Abstract
Introduction:
The epidemiology of common cancers in Kerman province, southeast of Iran, was assessed based upon results of the Kerman Population-Based Cancer Registry Program (KPBCR).
Methods:
in this retrospective study, all patients diagnosed with primary cancers and registered with the KPBCR were included. New cancer cases registered from 2014 were identified from pathological labs, medical reports of 48 health facilities providing cancer diagnosis or treatment services and the national death registry program. Data for patients who were referred to neighboring provinces to access health services were also collected from national referral registries. Results from autopsies was additionally extracted from regional forensic and legal medicine centers and added to the registry periodically. Age standardized incidence rates (ASRs) per 100,000 person-years for all cancers were computed, using direct-standardization and CanReg methodology. Mortality to incidence (M:I) ratios and microscopically verified (MV) proportions were calculated as quality measures.
Results:
A total of 2,838 cases of cancer were registered in Kerman province, 2014. Of these 45. 6% involved women (n=1,293). Individuals aged 60-64 years represented the largest proportion (11.6%) of the total cancer prevalence, followed by those aged 55-59 years (10.86%) and 65-69 years (8.99%). The ASRs for all cancers were 155.1 and 118.90 per 100,000, in men and women, respectively. In women, breast (ASR: 26.4), skin (ASR: 13.0), thyroid (ASR: 9.2), leukemia (ASR: 8.0) and colorectal (ASR: 7.70) were the most common cancers. In men, bladder (ASR: 24.70), skin (ASR: 16.80), lung (ASR: 14.6), leukemia (ASR: 14.50), and stomach (ASR: 10.8) were found to be the most frequent.
Conclusion:
This study provided latest evidence on epidemiology of cancer in the southeast of Iran that could be used to empower prevention and control interventions in a developing country.
Keywords: Cancer, incidence, Kerman
Introduction
Cancer is the second leading cause of death and disability (Titus-Ernstoff et al., 2006), with an estimated incidence of 17.5 million cases, 8.7 million deaths, and 208.3 million DALYs worldwide (Fitzmaurice et al., 2017). In 2015, prostate, lung and colorectal cancer were the most common cancers in men, with approximately 1.6 million, 1.4 million and 920,000 cases respectively. In women, breast, lung and colorectal cancers were the most common incident cancers with approximately 2.4 million, 733,000 and 640,000 cases respectively in the same year(Fitzmaurice et al., 2017). It is estimated that since 2005, the incidence of cancer has increased by 33% globally, mainly due to population growth, aging and changes in global age-specific rates (Fitzmaurice et al., 2015; Fitzmaurice et al., 2017). However, the incidence is not similar between and within countries and seems to be disproportionately higher in low and middle income countries (Titus-Ernstoff et al., 2006; Thun et al., 2009; Ferlay et al., 2015). It has been suggested that cancer may account up to 70% of annual deaths in developing countries (Titus-Ernstoff et al., 2006).
In Iran, cancer is the third leading cause of deaths after injuries and cardiovascular diseases, accounting for more than 53,000 annual deaths (International Agency for Research on Cancer, 2012). Current evidences suggest that stomach, breast, prostate, leukemia and lung are the most common incident cancers in both sexes in Iran (Ferlay et al., 2015). The cancers of stomach, lung, leukemia, esophagus and colorectal are the leading causes of death with the estimated number of 8.7, 6.59, 4.65, 3.26 and 3.07 thousands cases respectively (Ferlay et al., 2015). In this context, comprehensive cancer control programs are needed to set priorities for evidence-based preventive strategies.
National and regional cancer registries are both key to research into the epidemiology and etiology of cancer, to the planning and developing of cancer control programs and health services and to assessment their efficacy. Population-based cancer registries which collect data on all incident cases in a defined population provide cost-effective opportunities for assessing and controlling the impact of cancer (Wall, 2001). Exploring the nature and burden of cancer in a community, providing a source of data for etiological studies, monitoring and assessment of cancer prevention strategies are the primary uses of population-based cancer registries (Wall, 2001; Jedy-Agba et al., 2015). Kerman population-based cancer registry (KPBCR) has collaborated with a wide range of pathological labs and health providers to collect accurate data on all cancer cases in the community. Since its inception in 2008 the only report of cancer epidemiology in Kerman province was published based on data which gathered from 2004 to 2009 (Sadjadi et al., 2007). However, recent and accurate reports on incidence and patterns of age-specific rates are essential to define the priorities and preventive strategies and investigate the underlying causes of more prevalent cancers. In an attempt to provide an updated evidence on cancer epidemiology and incidence in southeast of Iran, this study summarizes data from 52 health providers and diagnostic centers using a population-based framework.
Materials and Methods
Geography
Kerman is the largest and most developed region in the southeast of Iran. It is the first-largest province of Iran with an area of 180,726 km2 (69,779 sq mi) that encompasses nearly 11 percent of the land area of Iran. Kerman is the capital city of Kerman Province, Iran. It is located at 30 degree latitude north and 57 degree longitude east. Kerman is 1,755 m (5,758 ft) above sea level, making it third in elevation among provincial capitals in Iran (Figure 1).
Figure 1.
