Abstract
Background:
Colorectal cancer (CRC) is widespread across the world. While conventional anticancer treatments can help the affected patients, cells of vital organs such as the kidney, lungs, bladder and nervous system may suffer from side effects of chemotherapeutic drugs, so that it is necessary to search for alternatives. From ancient times, attention has focused on medicinal plants and natural products. In the current work, Camellia sinensis, whose leaves are used to produce green tea was evaluated for anticancer effects in cell culture.
Materials and Methods:
A hydroalcoholic extract of Camellia sinensis young leaves was prepared by percolation and compared with Cisplatin as a known anticancer drug for effects on two cell lines: Caco-2, colon carcinoma cells, and mouse normal fibroblasts (L929). Cytotoxicity of 50, 100, 200, 400 and 800 µg/ml of Camellia sinensis extract was evaluated by MTT assay and aquaporin 5 (AQP5), detected as a biomarker for surviving cells using immunofluorescence microscopy.
Results:
MTT assays with hydroalcoholic extract of Camellia sinensis showed considerable inhibition of growth of Caco-2 cells, significant at 800 µg/ml (P<0.05), with little effect on L929 cells. Levels of aquaporin 5 protein decreased in Caco-2 cell culture following green tea extract treatment.
Conclusion:
According to the results of the current study, Camellia sinensis is a medicinal plant with potent anticancer influence which might be specific.
Keywords: Tea, Camellia sinensis, Caco-2 cells, Cisplatin, Aquaporin 5
Introduction
Cancers with a global distribution, are prominent diseases that are characterized by uncontrolled growth and spread of abnormal cells and cause of millions deaths in the present century (Siegel et al., 2017). The World Health Organization (WHO) predicts about 15 million new incidents of cancer by 2020 (McGuire, 2016). Between all types of cancers, colorectal cancers (CRC) are the third major incidence of cancer with a mortality of nearly 9.4% cancer cases annually (Grothey et al., 2004; Ansari et al., 2006). Also, CRC is the third most prevalent cancer among the Iranian population (Ansari et al., 2006). Chemotherapy, radiation therapy, hormonal therapy and surgery are the common treatments for all types of cancer, and due to resistance and adverse or toxic side effects of these treatments, it has become necessary to search for an alternative anticancer treatment (Shrivastava et al., 2005). Natural products retain vast pharmacological significance and have been considered as a main source of potential chemotherapeutic treatments (Roe et al., 2016).
About 60% of drugs commercially used for anticancer therapy are derived from plants (Gordaliza, 2007), such as Vinca rosea which is the source of vinblastine and vincristine (Banerjee and Basu, 1991), Taxus brevifolia which is the source of taxol (Wani et al., 1971), and Camptotheca acuminate which is the source of camptothecin (Wall et al., 1966). Camellia sinensis is one of the most common drinks consumed worldwide that is a rich source of nutritional flavonoids (Gomes et al., 1995; Thitimuta et al., 2017). Catechins, epigallocatechin-3-gallate, epigallocatechin, epicatechin-3-gallate and epicatechin are the five major flavonoids derived from Camellia sinensis (Fan et al., 2017). Flavonoids and polyphenol compounds have been reported to have favourable properties such as anticarcinogenic, antimutagenic, antimicrobial and anti-oxidant properties (Yıldırım et al., 2000). A study conducted by Ann Beltz et al. reported the widespread variety of mechanisms by flavonoids and polyphenols of Camellia sinensis that prevent cancer cell survival (Ann Beltz et al., 2016).
According to the adverse and inevitable side effects of conventional anticancer treatments and the inaccessibility of these drugs in most developing countries, Itis essential to utilize an effective, economical and easily accessible treatment. So, this study aimed to evaluate the anticancer effects of Camellia sinensis on the Caco-2 and L929 cell lines via an in vitro investigation.
Materials and Methods
Study Design
An experimental study was designed. This work was undertaken to examine the potential anticancer activity of Camellia sinensis against colorectal cancer cell line Caco-2.
