Abstract
Objectives
The aim of this in vitro study was to evaluate the biocompatibility of newly proposed root-end filling materials, Biodentine, Micro-Mega mineral trioxide aggregate (MM-MTA), polymethylmethacrylate (PMMA) bone cement, and Smart Dentin Replacement (SDR), in comparison with contemporary root-end filling materials, intermediate restorative material (IRM), Dyract compomer, ProRoot MTA (PMTA), and Vitrebond, using human periodontal ligament (hPDL) fibroblasts.
Materials and Methods
Ten discs from each material were fabricated in sterile Teflon molds and 24-hour eluates were obtained from each root-end filling material in cell culture media after 1- or 3-day setting. hPDL fibroblasts were plated at a density of 5 × 103/well, and were incubated for 24 hours with 1:1, 1:2, 1:4, and 1:8 dilutions of eluates. Cell viability was evaluated by XTT assay. Data was statistically analysed. Apoptotic/necrotic activity of PDL cells exposed to material eluates was established by flow cytometry.
Results
The Vitrebond and IRM were significantly more cytotoxic than the other root-end filling materials (p < 0.05). Those cells exposed to the Biodentine and Dyract compomer eluates showed the highest survival rates (p < 0.05), while the PMTA, MM-MTA, SDR, and PMMA groups exhibited similar cell viabilities. Three-day samples were more cytotoxic than 1-day samples (p < 0.05). Eluates from the cements at 1:1 dilution were significantly more cytotoxic (p < 0.05). Vitrebond induced cell necrosis as indicated by flow cytometry.
Conclusions
This in vitro study demonstrated that Biodentine and Compomer were more biocompatible than the other root-end filling materials. Vitrebond eluate caused necrotic cell death.
Keywords: Apicoectomy, Apoptosis, Cytotoxicity, Endodontics, Fibroblast
INTRODUCTION
Endodontic surgery is an alternative treatment when orthograde root canal therapy/retreatment fails or nonsurgical retreatment is not an option [1]. Root-end resection procedures include the use of a root-end filling material to provide an effective seal, and to facilitate the repair and regeneration of periradicular tissues. Retrograde filling materials are in close contact with vital tissues around the root apex for extended periods of time; therefore, ideally, reparative root-end filling materials should be noncytotoxic to the proximal tissues.
Various materials with different compositions have been preferred for retrograde filling. Among them, intermediate restorative material (IRM) has shown a high clinical success rate, similar to that of mineral trioxide aggregate (MTA) [2]. However, previous studies have also indicated decreased cell viability characteristics with the use of IRM [3]. Vitrebond, a light cured glass ionomer cement, has been reported to have favorable tissue response when compared with amalgam [4]. Therefore, it was previously considered to be an alternative root-end filling material [5]. Composite resin applied with a dentin bonding agent has also been reported to have good sealing ability when used as a retrograde filling [6]. However, avoiding moisture contamination is of primary importance when placing composite resin to the root-end. Compomers are mixture of composite resins and glass ionomer cements, and these materials are popular in restorative dentistry because of their good mechanical and aesthetic qualities. When placed subgingivally, compomers have exhibited biocompatibility with gingival tissues [7]. In addition, polymethylmethacrylate (PMMA) bone cement (AF cement, Laboratorios, Buenos Aires, Argentina) is designed for medical surgery applications [8]. It is very tolerant of a high moisture environment, and may be a promising root-end filling material; however, little is known about the potential cytotoxicity of PMMA bone cement when placed as a root-end filling material [9].
MTA has been widely used as a retrograde filling material because of its favorable biological properties [10]. Recently developed calcium silicate-based cements, such as Micro-Mega MTA (MM-MTA; Micro-Mega, Besançon, France) and Biodentine (Septodont, Saint-Maur-des-Fossés, France), have been proposed for use as pulp capping, root-end filling, and perforation repair materials. According to the manufacturer, MM-MTA has a reduced setting time of 20 minutes, and better physicochemical characteristics than other MTA formulations. However, Biodentine has been found to display biocompatibility similar to that of MTA [11,12]. To date, our knowledge is limited as to whether MM-MTA and/or Biodentine are sufficient for such clinical applications. Additionally, the apoptotic/necrotic effects of novel root-end filling materials on periodontal ligament (PDL) cells have not been well documented.
The aim of this in vitro study was to assess the cytotoxic effects of 4 new root-end filling materials, Biodentine, MM-MTA, PMMA bone cement, and Smart Dentin Replacement flowable composite resin (SDR; Dentsply, Konstanz, Germany), on PDL cells, and to compare them with contemporary root-end filling materials, IRM (Dentsply Caulk, Milford, DE, USA), Dyract compomer (Dentsply), ProRoot MTA (PMTA; Dentsply Tulsa Dental, Tulsa, OK, USA), and Vitrebond (3M ESPE, Seefeld, Germany), using the XTT cell proliferation kit and apoptosis assays.