Current Divisions of Kerman Province, Iran (Source: Health Department of Kerman University of Medical Sciences)
Population
According to the 2011 census, the total population of Kerman Province is about 2,938,988 (57.33% urban vs. 42.67% rural with the men: women ratio of 1,482,339:1,456,649 (Figure 2). The capital city, Kerman, has a population of 722,317. The men: women ratio is 364,450:357,867. The racial structure of Kerman Province is homogenous, that generally consisted of Caucasians.
Figure 2.
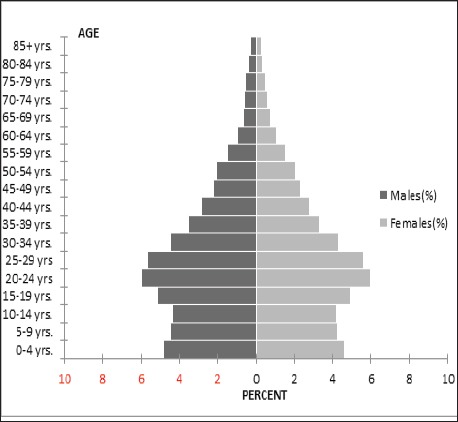
Population Pyramid of Kerman Province, 2010 (Source: Health Department of Kerman University of Medical Sciences)
Data Collection and Analysis
KPBCR collects data from pathological labs and clinic registers of 52 health facilities providing cancer diagnosis or treatment services. Data of patients, who referred to neighbor provinces for accessing health services, are also routinely collected from the three national referral registries, including Tehran, Fars and Yazd provinces. Data from cancer cases that had been diagnosed by autopsies are also extracted from the regional forensic and legal medicine centers and added to the registry periodically. Data from Death Certificates Notifications (DCN) are routinely extracted from national death registry program. Cancer cases that are only reported by death certificates without any previous pathological or clinical history are considered as Death Certificate Only (DCO).Figure 3 illustrates the flow diagram of data collection for KPBCR.
Figure 3.
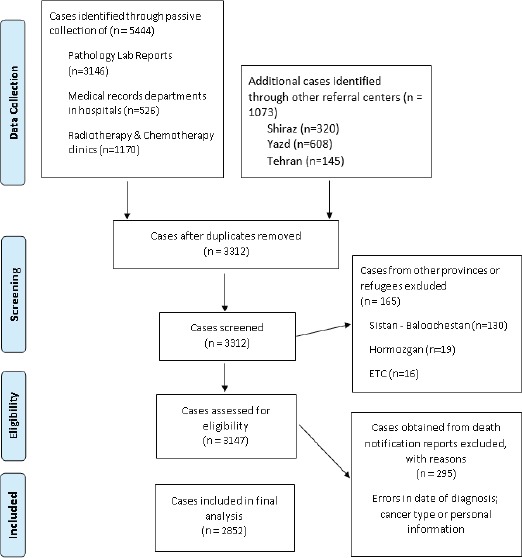
Flow Diagram of Kerman Population-Based Cancer Registry
Case that are currently International Agency for Research on Cancer/International Association of Cancer Registries’ criteria (IACR/IARC) was applied for diagnosis and reporting multiple primaries (Jensen, 1991).
The KPBCR uses ICD-O-3 coding system (Fritz et al., 2000). New cases are regularly checked for duplications according to personal information reported including national ID code, first name, last name, age, father’s name, topography and morphology of tumor, place of residence and date of diagnosis. The KPBCR database currently includes over 17,000 incident cancers since 2008.
In this retrospective study, all patients diagnosed with primary cancers and registered of KPBCR in 2014 were included. The age standardized incidence rate (ASRs) per 100,000 persons- years for all invasive cancers were computed, using the direct-standardization and CanReg methodology. Kerman population in 2011 and world population in 2000 were used as reference populations (Haub and Haub, 2000). Mortality to incidence Ratio (M: I) and morphologically verified (MV) ratios were calculated as completeness and accuracy measures. M:I is defined as a comparison of number of deaths, obtained from National Death Registry Program, and the number of incident cases of a specific cancer registered in 2014. Percentage of MV refers to cases that their diagnosis is based on histology or cytology (proportion of microscopically verified cases to incident cases) (Bray et al., 2014).
Principles of privacy and confidentiality were considered following International Association of Cancer Registries’ criteria for population-based cancer registries (Jensen, 1991; Bray et al., 2014). Data were analyzed using the methods provided by CanReg software.
Results
A total of 2838 new cases of cancer were registered in Kerman province in 2014. Of these, 45.56% were women (n=1293). Individuals aged 60-64 years represented the largest proportion (11.60%) of the total cancer prevalence, followed by patients aged 55-59 years (10.86%) and 65-69 years (8.99%) as the other largest groups of cancer cases.
The age-standardized incidence rates (standard population: world population) were 155.10 and 118.90, respectively. In women, breast (26.4 per 100,000), skin (13.0 per 100,000), thyroid (9.2 per 100,000), leukemia (8.00 per 100,000) and colon and rectum (7.70 per 100,000) were the most common cancers. In men, bladder (24.70 per 100,000), skin (16.80 per 100,000), Lung (14.6 per 100,000), leukemia (14.50 per 100,000) and Stomach (10.8 per 100,000) were reported as the top common cancers. Table 1 summarizes the estimated incidence of the leading cancer types, completeness and accuracy of reporting, by sex and primary site, in 2014.
Table 1.