Plant Material Collection, Identification
The aerial parts of fresh plants were collected from around Lahijan District, north of Iran, during March 2014. The plant recognized as Camellia sinensis in the Pharmacognosy Department of the Tehran University of Medical Sciences and the herbarium was registered as THE-6561. The young leaves shade dried at room temperature for 10 days and powdered to obtain 2-3 mm particle sizes. Fifty grams of powdered plants was macerated in 1,500 ml hydroalcoholic solution (50% water + 50% absolute ethanol (Merk, Germany) for 72 h. The extracts were filtered and concentrated in a rotary evaporator to obtain solid extracts and then freeze-dried (OPERON, Korea) to remove the solvent completely (Golami et al., 2016). For preparation of the extracts, essential media Dulbecco’s Modified Eagle’s Medium (DMEM; Himedia Labs, Mumbai, India) was used.
Essential oil analysis technique
GC examination was done by Thermoquest gas chromatograph by using a flame ionization sensor and Silica tube DB-1 column (30 m × 0.25 mm with film thickness of 0.25 lm). The injector temperature was 250 ºC and sensor temperatures were adjusted at 300 ºC Nitrogen was at a flow rate of 1.1 ml/min by means of hauler gas. GC-MS examination was done with Thermoquest-Finnigan gas chromatograph with fused silica capillary DB-1 column (60 m - 0.25 mm i.d.; film thickness 0.25 lm) joined with a TRACE mass (Manchester, UK). In this section, helium was used as a transporter gas with ionization voltage of 70 eV. Ion source temperature was 200 ºC and interface temperature was 250 ºC. Mass series was 35 to 456 amu and oven temperature program was the same as the GC.
Identification of compounds
The ingredients of the essential oils were diagnosed by calculation of their retaining directors under temperature-programmed conditions for n-alkanes (C6-C24) and the oil on a DB-1 column under the same chromatographic conditions. Diagnosis of each compound was done in comparison to their mass spectra with those of the internal reference mass spectra library. For quantification of compounds, comparative zone percentages obtained by FID were used without the use of correction factors (Ebrahimi et al., 2008).
Preparation of cancer cell lines
In the current work, the effects of Camellia sinensis were compared in the carcinoma colon (Caco-2) cell line which originated from human colonic adenocarcinoma and mouse normal fibroblast cell line L929 that were provided by the National Cell Bank of Iran (NCBI) affiliated with the Pasteur Institute of Iran. The cells were cultured at 37 ºC in a humidified incubator with CO2 (5 %) in flasks containing essential media Dulbecco’s Modified Eagle’s Medium (DMEM; Himedia Labs, Mumbai, India), 10% fetal bovine serum (Gibco, Paisley, England) and 1% penicillin and streptomycin (Sigma, Deisenhofen, Germany).
MTT assay
Caco-2 and L929 cells were respectively seeded into 96-well plates (Nunc; Intermed, Roskilde, Denmark) at a density of 4×104 and 5×104 per well (100 µl) in DMEM supplemented with 10% fetal bovine serum and 1% (Pen/Step) except for the last row which contained only 100 µl of DMEM which was considered as the blank. Cells then were incubated at 37 °C in a humidity of 95% and 5% CO2 for 24 h. Plant extracts in concentrations of 50, 100, 200, 400 and 800 µg/ml were added to each well in triplicate. Moreover, Cisplatin (Sigma, Poole, UK) as a conventional chemotherapy medication and non-treated cells (without extract) were used as positive and negative controls, respectively. After 48 h of incubation time, 10 μL of MTT (Sigma, UK) solution (5 mg/mL in PBS) was added to each well, including controls. After 3 h of incubation at 37°C the supernatant was removed and 100 µL of dimethyl sulfoxide (DMSO) that was purchased from Merck (Darmstadt, HE, Germany) was added. Finally, the absorbance at 570 nm was measured by a microtiter plate reader (BioTek ELX800, Winooski, Vermont, USA) (Lakshmi and Bai, 2016).
