared with 1 in 94 men and 1 in 112 women in the low SDI quintile. Globally, and for high SDI countries, colon and rectum cancer ranked third for cancer incidence and second for cancer deaths in 2015 as shown in Figures 4 and 5. Colon and rectum cancer incidence ranked lowest in low SDI countries as the eighth most common cancer and was the sixth leading cause for cancer mortality.
As can be seen in Web Tables 1 and 2, in 2015 ASIRs and ASDRs (95% UI) per 100 000 person-years for men were the lowest in South Asia: ASIR 8.2 (6.9–9.5), ASDR 6.3 (5.8–6.8); Central Sub-Saharan Africa: ASIR 8.7 (5.9–12.9), ASDR 9.5 (6.4–14.1); and Western Sub-Saharan Africa: ASIR 9.0 (7.4–12.2), ASDR 8.7 (7.2–11.1). Rates were highest in Australasia: ASIR 86.4 (76.1–98.5), ASDR 21.3 (19.9–22.9); high-income Asia Pacific: ASIR 78.7 (74.3–83.4), ASDR 21.8 (21.1–22.6); and Western Europe: ASIR 60.0 (56.7–63.3), ASDR 21.9 (20.9–22.8). For women, rates in 2015 were the lowest in Western Sub-Saharan Africa: ASIR 7.1 (5.6–9.8), ASDR 7.1 (5.6–10.1); South Asia: ASIR 7.1 (6.0–8.4), ASDR 5.7 (5.3–6.2); and Central Sub-Saharan Africa: ASIR 8.3 (5.3–12.3), ASDR 9.1 (5.5–14.0). They were the highest in Australasia: ASIR 64.9 (56.6–74.5), ASDR 15.3 (14.1–16.7); high-income Asia Pacific: ASIR 43.7 (40.9–46.8), ASDR 12.7 (12.2–13.3); and high-income North America: ASIR 42.8 (39.6–46.4), ASDR 13.4 (12.8–14.0). Colon and rectum cancer was the cancer with the highest incidence in 2015 for men in 6 countries (eFigure 9 in the Supplement). For women, colon and rectum cancer was the most common cause of cancer deaths in 5 countries (eFigure 12 in the Supplement).
Colon and rectum cancer has remained the fourth leading cause for cancer YLLs between 2005 and 2015 (Figure 6). As summarized in Table 2, between 2005 and 2015, incidence (95% UI) increased by 37% (32.1%–41.0%) from 1.2 million (1.19–1.24 million) to 1.7 million (1.6–1.7 million) cases. Most of this increase can be explained by an aging and growing population, however, even with the same population size and age structure, colon and rectum cancer cases would have increased by 5% between 2005 and 2015 reflecting a change in age-specific incidence rates.
Figure 9 shows similar trends in ASIRs between men and women for all levels of SDI except for the high-middle SDI quintile, where trends are decreasing in women but increasing in men. As can be seen in Web Table 1, ASIRs (95% UIs) have increased by 7% (1.8%–11.6%) between 2005 and 2015 for men but have remained stable for women at the global level: −0.2% (−4.3% to 4.4%). The largest increase occurred in low-middle SDI countries at 25% (10.3%–40.2%) for men and 13% (0.7%–27.4%) for women.
Figure 9. Trends in Age-Standardized Incidence Rates for Colon and Rectum Cancer, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
Between 2005 and 2015, age-standardized DALY rates for both sexes decreased by 8% (−10.2% to −6.2%) at the global level, with the largest decrease in high-SDI countries of 11% (−13.6% to −9.1%) and the largest (non significant) increase in the low SDI quintile of 9% (−6.0% to 27.8%) (Web Table 3).
4. Prostate Cancer
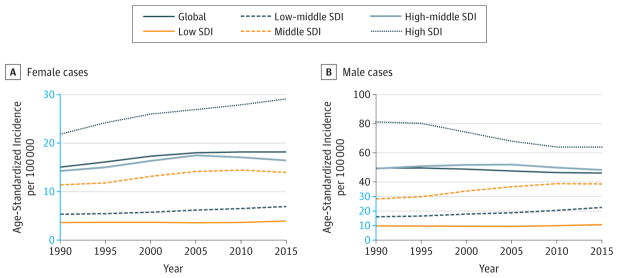
In 2015, there were 1.6 million (95% UI, 1.3–2.2 million) incident cases of prostate cancer and 366 000 (95% UI, 303 000–460 000) deaths. Prostate cancer caused 6.3 million (95% UI, 5.2–7.9 million) DALYs globally in 2015, with 82% coming from YLLs and 18% from YLDs (eFigure 13 in the Supplement). The odds of developing prostate cancer between ages 0 to 79 years was 1 in 14 at the global level and ranged from 1 in 47 men for low-middle SDI countries to 1 in 6 men in high SDI countries (eTable 16 in the Supplement).
ASIRs and ASDRs (95% UIs) for prostate cancer in 2015 were the lowest in South Asia: ASIR 11.5 (8.1–17.5), ASDR 7.2 (5.4–9.1); East Asia: ASIR 12.1 (8.6–16.9), ASDR 6.6 (5.0–8.5); and Central Sub-Saharan Africa: ASIR 20.5 (12.7–29.8), ASDR 17.6 (11.1–25.1). They were the highest in Australasia: ASIR 243.9 (162.6–336.6), ASDR 24.1 (17.3–31.8); high-income North America: ASIR 158.6 (126.0–250.6), ASDR 17.7 (14.4–27.4), and Western Europe: ASIR 151.0 (114.2–230.5), ASDR 20.8 (16.3–30.4) (Web Tables 1 and 2).
In 2015, prostate cancer was the cancer with the highest incidence for men in 103 countries or territories, and the leading cause of cancer deaths for men in 29 countries (eFigures 9 and 11 in the Supplement).
Prostate cancer ranked 14th in 2005 and 12th in 2015 for cancer YLLs (Figure 6) with an increase of 26% (95% UI, 22.0%–29.9%) in absolute YLLs between 2005 and 2015. As summarized in Table 2, the increasing incidence rates, together with an aging and growing population, have led to a 66% increase in prostate cancer cases since 2005 (974 000 in 2005, 1.6 million in 2015). Thirty-four percent of this increase can be attributed to a change in the age-specific rates.
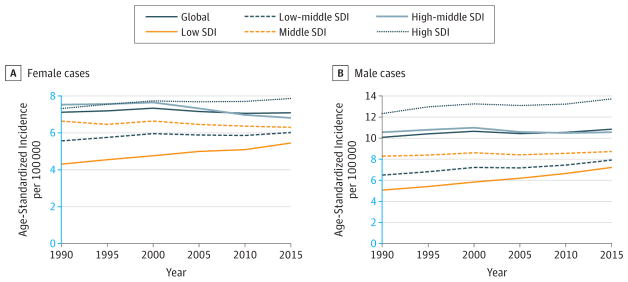
Prostate cancer ASIRs (95% UIs) for men were the lowest in low-middle SDI countries (17.6; 12.9–22.5) and the highest in high SDI countries (123.6; 92.6–181.7). ASIRs have been increasing in all SDI quintiles between 1990 and 2015, with the largest increase in the high-SDI countries (Figure 10). Age-standardized DALY rates (95% UIs) in men were the highest in low SDI countries (368.2; 249.3–476.1) and the second highest in high SDI countries (302.4; 231.0–432.0) (Web Table 3).
Figure 10.
Trends in Age-Standardized Incidence Rates for Prostate Cancer, 1990–2015
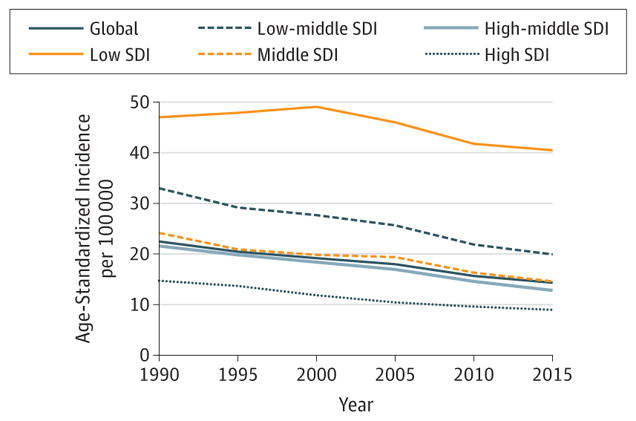
5. Stomach Cancer
In 2015, there were 1.3 million (1.2–1.4 million) incident cases of stomach cancer and 819000 ( 95%UI, 795 000–844 000) deaths world wide. Stomach cancer caused 17.4 million (95%UI, 16.9–18 million) DALYs in 2015 with 98% coming from YLLs and 2% coming from YLDs (eFigure 13 in the Supplement). One in 27 men and 1 in 68 women develop stomach cancer before age 79 years. The highest odds for men occurred in middle SDI countries (1 in 25), whereas the lowest occurred in low-middle SDI countries (1 in 48). For women, the highest odds were in low SDI countries (1 in 58) and the lowest in low-middle SDI countries (1 in 83) (eTable 16 in the Supplement). Globally and for high SDI countries, stomach cancer ranked fifth for cancer incidence and third for cancer deaths in 2015 (Figures 4 and 5). In high-middle, middle, low-middle, and low SDI countries, stomach cancer ranked third for incidence. For cancer mortality in high middle, middle, and low SDI countries, stomach cancer ranked third. For low-middle SDI countries it ranked second for cancer mortality.
ASIRs and ASDRs (95% UIs) for men in 2015 were lowest in high income North America: ASIR 11.7 (10.5–13.0), ASDR 5.2 (5.0–5.4);South Asia: ASIR 12.8 (10.9–14.9), ASDR 8.4 (7.8–9.1);and Australasia: ASIR 15.1 (12.9–18.1), ASDR 7.0 (6.5–7.5), as summarized in Web Tables 1 and 2. They were the highest in high-income Asia Pacific: ASIR 90.1 (83.5–96.9), ASDR 28.5 (27.5–29.5);East Asia: ASIR 46.2 (38.8–56.5), ASDR 33.5 (31.2–36.0); and Central Asia: ASIR 34.9 (32.6–37.2), ASDR 23.6 (22.2–25.0). For women, rates were the lowest in high-income North America: ASIR 6.1 (5.4–6.8), ASDR 2.9 (2.8–3.0); South Asia: ASIR 6.2 (5.1–7.4), ASDR 4.1 (3.7–4.5);and Southern Sub-Saharan Africa: ASIR 7.5 (6.2–8.8), ASDR 5.3 (4.6–6.2). They were the highest in high-income Asia Pacific: ASIR 31.5 (28.9–34.2), ASDR 10.6 (10.2–11.1);Andean Latin America: ASIR 20.9 (18.1–23.7), ASDR 15.2 (13.4–17.3); and East Asia ASIR 18.0 (15.0–21.3), ASDR 13.3 (12.3–14.2).