Incidence, Completeness of Reporting and Accuracy of Diagnosis in Kerman Province, According to Sex and Primary Site, 2014
| Quality & completeness of reporting | Accuracy | ||||||
|---|---|---|---|---|---|---|---|
| Primary site | ICD-O-3 | crude rate | ASR Kerman a | ASR (World Population 2012) (62) | DCO/I(%) | M/I | MV/I (%) |
| Men | |||||||
| All site | C00-80 | 104.22 | 155.1 | 204.9 | 12.49 | 0.34 | 71.2 |
| Bladder | C67 | 15.38 | 24.7 | 9.0 | 4.39 | 0.12 | 85.5 |
| Lung | C34 | 9.44 | 14.6 | 34.2 | 32.14 | 0.74 | 47.9 |
| Leukemia | C42 | 10.92 | 14.5 | 5.6 | 9.88 | 0.12 | 58.0 |
| Prostate | C61 | 7.08 | 11.5 | 30.6 | 21.90 | 0.40 | 62.9 |
| Stomach | C16 | 7.15 | 10.8 | 17.4 | 13.21 | 0.46 | 73.6 |
| Colorectum | C18-20 | 6.34 | 10.0 | 20.6 | 5.32 | 0.27 | 75.5 |
| Larynx | C32 | 5.26 | 8.7 | 3.9 | 5.13 | 0.24 | 70.5 |
| Women | |||||||
| All site | C00-80 | 88.76 | 118.9 | 165.2 | 8.74 | 0.27 | 76.6 |
| Breast | C50 | 20.32 | 26.4 | 43.1 | 3.72 | 0.15 | 82.4 |
| Thyroid | C73 | 8.37 | 9.2 | 6.1 | 0.00 | 0.02 | 100.0 |
| Leukemia | C42 | 6.65 | 8.0 | 3.9 | 4.12 | 0.04 | 64.9 |
| Colorectum | C18-20 | 5.14 | 7.7 | 14.3 | 4.00 | 0.15 | 70.7 |
| Stomach | C16 | 3.7 | 5.4 | 7.5 | 22.22 | 0.43 | 66.7 |
| Lung | C34 | 3.36 | 4.9 | 13.6 | 40.82 | 0.88 | 30.6 |
| Ovary | C56 | 2.54 | 3.4 | 6.1 | 10.81 | 0.30 | 73.0 |
ICD-O-3, International Classification of Disease Oncology, 3rd Revision; DCO/I, proportion of cases with the death certificate only to incident cases; M/I, number of mortality/number of incidence; MV/I, proportion of microscopically verified cases to incident cases. a Per 100 000 population.
Regarding quality and completeness of reporting, the overall DCO% for men was 0.34 and for women was 12.49% and 8.74% respectively. The M/I for men was 0.34 and for women was 0.27.Some variations were observed according to the cancer site. For accuracy of diagnosis, the overall MV% was 71.2% in men and 76.6% in women.
Figure 1, shows age-specific incidence rates for the seven major cancer sites for men and for the seven major cancer sites for women. In men, the age-specific incidence rate increased with age for bladder, lung, leukemia and prostate. Incidence rates increased in the 65–69 and 80-84-year-old groups for bladder and prostate cancers. For lung cancer, the incidence rate increased in the 65–69-year-old group and was the highest in all sites in 80–84-year-old. The incidence rate increased by the 75–79-year-old group for leukemia and decreased over that age group.
In women, the age-specific incidence rates increased with age for breast, thyroid, stomach, leukemia, colon and rectum cancers. For breast cancer, incidence rates peaked in approximately the 50–54-year-old group and decreased in the 70–74-year-old group. For thyroid and stomach cancers, incidence rates peaked in the 80+ year-old groups. For leukemia and colon and rectum cancers, incidence rates peaked in the 80-84-year old group and decreased over that age group dramatically.
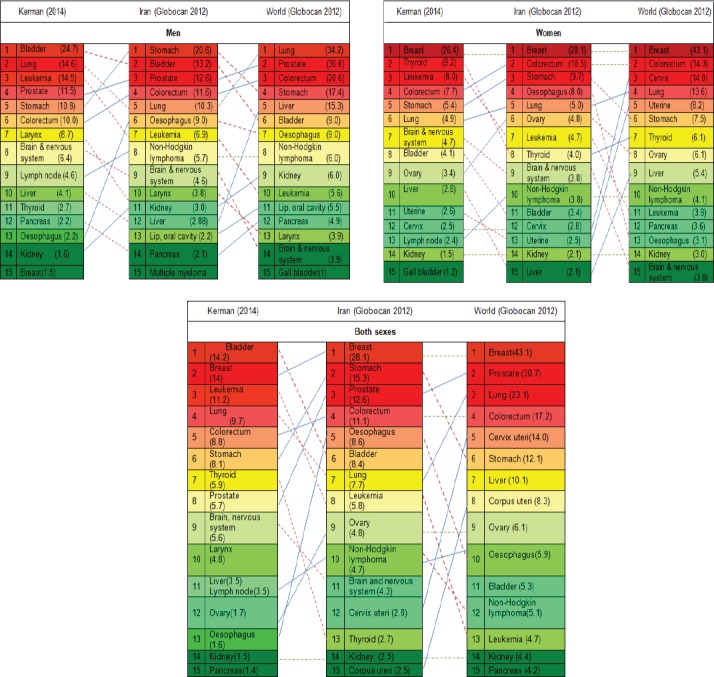
The men to women ratio for cancers of larynx and bladder were 7.9 and 6 respectively and the women to men ratio for thyroid cancer was 6.2 (Table 2).