MATERIALS AND METHODS
Cell culture
The ethics committee of the Selcuk University approved this study, and informed consent was provided prior to the collection of the PDL tissue samples. The human PDL cells were obtained from first premolars that were extracted for orthodontic purposes. After extraction, the teeth were immediately stored in Dulbecco's MEM/Ham's F-12 (1:1 mixture, DMEM/Ham's F-12 [1:1], Biochrom, Berlin, Germany). The PDL tissues from the middle third of the root surface were removed via thorough curetting. Extracted PDL tissue samples were cultured on 100-mm culture dishes (Corning, NY, USA) with the use of explant technique in DMEM/Ham's F-12 (1:1) supplemented with 10% fetal bovine serum (Superior FBS, Biochrom), 100 units/mL of penicillin, and 100 µg/mL of streptomycin (Biochrom). Then, the samples were incubated at 37°C for one week to observe PDL cells (passage zero). When the cells reached confluence, they were subcultured by using 0.25% trypsin/ethylenediaminetetraacetic acid (EDTA) (Biochrom). Passage numbers of 4–7 were used for this study.
Sample preparation
The test materials were prepared according to the manufacturer's instructions under aseptic conditions, and 10 discs from each material were fabricated in sterile Teflon molds (6 mm in diameter and 2 mm thick). Each sample disc was allowed to set for 1 day (n = 5) or 3 days (n = 5) at 37°C and 100% humidity. After setting, the samples were exposed to ultraviolet light for 20 minutes on each surface to ensure sterility [3] and then transferred to 24-well tissue culture plates (Corning). Then, 24-hour eluates were obtained from each material in cell culture media in general accordance with ISO 10993-5 [13]. The original eluates (1:1) were serially diluted with cell culture media to achieve a total of 4 concentrations of each eluate.
XTT assay
The human PDL cells were seeded in 96-well plates, at a density of 5 × 103 cells per well, and incubated for 24 hours to allow the attachment of the cells before the addition of the eluates. Then, the culture media was removed, and the fibroblasts were incubated for 24 hours with equal volumes (100 µL) of the 1:1, 1:2, 1:4, and 1:8 concentrations of the eluates. In the control wells, 100 µL of the culture medium was added. After 24 hours of cell exposure to each eluate, the cell viability was determined via XTT assay using a commercial kit (Serva Electrophoresis, Heidelberg, Germany). The eluates were then aspirated and the morphological changes in the PDL cells were immediately photographed under an inverted microscope (×10 magnification, Leica DM IL LED, Leica Microsystems GmbH, Wetzlar, Germany) before adding the XTT working solution. The XTT working solution was prepared as described previously [14]. After the aspiration of the eluates, 100 µL of the colorless culture media and 50 µL of the XTT working solution were added to each well. The metabolic activity of the mitochondria was measured using a microplate spectrophotometer (Epoch, BioTek Instrument Inc., Winooski, VT, USA). The optical density (OD) was determined at a wavelength of 460 nm, and the experiment was repeated 3 times. The cell viabilities were calculated as a percentage relative to the cell control. The impacts of the materials, eluate concentrations, and setting times on the cytotoxicity of the fibroblasts were statistically analyzed using a univariate analysis of variance followed by post hoc Tukey test (α = 0.05). Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA) was used as the analytical tool.
Flow cytometry
The PDL cells were seeded in 6-well plates at a density of 5 × 104 cells/mL, and allowed to attach for 24 hours. The 1:8 concentration eluates from the 3-day setting time samples were chosen for use in the flow cytometry. The eluates were obtained as described in the previous section. After exposing the PDL cells to the selected material eluates for 24 hours, the cells were detached, centrifuged, and resuspended at 1 × 104 cells/mL in the 1× binding buffer included in the Apoptosis and Necrosis Quantification kit (BD Pharmingen, San Diego, CA, USA). The cells were stained with Annexin V and 7AAD according to the flow cytometry protocol for a viability assay (BD Pharmingen), and incubated for 20 minutes at room temperature, with protection from the light. The stained cells were analyzed using a flow cytometer (BD Biosciences, San Jose, CA, USA) with 488 nm excitation. Then, the percentage distributions of the vital, early apoptotic, late apoptotic/necrotic, and dead cell populations were determined. Finally, the remaining undiluted eluates were collected and the pH values were measured with pH indicator strips (Merck, Darmstadt, Germany).