Verification of aquaporin-5 (AQP5) protein expression by immunofluorescence staining
To examine expression of AQP5 protein after 48 hours treatment with 800 µg/ml concentration hydro alcoholic green tea extract in duplicate, Caco-2 cells were fixed on glass microscope slides for 15 min with a cold ethanol- acetone mixture. As Esghaei et al., (2012) reported in her previous work, the slides were washed with PBS and then incubated at 37°C for 30 min with the rabbit monoclonal antibody to the aquaporin-5 protein (ab92320, Abcam, Cambridge, UK) diluted 1:100 in 3% BSA according to the manufacturers. Following rinsing with PBS, subsequently incubated at 37°C with FITC-labeled goat anti-rabbit IgG antibody (Cooper Biomedical, Inc., Malvern, PA, USA). After rinsing the slides were counterstained with Evans blue (2%; Sigma Chem. Co., St Louis, MO, USA) and mounted for visualization using a fluorescence microscope (Nikon Eclipse E600, Kawasaki, Japan).
Statistical analysis
SPSS version 20 software (SPSS Inc., Chicago, IL, USA) was used to perform statistical tests. Measurement data among the groups was compared using one way analysis of variance. P-values less than 0.05 were considered to demonstrate statistically significant differences.
Results
In the current study, the cytotoxic effect of hydroalcoholic extract of Camellia sinensis on the Caco-2 cancer cell line and one normal cell line (L929) was determined using MTT assay at a concentration range of 50-800 μg/ml after 48 h of treatment. Cell viability was assessed using MTT assay, the percentage of cell viability according to the following equation: The percentage of cell viability = OD of treated cells/OD of control cells×100
Each control and extract was assayed in triplicate and the percent of cell survival in the negative control was assumed 100 (Figure 1, 2).
Figure 1.
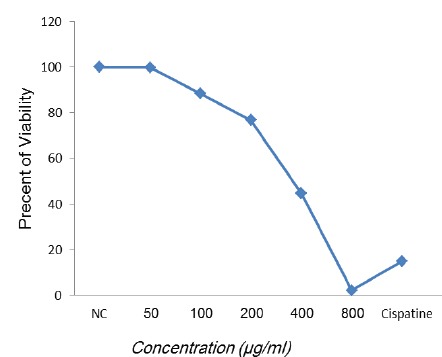
Effects of Different Concentrations of Camellia Sinensis Against Caco-2 Cell Line. Values Represent the Mean of Three Experiments
Figure 2.

Effects of Different Concentrations of Camellia Sinensis Against L929 Cell Line. Values Represent the Mean of Three Experiments
The in vitro cytotoxic activities of each plant extract are shown in Table 1.
Table 1.
Absorbance of Camellia Sinensis in Different Concentrations on the Caco-2 and L929 Cell Lines in Comparison with Cis-platine as Positive Control in an in Vitro Study
| Cell line | Negative control | 50 µg/ml | 100 µg/ml | 200 µg/ml | 400 µg/ml | 800 µg/ml | Cis platine | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Caco-2 | Mean | 0.18 | 0.143 | 0.303 | 0.423 | 0.767 | 1.217 | 1.08 | <0.05 |
| Std. Deviation | 0.020 | 0.006 | 0.045 | .045 | 0.021 | 0.040 | 0.090 | ||
| L929 | Mean | 0.377 | 0.32 | 0.367 | 0.26 | 0.307 | 0.307 | 1.46 | >0.05 |
| Std. Deviation | 0.045 | 0.035 | 0.061 | 0.020 | 0.031 | 0.040 | 0.053 |
According to Table 1 and Figure 1, the concentration of 800 μg/ml was more effective than other concentrations and in comparison with to Cisplatin as a positive control was significant (P<0.05). The results of one-way ANOVA indicated that there are significant difference between groups and within groups.