Table 2.
Age Standardized Incidence of 15 Incident Cancers (Excluding Skin Cancer) in Kerman, Iran and world Per 100,000 Populations
Discussion
The estimated incidence of cancers in Kerman was 136 per 100,000 populations in 2014 which is corresponding to 2834 new cases of cancer. Totally the most common five affected sites excluding skin were bladder, breast, leukemia, lung and colorectal. However, the incidence of cancers by type differs across sex groups. In men, the most common affected sites were bladder followed by lung, leukemia, prostate and stomach; while in women, the breast was the most common site of cancer followed by thyroid, stomach, leukemia and colorectal.
Our result indicates that the pattern of cancers in Kerman is slightly different than total pattern of cancers in Iran and in the world (Table 2): excluding skin cancers, bladder cancer with the ASR of 14.2 is the most prevalent cancer in Kerman while the sixth rank cancer in Iran (International Agency for Research on Cancer, 2012). Surprisingly, the incidence in men population is 6 times more prevalent than women which is higher than corresponding sex ratio in Iran (3.7) (Pakzad et al., 2015) and the global estimates (3.9) (Fitzmaurice et al., 2017)(Table 2).The current evidence indicates that the incidence of bladder cancer is increasing in Asian countries while the trend is declining in Europe and north America (Pakzad et al., 2015). Variety of risk factors including cigarette smoking, opium consumption, higher BMI and lower consumption of fruits and vegetables have been associated with bladder cancer (Jafari-Koshki et al., 2017). The result of a meta-analysis in Iran indicates that around 12.3%-35.5% of Iranian men and 0.6%- 9.8% Iranian women smoke cigarette (Moosazadeh et al., 2013). While the findings from a population-based household survey in Kerman report an estimated prevalence of 15.5% in men and 0.8% in women which is lower than the national estimates (Salimzadeh et al., 2016). So it seems that tobacco smoking couldn’t justify the higher incidence of bladder cancer in Kermanees population. Among other risk factors of bladder cancer, opium consumption may be somehow a potential risk factor for the distinct pattern of bladder cancer in Kerman. The result of an ecological study on 160 countries showed a significant relationship between opiate use and bladder cancer (Rashidian et al., 2016). According to the latest population size estimation of drug users in Iran, Kerman ranked fifth in terms of opium consumption and ranked third in terms of Shire (combination of Opium residue and pure opium) consumption (Baneshi MR, 2013). As most of opium consumers are men, this may be explaining the higher sex ratio of bladder cancer in Kerman compared to the whole country.
Breast cancer is the second common cancer in both sexes and the first common cancer in women population of Kerman. The corresponding pattern is similar to national and somehow global estimates (Table 2). Variety of risk factors including genetic susceptibility, history of no live birth, body mass index (BMI) more than 30, age at first pregnancy more than 30 years old, meat consumption more than three times per week, diabetes mellitus, smoking and alcohol consumption has been associated with breast cancer (Anothaisintawee et al., 2013; Castro and Castro, 2014; Namiranian et al., 2014). Except genetic susceptibility, other risk factors of breast cancer are modifiable. Evidence suggests that breast cancer risk accumulation starts from childhood. It means that exposures during childhood and adolescence affect a woman’s long-term risk of breast cancer (Colditz et al., 2014). So it would be very important that interventions for breast cancer risk reduction start from early life. However, the awareness and risk perception of Iranians toward healthy life style is poor and in some degrees affected by some myths, misconception and presence of stigma (Khazaee-Pool et al., 2014; Sadighi et al., 2014). Therefore, establishing a comprehensive knowledge raising programs which start from childhood are urgently needed for primary prevention of breast cancer in Iran. In addition, secondary prevention of breast cancer by offering the accessible and formidable strategies of early diagnosis would be an effective strategy to decrease the mortality and burden of disease in low and middle income settings and should be extended in Iran (Dey, 2014; Demment et al., 2015; Altobelli et al., 2017).
Figure 4.
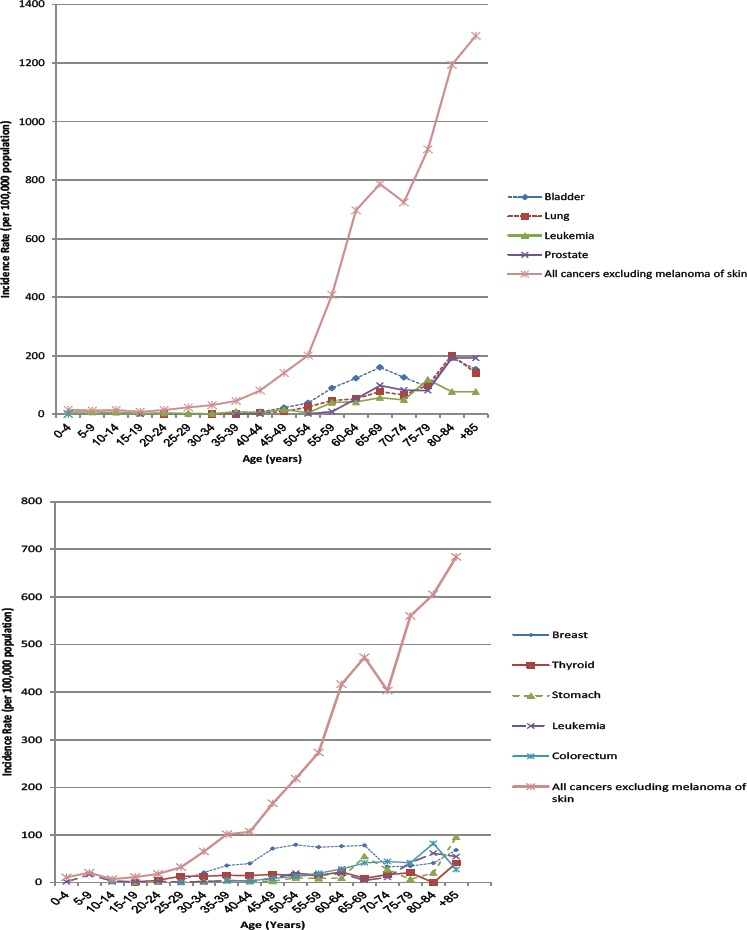
Age-specific Incidence Rate per Population of 100,000 in 2014 for Major Cancer Sites (only Invasive) in Men (a) and Women (b).