RESULTS
Cell morphology analysis
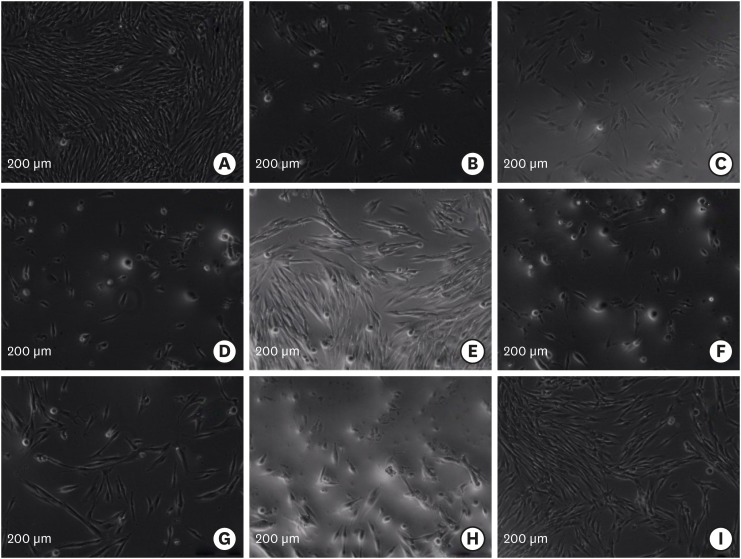
In the control groups, the untreated PDL cells were attached and spread over the tissue culture plate well. They exhibited spindle shapes with elongated cytoplasmic extensions (Figure 1A). In the IRM, Vitrebond, SDR, PMMA, and MM-MTA groups, some of the cells had become rounded and the morphology of the cells tended to show an altered appearance with retraction (Figure 1B-1D, 1F, and 1H). Additionally, some particulates were observed adjacent to the cells exposed to the Vitrebond eluates. The cells exposed to the Biodentine and Dyract compomer eluates were fully spread out, showing the typical morphology of fibroblasts (Figure 1E and 1I). In addition, the exposure of the PDL cells to the PMTA for 24 hours induced few morphological changes (Figure 1G).
Figure 1. Morphological observations of periodontal ligament cells exposed to the cement eluates (3-day set, 1:8) for 24 hours (inverted microscopy, ×10). (A) Cell control; (B) intermediate restorative material (IRM); (C) Vitrebond; (D) Smart Dentin Replacement (SDR); (E) Dyract Compomer; (F) polymethylmethacrylate (PMMA) bone cement; (G) ProRoot mineral trioxide aggregate (PMTA); (H) Micro-Mega mineral trioxide aggregate (MM-MTA); (I) Biodentine.
Cytotoxicity
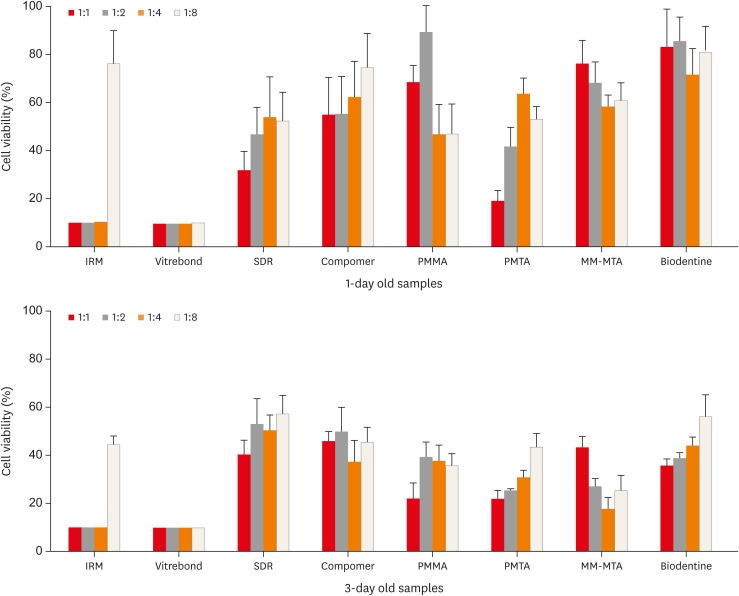
The cell viability depended significantly on the type of the material, setting time, and concentration of the eluate that the cells were exposed to (p < 0.05). The results regarding the effects of the materials, setting times, and eluate concentrations on the PDL cell viability are summarized in Figure 2. Overall, the Vitrebond and IRM were significantly more cytotoxic than the other root-end filling materials (p < 0.05). Those cells exposed to the Biodentine and Dyract compomer eluates showed the highest fibroblast survival rates, while the PMTA, MM-MTA, SDR, and PMMA groups exhibited similar cell viabilities. The 3-day samples were more cytotoxic than the 1-day samples for the Biodentine, IRM, PMTA, MM-MTA, and PMMA groups (p < 0.05). However, there was no statistical difference between the 1-day setting and 3-day setting times in the Vitrebond, Dyract compomer, and SDR groups. The 1:1 dilution eluates were significantly more cytotoxic than the eluates at the 1:2, 1:4, and 1:8 dilutions (p < 0.05).
Figure 2. The relative cell viability of human periodontal ligament cells exposed to eluates of test materials as measured by the XTT assay.
