Abstract
Background:
Among the proposals for joint disease mapping, the shared component model has become more popular. Another advance to strengthen inference of disease data is the extension of purely spatial models to include time aspect. We aim to combine the idea of multivariate shared components with spatio-temporal modelling in a joint disease mapping model and apply it for incidence rates of seven prevalent cancers in Iran which together account for approximately 50% of all cancers.
Methods:
In the proposed model, each component is shared by different subsets of diseases, spatial and temporal trends are considered for each component, and the relative weight of these trends for each component for each relevant disease can be estimated.
Results:
For esophagus and stomach cancers the Northern provinces was the area of high risk. For colorectal cancer Gilan, Semnan, Fars, Isfahan, Yazd and East-Azerbaijan were the highest risk provinces. For bladder and lung cancer, the northwest were the highest risk area. For prostate and breast cancers, Isfahan, Yazd, Fars, Tehran, Semnan, Mazandaran and Khorasane-Razavi were the highest risk part. The smoking component, shared by esophagus, stomach, bladder and lung, had more effect in Gilan, Mazandaran, Chaharmahal and Bakhtiari, Kohgilouyeh and Boyerahmad, Ardebil and Tehran provinces, in turn. For overweight and obesity component, shared by esophagus, colorectal, prostate and breast cancers the largest effect was found for Tehran, Khorasane-Razavi, Semnan, Yazd, Isfahan, Fars, Mazandaran and Gilan, in turn. For low physical activity component, shared by colorectal and breast cancers North-Khorasan, Ardebil, Golestan, Ilam, Khorasane-Razavi and South-Khorasan had the largest effects, in turn. The smoking component is significantly more important for stomach than for esophagus, bladder and lung. The overweight and obesity had significantly more effect for colorectal than of esophagus cancer.
Conclusions:
The presented model is a valuable model to model geographical and temporal variation among diseases and has some interesting potential features and benefits over other joint models.
Keywords: Spatial statistics, disease mapping, bayesian modelling, shared component model, Iran, cancer
Introduction
Disease mapping is defined as the spatial analysis, estimation and presentation of disease incidence, prevalence, survival or mortality data. It has seen a tremendous growth in the last few decades that have led to the use of complex models enabling the study of possible associations between the disease rates and spatially varying covariates (Lawson et al., 2000; Lawson et al., 2003).
However most of the work in the field of disease mapping studies has been carried out at the univariate level considering spatial modelling of a single dis- ease. Nevertheless many diseases share common risk factors and it can be advantageous both from the epidemiological and the statistical point of view to apply models which combine information from related diseases (Knorr-Held and Best, 2001). Therefore, multivariate disease mapping has emerged, which is defined as the joint modelling of the spatial occurrence of two or more diseases or health outcomes (Assunção and Castro, 2004).
Employing this approach we can expect more information is available com- pared to considering each disease separately. This can lead to benefits like the ability to highlight shared and divergent geographical patterns of the different diseases. The precision and efficiency of estimates can be improved, leading to a better identification of hot-spots for less prevalent diseases and improved prediction of those diseases. Joint disease models allow to suggest possible risk factors associated with the diseases, providing stronger and more convincing evidence for the underlying risk surface. Finally, the understanding of relationships among diseases and ease of interpretation can be increased (Dabney and Wakefield, 2005; Dreassi, 2007; Downing et al., 2008, Held et al., 2005; Knorr-Held and Best, 2001).
The shared component model has become more popular in recent years and several types of this model for different data structures have been introduced. This model is a Bayesian hierarchical latent variable model where the relative risk of each of the two or more diseases is split into some different spatially structured latent components. Each component is shared by different subsets of diseases and the area-specific values of these shared components as well as the relative contribution (weight) of the component to each relevant disease may be estimated (Downing et al., 2008; Dabney and Wakefield, 2005; Mahaki et al., 2011).
Another recent advance to strengthen inference in the subject of disease mapping has been the extension of purely spatial models to include time and also space-time interaction (Jafari-Koshki et al., 2014; Knorr-Held and Besag, 1998; Oleson et al., 2008; Rastaghi et al., 2015). Such analyses may have additional benefits over purely spatial disease mapping. The ability to study and identify the persistence or systematic evolvement of geographical patterns over time provides more convincing evidence of true variations than a single cross-sectional analysis (Bernardinelli et al., 1995; Knorr-Held and Besag, 1998)
However, only a few proposed spatio-temporal models could address analyzing multiple diseases jointly. There is a growing need to combine methods for spatial-only and temporal-only analysis of multivariate data, to enable simultaneous investigation of space-time variations in multiple health outcomes (Oleson et al., 2008; Richardson et al., 2006).