Moreover, the effects of Camellia sinensis on the L929 cell line indicated that there is not any significant difference between the effects of Camellia sinensis and negative control (Figure 2).
The results of this study showed that Cisplatin was the most effective among all concentrations of Camellia sinensis.
In this study, Caco-2 cells were treated with 800 µg/ml concentration hydro alcoholic green tea extract. According to the results of this qualitative method, AQP5 is expressed untreated cells control more than treated Caco-2 cells l (Figure 3).
Figure 3.

Aquaporin5 Expression was Detected by Fluorescence Microscopy in Untreated (A) and Treated with 800 µg/ml Concentration Hydro Alcoholic Green Tea Extract (B) Caco-2 cells. L929cells (C) were used as negative control. All cells were stained with the rabbit monoclonal antibody to the aquaporin 5 protein (ab92320, Abcam), which is directed against Aquaporin 5.
Discussion
These days globally research works show that millions of people are suffering from colorectal cancers and like other neoplastic diseases has become the main challenge for humans. Recently, several studies have been undertaken in the treatments of different colorectal cancers, such treatments which have not been efficient enough to be used as patented drugs for patients (Siegel et al., 2017). Toxicity of conventional drugs are their main limitations, and plants may serve as potent chemotherapeutic agents with less toxicity to normal mammalian tissues and at low cost (Van Wyk and Wink, 2017). In the current work, an effort was made to define and ascertain the anti-proliferation effect of hydroalcoholic extracts of Camellia sinensis in the Caco-2 cell line as a model for colorectal cancer. The results of this study showed a significant difference between the effects of Camellia sinensis and negative control in that it was shown that Camellia sinensis is effective against cancer cells. Moreover, the cytotoxicity was evaluated on the L929 cell line as a normal epithelial cell in the mouse model. According to the Database for the Flavonoid Content of Selected Foods (USDA), 60% of the ingredients of Camellia sinensis extract is allocated to the polyphenols (Pedro et al., 2016).
According to GC examination, most of the compounds identified were health advancing and physiologically significant. Palmitic acid, hexahydrofarnesyl acetone and decane are the main components of Camellia sinensis. Palmitic acid is the first fatty acid produced during lipogenesis, which is responsible for converting acetyl-ACP to malonyl-ACP on the growing acyl chain, thus preventing further palmitate generation. Hexahydrofarnesyl acetone belongs to the family of Sesquiterpenes. These are terpenes with three consecutive isoprene units. Decane is an alkane hydrocarbon that is mainly detected in the edible Korean chamchwi plant (Chung et al., 1993). Terpenoids have a significant role in biological activities. In the current work, Terpenoids as like as Geraniol Thymol, Caryacrol, E-a-Lonone, 1-Dodecanol and E-β-Ionone, are the main parts of Camellia sinensis that may be responsible for such biological effects as anti-tumor and antimicrobial effects. Several investigations have suggested the anticancer effects of Camellia sinensis on lung, skin, esophagus, liver and stomach cancers (Chen et al., 2009). Ahmad et al., (1997) evaluated the effects of polyphenols extracted Camellia sinensis on A431, HaCaT and DU145 cell lines (human epidermoid, keratinocyte, prostate carcinoma cells, respectively) and reported the induction of apoptosis in these cells. In a similar study conducted by Lassed et al., (2017) Camellia sinensis leaf extracts were affected on the PC-3 (human metastatic prostate cancer) cell line in an in vitro study and showed significant efficacy of Camellia sinensis extracts on the PC-3 cell line. In the current work, the anticancer effects of Camellia sinensis in concentrations of 800 μg/ml were greater than those of Cisplatin as a conventional anticancer drug (p < 0.005). In most types of cancers; chemotherapies are the first choice of treatment with high efficacy. Standard treatments have been effective on cancer cells only, but today some conventional drugs affect normal cells and consequently cause some adverse side effects, such as headache, hair loss, nausea, vomiting, fatigue, nausea, and even death (Kurkjian et al., 2017).