Lung cancer in Kerman is the third ranked cancer in both sexes and the second common cancer among men which shows a different pattern compared to the national estimates (seventh rank of both sex and fifth rank of men cancer) (Table 2). Risk factors such as cigarette smoking, water-pipe and opium consumption, air-pollution, exposure to radiation, obesity, unhealthy diet and some environmental and work-place exposures have been associated with lung cancer (Ridge et al., 2013; Kamangar et al., 2014; Hamra et al., 2015; Hidayat et al., 2016; Sun et al., 2016; Montazeri et al., 2017).The Kerman province is one of the richest mineral provinces in the country and the city of Kerman is surrounded by variety of active mines including copper, coal, Iron and titanium (2015). Some evidence indicate that exposure to such industries increases the risk of lung cancer (Seidler et al., 2014). In addition indirect exposure to heavy metals as the consequence of water pollution due to mining and related industries has detrimental effects on public health and should be evaluated in future researches (Pirsaheb et al., 2015). Furthermore, higher prevalence of opium consumption in Kerman may justify the higher incidence of lung cancer in Kerman which needs further researches to be evaluated.
Another factor that is highly suspected to be responsible for the observed pattern of lung cancer is consumption of water-pipe. The result of a current systematic review shows that water-pipe smoking increases the risk of lung cancer (Waziry et al., 2017). While on average between 2% to 23% of Iranian currently use water-pipe (Meysamie et al., 2010; Ansari-Moghaddam et al., 2016; Hessami et al., 2017), the prevalence in Kerman is reported 19% in university students (Sabahy et al., 2011; Hessami et al., 2017) and 29% in general adult population (Danaei et al., 2017) that seem to be higher than national estimates (International Agency for Research on Cancer, 2012). Although water pipe has been associated with lung cancer in various researches, the causal role of water-pipe smoking in lung cancer in Kermanees is questionable and should be studied more in analytic studies: Most of water-pipe consumers are at young ages while most cases of lung cancer occur in middle ages. The lower incidence of lung cancer in Iran compared to global estimates may be probably because of modest prevalence of smoking in Kerman. Opium and water-pipe consumption have been associated with cancers of larynx, stomach, esophagus, pharynx and oral cavity (Maziak, 2013; Kamangar et al., 2014; Rashidian et al., 2016; Montazeri et al., 2017). The observed different pattern of these cancers compared to national pattern suggests that other risk factors such as dietary and environmental factors may have stronger role than opium or water-pipe in causal pathway of disease.
Further discrepancy between Kerman and national/global estimates is related to the incidence of leukemia which is the third common cancer in Kerman, while the eight rank both in Iran and in the world (International Agency for Research on Cancer, 2012; Fitzmaurice et al., 2017). A variety of risk factors has been associated with leukemia including Ionizing radiation, electromagnetic field exposure, hydrocarbons, socioeconomic factors, immunity and infection, certain viruses such as Epstein-Barr Virus, HIV, agricultural pesticides and chemical fertilizers (Turner et al., 2011; Terwilliger and Abdul-Hay, 2017). The association between environmental factors and leukemia has been well investigated. Although the underlying factors related to the increased incidence of leukemia in Kerman are not clear, some environmental factors are highly suspicious. Kerman is known by its two main agricultural poles: Rrafsanjan which is the main producer of pistachio In Iran and Jiroft which usually considered as the main agricultural areas in Kerman province. Some informal reports imply the improper use of fertilizers and pesticides in these regions which may explains the higher incidence of leukemia in Kerman compared to other parts of country. Improper use of pesticide and fertilizers are partly as the result of low level of education, lack of knowledge and economical barriers in farmers and to some extent lack of proper monitoring of supervisory organizations (Sharifi et al., 2010; Loloei et al., 2014). Therefore, appropriate education of farmers and workers and strengthening the monitoring systems may play an important role in prevention of cancers.