IRM, intermediate restorative material; SDR, Smart Dentin Replacement; PMMA, polymethylmethacrylate; PMTA, ProRoot mineral trioxide aggregate; MM-MTA, Micro-Mega mineral trioxide aggregate.
pH analysis
The pH values of the undiluted eluates of the test materials (3-day setting time) are shown in Table 1. The MM-MTA cement showed the highest pH (alkaline), while the Vitrebond presented the lowest pH (acidic).
Table 1. pH values of test materials' (3-day set) undiluted eluates.
| Test group | pH values of eluates |
|---|---|
| IRM | 8–9 |
| Vitrebond | 6 |
| SDR | 8 |
| Compomer | 8 |
| PMMA | 8 |
| PMTA | 9–10 |
| MM-MTA | 11 |
| Biodentine | 9 |
IRM, intermediate restorative material; SDR, Smart Dentin Replacement; PMMA, polymethylmethacrylate; PMTA, ProRoot mineral trioxide aggregate; MM-MTA, Micro-Mega mineral trioxide aggregate.
Apoptosis/necrosis
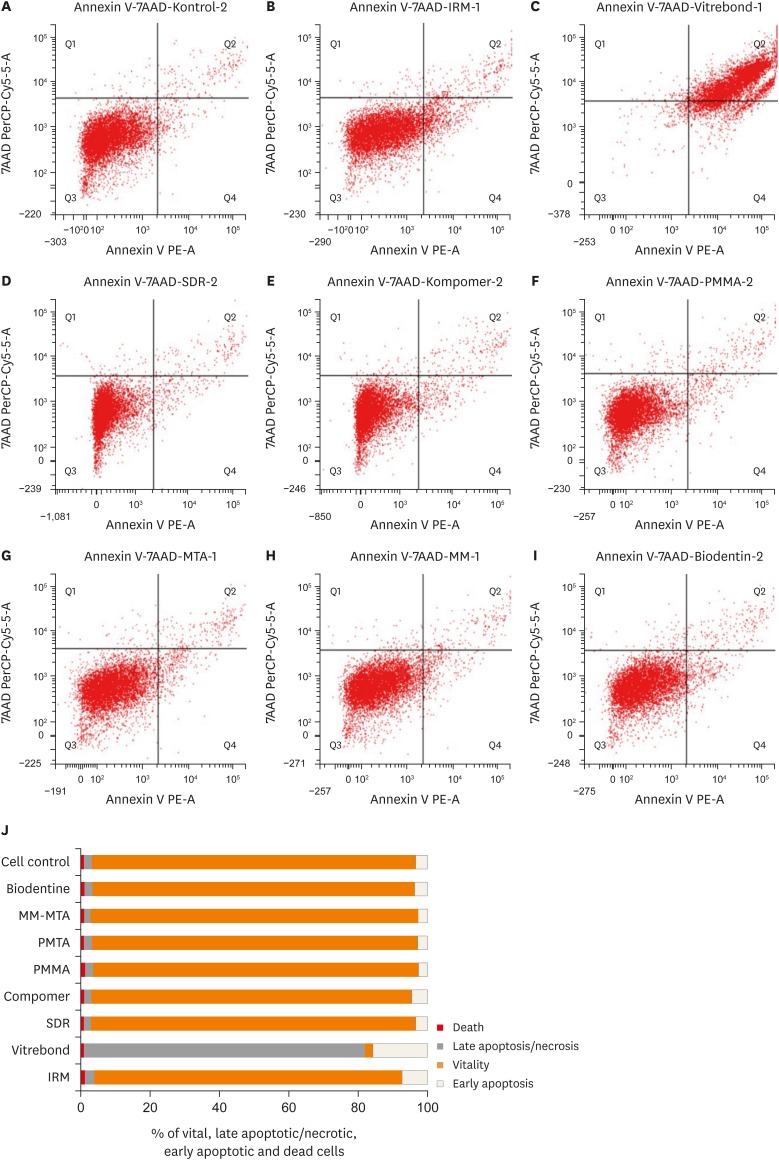
The representative 2-dimensional flow cytometry dot plot of the data from the test groups and cell control group, and the percentage distributions of the vital, early apoptotic, late apoptotic/necrotic, and dead cells are illustrated in Figure 3. The Vitrebond eluates induced an increase in the percentage of necrotic fibroblasts. However, the PDL cells were dominantly vital after exposure to the Biodentine, IRM, PMTA, MM-MTA, SDR, Dyract compomer, and PMMA bone cement eluates.
Figure 3. Representative 2-dimensional dot plots of the flow cytometry data derived from Annexin V and 7AAD stained human periodontal ligament cells in (A) untreated cell control group, and after exposure to eluates from (B) intermediate restorative material (IRM), (C) Vitrebond, (D) Smart Dentin Replacement (SDR), (E) Dyract Compomer, (F) polymethylmethacrylate (PMMA), (G) ProRoot mineral trioxide aggregate (PMTA), (H) Micro-Mega mineral trioxide aggregate (MM-MTA), and (I) Biodentine. The dot plot represents the distribution of vital (lower left), early apoptotic (lower right), late apoptotic/necrotic (upper right) and dead cell populations (upper left), respectively. (J) A bar graph representing the percentage of vital, late apoptotic/necrotic, early apoptotic and dead cell populations after exposure to test material eluates.