Stomach cancer was highest in absolute incidence in 2015 for men in 26 countries and territories and was the leading cause of cancer deaths in 11 countries (eFigures 9 and 11 in the Supplement). For women it was the leading cause of cancer deaths in 4 countries (eFigure 12 in the Supplement).
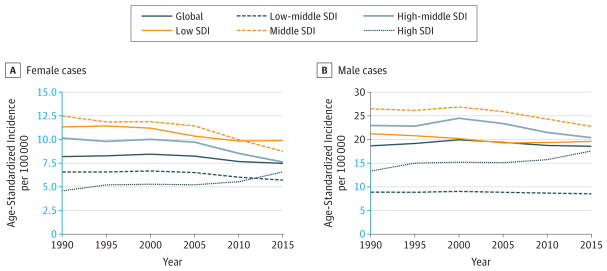
Stomach cancer has remained the third highest cause for crude cancer YLLs between 2005 and 2015, with a 7% decrease in absolute YLLs due to stomach cancer (Figure 6). If the population age structure and size had remained the same in 2015 as it was in 2005, incidence would have dropped by 21% due to decreasing rates (Table 2). ASIRs have dropped substantially since 1990 at the global level and for all SDI quintiles except the low SDI quintile (Figure 11).
Figure 11. Trends in Age-Standardized Incidence Rates for Stomach Cancer, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
Between 2005 and 2015, age-standardized DALYs for both sexes decreased by 27% (95% UI, −29.4% to −24.5%) globally, with the largest decrease in high-middle SDI countries of 32% (95% UI, −35.8% to −27.5%) (Web Table 3).
6. Liver Cancer
In 2015, there were 854 000 (95% UI, 768 000–961 000) incident cases for liver cancer globally and 810 000 (750 000–863 000) deaths. Liver cancer caused 20.6 million (19–22 million) DALYs in 2015 with 99% coming from YLLs and 1% coming from YLDs (eFigure 13 in the Supplement). Liver cancer was more common in men, with 1 in 45 men developing liver cancer before age 79 years compared with 1 in 113 women at the global level. The highest odds of developing liver cancer was in middle SDI countries, with 1 in 38 men and 1 in 96 women developing liver cancer, whereas the lowest odds were seen in low-middle SDI countries, with 1 in 98 men and 1 in 144 women developing liver cancer during their lifetime (eTable 16 in the Supplement). Globally, liver cancer ranked sixth for cancer incidence and fourth for cancer deaths in 2015, as shown in Figures 4 and 5. In low SDI countries, it ranked fourth for cancer incidence and first for cancer mortality, whereas in middle and high-middle SDI countries it ranked fourth and sixth, respectively, for cancer incidence but second for cancer mortality.
ASIRs (95% UIs) (per 100 000 person-years) were the highest in middle SDI countries in 2015 (15.6; 13.2–18.8), followed by low SDI countries (14.5; 11.5–17.1), high-middle (13.7; 11.6–16.3), high (11.7; 10.8–12.7), and low-middle SDI countries (7.1; 6.2–8.3). ASDRs in 2015 for both sexes were the highest in the low SDI quintile (16.6; 13.2–19.7), followed by middle SDI countries (15.8; 14.5–17.5), high-middle SDI countries (14.5; 12.9–15.8), high SDI countries (7.9; 7.6–8.2), and low-middle SDI countries (7.5; 6.7–8.7) (Web Tables 1 and 2).
In 2015, ASIRs and ASDRs (95% UIs) for men were the lowest in South Asia: ASIR 4.7 (3.8–6.2), ASDR 5.1 (4.5–5.9); Southern Latin America: ASIR 6.1 (5.4–7.3), ASDR 6.8 (6.2–7.5); and Tropical Latin America: ASIR 6.5 (5.5–8.2), ASDR 7.4 (6.6–8.2). They were the highest in high-income Asia Pacific: ASIR 40.1 (34.4–48.3), ASDR 23.8 (22.5–25.2); East Asia: ASIR 36.4 (28.6–48.0), ASDR 39.3 (35.4–44.1); and Central Sub-Saharan Africa: ASIR 24.4 (13.4–42.7), ASDR 29.4 (15.9–50.3). For women, rates were the lowest in South Asia: ASIR 3.2 (2.4–4.5), ASDR 3.1 (2.6–3.6); Australasia: ASIR 3.6 (2.4–6.2), ASDR 2.3 (2.0–2.6); and Southern Latin America: ASIR 4.0 (3.3–5.2), ASDR 3.9 (3.5–4.3) and the highest in high-income Asia Pacific: ASIR 14.2 (10.9–19.6), ASDR 7.4 (7.0–7.8); East Asia: ASIR 12.5 (9.0–18.2), ASDR 12.1 (10.3–14.1); and Western Sub-Saharan Africa: ASIR 10.9 (7.0–15.8), ASDR 10.9 (7.0–15.8) (Web Tables 1 and 2).
Liver cancer was the most commonly diagnosed cancer in 2015 for men in 11 countries (eFigure 9 in the Supplement) and the most common cause of cancer deaths in 40 countries (eFigure 11 in the Supplement). Liver cancer was the most commonly diagnosed cancer for women in Mongolia (eFigure 10 in the Supplement) in 2015 and the leading cause of cancer deaths for women in 5 countries in 2015 (eFigure 12 in the Supplement).
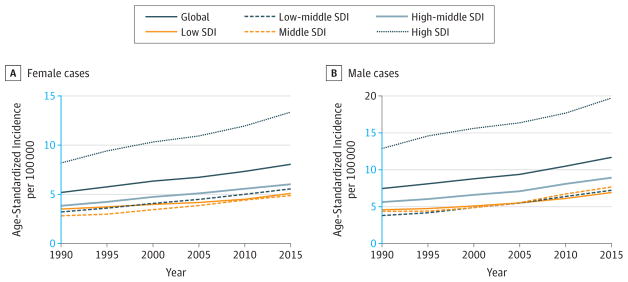
Liver cancer remained the second leading cause of cancer YLLs between 2005 and 2015 (Figure 6). Aging and population growth were the drivers of the increase from 709 000 (616 000–782 000) cases in 2005 to 854000 (768 000–961 000) cases in 2015, as summarized in Table 2. If the population age structure and size had remained the same in 2015 as they were in 2005, 8% fewer cases of liver cancer would have been diagnosed in 2015 than in 2005. Globally, ASIRs slowly decreased since the late 1990s (Figure 12). This global trend, however, masks an increase in low and high SDI countries since 1990. ASIRs have been increasing for low SDI countries since 1990; in high SDI countries, rates decreased until the early 2000s for men and the late 2000s for women and then increased. Between 2005 and 2015, age-standardized DALY rates for liver cancer decreased for both sexes by 17%(95%UI, −21.4% to −8.7%) at the global level, with the largest decrease in high-middle SDI countries of 24%(95%UI, −30.6% to −11.3%) (Web Table 3).
Figure 12. Trends in Age-Standardized Incidence Rates for Liver Cancer, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
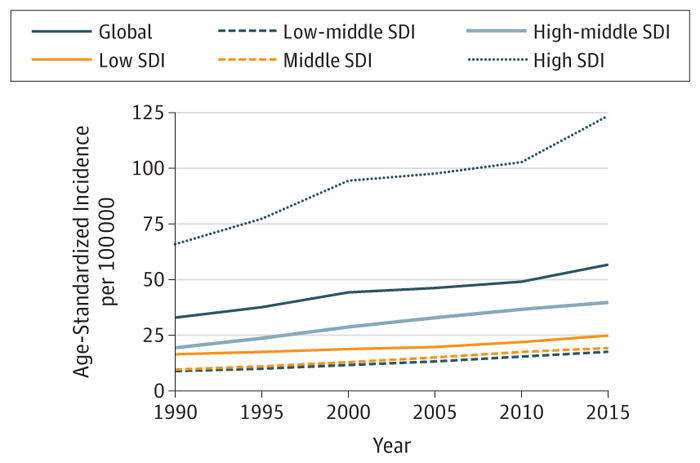
7. Non-Hodgkin Lymphoma
In 2015, there were 666 000 (95%UI, 584 000–710 000) incident cases of NHL and 231 000 (95%UI, 196 000–244 000) deaths. Non-Hodgkin lymphoma caused 6.3 million (95%UI, 5.4–6.6 million) DALYs in 2015, with 95% coming from YLLs and 5% from YLDs (eFigure 13 in the Supplement). One in 78 men and 1 in 110 women at the global level developed NHL between birth and age 79 years. The highest odds for developing NHL were in high SDI countries with 1 in 44 men and 1 in 63 women developing NHL. The lowest odds occurred in low SDI countries, with 1 in 148 men and 1 in 190 women developing NHL.
Globally, for both sexes combined in 2015, NHL ranked seventh for cancer incidence and 11th for cancer deaths (Figures 4 and 5). The highest rank for the incidence of NHL was in low SDI countries, where it was fifth. However, NHL cancer in low SDI countries ranked only 11th for death.
Web Tables 1 and 2 illustrate that incidence and death rates in 2015 for men were the lowest in Central Sub-Saharan Africa: ASIR 5.5 (3.4–8.9), ASDR 3.4 (2.1–5.5); Oceania: ASIR 5.8 (4.1–9.7), ASDR 3.0 (2.1–4.7); and South Asia: ASIR 6.0 (4.7–7.4), ASDR 2.9 (2.4–3.2). They were the highest in high-income North America: ASIR 28.5 (24.2–35.0), ASDR 7.7 (6.3–8.9); Australasia: ASIR 25.3 (20.4–31.4), ASDR 6.9 (5.4–8.1); and Western Europe: ASIR 20.0 (15.9–23.0), ASDR 5.7 (4.3–6.3). For women, incidence rates in 2015 were the lowest in Central Asia: ASIR 4.1 (3.4–4.6), ASDR 1.5 (1.3–1.6); North Africa and Middle East: ASIR 4.4 (3.8–5.5), ASDR 2.0 (1.8–2.6); and South Asia: ASIR 4.4 (3.0–5.8), ASDR 2.1 (1.6–2.4). They were the highest in high-income North America: ASIR 20.1 (17.4–26.6), ASDR 5.0 (4.6–6.1); Australasia: ASIR 18.8 (15.5–24.1), ASDR 4.7 (4.0–5.5); and Western Europe: ASIR 13.7 (11.7–15.9), ASDR 3.6 (2.9–4.0).