In this study we aim to combine the idea of multivariate shared components with spatio-temporal modelling in a joint disease mapping model. To this end, each of the shared components in the proposed model considers spatial and temporal dimensions. Each component is shared between a subset of the diseases and therefore represents a different latent variable. The model can be applied for any desired number of diseases, time periods and spatial areas.
Cancer is the third cause of mortality after cardiovascular disease and car accident in Iran. The rate of cancer is expected to be increased in future due to improve in life expectancy and changing in the lifestyle (Khoshkar et al., 2015). Hence, cancer is an important problem in public health in Iran and knowledge about the temporal trend and spatial pattern of its incidence, prevalence and mortality rates, will lead to better health policies.
According to the last cancer registry reports by Iran Ministry of Health, esophagus, stomach, bladder, colorectal, lung, prostate and breast cancers are amongst the 10 most prevalent cancers in Iran and together account for approximately 50% of all cancers (Iran Cancer Registry Report, 2005-2006, 2006-2007, 2007-2008, 2009-2010).
A lot of studies with different methods have been conducted about the mapping of cancers in Iran (Mahaki et al., 2011; Rastaghi et al,. 2018; Nasrazadani et al,. 2018; Asmarian et al., 2013). These studies have mainly used traditional models to estimate the relative risk of cancer and a few of them take time aspect into account (Jafari-Koshki et al., 2014; Ahmadipanahmehrabadi et al., 2018).
Considering the shared risk factors between the seven mentioned cancers and due to ageing process of Iranian population, increase in the cancer rates, considerable geographical variation in differing provinces, it is necessary to provide knowledge about the geographical distribution at provincial level to recognize the regions with high incidence to improve prevention process applying advanced statistical models. The present study aims to use joint spatio-temporal shared component model to investigate shared and distinct temporal and spatial shared components regarding the seven prevalent cancers.
In the present study we focus on applying joint spatio-temporal shared component model for incidence rates of the mentioned seven prevalent cancers in Iran to explore their spatial and temporal patterns, and to estimate the relative weight of the four shared component for each cancer in time periods and geographical areas.
We outline the data, joint spatio-temporal shared component models, assignment of prior distributions and finally computation and model comparison in section 2. We then describe the model comparison results and also estimates obtained for the best model in the section 3. Finally in section 4, we present statistical and epidemiological conclusion and discussion.
Materials and Methods
Data
Incidence data for seven cancers including esophagus (ICD10 code C15), stomach (C16), bladder (C67), colorectal (C18-C20, C26), lung (C34), prostate (C61) and breast (C50) cancer in 30 provinces of Iran in 5 years, 2005-2009, were considered in this analysis. The data have been collected and made available by the Iranian Ministry of Health and Medical Education (Iran Cancer Registry Report, 2005-2006, 2006-2007, 2007-2008, 2009-2010).
Joint Spatio-temporal Shared Component Models
Let Yijk be the observed number of index cases for cancer type k=1,…,7, grouped within province i=1,…,30 and time period j = 1,…, 5. The expected number of cases Eijk in each province and each year were calculated by multiplying the national crude incidence rate and the estimate of the province population for the corresponding year. The latter was based on 2006 census conducted by Statistical Center of Iran.
We propose a Bayesian hierarchical model to provide improved estimates of area and time period-specific cancer relative risks (RR). In this, similarities and differences in risk profiles of the diseases were captured by the shared and disease-specific components using a shared component model, with space-time interactions (Held et al., 2005; Downing et al., 2008). As an initial step, it is convenient to assume that the number of incidences Yijk are conditionally independent Poisson random variables:
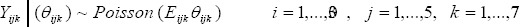
where Θijk represent the true, but unknown underlying relative risks. This Poisson model is widely used for cancer mapping, and arises as an approximation to the binomial distribution for rare and noninfectious diseases. Then following Richardson et al., (2006), we modeled the variability of the observed incidence counts around the relative risks:
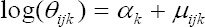
With αk the intercept for cancer k. The cancers used here are esophagus (k = 1), stomach (k = 2), bladder (k = 3), colorectal (k = 4), lung (k = 5), prostate (k = 6) and breast (k = 7) cancers, respectively.
We introduce a space-time structure on the log scale by considering different hypotheses on the joint structure of μijk.