The L929 cell line considered as a normal fibroblast in the mouse was used in this study as control group to assess cytotoxicity using MTT assay. Camellia sinensis in concentrations of 50 to 800 μg/ml did not have not any cytotoxicity effects on the L929 cell line and there was a significant difference to the positive control (P < 0.005). This means that Camellia sinensis is safe for mammalian cells and is toxic for cancer cells and it is the main target for the WHO.
The aquaporin 5 is a water channel protein that exhibit several properties related to tumor development. As the expression of AQP5 protein in intestinal cells is a strong prognostic biomarker for colorectal cancer. The aim of the present study was to determine whether or not the expression of AQP5 in Caco-2 cells after treating with 800 µg/ml concentration hydro alcoholic green tea extract. The results showed the protein levels of aquaporin-5 decreased in Caco-2 cell culture following green tea extract treatment (Shan et al., 2014; Endoh et al., 2017).
According to the results of this study and those of most similar works, Camellia sinensis is a potent anticancer without any side effects which supports these features; complementary experiences and investigation on animal models to reach a better response to treatment are recommended.
Limitations of the study
The hydroalcoholic extract of Camellia sinensis demonstrates a dose-dependent cytotoxic effect on the colorectal cancer cell line in our experimental observations.
However, the study lacks in its ability to determine the toxic effect of the extract to any class of compounds. Because of, using full extract has the potential bias for identifying the active cytotoxic compound(s). Our findings will be strengthened and clarified via structural analysis and component validation of the extract. So, further detailed phytochemical, in vivo studies and pharmacological research should be the next step in the identification of active anticancer compounds of plants, particularly Camellia sinensis which is currently ongoing.
References
- 1.Ann Beltz L, Kay Bayer D, Lynn Moss A, Mitchell Simet I. Mechanisms of cancer prevention by green and black tea polyphenols. Anti-Cancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad N, Feyes DK, Agarwal R, Mukhtar H, Nieminen A-L. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad N, Mukhtar H. Green tea polyphenols and cancer:biologic mechanisms and practical implications. Nutr Rev. 1999;57:78–83. doi: 10.1111/j.1753-4887.1999.tb06927.x. [DOI] [PubMed] [Google Scholar]
- 4.Ansari R, Mahdavinia M, Sadjadi A, et al. Incidence and age distribution of colorectal cancer in Iran:results of a population-based cancer registry. Cancer Lett. 2006;240:143–7. doi: 10.1016/j.canlet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee R, Basu T. Hypoglycemic and antihyperglycemic effecr of leayes of vinca rosea linn. Indian J Physiol Pharmacol. 1991;35:145–51. [PubMed] [Google Scholar]
- 6.Chen Q, Zhao J, Chaitep S, Guo Z. Simultaneous analysis of main catechins contents in green tea (Camellia sinensis (L.)) by Fourier transform near infrared reflectance (FT-NIR) spectroscopy. Food Chem. 2009;113:1272–7. [Google Scholar]
- 7.Chung Ty, Eiserich JP, Shibamoto T. Volatile compounds isolated from edible Korean chamchwi (Aster scaber Thunb) J Agric Food Chem. 1993;41:1693–7. [Google Scholar]
- 8.Ebrahimi SN, Hadian J, Mirjalili M, et al. Essential oil composition and antibacterial activity of Thymus caramanicus at different phenological stages. Food Chem. 2008;110:927–31. doi: 10.1016/j.foodchem.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 9.Endoh K, Matsui Y, Takeshita M, Kuriki K. Actual daily intakes of tea catechins and thier estimation according to four season 3 day weighed dietary records and a short food frequency questionnaire among Japanese men and women. Asian Pac J cancer Prev. 2017;18:2875–81. doi: 10.22034/APJCP.2017.18.10.