Non-Hodgkin lymphoma ranked 11th for cancer YLLs in 2005 and in 2015 (Figure 6). Cases of NHL increased by 56% between 2005 and 2015 (Table 2). Population growth and population aging would have increased incidence by 13% each. Rising age-specific incidence rates with stable population size and structure between 2005 and 2015 would have increased cases by 29%. Figure 13 shows the slight increase in ASIRs between 1990 and 2015 graphically with very similar trends for men and women and all SDI quintiles. On the global level, ASIRs per 100 000 person-years (95% UI) for both sexes for NHL have increased by 23%(13.1%–29.4%) between 2005 and 2015, from 8.0 (7.2–8.5) to 9.8 (8.5–10.4), with the largest increase in middle SDI countries: 33%(11.3%–52.1%) (Web Table 1). During this timeframe, age-standardized DALY rates (95% UIs) for both sexes increased at the global level (1.3% increase; −8.5% to 7.0%), although this increase was not statistically significant. Large, but not significant decreases of 6% (−10.2% to 0.1%) occurred in high SDI countries, and the largest, but also non significant, increase occurred in low-middle SDI countries (7%; −9.4% to 17.9%) (Web Table 3).
Figure 13. Trends in Age-Standardized Incidence Rates for Non-Hodgkin Lymphoma, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
8. Leukemia
In 2015 there were 606 000 (95% UI, 573 000–643 000) new cases of leukemia world-wide and 353 000 (95% UI, 345 000–363 000) deaths. In 2015, leukemia caused 12.0 million (95%UI, 11.6–12.5 million) DALYs globally, with 97% coming from YLLs and 3% from YLDs (eFigure 13 in the Supplement). One in 87 men compared with 1 in 137 women developed leukemia between ages 0 and 79 years at the global level. The highest odds were seen in the high SDI quintile, with 1 in 64 men and 1 in 116 women developing leukemia. The lowest odds occurred in low SDI countries, with 1 in 124 men and 1 in 164 women developing leukemia (eTable 16 in the Supplement).
Leukemia ranked eighth for cancer incidence and ninth for cancer deaths at the global level in 2015 (Figures 4 and 5). Leukemia incidence was ranked highest for low-SDI and low-middle SDI countries at sixth place (leukemia was ninth and eighth for cancer deaths in low-SDI and low-middle SDI countries, respectively). Leukemia was ranked lowest in high-SDI countries at 13th place (eighth for cancer deaths).
In 2015, ASIRs and ASDRs (95% UIs) for men were the lowest in Eastern Sub-Saharan Africa: ASIR 6.5 (5.0–8.3), ASDR 3.8 (3.1–4.8); South Asia: ASIR 7.0 (5.8–8.4), ASDR 4.1 (3.8–4.4); and Central Sub-Saharan Africa: ASIR 7.2 (4.5–10.8), ASDR 4.4 (2.9–6.5). They were the highest for men in high-income North America: ASIR 17.1 (15.6–18.9), ASDR 8.9 (8.5–9.4); Australasia: ASIR 16.1 (12.6–21.0), ASDR 8.8 (7.7– 10.0); and Western Europe: ASIR 14.9 (13.7–16.5), ASDR 8.6 (8.2–9.1). For women, they were the lowest in Eastern Sub-Saharan Africa: ASIR 4.6 (3.3–6.2), ASDR 2.7 (2.1–3.5); South Asia: ASIR 4.7 (3.8–5.8), ASDR 2.8 (2.6–3.1);and Western Sub-Saharan Africa: ASIR 5.5 (4.1–7.8), ASDR 3.2 (2.5–4.3). Rates were the highest in high-income North America: ASIR 10.0 (8.9–11.2), ASDR 4.9 (4.7–5.2); Southeast Asia: ASIR 9.6 (8.1–11.2), ASDR 5.6 (4.9–6.3); and North Africa and Middle East: ASIR 8.9 (7.9–10.1), ASDR 5.1 (4.6–5.6) (Web Tables 1 and 2).
Leukemia led incident cases in 2015 for men in 5 countries (eFigure 9 in the Supplement). It remained the sixth leading cause of cancer YLLs between 2005 and 2015, with a 6% (95% UI, 2.5%–9.9%) increase in absolute YLLs and an 8% (95% UI, −11.1% to −4.9%) decrease in age-standardized YLLs (Figure 6).
Between 2005 and 2015, incident cases at the global level increased from 481 000 (95% UI, 456 000–512 000) to 606 000 (95%UI, 573 000–643 000)(total increase of 26%(95%UI, 19.6%–33.2%); population growth and aging were the drivers behind this increase. Had the population growth and age-specific rates remained the same as in 2005, there would be only 3% more cases of leukemia in 2015 (Table 2). Increasing trends in ASIRs are similar for all SDI quintiles except for countries in the high-middle SDI group, where rates have decreased since the 2000s (Figure 14).
Figure 14. Trends in Age-Standardized Incidence Rates for Leukemia, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
Between 2005 and 2015, age-standardized DALY rates (95% UIs) for both sexes decreased by 8% (−10.8% to −4.6%) at the global level, with the largest decrease in high-middle SDI countries at 12%(−16.6% to −8.6%), and the largest increase in low SDI countries at 9%(−3.9 to 22.8), although this increase was not significant (Web Table 3).
9. Bladder Cancer
In 2015, there were 541 000 (95%UI, 517 000–567 000) incident cases for bladder cancer globally and 188 000 (95%UI, 183 000–193 000) deaths. Bladder cancer caused 3.4 million (95% UI, 3.3–3.5 million) DALYs in 2015, with 92% coming from YLLs and 8% from YLDs (eFigure 13 in the Supplement). Bladder cancer was more common in men, with 1 in 59 men being diagnosed before age 79 years compared with 1 in 239 women. The odds of developing bladder cancer during a lifetime were the highest in high-SDI countries (1 in 36 men and 1 in 165 women) and the lowest in low-SDI countries (1 in 122 men and 1 in 310 women) (eTable 16 in the Supplement). Globally, bladder cancer ranked ninth for cancer incidence and 13th for cancer deaths in 2015, as shown in Figures 4 and 5. It ranked the highest in high-SDI countries at position 8 (11th for mortality).
In 2015, ASIRs and ASDRs (95% UIs) for men were the lowest in Oceania: ASIR 4.5 (3.6–5.8), ASDR 2.3 (1.9–2.9); Andean Latin America: ASIR 5.8 (4.7–7.2), ASDR 2.1 (1.9–2.4); and Central Latin America: ASIR 5.9 (5.0–6.8), ASDR 2.4 (2.2–2.5). They were the highest in high-income North America: ASIR 31.6 (28.5–35.1), ASDR 6.1 (5.8–6.4); Western Europe: ASIR 26.0 (24.1–27.9), ASDR 8.6 (8.1–9.1); and Central Europe: ASIR 24.1 (21.9–26.6), ASDR 9.4 (8.6–10.1). For women, incidence rates in 2015 were the lowest in Oceania: ASIR 2.1 (1.6–2.8), ASDR 1.2 (0.9–1.5); Southeast Asia: ASIR 2.2 (1.8–2.7), ASDR 1.2 (1.0–1.4); and Andean Latin America: ASIR 2.3 (1.8–2.8), ASDR 1.1 (0.9–1.2). They were the highest in high-income North America: ASIR 7.7 (6.7–8.8), ASDR 1.8 (1.7–1.9);Western Europe: ASIR 5.7 (5.2–6.3), ASDR 2.0 (1.9–2.2); and Southern Sub-Saharan Africa: ASIR 5.1 (4.0–6.6), ASDR 2.4 (2.0–2.9) (Web Tables 1 and 2).
Bladder cancer was the most commonly diagnosed cancer in 2015 for men in Egypt (eFigure 9 in the Supplement). Globally, it dropped from the 17th to the 18th leading cause of cancer YLLs between 2005 and 2015 (Figure 6). Aging and population growth were the drivers of the increase: from 413 000 (95% UI, 403 000–424 000) cases in 2005 to 541 000 (95% UI, 517 000–567 000) cases in 2015 (Table 2). If population age structure and size had remained the same in 2015 as they were in 2005, bladder cancer incidence would have been stable. However, population growth and aging led to a 31% increase in incident cases. Worldwide, as well as in high and high-middle SDI countries, ASIRs peaked in the late 1990s in both sexes followed by a slow decrease (Figure 15). Rates increased in the low and low-middle quintiles. Between 2005 and 2015, age-standardized DALY rates (95% UIs) for both sexes for bladder cancer decreased by 9% (−11.5% to −6.2%) at the global level, with the largest decrease in high-middle SDI countries by 13% (−17.0% to −9.7%), and the largest (although nonsignificant) increase in low SDI countries of 3%(−8.8% to 18.2%) (Web Table 3).
Figure 15. Trends in Age-Standardized Incidence Rates for Bladder Cancer, 1990–2015.
The y-axes differ in scale between male and female graphs to reflect differing incidence rates between sexes. The colored section of the higher-scale y-axis represents the entirety of the lower-scale y-axis.
10. Cervical Cancer
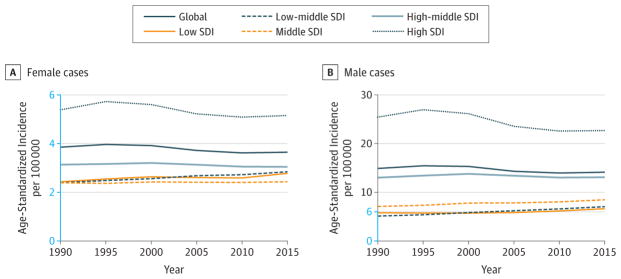
In 2015, 526 000 (95%UI, 483 000–571 000) women developed cervical cancer worldwide, and it caused 239 000 (95% UI, 225 000–252 000) deaths (Table 1). Cervical cancer caused 7 million (95% UI, 6.5–7.4 million) DALYs, with 96% coming from YLLs and 4% from YLDs (eFigure 13 in the Supplement).
One in 68 women developed cervical cancer between birth and age 79 years at the global level (eTable 16 in the Supplement). The odds were the highest in low SDI countries, with 1 in 24 women developing cervical cancer, and the lowest in high SDI countries, where 1 in 115 women developed cervical cancer during a lifetime.
In 2015, ASIRs and ASDRs per 100000 person-years (95% UIs) for women were the lowest in Australasia: ASIR 5.6 (4.8–6.5), ASDR 2.4 (2.2–2.7); North Africa and Middle East: ASIR 7.5 (5.8–9.3), ASDR 3.3 (2.8–3.9);and high-income North America: ASIR 7 .6 (6.7–8.6), ASDR 2.9 (2.8–3.1); and the highest in Central Sub-Saharan Africa: ASIR 47.4 (25.9–82.4), ASDR 24.7 (13.8–39.9);Southern Sub-Saharan Africa: ASIR 46.8 (35.0–62.3), ASDR 27.0 (21.7–34.0);and Oceania: ASIR 42.3 (22.7–70.2), ASDR 15.6 (9.2–23.1) (Web Tables 1 and 2).