DISCUSSION
Biocompatibility is one of the most desired properties of a root-end filling material that is placed in contact with vital periradicular tissues for a prolonged period of time. Non-biocompatible retrograde filling materials may lead to the degeneration of adjacent tissues and delay periradicular healing.
The inverted microscopic observations of the PDL cells exposed to the material eluates presented the interactions between the cells and retrograde fillings visually. Our observations indicated that the PDL cells were healthy when exposed to the Biodentine and Dyract compomer eluates, while the IRM, Vitrebond, SDR, PMMA, and MM-MTA eluates caused retraction in the PDL cells. However, minimal morphological alterations were established in the PMTA group. The microscopic photographs taken during the experiment were consistent with the results of the XTT assay. Unlike the other groups, some particulates were released to the cell culture system in the Vitrebond group. The numerous and intense particles that were present in the Vitrebond eluate may have adversely affected cell growth.
The present study revealed that the IRM was one of the most cytotoxic materials. In addition, several other studies have reported high cytotoxicity in the IRM, probably due to the presence of eugenol, which has been shown to be toxic to fibroblasts [15,16]. Contrarily, a previous in vivo study reported high clinical performance for the IRM, comparable to that of MTA [2]. We speculated that the higher concentrations of the IRM eluate (1:1, 1:2, and 1:4) would cause decreased cell viability, but the cell viability increased when the cells were exposed to the 1:8 concentration of the IRM eluate. The dilution of the eluates mimics the clinical situation because the cytotoxic effects of eugenol are lessened with the systemic circulation. The IRM may cause only temporary effects on the cell behavior, which may explain the long-term clinical success of the IRM.
Based on the findings of the present study, Vitrebond was the most cytotoxic material (like IRM). These results agreed with those of previous reports in which Vitrebond has exhibited high cytotoxicity [11,17]. The aluminum and fluoride release from the material and the acidity of the eluate may be possible reasons for Vitrebond's toxicity [18]. Our results were also confirmed by Camargo et al. [19], who found that the original Vitrebond eluate caused a 7-fold increase in reactive oxygen species (ROS) production, which may explain one possible cellular toxicity mechanism.
One of the test materials used in this study was SDR, a low shrinkage flowable composite resin designed to be a base under direct restorations and/or a pit and fissure sealant. SDR has been compared with other commercial flowable composites in terms of its cytotoxicity to dental stem cells, and it has been suggested that SDR can be used as a dental restorative material [20]. However, there has been no study evaluating SDR for retrograde filling. Our findings indicated that the SDR exhibited mild toxicity, similar to that of the PMTA. Previously, the monomer content of composite resins has shown cytotoxic effects in high concentrations [21]; therefore, SDR's cytotoxicity may be closely related to its monomer constituent.
Based on the present study, compomers seem to be less cytotoxic materials. However, inconsistent observations have been made in previous studies showing that the toxic potential of compomers is very likely due to the leachable components of the compomers, like bisphenol A glycidyl methacrylate (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA), and urethane dimethacrylate (UDMA) [22,23]. The use of different commercial materials and different methodologies may partially explain the conflicting results. Our present results also suggested that the Dyract compomer was as biocompatible as Biodentine. However, no other study has compared Biodentine and a compomer with regard to the biocompatibility, other than the present study. Therefore, the biocompatibility of compomers needs to be elucidated in future in vivo experiments.
The PMMA caused a cell reduction similar to those of the MM-MTA, SDR, and PMTA. Previous studies evaluating PMMA as a retrograde filling generally examined this material in terms of its sealing ability. The findings obtained in the present study are in general agreement with the results of Badr [9], which reported the similar cytotoxic effects of PMMA and MTA on fibroblasts. The residual methylmethacrylate monomer may be responsible for the decreased biocompatibility of the PMMA bone cement [24].
The PMTA was statistically ranked as moderate among the other test groups with regard to the cell viability. Although they were not statistically significant, the cell viability values of the PMTA group were lower than those of the MM-MTA, SDR, and PMMA bone cement groups. Our findings are consistent with a previous observation that PMTA has cytotoxic effects on macrophages and fibroblasts [15]. Moreover, the cytotoxicity of the PMTA could be attributed to the toxic heavy metals that were found in the PMTA eluate [25]. The high pH of the PMTA eluate, which may cause cell denaturation, may be another explanation for PMTA's cytotoxicity [15].