The incidence of thyroid cancer in Kermanees women is also distinctly higher than national estimates. While it is the second rank of women cancers in Kerman, it’s at the eighth rank of women cancers globally (Fitzmaurice et al., 2017) and ninth rank in developing countries (Torre et al., 2015). The sex ratio in Kerman is 6.2 while the estimated ratio in Iran and global is 2.9 and 1.2 respectively (Table 2). Gender variations in thyroid cancer are a common issue all around the world (Yao et al., 2011). For example in women, the incidence of papillary and follicular thyroid carcinoma is three-times higher than men while the incidence of anaplastic thyroid carcinoma in men is two-times higher than women. As papillary and follicular carcinoma are more common than anaplastic carcinoma (Safavi et al., 2016), this may justify the higher incidence of thyroid cancer in women. On the other side, higher incidence of thyroid cancer in women may be partly due to over-diagnosis of thyroid cancer during the recent years. As benign thyroid disease are more prevalent in women than men, women are more likely to undergo diagnostic and imaging techniques which consequently increase the diagnosis of cancer (Yao et al., 2011). Furthermore, the care seeking behavior in women is more than men which increase the chance of diagnosis. According to the WHO report, up to 50-90% of thyroid cancers in women in high-income countries estimated to be over-diagnoses. It means that some indolent forms of thyroid forms that would neither be symptomatic during a persons’ lifetime, nor reduce lifespan are being detected as cancer (Jegerlehner et al., 2017) which in turn exposes patients to the side effects of treatment, without increasing the overall survival (Davies, 2016). Besides to over-diagnosis, variety of hormonal and reproductive factors may explain gender variations of thyroid cancer (Rahbari et al., 2010). The higher sex ratio of thyroid cancer in Kerman compared to the national estimates is an issue that should be addressed in future studies. The results of the first report of population based cancer registry in Kerman indicated that between 1996-2000 the women to men ratio of thyroid cancer was 4 which is lower than current ratio in Kerman (Sadjadi et al., 2007). A potential explanation for recent increase in this ratio may include application of new diagnostic techniques and increased number of endocrinologist and surgeon during the recent years in the province.
Another striking pattern in our study was relatively higher incidence of larynx cancer compared to both global and national estimates. While larynx cancer is the 10th common cancer in both sexes and 7th common cancer in men in Kerman, it doesn’t lie among the ten most common cancers in Iran and even in the world. The sex ratio of larynx cancer in our study (7.9) was also higher than national (6.3) and global (4.6) estimates. The incidence of this cancer in comparison to other cancers of head and neck is also high. For example while the incidence of larynx cancer in both sex and in men is 4.8 and 8.7 per 100,000 population, the corresponding incidence for cancers of lip and oral cavity is 1.2 and 1.4 and for cancers of nasopharynx 0.3 and 0.4 respectively (Health depatment of Kerman university of medical sciences, 2017). The main common risk factors for all of these cancers are cigarette smoking. As we stated earlier, lower prevalence of cigarette smoking compared to national prevalence weaken the hypothesis regarding the role of cigarette smoking in high incidence of larynx cancer in Kerman. Instead, The role of opium smoking in the development of larynx cancer has been studied well (Mousavi et al., 2003; Bakhshaee et al., 2017) and is highly suspected . However, the causal role of opium smoking on cancer of larynx should be studied more in future research.
The main strength of the study is that we presented the updated data on cancer incidence and epidemiology in southeast of Iran with the large population and quality of data. However, the study has some limitations that should be acknowledged. The data reported in this paper refers to the patients registered in 2013. This limited time-window may produce a poor representative cohort of patients, however current findings represent similar patterns of cancer incidence compared with previously published data. In addition, because this is a report for one year and might be inflated by prevalent cases, especially because of high rate of clinical cases and low MV% for lung, leukaemia, and prostate cancers. Since data is partly based on medical records kept in public hospitals and medical centers, the accuracy of cancer diagnosis may be affected by the poor quality and incompleteness of reports. For more cancers, the DCO% was more than 5% which may affect the quality of information. While the national population-based cancer registry program has not been comprehensively established in all areas of the country, information about patients who may have sought treatment elsewhere outside the Kerman province was not entirely available and some cases may have been missed. However, the authors did the best to collect information about such patients from some national referral centers in Yazd, Shiraz and Tehran to minimize the number of such cases. Due to lack of data, staging of tumors was not available for registered patients. Furthermore, because of administrative challenges and follow-up limitations, survival rates in different subgroups of patients were not inspected.
In conclusion, our results showed that pattern of cancer in Kerman province is somehow different from national pattern. High quality analytic studies to capture responsible factors and mechanism of such different pattern are urgently needed. In addition, local risk reduction interventions such as knowledge raising programs and establishment of preventive medicine centers is highly encouraged. Inter-sectional cooperation such as monitoring of foods and agricultural products as well as extreme supervision to decrease the hazardous effects of local industries should be reinforced. While the current insurance policies in Iran mainly focus on treatment and tertiary prevention of cancers, the revision of policies to cover the costs of primary and secondary prevention of cancer and non-communicable diseases is a priority that should be taken into account in future planning.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors wish to acknowledge support staff of pathology labs and medical records departments in providing cancer statistics. The authors would like to take this opportunity to acknowledge Ms. Shima Hajalizadeh and Ms. Maryam Zare for their technical support.
References
- 1.Visit Kerman:A paradise in the heart of desert [Online] 2015. [[Accessed 17/09/2017]]. Available: http://www.kerman-info.ir/economy-and-industry .