In 2015, cervical cancer was the most commonly diagnosed cancer for women in 11 countries (eFigure 10 in the Supplement) and the most common cause of cancer deaths for women in 50 countries (eFigure 12 in the Supplement).
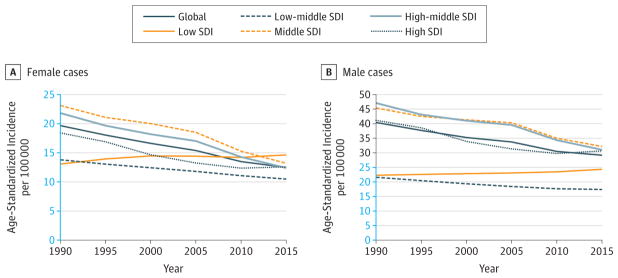
Cervical cancer dropped from the ninth to the tenth leading cause for cancer YLLs between 2005 and 2015, with a 19% (95% UI, −25.8% to −12.0%) decrease in age-standardized YLLs (Figure 6). Total incidence would have decreased by 26% if the population size and age structure had remained the same as in 2005 due to decreasing incidence rates (Table 2). ASIRs decreased globally for all SDI quintiles (Figure 16). Between 2005 and 2015, age standardized DALYs decreased globally in women by 19% (95% UI, −23.9% to −12.2%) with rates decreasing by 17% to 23% in low-middle, middle, high-middle, and high SDI countries, but only by 13% (95% UI, −32.6% to 10.0%) in low SDI countries (Web Table 3).
Figure 16.
Trends in Age-Standardized Incidence Rates for Cervical Cancer, 1990–2015
Trends in Incidence for Less Common Cancers
Incidence (95%UI) for both sexes increased substantially between 2005 and 2015 for certain cancers, as summarized in Table 2. Thyroid cancer cases almost doubled between 2005 and 2015, from 168 000 (160 000–178 000) to 334 000 (310 000–353 000) cases. Seventy-one percent of this change can be explained by an increase in age-specific incidence rates (Table 2). At the same time, the age-standardized YLL rate for thyroid cancer decreased significantly by 7%(95%UI, −15.0% to −2.3%) (Figure 6). Melanoma cases increased from 225 000 (187 000–289 000) in 2005 to 352 000 (282 000–445 000) in 2015, a 56% (95% UI, 48.0%–63.9%) increase. Twenty-eight percent of the change can be explained by an increase in the age-specific incidence rates (Table 2). Kidney cancer cases increased by 53%(95%UI, 45.7%–59.7%) between 2005 and 2015 (from 279 000 (271 000–288 000) to 425 000 (405 000–447 000), with age-specific rates contributing 23% to this total increase. Mesothelioma has increased from 26 000 (25 000–27 000) to 37000 (35 000–29 000) cases between 2005 and 2015, a 40% (33.4%–47.0%) increase, of which 9% can be attributed to a rise in age-specific rates.
Discussion
Between 2005 and 2015, the proportion of deaths from noncommunicable diseases (NCDs) increased from 65% in 2005 to 71% in 2015 at the global level.1 Fourteen percent of all deaths in 2005 were due to cancer, which increased to 16% in 2015.1 Seven percent of all DALYs in 2005 were due to cancer, which increased to 9% in 2015.19 Deaths due to communicable, maternal, neonatal, and nutritional diseases decreased from 26% in 2005 to 20% in 2015.1 These numbers are evidence that NCDs may be a barrier to future development.20 The international health community has responded to this threat, with major milestones being the 2011 United Nations political declaration on NCD prevention and control,21 the World Health Organization Global Action Plan for the Prevention and Control of NCDs 2013–2020,22 and the integration of NCDs in the Sustainable Development Goals.11
The GBD 2015 study identifies some progress in meeting the targets of the Sustainable Development Goals.23 Between 2005 and 2015, many countries experienced a decrease in cancer mortality despite increasing incidence rates. Countries with increasing cancer mortality rates were dominantly in Sub-Saharan Africa where, with few exceptions, the complex health care infrastructure required to treat cancer is generally lacking.24 Efforts are ongoing to expand the existing resources in the region to allow for improved cancer care.25–28 Cancer prevention efforts may, however, be as important as delivery of care, given the profile of cancer in low SDI countries where the top 3 leading causes of cancer mortality (liver cancer, cervical cancer, and stomach cancer) are largely preventable.
Prevention and treatment of chronic hepatitis B and C, which account for the majority of liver cancer deaths, would reduce the incidence and mortality of liver cancer.29 The World Health Organization has adopted a global health sector strategy on viral hepatitis that features a 2030 target of a 65% reduction in mortality related to hepatitis B and C. This is to be achieved by reducing the occurrence of new chronic hepatitis B and C infections by 90% through increased newborn hepatitis B immunization coverage, blood and injection safety, harm reduction, and by an 80% treatment rate for chronic hepatitis Band C.30 Our results show that trends for liver cancer incidence differ among the SDI quintiles. ASIRs have been decreasing at the global level and for most SDI quintiles since at least 2000. However, rates for high SDI countries have increased since 1990, and rates for low SDI countries increased in the most recent observations (from 2010 to 2015). These findings are consistent with observations in some high-income countries, where obesity, diabetes, and hepatitis Care thought to be major contributors to rising incidence rates.31,32
Human papillomavirus vaccination is universally recommended by health authorities and is expected to reduce cervical cancer incidence over the next decades if vaccination uptake is successful. In the meantime, screen and treat approaches that have been shown to reduce cervical cancer mortality in high-income countries should be implemented in regions with a high burden of cervical cancer.33–35 The stark inequity between high SDI countries where cervical cancer is the 18th leading cause of cancer deaths compared with low SDI countries where it ranks second (Figure 5) is widely recognized by the international community; an emerging political commitment to reducing this gap will hopefully translate into a decreased burden of cervical cancer in the most affected countries.36 Our results show that progress in reducing cervical cancer burden was the slowest in low SDI countries where the decrease in ASIRs and ASDRs was the lowest; a 12% decrease in ASIR occurred for countries at low-SDI compared to a greater than 14% decrease in all other SDI quintiles, and a 14% decrease in ASDR in low SDI compared to the other SDI quintiles, with decreases in ASDR of greater than 16%.
Stomach cancer rates have been declining globally for decades.37 However, our results demonstrate that this trend has not been uniform among the SDI quintiles. ASIRs in the lowest SDI quintile increased throughout the GBD study timeframe (1990 to 2015). In the highest SDI quintile, rates declined until 2010 but have since increased. One possible explanation is the increasing trend of gastric cancer of the cardia in high-income countries owing to risk factors such as obesity, although the mechanism explaining this association is not fully understood.37–39 Treatment for Helicobacter pylori infection, which causes about 78% of all stomach cancers, reduces gastric cancer incidence, and the International Agency for Research on Cancer (IARC) recommends that countries investigate whether population based screening and treatment programs for Hpylori are indicated. 40,41 Despite this recommendation, few countries with high incidence of stomach cancer have implemented population wide screening programs. Although studies have shown reductions in stomach cancer mortality in screened populations, the target group, onset, and modality of screening programs remain controversial.37,42–46
Prevention and treatment of carcinogenic infections that lead to these observed cancer patterns in low SDI countries have the potential to decrease future cancer burden as well as reducing associated diseases like cirrhosis in endemic areas.47 In addition, limiting transmission can lead to prevention efforts that are sustained beyond 1 generation.10
The dominance of infection-related cancers in low SDI countries is an exceptional pattern compared with the leading causes of cancer deaths for countries in higher SDI quintiles, where TBL, colorectal, stomach, liver, and breast cancer lead the rankings. The importance of tobacco control as a crucial cancer control strategy should therefore not be underestimated, especially since tobacco control has health benefits reaching far beyond cancer prevention.48 Effective primary and secondary prevention strategies are available for colorectal cancer with many risk factors being attributed to “Western life styles.”49 Increased physical exercise, avoidance of processed meat, alcohol, and tobacco, as well as aspirin use have all been associated with reduced colorectal cancer incidence.49–52 Multiple modalities for colorectal screening are known to reduce colorectal mortality in high-income countries.53,54 Means of implementation in other health care settings is currently being investigated.55 Primary prevention of breast cancer, which is the leading cause of cancer incidence, death, and DALYs in women, should be emphasized. However, even in the setting of population wide primary prevention efforts, it is estimated that only between 20% and 50% of breast cancer can be prevented.48,56 Therefore, breast cancer control strategies must also focus on early detection in addition to effective treatment. Early detection strategies can range from improving breast cancer awareness and clinical breast examination in basic resource settings to mammography screening.57 Clearly, cancer control strategies focused on early detection should also target access to the full spectrum of care including surgical, medical, and radiation oncology; affordable chemotherapeutics and supportive drugs; and survivorship support and palliative and hospice care.58 This is imperative not only for breast cancer but for comprehensive cancer care in general.
The variation in the leading causes of cancer incidence and mortality between countries documented in the GBD study are remarkable. The largest ranges in rankings for cancer mortality were found in cervical cancer, acute lymphoid leukemia, and nasopharynx cancer followed by esophageal, lip and oral cavity, gallbladder and biliary tract cancer, and melanoma (eFigure 8 in the Supplement). For cancer incidence, the largest divergence was seen in esophageal cancer, cervical cancer, melanoma, acute lymphoid leukemia, kidney, larynx, and gallbladder and biliary tract cancer (eFigure 7 in the Supplement). For most of these cancers, the observed pattern can be explained by known risk factors or by access to care. In acute lymphoidleukemia, for example, it is well known that the survival rate for children differs substantially between countries from around 15% to over 90%, suggestive of disparities in treatment.59 For nasopharynx cancer it is also well known that incidence differs drastically between populations; this can in part be explained by early infection with Epstein-Barr virus as well as by a high intake of salt-preserved food.60 Melanoma is a much more common in populations with lighter skin types.61 Esophageal cancer is a similar example of widely differing incidence rates attributable to risk factor profiles. Risk factors for esophageal cancer vary depending on the histologic characteristics of squamous cell carcinoma vs adeno carcinoma. For example, it is postulated that the diverse incidence of esophageal squamous cell carcinoma, the dominant histologic type in high-endemic areas, is driven by chronic cell damage from risk factors like smoking, alcohol consumption, heat damage, and nutritional deficiency.62,63 Risk factors for esophageal adenocarcinoma, which is increasing in traditionally low-endemic areas, include a high body mass index, smoking, reflux disease, and a diet low in fruits and vegetables.64,65
A detailed analysis of the reasons behind the observed variation in cancer burden goes beyond the scope of this analysis. However, the cited examples show that the descriptive epidemiology approach of the GBD study can identify patterns of cancer burden that can be hypothesis generating. This is especially true when cancer burden is analyzed in conjunction with nonmalignant diseases that might share similar risk factors—a potential provided by the comprehensive nature of the GBD approach.