There have been few reports researching the cytotoxicity of MM-MTA, which was produced as an alternative to PMTA [26]. Similar to our results, it has been showed previously that PMTA and MM-MTA are similar to each other, presenting a higher cell viability than that of IRM [26]. Kum et al. [27] reported that MM-MTA includes aluminum, arsenic, beryllium, cadmium, chromium, and iron at minimum levels. We can assume that PMTA and MM-MTA contain similar levels of heavy metals, based on previous evidence [27] which can explain the similar biocompatibilities of PMTA and MM-MTA. Furthermore, the original MM-MTA eluate was found to have the highest pH value, which may negatively affect the cell proliferation.
As a new calcium silicate based cement, Biodentine has attracted attention in the field of endodontic surgery. Our present results suggest that Biodentine shows the greatest cell viability. The liquid content of Biodentine includes calcium chloride, which was added to the composition of Portland cement to induce cell growth by releasing calcium from the materials [28]. This calcium chloride seems to contribute to the biocompatibility of Biodentine. Our cytotoxicity findings also indicated that Biodentine is preferable to PMTA. However, the present results are in contrast with the results of other studies that have described similar biocompatibilities for Biodentine and PMTA, based on different methodologies [11,12]. The superior biocompatibility of Biodentine may be explained by the different chemical compositions of these materials. For example, zirconium oxide is included as a radiopacifier in Biodentine, while bismuth oxide provides the radiopacity for the PMTA. One previous study reported that bismuth oxide did not induce cell growth [29], while zirconium oxide was found to be nontoxic to rat fibroblast cells [30]. Another explanation for the lower cytotoxicity of the Biodentine may be the active biosilicate technology that eliminates the metal impurities inherent in MTA cements [31]. A recent study reported that traces of arsenic, lead, and chrome were present in Biodentine eluates [32]. Another study revealed that Biodentine released heavy metals including arsenic, copper, iron, manganese, and zinc [33]. However, Camilleri et al. [32] stated that the amount of arsenic detected in the Biodentine samples was negligible; therefore, Biodentine could be considered a safer material.
The different setting times did not have significant effects on the cell viability in the Vitrebond, SDR, and compomer eluate groups in which the setting reaction was completed via light. However, the 1-day setting time samples were more biocompatible than the 3-day samples in the IRM, Biodentine, PMTA, MM-MTA, and PMMA bone cement groups. Older IRM specimens may exhibit higher toxicity due to the long-term release of eugenol [34]. Camilleri et al. [29] indicated that the 28-day setting MTA cements showed poorer cell growth than the 1-day setting MTA cements. Calcium hydroxide is produced as a result of the primer hydration reaction of the tricalcium silicate cements, which may positively affect the cell growth, but the level of calcium hydroxide diminishes as the material sets [29]. This fact could explain the better biocompatibility of the fresh Biodentine, PMTA, and MM-MTA samples. Moreover, a recent study has reported that the long-term ageing of PMMA based cements enhanced the cytotoxicity of the material [35]. The long-term release of the residual monomer from the PMMA could be responsible for the higher toxicity of the 3-day samples of the PMMA bone cement [35].
The XTT findings of this study revealed that the cell viability was significantly associated with the concentration of the test material eluates, since the higher concentrations were more cytotoxic than the lower concentrations. We aimed to assess the dose-dependent effects and to simulate the clinical conditions by using different concentrations of the material eluates. It has been reported that as the toxic components of the root-end filling materials become diluted, the toxic influences are lessened by the systemic circulation [36]. In this study, 2 different biocompatibility tests were used with the material eluates. The XTT assay provided information about the cell viability, while the mode of cell death (apoptosis) was established with flow cytometry. The XTT results were evaluated and those eluate concentrations giving 50%–60% cell viability were selected for the flow cytometry analysis. This was done to establish both the vital and dead cells, to distinguish early and late apoptosis, and to determine the stages of cell death if apoptosis occurred. It appeared that the 1:8 concentration eluates of the 3-day specimens of most of the groups presented cell viabilities of 50%–60%. If the setting times and eluate concentrations with the lower cell viability values were selected, the cell death caused by the cytotoxic materials, such as Vitrebond, could not be shown with flow cytometry.
Our flow cytometry findings indicated that the Vitrebond caused cell necrosis, while the cells exposed to the eluates of all of the other groups remained vital. The cell viability values for the Vitrebond group with the XTT were higher than the values shown via flow cytometry. In this research, 2 methods were used: XTT, which measures mitochondrial metabolic activity, and flow cytometry, which establishes the permeability of the cell membrane. Cell proliferation and cell death offer different aspects of the cell functions. However, further studies with different concentrations of the eluates of the root-end filling materials are required to clarify the mode of cell death induced by them.
CONCLUSIONS
Within the limitations of this in vitro study, it can be concluded that the Biodentine and compomers were more biocompatible than the other root-end filling materials. Moreover, the cell death induced by the Vitrebond was more pronounced and dominantly necrotic.