- 2.Altobelli E, Rapacchietta L, Angeletti PM, et al. Breast cancer screening programmes across the WHO European Region:differences among countries based on national income level. Int J Environ Res Public Health. 2017;14:452. doi: 10.3390/ijerph14040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, et al. Risk factors of breast cancer:a systematic review and meta-analysis. Asia Pac J Public Health. 2013;25:368–87. doi: 10.1177/1010539513488795. [DOI] [PubMed] [Google Scholar]
- 4.Ansari-Moghaddam A, Rakhshani F, Shahraki-Sanavi F, et al. Prevalence and patterns of tobacco, alcohol, and drug use among Iranian adolescents:A meta-analysis of 58 studies. Child Youth Serv Rev. 2016;60:68–79. [Google Scholar]
- 5.Bakhshaee M, Raziee HR, Afshari R, et al. Opium addiction and risk of laryngeal and esophageal carcinoma. Iran J Otorhinolaryngol. 2017;29:19. [PMC free article] [PubMed] [Google Scholar]
- 6.Bray F, Znaor A, Cueva P, et al. Planning and developing population-based cancer registration in low-and middle-income settings, IARC (International Agency for Research on Cancer) 2014 [PubMed] [Google Scholar]
- 7.Castro GD, Castro JA. Alcohol drinking and mammary cancer:Pathogenesis and potential dietary preventive alternatives. World J Clin Oncol. 2014;5:713. doi: 10.5306/wjco.v5.i4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early:prevention must also. Breast Cancer Res Treat. 2014;145:567–79. doi: 10.1007/s10549-014-2993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danaei M, Jabbarinejad-Kermani A, Mohebbi E, et al. Waterpipe tobacco smoking prevalence and associated factors in the southeast of Iran. Addict Health. 2017;9:72–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Demment MM, Peters K, Dykens JA, et al. Developing the evidence base to inform best practice:a scoping study of breast and cervical cancer reviews in low-and middle-income countries. PLoS One. 2015;10:e0134618. doi: 10.1371/journal.pone.0134618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey S. Preventing breast cancer in LMICs via screening and/or early detection:the real and the surreal. World J Clin Oncol. 2014;5:509. doi: 10.5306/wjco.v5.i3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–80. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 13.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015:a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol, 2015;1:505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology. Geneva: World Health Organization; 2000. [Google Scholar]
- 16.Hamra GB, Laden F, Cohen AJ, et al. Lung cancer and exposure to nitrogen dioxide and traffic:a systematic review and meta-analysis. Environ Health Perspect. 2015;123:1107. doi: 10.1289/ehp.1408882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haub C, Haub C. World population date sheet of the population reference bureau, population reference Bureau. 2000 [Google Scholar]
- 18.Hessami Z, Masjedi MR, Ghahremani R, et al. Evaluation of the prevalence of waterpipe tobacco smoking and its related factors in Tehran, Islamic Republic of Iran/Évaluation de la prévalence de la consommation de tabac par pipe àeau et de ses facteurs associés àTéhéran, République islamique d'Iran. East Mediterr Health J. 2017;23:94. doi: 10.26719/2017.23.2.94. [DOI] [PubMed] [Google Scholar]
- 19.Hidayat K, Du X, Chen G, et al. Abdominal obesity and Lung Cancer Risk:Systematic Review and Meta-Analysis of prospective studies. Nutrients. 2016;8:810. doi: 10.3390/nu8120810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafari-Koshki T, Arsang-Jang S, Mahaki B. Bladder cancer in Iran:Geographical distribution and risk factors. Iran J Cancer Prev. 2017;10:5610–17. [Google Scholar]
- 21.Jedy-Agba EE, Oga EA, Odutola M, et al. Developing national cancer registration in developing countries–case study of the Nigerian National System of Cancer Registries. Front Public Health. 2015;3:1–10. doi: 10.3389/fpubh.2015.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jegerlehner S, Bulliard J-L, Aujesky D, et al. Overdiagnosis and overtreatment of thyroid cancer:A population-based temporal trend study. PLoS One. 2017;12:e0179387. doi: 10.1371/journal.pone.0179387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen OM. Cancer registration:principles and methods, IARC. 1991 [PubMed] [Google Scholar]
- 24.Kamangar F, Shakeri R, Malekzadeh R, et al. Opium use:an emerging risk factor for cancer? Lancet Oncol. 2014;15:e69–e77. doi: 10.1016/S1470-2045(13)70550-3. [DOI] [PubMed] [Google Scholar]
- 25.Khazaee-Pool M, Montazeri A, Majlessi F, et al. Breast cancer-preventive behaviors:exploring Iranian women's experiences. BMC Womens Health. 2014;14:41. doi: 10.1186/1472-6874-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loloei M, Zolala F, Razzaghi A. Farmers'pesticide using behaviors:A case study on Pistachio farms in Kerman, Iran. Health Scope. 2014;3:14101–7. [Google Scholar]