Limitations
As in prior GBD studies, our estimates depend on the quality and quantity of the data sources available to inform the estimates. Because of the lag time for data reporting, estimates for 2015 are mainly based on data and trends from recent years. For many countries, data sources for informing cancer burden estimation are still sparse, and the GBD estimates rely heavily on covariate selection in the models and regional patterns. Only an estimated 38% of deaths world wide were registered in 2012.66 Alternative data sources for cancer mortality, such as verbal autopsy, are substantially less reliable.67 To overcome the limitation from lack of data sources, the GBD study includes cancer incidence data from registries in the mortality estimation. Cancer registration has a long tradition in many countries. However, in regions where the burden of cancer is expected to grow significantly due to anticipated population growth and aging, cancer registries of ten only cover a small fraction of the population, are of low quality, or do not exist.68 Compared with almost complete coverage of the population with high-quality cancer registration in Nordic countries or North America, less than 10% of the population in South America, Asia, and Africa are covered by high-quality cancer registries.68 With technological innovation, including an improved ability to identify and link different types of data sources, cancer surveillance will hopefully become a routine component of every health care system. Endeavors like the Global Initiative for Cancer Registry Development (http://gicr.iarc.fr) play a major role in this task.
Cancer mortality estimates are predominantly based on vital registration data, cancer registry data, and to a much lesser extent other data sources. If a large proportion of deaths are miscoded, the redistribution of these so-called garbage codes can substantially affect mortality estimates. Since GBD cancer incidence estimates are based on mortality estimates, garbage code redistribution directly affects cancer incidence. Misclassification of metastatic sites as primary cancer sites (eg, liver, lung, brain) is another source of potential bias, especially in locations with limited diagnostic resources. Changes in coding practices or coding systems may also have an effect, even though mapping to the GBD causes list includes adjustments to account for different coding systems.69
Cervical and uterine cancer incidence rates are potentially overestimated in the GBD in locations where hysterectomies are common, since rates are calculated without adjustments for the population at risk.
For GBD 2015, we have updated the MI (mortality to incidence) ratios and used out-of-sample validation of a set of potential models to choose the most appropriate MI model. However, in young age groups with sparse data, and in areas where no matching mortality and incidence data exist (which is the case for most countries in Sub-Saharan Africa), the MI model is based on the combination of trends for older age groups as well as covariate selection.
The addition of the leukemia subtypes for the GBD 2015 study is a necessary step; aggregating “leukemia” into a single entity is appropriate from neither a public health nor a clinical perspective. However, when interpreting the results, it is important to recognize that those data sources for the MI ratios that determine incidence for leukemia subtypes are mostly from high-income countries. Another caveat is that leukemia subtypes are scaled to sum to the leukemia “parent” cause, which can lead to incidence estimates for leukemia subtypes that are different than what would be expected based on the MI ratio alone. Changes in classification of leukemia subtypes over time can also have an effect on the GBD estimates. With the wider availability of cancer registry data, including childhood cancer registries, it is expected that estimates will continue to be adjusted in future iterations of the GBD.
Conclusions
Despite significant reductions in cancer mortality in many countries, cancer poses a barrier to future development. Incidence is expected to increase, straining resources even in countries with advanced health care systems. An expanding arsenal of cancer prevention and treatment interventions, together with a political commitment to address NCDs, offers hope that this threat can be controlled. The GBD study enables timely tracking of progress toward defined targets.23 Since most cancer prevention efforts have a much broader impact than just reducing cancer incidence, the ability of the GBD to put single diseases into the perspective of population health is unique and of the utmost importance.
Supplementary Material
Key Points.
Question
What is the burden of cancer between 1990 and 2015 at the global, regional, and national level measured in incidence, mortality, years lived with disability, years of life lost, and disability-adjusted life-years (DALYs) by sex and age?
Findings
Using the Global Burden of Disease (GBD) methodology, we estimated that in 2015, there were 17.5 million cancer cases, 8.7 million deaths, and 208.3 million DALYs. Between 2005 and 2015, incident cancer cases increased by 33%, of which 12.6% were due to population growth, 16.4% due to an aging population, and 4.1% due to increasing age-specific incidence rates.
Meaning
Cancer control, which requires a detailed understanding of the cancer burden as provided in the GBD, is of utmost importance given the rise in cancer incidence due to epidemiological and demographic transition.
Acknowledgments
Funding/Support: The Institute for Health Metrics and Evaluation received funding from the Bill and Melinda Gates Foundation. Dr Fitzmaurice was supported by National Institutes of Health grant 5T32HL007093-40.
The Global Burden of Disease Cancer Collaboration
Christina Fitzmaurice, MD, MPH; Christine Allen, BA; Ryan M. Barber, BS; Lars Barregard, PhD, MD; Zulfiqar A. Bhutta, PhD; Hermann Brenner, MD, PhD; Daniel J. Dicker, BS; Odgerel Chimed-Orchir; Rakhi Dandona, PhD; Lalit Dandona, MD, MPH; Tom Fleming, BS; Mohammad H. Forouzanfar, PhD; Jamie Hancock, MLS; Roderick J. Hay, DM; Rachel Hunter-Merrill, MA; Chantal Huynh, BA; H. Dean Hosgood, PhD, MPH; Catherine O. Johnson, PhD; Jost B. Jonas, MD; Jagdish Khubchandani, PhD, MD, MPH; G. Anil Kumar, PhD; Michael Kutz, BS; Qing Lan, PhD, MD; Heidi J. Larson, PhD; Xiaofeng Liang, MD, MSc; Stephen S. Lim, PhD; Alan D. Lopez, PhD; Michael F. MacIntyre, EdM; Laurie Marczak, PhD; Neal Marquez, BS; Ali H. Mokdad, PhD; Christine Pinho, BA; Farshad Pourmalek, MD, PhD, MPH; Joshua A. Salomon, PhD; Juan Ramon Sanabria, MD; Logan Sandar, BS; Benn Sartorius, PhD; Stephen M. Schwartz, PhD; Katya A. Shackelford, BA; Kenji Shibuya, MD; Jeff Stanaway, PhD; Caitlyn Steiner, MPH; Jiandong Sun, PhD; Ken Takahashi, MD; Stein Emil Vollset, DrPH; Theo Vos, PhD; Joseph A. Wagner, BS; Haidong Wang, PhD; Ronny Westerman, PhD; Hajo Zeeb, PhD; Leo Zoeckler, BA; Foad Abd-Allah; Muktar Beshir Ahmed, MPH; Samer Alabed, MD; Noore K. Alam, MPH; Saleh Fahed Aldhahri, MD; Girma Alem, MS; Mulubirhan Assefa Alemayohu, MPH; Raghib Ali, FRCP, MSc; Rajaa Al-Raddadi, PhD; Azmeraw Amare, MPH; Yaw Amoako, MD, FWACP; Al Artaman, MD, PhD; Hamid Asayesh, PhD; Niguse Atnafu, MS; Ashish Awasthi, PhD; Huda Ba Saleem, PhD; Aleksandra Barac, PhD; Neeraj Bedi, MD; Isabela Bensenor, MD, PhD; Adugnaw Berhane, MD, PhD; Eduardo Bernabé, PhD; Balem Betsu, MS; Agnes Binagwaho, MD, PhD; Dube Boneya, MPH; Ismael Campos-Nonato, MD, PhD; Carlos Castañeda-Orjuela, MD; Ferrán Catalá-López, PhD; Peggy Chiang, PhD; Chioma Chibueze, MD, PhD; Abdulaal Chitheer, FETP; Jee-Young Choi, PhD; Benjamin Cowie, MD, PhD; Solomon Damtew, MPH; José das Neves, PhD; Suhojit Dey, PhD; Samath Dharmaratne, MD; Preet Dhillon, PhD; Eric Ding, ScD; Tim Driscoll, PhD; Donatus Ekwueme, PhD; Aman Yesuf Endries, MPH; Maryam Farvid, PhD; Farshad Farzadfar, MD; Joao Fernandes, PhD; Florian Fischer, MPH; Tsegaye Tewelde G/hiwot, MPH; Alemseged Gebru, MPH; Sameer Gopalani, MPH; Alemayehu Hailu, MPH; Masako Horino, MPH; Nobuyuki Horita, MD, PhD; Abdullatif Husseini, PhD; Inge Huybrechts, PhD; Manami Inoue, MD, PhD; Farhad Islami, MD, PhD; Mihajlo Jakovljevic, MD, PhD; Spencer James, MD; Mehdi Javanbakht, PhD; Sun Ha Jee, PhD; Amir Kasaeian, PhD; Muktar Sano Kedir, MS; Yousef S. Khader, ScD; Young-Ho Khang, MD, PhD; Daniel Kim, MD, DrPH; James Leigh, MD, PhD; Shai Linn, MD, PhD; Raimundas Lunevicius, MD, PhD; Hassan Magdy Abd El Razek, MBBCH; Reza Malekzadeh, MD; Deborah Carvalho Malta, MD, PhD; Wagner Marcenes, PhD; Desalegn Markos, MS; Yohannes A. Melaku, MPH; Kidanu G Meles, MPH; Walter Mendoza, MD; Desalegn Tadese Mengiste, MS; Tuomo J. Meretoja, MD, PhD; Ted R. Miller, PhD; Karzan Abdulmuhsin Mohammad, PhD; Alireza Mohammadi, PhD; Shafiu Mohammed, PhD; Maziar Moradi-Lakeh, MPH, MD; Gabriele Nagel, MD, PhD; Devina Nand, MPH; Quyen Le Nguyen, MD; Sandra Nolte, PhD; Felix A. Ogbo, MD; Kelechi E. Oladimeji, MPH; Eyal Oren, PhD; Mahesh Pa, DNB; Eun-Kee Park, PhD; David M Pereira, PhD; Dietrich Plass, DrPH; Mostafa Qorbani, PhD; Amir Radfar, MD; Anwar Rafay, MS; Mahfuzar Rahman, MD, PhD; Saleem M. Rana, PhD; Kjetil Søreide, PhD; Maheswar Satpathy, PhD; Monika Sawhney, PhD; Sadaf G. Sepanlou, MD, PhD; Masood Ali Shaikh, MD; Jun She, MD, PhD; Ivy Shiue, PhD; Hirbo Roba Shore, MPH; Mark G. Shrime, MD, PhD; Samuel So, MBBS; Samir Soneji, PhD; Vasiliki Stathopoulou, PhD; Konstantinos Stroumpoulis, MD, PhD; Muawiyyah Babale Sufiyan, MBA; Bryan L. Sykes, PhD; Rafael Tabarés-Seisdedos, MD, PhD; Fentaw Tadese, MPH; Bemnet Amare Tedla, BS; Gizachew Assefa Tessema, MPH; J. S. Thakur, MD; Bach Xuan Tran, PhD; Kingsley Nnanna Ukwaja, MD; Benjamin S. Chudi Uzochukwu, FWACP; Vasiliy Victorovich Vlassov, MD; Elisabete Weiderpass, MD, PhD; Mamo Wubshet Terefe, PhD; Henock Gebremedhin Yebyo, MS; Hassen Hamid Yimam, MPH; Naohiro Yonemoto, MPH; Mustafa Z. Younis, DrPH; Chuanhua Yu, PhD; Zoubida Zaidi, MD, PhD; Maysaa El Sayed Zaki, MD, PhD; Zerihun Menlkalew Zenebe, MS; Christopher J. L. Murray, MD, DPhil; Mohsen Naghavi, PhD.