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esghaei M, Monavari SHR, Tavassoti-Kheiri M. Expression of the influenza M2 protein in three different eukaryotic cell lines. J Virol Methods. 2012;179:161–5. doi: 10.1016/j.jviromet.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Fan D-m, Fan K, Yu C-p, Lu Y-t, Wang X-c. Tea polyphenols dominate the short-term tea (Camellia sinensis) leaf litter decomposition. J Zhejiang Univ Sci. 2017;18:99–108. doi: 10.1631/jzus.B1600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golami S, Rahimi-Esboei B, Mousavi P, et al. Survey on efficacy of chloroformic extract of Artemisia annua against Giardia lamblia trophozoite and cyst in vitro. J Parasit Dis. 2016;40:88–92. doi: 10.1007/s12639-014-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes A, Vedasiromoni J, Das M, Sharma R, Ganguly D. Anti-hyperglycemic effect of black tea (Camellia sinensis) in rat. J Ethnopharmacol. 1995;45:223–6. doi: 10.1016/0378-8741(95)01223-z. [DOI] [PubMed] [Google Scholar]
- 14.Gordaliza M. Natural products as leads to anticancer drugs. Clinl Translat Oncol. 2007;9:767–76. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 15.Grothey A, Sargent D, Goldberg RM, Schmoll H-J. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–14. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Kurkjian N, Tucker P, Ostermeyer B, Valentine A. Chemotherapy, immunotherapy, and psychotropic use in cancer patients:A review of psychiatric side effects. Psychiatr Ann. 2017;47:200–5. [Google Scholar]
- 17.Lakshmi V, Bai GVS. In vitro anticancer activity of Clerodendrum phlomidis leaves and its silver nanoparticles on human breast cancer cell line (MCF-7) Asian J Innovat Res. 2016;1:01–5. [Google Scholar]
- 18.Lassed S, Deus CM, Djebbari R, et al. Protective effect of green tea (Camellia sinensis (L.). Kuntze) against prostate cancer:From In Vitro Data to Algerian Patients. Evid.-Based Complementary Altern. Med. 2017;2017 doi: 10.1155/2017/1691568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire S. World cancer report 2014. Geneva, Switzerland:World Health Organization, international agency for research on cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–9. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedro AC, Granato D, Rosso ND. Extraction of anthocyanins and polyphenols from black rice (Oryza sativa L.) by modeling and assessing their reversibility and stability. Food Chem. 2016;191:12–20. doi: 10.1016/j.foodchem.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 21.Roe K, Visovatti MK, Brooks T, et al. Use of complementary therapies for side effect management in breast cancer:evidence and rationale. Breast Cancer Manag. 2016;5:125–38. [Google Scholar]
- 22.Shan T, Cui X, Li W, Lin W, Li Y. AQP5:a novel biomarker that predicts poor clinical outcome in colorectal cancer. Oncol Rep. 2014;32:1564–70. doi: 10.3892/or.2014.3377. [DOI] [PubMed] [Google Scholar]
- 23.Shrivastava SK, Engineer R, Rajadhyaksha S, Dinshaw KA. HIV infection and invasive cervical cancers, treatment with radiation therapy:toxicity and outcome. Radiother Oncol. 2005;74:31–5. doi: 10.1016/j.radonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 25.Thitimuta S, Pithayanukul P, Nithitanakool S, et al. Camellia sinensis L. extract and its potential beneficial effects in antioxidant, anti-inflammatory, anti-hepatotoxic, and anti-tyrosinase activities. Mol. 2017;22:401. doi: 10.3390/molecules22030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Wyk B-E, Wink M. Medicinal plants of the world. 2017:2. [Google Scholar]
- 27.Wall ME, Wani M, Cook C, et al. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata1, 2. J Am Chem Soc. 1966;88:3888–90. [Google Scholar]
- 28.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–7. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 29.Yıldırım A, Mavi A, Oktay M, et al. Comparison of antioxidant and antimicrobial activities of Tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.) and Black tea (Camellia sinensis) extracts. J Agric Food Chem. 2000;48:5030–4. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]