- 27.Maziak W. The waterpipe:an emerging global risk for cancer. Cancer Epidemiol. 2013;37:1–4. doi: 10.1016/j.canep.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meysamie A, Ghaletaki R, Haghazali M, et al. Pattern of tobacco use among the Iranian adult population:results of the national Survey of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007) Tob Control. 2010;19:125–8. doi: 10.1136/tc.2009.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montazeri Z, Nyiraneza C, El-Katerji H, et al. Waterpipe smoking and cancer:systematic review and meta-analysis. Tob Control. 2017;26:92–7. doi: 10.1136/tobaccocontrol-2015-052758. [DOI] [PubMed] [Google Scholar]
- 30.Moosazadeh M, Ziaaddini H, Mirzazadeh A, et al. Meta-analysis of smoking prevalence in Iran. Addict Health. 2013;5:140. [PMC free article] [PubMed] [Google Scholar]
- 31.Mousavi MRA, Damghani MA, Haghdoust AA, et al. Opium and risk of laryngeal cancer. Laryngoscope. 2003;113:1939–43. doi: 10.1097/00005537-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Namiranian N, Moradi-Lakeh M, Razavi-Ratki SK, et al. Risk factors of breast cancer in the Eastern Mediterranean Region:a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:9535–41. doi: 10.7314/apjcp.2014.15.21.9535. [DOI] [PubMed] [Google Scholar]
- 33.Pakzad R, Mohammadian-Hafshejani A, Mohammadian M, et al. Incidence and mortality of bladder cancer and their relationship with development in Asia. Asian Pac J Cancer Prev. 2015;16:7365–74. doi: 10.7314/apjcp.2015.16.16.7365. [DOI] [PubMed] [Google Scholar]
- 34.Pirsaheb M, Khamutian R, Pourhaghighat S. Review of heavy metal concentrations in Iranian water resources. Int J Health Life Sci. 2015;1:35–45. [Google Scholar]
- 35.Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6:1771–9. doi: 10.2217/fon.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rashidian H, Zendehdel K, Kamangar F, et al. An ecological study of the association between opiate use and incidence of cancers. Addict Health. 2016;8:252. [PMC free article] [PubMed] [Google Scholar]
- 37.Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Seminars in interventional radiology. Thieme Med Pub. 2013;10:93–8. doi: 10.1055/s-0033-1342949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabahy A, Divsalar K, Bahreinifar S, et al. Waterpipe tobacco use among Iranian university students:correlates and perceived reasons for use. Int J Tuberc Lung Dis. 2011;15:844–7. doi: 10.5588/ijtld.10.0744. [DOI] [PubMed] [Google Scholar]
- 39.Sadighi J, Montazeri A, Jahangiri K. Towards a holistic approach to healthy diet:Evidence from Iranian health perception study. Iran J Public Health. 2014;43:828. [PMC free article] [PubMed] [Google Scholar]
- 40.Sadjadi A, Zahedi M, Nouraie M, et al. The first population-based cancer survey in Kerman Province of Iran. Iran J Public Health. 2007;36:26–34. [Google Scholar]
- 41.Safavi A, Azizi F, Jafari R, et al. Thyroid cancer epidemiology in Iran:a time trend study. Asian Pac J Cancer Prev. 2016;17:407–12. doi: 10.7314/apjcp.2016.17.1.407. [DOI] [PubMed] [Google Scholar]
- 42.Salimzadeh H, Najafipour H, Mirzaiepour F, et al. Prevalence of active and passive smoking among adult population:findings of a population-based survey in Kerman (KERCADRS), Iran. Addict Health. 2016;8:16. [PMC free article] [PubMed] [Google Scholar]
- 43.Seidler A, Brüning T, Taeger D, et al. Cancer incidence among workers occupationally exposed to dinitrotoluene in the copper mining industry. Int Arch Occup Environ Health. 2014;87:117–24. doi: 10.1007/s00420-012-0842-9. [DOI] [PubMed] [Google Scholar]
- 44.Sharifi O, Sadati SA, Ghobadi FR, et al. Barriers to conversion to organic farming:A case study in Babol County in Iran. Afr J Agric Res. 2010;5:2260–7. [Google Scholar]
- 45.Sun Y, Li Z, Li J, et al. A healthy dietary pattern reduces lung cancer risk:A systematic review and meta-analysis. Nutrients. 2016;8:134. doi: 10.3390/nu8030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia:a comprehensive review and 2017 update. Blood Cancer J. 2017;7:e577. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thun MJ, DeLancey JO, Center MM, et al. The global burden of cancer:priorities for prevention. Carcinogenesis. 2009;31:100–10. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Titus-Ernstoff L, Perry AE, Spencer SK, et al. Multiple primary melanoma:two-year results from a population-based study. Arch Dermatol. 2006;142:433–8. doi: 10.1001/archderm.142.4.433. [DOI] [PubMed] [Google Scholar]
- 49.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 50.Turner MC, Wigle DT, Krewski D. Residential pesticides and childhood leukemia:a systematic review and meta-analysis. Cien Saude Colet. 2011;16:1915–31. doi: 10.1590/s1413-81232011000300026. [DOI] [PubMed] [Google Scholar]
- 51.Wall S. Cancer epidemiology:principles and methods. isabel dos Santos Silva. IARC Press, Lyon, France, 1999. No. of pages:ix+442. Price:£40.00 ISBN 92-832-0405-0. Stat Med. 2001;20:821–2. [Google Scholar]
- 52.Waziry R, Jawad M, Ballout RA, et al. The effects of waterpipe tobacco smoking on health outcomes:an updated systematic review and meta-analysis. Int J Epidemiol. 2017;46:32–43. doi: 10.1093/ije/dyw021. [DOI] [PubMed] [Google Scholar]
- 53.Yao R, Chiu CG, Strugnell SS, et al. Gender differences in thyroid cancer:a critical review. Expert Rev Endocrinol Metab. 2011;6:215–43. doi: 10.1586/eem.11.9. [DOI] [PubMed] [Google Scholar]