Affiliations of The Global Burden of Disease Cancer Collaboration
Division of Hematology, Department of Medicine, University of Washington, Seattle (Fitzmaurice); Institute for Health Metrics and Evaluation, University of Washington, Seattle (Fitzmaurice, Allen, Barber, Dicker, L. Dandona, Fleming, Forouzanfar, Hancock, Hunter-Merrill, Huynh, Johnson, Kutz, Lim, MacIntyre, Marczak, Marquez, Mokdad, Pinho, Sandar, Shackelford, Stanaway, Steiner, Vos, Wagner, Wang, Zoeckler, Murray, Naghavi); University of Gothenburg, Gothenburg, Sweden (Barregard); Aga Khan University, Pakistan (Bhutta); German Cancer Research Center, Heidelberg, Germany (Brenner); University of Occupational and Environmental Health, Fukuoka, Japan (Chimed-Orchir); Public Health Foundation of India, New Delhi, India (R. Dandona, Kumar); International Foundation for Dermatology, London, England (Hay); Department of Epidemiology and Population Health, Global Health Center, Albert Einstein College of Medicine, New York, New York (Hosgood); Department of Ophthalmology, Ruprecht-Karls-University Heidelberg, Mannheim, Germany (Jonas); Ball State University, Muncie, Indiana (Khubchandani); National Cancer Institute, Rockville, Maryland (Lan); London School of Hygiene and Tropical Medicine, London, England (Larson); Chinese Center for Disease Control and Prevention, Beijing, China (Liang); School of Population and Global Health, University of Melbourne, Melbourne, Australia (Lopez); University of British Columbia, Vancouver, British Columbia (Pourmalek); Department of Global Health and Population, Harvard University, Cambridge, Massachusetts (Salomon); School of Medicine, Marshall University, Huntington, West Virginia (Sanabria); University of KwaZulu-Natal, Durban, South Africa (Sartorius); Fred Hutchinson Cancer Research Center, Seattle, Washington (Schwartz); University of Tokyo, Tokyo, Japan (Shibuya, Inoue); Queensland University of Technology, Brisbane, Queensland, Australia (Sun); University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan (Takahashi); Norwegian Institute of Public Health, Bergen, Norway (Vollset); Federal Institute for Population Research, Hessen, Germany (Westerman); Leibniz Institute for Prevention Research and Epidemiology, Bremen, Germany (Zeeb); Department of Neurology, Cairo University, Cairo, Egypt (Abd-Allah); College of Health Sciences, Department of Epidemiology, Jimma University, Jimma, Ethiopia (Ahmed); College of Health Sciences, Department of Epidemiology, University of Sheffield, Sheffield, England (Alabed); Queensland Health Herston, Brisbane, Queensland, Australia (Alam); Department of Otolaryngology–Head and Neck Surgery, King Saud University, Riyadh, Saudi Arabia (Aldhahri); Debre Markos University, Debre Markos, Ethiopia (Alem, Boneya); School of Public Health, Mekelle University, Mekelle, Ethiopia (Alemayohu); Cancer Epidemiology Unit, University of Oxford, Oxford, England (Ali); Public Health Directorate, Department of Preventive Medicine, Ministry of Health, Jeddah, Makkah, Saudi Arabia (Al-Raddadi); School of Medicine, University of Adelaide, Adelaide, Australia (Amare); Bahir Dar University, College of Medicine and Health Sciences, Bahir Dar, Ethiopia (Amare); Department of Medicine, Komfo Anokye Teaching Hospital Ghana, Kumasi, Ghana (Amoako); Department of Community Health Science, University of Manitoba, Winnipeg, Manitoba, Canada (Artaman); Department of Medical Emergency, School of Paramedic, Qom University of Medical Sciences, Qom, Iran (Asayesh); College of Medicine and Health Sciences, Department of Nursing, Mizan Tepi University, Mizan Teferi, Ethiopia (Atnafu); Department of Biostatistics, Nayati Multi Super Speciality Hospital, Mathura, India (Awasthi); Department of Community Medicine, Aden Cancer Registry, and Research Center Faculty of Medicine and Health Sciences, Aden University, Aden, Yemen (Saleem); Clinic for Infectious and Tropical Diseases, Clinical Center of Serbia, Faculty of Medicine University of Belgrade, Belgrade, Serbia (Barac); Department of Epidemiology, Tropical Disease Unit, College of Public Health and Tropical Medicine, Jazan, Saudi Arabia (Bedi); Department of Internal Medicine, University of São Paul, São Paul, Brazil (Bensenor); College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia) (Berhane); Division of Population and Patient Health, King’s College London Dental Institute, London, England (Bernabé, Marcenes); Mekelle University, Tigray, Ethiopia (Betsu); University of Global Health Equit, Kigali, Rwanda (Binagwaho); Department of Global Health and Social Medicine, Harvard Medical School, Harvard University, Cambridge, Massachusetts (Binagwaho); National Institute of Public Health, Morelos, Mexico (Campos-Nonato); Instituto Nacional de Salud Bogota, Bogota, Colombia (Castañeda-Orjuela); Department of Medicine, University of Valencia/INCLIVA Health Research Institute and CIBERSAM, Valencia, Spain (Catalá-López, Tabarés-Seisdedos); Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Ontario, Canada (Catalá-López); Clinical Governance Unit, Gold Coast Health, Southport, Queensland, Australia (Chiang); National Center for Child Health and Development, Tokyo, Japan (Chibueze); Ministry of Health, Baghdad, Iraq (Chitheer); Seoul National University Hospital, Seoul, South Korea (Choi); WHO Collaborating Centre for Viral Hepatitis, Doherty Institute, Melbourne, Australia (Cowie); School of Public Health, College of Health Sciences and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia) (Damtew); i3S–Instituto de Investigação e Inovação em Saúde, University of Porto, Porto, Portugal (das Neves); Indian Institute of Public Health–Delhi, Public Health Foundation of India, Gurgaon, India (Dey); Department of Community Medicine, Faculty of Medicine, University of Peradeniya, Peradeniya, Sri Lanka (Dharmaratne); Centre for Chronic Conditions and Injuries, Public Health Foundation of India, Gurgaon, India (Dhillon); Department of Nutrition, Harvard University, Boston, Massachusetts (Ding); Sydney School of Public Health, University of Sydney, Sydney, New South Wales, Australia (Driscoll); Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia (Ekwueme); Arba Minch University, Arba Minch, Ethiopia (Endries); Department of Nutrition, T.H. Chan School of Public Health, Boston, Massachusetts (Farvid); Harvard/MGH Center on Genomics, Vulnerable Populations, and Health Disparities, Mongan Institute for Health Policy, Massachusetts General Hospital, Boston, Massachusetts (Farvid); Non-Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran (Farzadfar); Center for Biotechnology and Fine Chemistry–Associate Laboratory, Faculty of Biotechnology, Catholic University of Portugal, Porto, Portugal (Fernandes); Bielefeld University, Bielefeld, Germany (Fischer); Department of Epidemiology and Biostatistics, Jimma University, Jimma, Ethiopia (G/hiwot); Mekelle University, Mekelle, Ethiopia (Gebru, Melaku, Mengiste, Yebyo); Government of the Federated States of Micronesia, Palikir, Federated States of Micronesia (Gopalani); Addis Ababa University, Addis Ababa, Ethiopia (Hailu); Nevada Division of Public and Behavioral Health, Carson City (Horino); Yokohama City University Graduate School of Medicine, Yokohama, Japan (Horita); Institute of Community and Public Health, Birzeit University, Birzeit, Palestine (Husseini); International Agency for Research on Cancer, Lyon, France (Huybrechts); Surveillance and Health Services Research, American Cancer Society, Atlanta, Georgia (Islami); University of Kragujevac, Kragujevac, Serbia (Jakovljevic); Emergency Medicine, Denver Health/University of Colorado, Denver (James); Health Economics Group, Institute of Health and Society, Newcastle University, Newcastle Upon Tyne, England (Javanbakht); Yonsei University, Seoul, South Korea (Jee); Hematology–Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran (Kasaeian); Department of Pharmacy, College of Health Sciences, Mizan Tepi University, Mizan Teferi, Ethiopia (Kedir); Department of Community Medicine, Public Health and Family Medicine, Jordan University of Science and Technology Irbid, Irbid, Jordan (Khader); Department of Health Policy and Management, Seoul National University, Seoul, South Korea (Khang); Institute of Health Policy and Management, Seoul National University Medical Research Center, Seoul, South Korea (Khang); Department of Health Science, Northeastern University, Boston, Massachusetts (Kim); Asbestos Disease Research Institute, University of Sydney, Sydney, Australia (Leigh); School of Public Health, Faculty of Social Welfare and Health, University of Haifa, Haifa, Israel (Linn); Aintree University Hospital National Health Service Foundation Trust, Liverpool, England (Lunevicius); Surgery Department, Mansoura Faculty of Medicine, Mansoura, Egypt (El Razek); Non-Communicable Diseases Research Center, Shiraz University of Medical Sciences, Shiraz, Fars, Iran (Malekzadeh); Universidade Federal de Minas Gerais, Belo Horizonte, Brazil (Malta); College of Health Sciences, Arsi University, Assela, Ethiopia (Markos); Department of Epidemiology, College of Health Science, School of Public Health, Mekelle University, Mekelle, Ethiopia (Meles); Peru Country Office, United Nations Population Fund, Lima, Peru (Mendoza); Comprehensive