ACKNOWLEDGEMENTS
The authors thank 3M ESPE, Dentsply, and Septodont for providing some of the materials for this research project. We also thank Serhan Akman for his generous help with statistical analysis.
Footnotes
Funding: This article is based on the first part of the PhD thesis of Dr. Makbule Bilge Akbulut and the financial support of Selcuk University Scientific Research Projects Coordination is gratefully acknowledged (Grant No. 12202016).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: Akbulut MB, Eldeniz AU.
- Data curation: Akbulut MB.
- Formal analysis: Akbulut MB.
- Funding acquisition: Eldeniz AU.
- Investigation: Akbulut MB, Arpaci PU, Eldeniz AU.
- Methodology: Akbulut MB, Arpaci PU.
- Project administration: Eldeniz AU.
- Resources: Akbulut MB, Eldeniz AU.
- Software: Akbulut MB, Arpaci PU.
- Supervision: Eldeniz AU.
- Validation: Arpaci PU, Eldeniz AU.
- Visualization: Akbulut MB.
- Writing - original draft: Akbulut MB.
- Writing - review & editing: Akbulut MB, Arpaci PU, Eldeniz AU.
References
- 1.Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006;32:601–623. doi: 10.1016/j.joen.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Chong BS, Pitt Ford TR, Hudson MB. A prospective clinical study of Mineral Trioxide Aggregate and IRM when used as root-end filling materials in endodontic surgery. Int Endod J. 2003;36:520–526. doi: 10.1046/j.1365-2591.2003.00682.x. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod. 2011;37:793–798. doi: 10.1016/j.joen.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Chong BS, Pitt Ford TR, Kariyawasam SP. Short-term tissue response to potential root-end filling materials in infected root canals. Int Endod J. 1997;30:240–249. doi: 10.1046/j.1365-2591.1997.00077.x. [DOI] [PubMed] [Google Scholar]
- 5.Mazumdar D, Ray P, Wang CK, Dhanuka S. An investigation into the irritant properties of some retrograde filling materials-an in vivo study. J Conserv Dent. 2005;8:4–13. [Google Scholar]
- 6.McDonald NJ, Dumsha TC. A comparative retrofill leakage study utilizing a dentin bonding material. J Endod. 1987;13:224–227. doi: 10.1016/S0099-2399(87)80095-X. [DOI] [PubMed] [Google Scholar]
- 7.Dragoo MR. Resin-ionomer and hybrid-ionomer cements: Part II. Human clinical and histologic wound healing responses in specific periodontal lesions. Int J Periodontics Restorative Dent. 1997;17:75–87. [PubMed] [Google Scholar]
- 8.Stańczyk M, van Rietbergen B. Thermal analysis of bone cement polymerisation at the cement-bone interface. J Biomech. 2004;37:1803–1810. doi: 10.1016/j.jbiomech.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Badr AE. Marginal adaptation and cytotoxicity of bone cement compared with amalgam and mineral trioxide aggregate as root-end filling materials. J Endod. 2010;36:1056–1060. doi: 10.1016/j.joen.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995;21:603–608. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 11.Corral Nuñez CM, Bosomworth HJ, Field C, Whitworth JM, Valentine RA. Biodentine and mineral trioxide aggregate induce similar cellular responses in a fibroblast cell line. J Endod. 2014;40:406–411. doi: 10.1016/j.joen.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhou HM, Shen Y, Wang ZJ, Li L, Zheng YF, Hakkinen L, Haapasalo M. In vitro cytotoxicity evaluation of a novel root repair material. J Endod. 2013;39:478–483. doi: 10.1016/j.joen.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 13.International Organization for Standardization. ISO 10993-5 Biological evaluation of medical devices - Part 5: tests for in vitro cytotoxicity. 3rd ed. Geneva: International Organization for Standardization; 2009. [Google Scholar]
- 14.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 15.Haglund R, He J, Jarvis J, Safavi KE, Spangberg LS, Zhu Q. Effects of root-end filling materials on fibroblasts and macrophages in vitro . Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:739–745. doi: 10.1067/moe.2003.231. [DOI] [PubMed] [Google Scholar]
- 16.Sousa CJ, Loyola AM, Versiani MA, Biffi JC, Oliveira RP, Pascon EA. A comparative histological evaluation of the biocompatibility of materials used in apical surgery. Int Endod J. 2004;37:738–748. doi: 10.1111/j.1365-2591.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson JW, Czarnecka B. The biocompatibility of resin-modified glass-ionomer cements for dentistry. Dent Mater. 2008;24:1702–1708. doi: 10.1016/j.dental.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Savarino L, Cervellati M, Stea S, Cavedagna D, Donati ME, Pizzoferrato A, Visentin M. In vitro investigation of aluminum and fluoride release from compomers, conventional and resin-modified glass-ionomer cements: a standardized approach. J Biomater Sci Polym Ed. 2000;11:289–300. doi: 10.1163/156856200743706. [DOI] [PubMed] [Google Scholar]