Cancer Center, Breast Surgery Unit, Helsinki University Hospital, Helsinki, Finland (Meretoja); Pacific Institute for Research and Evaluation, Calverton, Maryland (Miller); Center for Population Health Research, The Curtin University, Calverton, Maryland (Miller); Faculty of Education, Ishik University, Erbil, Iraq (Mohammad); Faculty of Education, University of Salahaddin, Erbil, Iraq (Mohammad); Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran (Mohammadi); Ahmadu Bello University, Zaria, Nigeria (Mohammed); Department of Community Medicine, Preventive Medicine and Public Health Research Center, Iran University of Medical Sciences, Tehran, Iran (Moradi-Lakeh); Ulm University, Ulm, Germany (Nagel); Ministry of Health, Suva, Fiji (Nand); Duy Tan University, Da Nang Vietnam (Le Nguyen); Charité Universitätsmedizin, Berlin, Germany (Nolte); Centre for Health Research, Western Sydney University, Sydney, Australia (Ogbo); Department of Public Health Medicine, College of Health Science, Howard College Campus, University of KwaZulu-Natal, Durban, South Africa (Oladimeji); Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health University of Arizona, Tucson (Oren); Department of Pulmonary Medicine, JSS Medical College, JSS University, Mysore, India (Pa); Kosin University, Busan, South Korea (Park); Universidade do Porto, Porto, Portugal (Pereira); Department of Environmental Hygiene, German Environment Agency, Berlin, Germany (Plass); Alborz University of Medical Sciences, Karaj, Iran (Qorbani); College of Graduate Heath Study, A. T. Still University, Kirksville, Missouri (Radfar); Epidemiology and Biostatistics, Contech International Health Consultants, Lahore Pakistan (Rafay); BRAC, Dhaka, Bangladesh (Rahman); Contech School of Public Health, Lahore, Pakistan (Rana); Department of Gastrointestinal Surgery, Stavanger University Hospital, Stavanger, Norway (Søreide); Department of Clinical Medicine, University of Bergen, Bergen, Norway (Søreide); Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, India (Satpathy); Department of Public Health, College of Health Professions, Marshall University, Huntington, West Virginia (Sawhney); Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran (Sepanlou); Independent consultant, Karachi Pakistan (Shaikh); Fudan University Shanghai, China (She); Northumbria University, Newcastle Upon Tyne, England (Shiue); College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia (Shore); Program in Global Surgery and Social Change, Harvard Medical School, Boston, Massachusetts (Shrime); Asian Liver Center, Stanford University, Palo Alto, California (So); The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, Lebanon, New Hampshire (Soneji); Norris Cotton Cancer Center, Dartmouth Medical School, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire (Soneji); Attikon University Hospital, Athens, Greece (Stathopoulou); Alexandra General Hospital of Athens, Athens, Greece (Stroumpoulis); Department of Community Medicine Faculty of Medicine, Ahmadu Bello University, Zaria, Nigeria (Sufiyan); Department of Criminology, Law & Society, University of California Irvine, Irvine (Sykes); Department of Public Health, Wollo University, Dessie, Ethiopia (Tadese); University of Gondar, Gondar, Ethiopia (Tedla); Institute of Public Health, University of Gondar, Gondar, Ethiopia (Tessema); School of Public Health, University of Adelaide, Adelaide, Australia (Tessema); Post Graduate Institute of Medical Education and Research, Chandigarh, India (Thakur); Johns Hopkins University Baltimore, Maryland (Tran); Department of Medicine, Federal Teaching Hospital, Abakaliki Nigeria (Ukwaja); Institute of Public Health, College of Medicine, University of Nigeria Nsukka, Enugu, Nigeria (Uzochukwu); Center for Health Policy, National Research University, Higher School of Economics, Moscow, Russia (Vlassov); Department of Research, Group of Etiological Cancer Research, Cancer Registry of Norway, Institute of Population-Based Cancer Research, Oslo (Weiderpass); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (Weiderpass); Genetic Epidemiology Group, Folkhälsan Research Center, Helsinki, Finland (Weiderpass); Department of Community Medicine, University of Tromsø, The Arctic University of Norway, Tromsø, Norway (Weiderpass); Department of Public Health, St. Paul’s Hospital Millenium Medical College, Addis Ababa, Ethiopia (Wubshet Terefe); Mizan Tepi University, Mizan Teferi, Ethiopia (Yimam); Kyoto University, Kyoto, Japan (Yonemoto); Department of Health Policy and Management, Jackson State University, Jackson, Mississippi (Younis); Wuhan University, Wuhan, Hubei, China (Yu); Department of Epidemiology, University Hospital of Setif, Algeria (Zaidi); Clinical Pathology Department, Mansoura Faculty of Medicine, Mansoura, Egypt (Zaki); College of Health Sciences, Department of Midwidery, Mekelle University, Mekelle, Ethiopia (Zenebe).
Author Contributions
Drs Naghavi and Fitzmaurice had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Fitzmaurice, Dicker, Forouzanfar, Jonas, Liang, Mokdad, Stanaway, Vos, Alem, Barac, Ding, James, Jee, Leigh, Marcenes, Meles, Mohammad, Satpathy, Sawhney, Soneji, Tessema, Younis, Zaidi, Murray, Naghavi.
Acquisition, analysis, or interpretation of data: Fitzmaurice, Allen, Barber, Barregard, Bhutta, Brenner, Dicker, Fleming, Hancock, Hay, Hunter-Merrill, Huynh, Hosgood, III, Johnson, Jonas, Khubchandani, Kutz, Lan, Larson, Lim, Marczak, Marquez, Mokdad, Pourmalek, Salomon, Sanabria, Sartorius, Schwartz, Shackelford, Stanaway, Sun, Takahashi, Vollset, Vos, Wagner, Westerman, Zeeb, Abd-Allah, Ahmed, Alabed, Aldhahri, Alem, Alemayohu, Ali, Al-Raddadi, Amare, Amoako, Artaman, Asayesh, Atnafu, Awasthi, Ba Saleem, Bedi, Bensenor, Berhane, Bernabé, Betsu, Binagwaho, Boneya, Campos-Nonato, Castañeda-Orjuela, Catalá-López, Chibueze, Chitheer, Choi, Cowie, Damtew, das Neves, Dey, Dharmaratne, Dhillon, Driscoll, Ekwueme, Endries, Farvid, Farzadfar, Fernandes, Fischer, G/hiwot, Gebru, Gopalani, Hailu, Horino, Horita, Husseini, Huybrechts, Inoue, Islami, Jakovljevic, Javanbakht, Jee, Kasaeian, Khader, Khang, Kim, Leigh, Linn, Lunevicius, Magdy Abd El Razek, Malekzadeh, Malta, Shifti, Melaku, Mendoza, Meretoja, Miller, Mohammadi, Mohammed, Kedir, Moradi-Lakeh, Nagel, Nand, Nguyen, Nolte, Ogbo, Oladimeji, Oren, P A, Park, Pereira, Plass, Qorbani, Radfar, Rafay, Rahman, Rana, Søreide, Satpathy, Sawhney, Sepanlou, Shaikh, She, Shiue, Shrime, So, Stathopoulou, Stroumpoulis, Sufiyan, Sykes, Tabarés-Seisdedos, Tadese, Tedla, Thakur, Tran, Ukwaja, Uzochukwu, Vlassov, Weiderpass, Yebyo, Yimam Hassen, Yonemoto, Younis, Yu, Zaidi, Zaki, Zenebe, Naghavi.
Drafting of the manuscript: Fitzmaurice, Allen, Fleming, Hancock, Hunter-Merrill, Huynh, Marczak, Marquez, Mokdad, Sanabria, Shackelford, Barac, Boneya, Horino, Magdy Abd El Razek, Mohammad, Pereira, Satpathy, Sawhney, Vlassov, Zaki.
Critical revision of the manuscript for important intellectual content: Fitzmaurice, Allen, Barber, Barregard, Bhutta, Brenner, Dicker, Forouzanfar, Hay, Hosgood, III, Johnson, Jonas, Khubchandani, Kutz, Lan, Larson, Liang, Lim, Marczak, Mokdad, Pourmalek, Salomon, Sanabria, Sartorius, Schwartz, Stanaway, Sun, Takahashi, Vollset, Vos, Wagner, Westerman, Zeeb, Abd-Allah, Ahmed, Alabed, Aldhahri, Alem, Alemayohu, Ali, Al-Raddadi, Amare, Amoako, Artaman, Asayesh, Atnafu, Awasthi, Ba Saleem, Barac, Bedi, Bensenor, Berhane, Bernabé, Betsu, Binagwaho, Boneya, Campos-Nonato, Castañeda-Orjuela, Catalá-López, Chibueze, Chitheer, Choi, Cowie, Damtew, das Neves, Dey, Dharmaratne, Dhillon, Ding, Driscoll, Ekwueme, Endries, Farvid, Farzadfar, Fernandes, Fischer, G/hiwot, Gebru, Gopalani, Hailu, Horino, Horita, Husseini, Huybrechts, Inoue, Islami, Jakovljevic, James, Javanbakht, Jee, Kasaeian, Khader, Khang, Kim, Leigh, Linn, Lunevicius, Magdy Abd El Razek, Malekzadeh, Malta, Marcenes, Shifti, Melaku, Meles, Mendoza, Meretoja, Miller, Mohammadi, Mohammed, Kedir, Moradi-Lakeh, Nagel, Nand, Nguyen, Nolte, Ogbo, Oladimeji, Oren, P A, Park, Pereira, Plass, Qorbani, Radfar, Rafay, Rahman, Rana, Søreide, Satpathy, Sawhney, Sepanlou, Shaikh, She, Shiue, Shrime, So, Soneji, Stathopoulou, Stroumpoulis, Sufiyan, Sykes, Tabarés-Seisdedos, Tadese, Tedla, Tessema, Thakur, Tran, Ukwaja, Uzochukwu, Vlassov, Weiderpass, Yebyo, Yimam Hassen, Yonemoto, Younis, Yu, Zaidi, Zaki, Zenebe, Murray, Naghavi.
Statistical analysis: Fitzmaurice, Allen, Barber, Dicker, Fleming, Forouzanfar, Hunter-Merrill, Huynh, Jonas, Khubchandani, Kutz, Lim, Marquez, Mokdad, Salomon, Stanaway, Sun, Vos, Alem, Amare, Asayesh, Boneya, Ding, Endries, Farzadfar, G/hiwot, Hailu, James, Kasaeian, Khader, Magdy Abd El Razek, Mohammed, Kedir, Moradi-Lakeh, Nguyen, Qorbani, Satpathy, Sawhney, Shaikh, Sykes, Ukwaja, Vlassov, Yebyo, Yu, Zaki, Naghavi.
Obtained funding: Mokdad, Jee, Weiderpass, Murray.
Administrative, technical, or material support: Allen, Hancock, Hay, Johnson, Liang, Marczak, Mokdad, Shackelford, Wagner, Abd-Allah, Amoako, Asayesh, Awasthi, Barac, Bedi, Bensenor, Berhane, Castañeda-Orjuela, Catalá-López, das Neves, Dhillon, Fernandes, Kasaeian, Marcenes, Meles, Meretoja, Mohammadi, Mohammed, Nand, Ogbo, Oren, P A, Pereira, Rahman, Satpathy, Sawhney, Shaikh, She, Tedla, Tran, Ukwaja, Weiderpass, Younis.