- 19.Camargo SE, Camargo CH, Hiller KA, Rode SM, Schweikl H, Schmalz G. Cytotoxicity and genotoxicity of pulp capping materials in two cell lines. Int Endod J. 2009;42:227–237. doi: 10.1111/j.1365-2591.2008.01506.x. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez IA, Ferrara CA, Campos-Sanchez F, Alaminos M, Echevarria JU, Campos A. An in vitro biocompatibility study of conventional and resin-modified glass ionomer cements. J Adhes Dent. 2013;15:541–546. doi: 10.3290/j.jad.a29588. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hiyasat AS, Darmani H, Milhem MM. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Investig. 2005;9:21–25. doi: 10.1007/s00784-004-0293-0. [DOI] [PubMed] [Google Scholar]
- 22.Geurtsen W, Spahl W, Leyhausen G. Residual monomer/additive release and variability in cytotoxicity of light-curing glass-ionomer cements and compomers. J Dent Res. 1998;77:2012–2019. doi: 10.1177/00220345980770121001. [DOI] [PubMed] [Google Scholar]
- 23.Tunç ES, Ozer L, Sari S, Cetiner S. Cytotoxic effects of halogen- and light-emitting diode-cured compomers on human pulp fibroblasts. Int J Paediatr Dent. 2009;19:55–60. doi: 10.1111/j.1365-263X.2008.00953.x. [DOI] [PubMed] [Google Scholar]
- 24.Stafford GD, Brooks SC. The loss of residual monomer from acrylic orthodontic resins. Dent Mater. 1985;1:135–138. doi: 10.1016/s0109-5641(85)80005-1. [DOI] [PubMed] [Google Scholar]
- 25.Schembri M, Peplow G, Camilleri J. Analyses of heavy metals in mineral trioxide aggregate and Portland cement. J Endod. 2010;36:1210–1215. doi: 10.1016/j.joen.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Chang SW, Lee SY, Kum KY, Kim EC. Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. J Endod. 2014;40:113–118. doi: 10.1016/j.joen.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Kum KY, Kim EC, Yoo YJ, Zhu Q, Safavi K, Bae KS, Chang SW. Trace metal contents of three tricalcium silicate materials: MTA Angelus, Micro Mega MTA and Bioaggregate. Int Endod J. 2014;47:704–710. doi: 10.1111/iej.12208. [DOI] [PubMed] [Google Scholar]
- 28.Gandolfi MG, Perut F, Ciapetti G, Mongiorgi R, Prati C. New Portland cement-based materials for endodontics mixed with articaine solution: a study of cellular response. J Endod. 2008;34:39–44. doi: 10.1016/j.joen.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J. 2004;37:699–704. doi: 10.1111/j.1365-2591.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- 30.Dion I, Rouais F, Baquey C, Lahaye M, Salmon R, Trut L, Cazorla JP, Huong PV, Monties JR, Havlik P. Physico-chemistry and cytotoxicity of ceramics: part I: characterization of ceramic powders. J Mater Sci Mater Med. 1997;8:325–332. doi: 10.1023/a:1018520630500. [DOI] [PubMed] [Google Scholar]
- 31.Strassler HE, Levin R. Biodentine tricalcium-silicate cement. Inside Dent. 2011;7:98–100. [Google Scholar]
- 32.Camilleri J, Kralj P, Veber M, Sinagra E. Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int Endod J. 2012;45:737–743. doi: 10.1111/j.1365-2591.2012.02027.x. [DOI] [PubMed] [Google Scholar]
- 33.Jang YE, Lee BN, Koh JT, Park YJ, Joo NE, Chang HS, Hwang IN, Oh WM, Hwang YC. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor Dent Endod. 2014;39:89–94. doi: 10.5395/rde.2014.39.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markowitz K, Moynihan M, Liu M, Kim S. Biologic properties of eugenol and zinc oxide-eugenol. A clinically oriented review. Oral Surg Oral Med Oral Pathol. 1992;73:729–737. doi: 10.1016/0030-4220(92)90020-q. [DOI] [PubMed] [Google Scholar]
- 35.Uzun IH, Tatar A, Hacimuftuoglu A, Saruhan F, Bayindir F. In vitro evaluation of long-term cytotoxic response of injection-molded polyamide and polymethyle metacrylate denture base materials on primary fibroblast cell culture. Acta Odontol Scand. 2013;71:1267–1272. doi: 10.3109/00016357.2012.757648. [DOI] [PubMed] [Google Scholar]
- 36.Wei W, Qi YP, Nikonov SY, Niu LN, Messer RL, Mao J, Primus CM, Pashley DH, Tay FR. Effects of an experimental calcium aluminosilicate cement on the viability of murine odontoblast-like cells. J Endod. 2012;38:936–942. doi: 10.1016/j.joen.2012.03.020. [DOI] [PubMed] [Google Scholar]