Supervision: Fitzmaurice, Allen, Liang, Mokdad, Shackelford, Vos, Westerman, Barac, Fischer, Horita, Jakovljevic, Jee, Nagel, Tabarés-Seisdedos, Weiderpass, Murray, Naghavi.
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank Ubai Alsharif, MPH, DMD, Charité Universitätsmedizin, Berlin, Germany, for contributions to analysis and interpretation of data and critical revision of the manuscript; we also thank Gholamreza Roshandel, MD, MPH, PhD, Golestan University of Medical Sciences, Golestan Research Center of Gastroenterology and Hepatology, Gorgan, Iran, for contributions to analysis and interpretation of data; drafting of the manuscript; and critical revision of the manuscript. They received no compensation for their contributions.
Additional Information: With 3 exceptions, author names are listed in 2 alphabetical series: the first series lists the core authors, who contributed most substantially to the work; the second series lists all of the other authors. Dr Fitzmaurice, as the corresponding author, is listed first; senior author Dr Naghavi is listed last; and Dr Murray is listed second to last owing to his critical involvement in the Global Burden of Disease enterprise.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowy DR, Collins FS. Aiming high: changing the trajectory for cancer. N Engl J Med. 2016;374(20):1901–1904. doi: 10.1056/NEJMp1600894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grady D. A sickened body as cancer weapon. The New York Times. 2016 Jul 30; [Google Scholar]
- 4.Hanahan D. Rethinking the war on cancer. Lancet. 2014;383(9916):558–563. doi: 10.1016/S0140-6736(13)62226-6. [DOI] [PubMed] [Google Scholar]
- 5.Horton S, Gauvreau CL. Cancer in Low- and Middle-Income Countries: An Economic Overview. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: Disease Control Priorities. 3. Vol. 3. Washington, DC: The International Bank for Reconstruction and Development/The World Bank; 2015. [Accessed July 31, 2016]. http://www.ncbi.nlm.nih.gov/books/NBK343620/ [PubMed] [Google Scholar]
- 6.Levit L, Balogh E, Nass S, Ganz PA, editors. Committee on Improving the Quality of Cancer Care. Addressing the Challenges of an Aging Population, Board on Health Care Services, Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [Accessed August 5, 2016]. https://www.nap.edu/catalog/18359. [PubMed] [Google Scholar]
- 7.Coleman MP. Cancer survival: global surveillance will stimulate health policy and improve equity. Lancet. 2014;383(9916):564–573. doi: 10.1016/S0140-6736(13)62225-4. [DOI] [PubMed] [Google Scholar]
- 8.Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303(12):1159–1166. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stringhini S, Berkman L, Dugravot A, et al. Socioeconomic status, structural and functional measures of social support, and mortality: The British Whitehall II Cohort Study, 1985–2009. Am J Epidemiol. 2012;175(12):1275–1283. doi: 10.1093/aje/kwr461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014;383(9916):549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 11.United Nations. [Accessed September 1, 2016];Sustainable Development Goals. https://sustainabledevelopment.un.org/
- 12.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzmaurice C, Dicker D, Pain A, et al. Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens GA, Alkema L, Black RE, et al. GATHER Working Group. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. PLoS Med. 2016;13(6):e1002056. doi: 10.1371/journal.pmed.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 16.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 17.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10(1):1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark H. NCDs: a challenge to sustainable human development. Lancet. 2013;381(9866):510–511. doi: 10.1016/S0140-6736(13)60058-6. [DOI] [PubMed] [Google Scholar]
- 21.United Nations. [Accessed October 27, 2016];High-level meeting on prevention and control of non-communicable diseases. 2011 http://www.un.org/en/ga/ncdmeeting2011/
- 22.World Health Organization. [Accessed October 27, 2016];Global Action Plan for the prevention and control of NCDs 2013–2020. http://www.who.int/nmh/events/ncd_action_plan/en/
- 23.Lim S GBD 2015 SDG Collaborators. Measuring the health-related Sustainable Development Goals in 188 countries: a baseline analysis from the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1813–1850. doi: 10.1016/S0140-6736(16)31467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefan DC. Cancer Care in Africa: an overview of resources. J Glob Oncol. 2015;1(1):30–36. doi: 10.1200/JGO.2015.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelband H, Sankaranarayanan R, Gauvreau CL, et al. Disease Control Priorities-3 Cancer Author Group. Costs, affordability, and feasibility of an essential package of cancer control interventions in low-income and middle-income countries: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387(10033):2133–2144. doi: 10.1016/S0140-6736(15)00755-2. [DOI] [PubMed] [Google Scholar]
- 26.Chalkidou K, Marquez P, Dhillon PK, et al. Evidence-informed frameworks for cost-effective cancer care and prevention in low, middle, and high-income countries. Lancet Oncol. 2014;15(3):e119–e131. doi: 10.1016/S1470-2045(13)70547-3. [DOI] [PubMed] [Google Scholar]
- 27.Ilbawi AM, Anderson BO. Global cancer consortiums: moving from consensus to practice. Ann Surg Oncol. 2015;22(3):719–727. doi: 10.1245/s10434-014-4346-6. [DOI] [PubMed] [Google Scholar]
- 28.Carlson JW, Lyon E, Walton D, et al. Partners in pathology: a collaborative model to bring pathology to resource poor settings. Am J Surg Pathol. 2010;34(1):118–123. doi: 10.1097/PAS.0b013e3181c17fe6. [DOI] [PubMed] [Google Scholar]
- 29.Qu C, Chen T, Fan C, et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: a cluster randomized controlled trial. PLoS Med. 2014;11(12):e1001774. doi: 10.1371/journal.pmed.1001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. [Accessed September 9, 2016];Global health sector strategy on viral hepatitis 2016–2021. 2016 Jun; http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/
- 31.Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer. 2016;139(7):1534–1545. doi: 10.1002/ijc.30211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364(9430):249–256. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 34.Tsu V, Jerónimo J. Saving the world’s women from cervical cancer. N Engl J Med. 2016;374(26):2509–2511. doi: 10.1056/NEJMp1604113. [DOI] [PubMed] [Google Scholar]
- 35.Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009;45(15):2640–2648. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Action CC. Investing in cervical cancer prevention 2015–2020; meeting report; London. November 3 & 4, 2015; [Accessed August 13, 2016]. http://www.cervicalcanceraction.org/pubs/pubs.php. [Google Scholar]
- 37.de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42(2):219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45(16):2867–2873. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Steffen A, Huerta J-M, Weiderpass E, et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2015;137(3):646–657. doi: 10.1002/ijc.29432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174–g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Agency for Research on Cancer. [Accessed October 27, 2016];Helicobacter pylori eradication as a strategy for preventing gastric cancer. http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php.
- 42.Choi KS, Jun JK, Suh M, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015;112(3):608–612. doi: 10.1038/bjc.2014.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee K-J, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S JPHC Study Group. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118(9):2315–2321. doi: 10.1002/ijc.21664. [DOI] [PubMed] [Google Scholar]
- 44.Leung WK, Wu MS, Kakugawa Y, et al. Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto A, Kuriyama S, Nishino Y, et al. Lower risk of death from gastric cancer among participants of gastric cancer screening in Japan: a population-based cohort study. Prev Med. 2007;44(1):12–19. doi: 10.1016/j.ypmed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Mizoue T, Yoshimura T, Tokui N, et al. Japan Collaborative Cohort Study Group. Prospective study of screening for stomach cancer in Japan. Int J Cancer. 2003;106(1):103–107. doi: 10.1002/ijc.11183. [DOI] [PubMed] [Google Scholar]
- 47.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609–e616. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 48.Forouzanfar MH, Murray CJL, Afshin A GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–2043e10. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2(6):762–769. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clague J, Bernstein L. Physical activity and cancer. Curr Oncol Rep. 2012;14(6):550–558. doi: 10.1007/s11912-012-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolk A. Potential health hazards of eating red meat. J Intern Med. 2016 Sep; doi: 10.1111/joim.12543. [DOI] [PubMed] [Google Scholar]
- 53.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576–2594. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 55.Siripongpreeda B, Mahidol C, Dusitanond N, et al. High prevalence of advanced colorectal neoplasia in the Thai population: a prospective screening colonoscopy of 1,404 cases. BMC Gastroenterol. 2016;16(1):101. doi: 10.1186/s12876-016-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colditz GA, Bohlke K. Priorities for the primary prevention of breast cancer. CA Cancer J Clin. 2014;64(3):186–194. doi: 10.3322/caac.21225. [DOI] [PubMed] [Google Scholar]
- 57.Anderson BO, Lipscomb J, Murillo RH, Thomas DB. Breast Cancer. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: Disease Control Priorities. 3. Vol. 3. Washington, DC: The International Bank for Reconstruction and Development/The World Bank; 2015. [Accessed September 14, 2016]. http://www.ncbi.nlm.nih.gov/books/NBK343636/ [Google Scholar]
- 58.Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12(4):387–398. doi: 10.1016/S1470-2045(11)70031-6. [DOI] [PubMed] [Google Scholar]
- 59.Allemani C, Weir HK, Carreira H, et al. CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 61.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III, family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41(14):2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 62.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 63.Loomis D, Guyton KZ, Grosse Y, et al. International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016;17(7):877–878. doi: 10.1016/S1470-2045(16)30239-X. [DOI] [PubMed] [Google Scholar]
- 64.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92(3):151–159. doi: 10.1002/jso.20357. [DOI] [PubMed] [Google Scholar]
- 66.Mikkelsen L, Phillips DE, AbouZahr C, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386(10001):1395–1406. doi: 10.1016/S0140-6736(15)60171-4. [DOI] [PubMed] [Google Scholar]
- 67.Flaxman AD, Vahdatpour A, James SL, Birnbaum JK, Murray CJ Population Health Metrics Research Consortium (PHMRC) Direct estimation of cause-specific mortality fractions from verbal autopsies: multisite validation study using clinical diagnostic gold standards. Popul Health Metr. 2011;9(1):35. doi: 10.1186/1478-7954-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bray F. The evolving scale and profile of cancer worldwide: much ado about everything. Cancer Epidemiol Biomarkers Prev. 2016;25(1):3–5. doi: 10.1158/1055-9965.EPI-15-1109. [DOI] [PubMed] [Google Scholar]
- 69.Ahern RM, Lozano R, Naghavi M, Foreman K, Gakidou E, Murray CJ. Improving the public health utility of global cardiovascular mortality data: the rise of ischemic heart disease. Popul Health Metr. 2011;9(1):8. doi: 10.1186/1478-7954-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.