We consider four variations of the joint spatio-temporal shared component model to estimate relative risks of the diseases in space and time. The models differ in their assumption of the space- time structure and the inclusion or not of a heterogeneity term. We start with a simple additive decomposition of the shared part without heterogeneity and space-time interaction terms (model A),
Model A: It contains no spatio-temporal interaction terms and no heterogeneity either
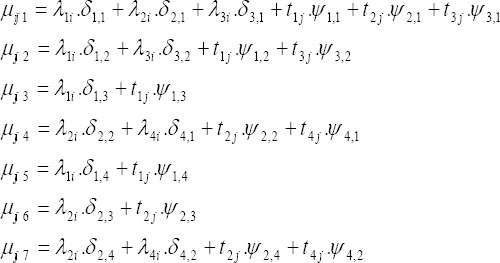
with λli and tlj with l = 1, 2, 3, 4 represent the spatial and temporal effects of shared smoking component (l = 1) common to esophagus, stomach, bladder and lung cancers, overweight and obesity component (l = 2) relevant to esophagus, colorectal, prostate and breast cancers, inadequate fruits and vegetables consumption (l = 3) for esophagus and stomach cancers and low physical activity factors (l = 4) common to colorectal and breast cancers respectively which capture the differential spatial and temporal effects among the relevant cancers. The unknown parameters δ and ψ are included to allow for different risk gradients of the shared spatial and temporal components for the relevant diseases and they represent the relative weight of the contribution of the shared terms to the risk of the relevant cancers, and are set to zero if the component is not relevant to the specific cancer.
In model B we include cancer-specific heterogeneity (ξij1,…,εj7) to capture possible variations not explained by the terms included in model A. In model C we add space-time interaction terms common to all diseases (ξij) to model A. The shared space-time interaction effects capture deviations from space and time main effects and may highlight space-time clusters of risk. Finally we build Model D by combining models B and C.
Model D: Space-time interaction terms, common to all diseases (ξij) are added to model B
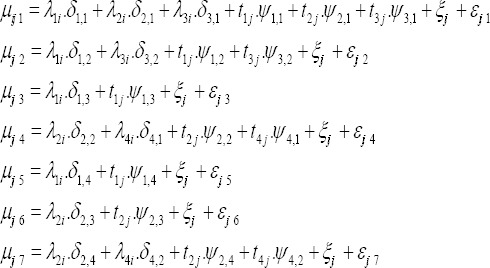
Specification of Priors
In a Bayesian framework prior distributions must be defined on all unknown parameters, whether fixed or random. We require priors that combine the framework introduced by (Besag et al., 1991) (known as BYM model) to link risk in space at every time period and time series techniques to link risk in time at every area. In this study, we assume conditional autoregressive (CAR) normal prior distribution to capture local dependence in space for the shared spatial random effects λi = (λi1,…, λi30) i.e., λl~N(0, Q−1) (Oleson et al., 2008; Richardson et al., 2006). Similarly, for the shared temporal effects tj, in order to capture local dependence in time, we use first order random walk priors, that is, the one dimensional version of the CAR normal prior distribution, where the neighborhood matrix is defined by the temporal neighbors of period j as periods j1 and j + 1 near 1 and none of them were significant. For the disease specific heterogeneity terms, we assign a zero-mean multivariate normal distribution with covariance matrix Σ to allow for correlation between the relevant diseases in each space-time unit.
For the disease specific intercepts αk we use a non-informative flat prior and for the logarithm of spatial and temporal scale parameters (log(δ) and log(ψ)) we assign multivariate normal distribution with mean 0 and variance 5 (Oleson et al., 2008; Richardson et al., 2006).
For all the precision parameters of the spatial and temporal CAR priors we follow Wakefield (Wakefield et al., 2001) and use weakly informative independent hyper- prior Gamma (0.5, 0.0005) distributions. The inverse of the covariance matrix Σ-1 is given a Wishart (S, 7) prior distribution, where the scale parameter S is a 7- dimensional identity matrix. There are various choices of prior distributions for the space-time interaction effects. In the present study, we only have 5 periods; too few to show any reliable space-time jumps in risk of long latency chronic disease such as cancers. Thus, we assume a simple exchangeable hierarchical structure for the interaction terms (Oleson et al., 2008; Richardson et al., 2006).
Computation and model comparison
In order to estimate the parameters, we employ Markov Chain Monte Carlo (MCMC) techniques, using the software WinBUGS (Lunn et al., 2000). Posterior inference is based on a total of 50,000 simulated draws keeping every 10th, after discarding the first 20,000 iterations as a burn-in sequence. To assess the convergence of our MCMC sampler, we use the diagnostics of Gelman and Rubin (1992) as well as graphical checks of the sample paths. Also, to produce the maps we use geographical information system (GIS).
Models comparison is done via the Deviance Information Criterion (DIC). DIC is computed as sum of deviance D¯ and number of effective parameters pD. The deviance is the Bayesian model fit, computed as the estimated expected posterior of minus two times log-likelihood of the observed data. Hierarchical models always have a high number of (correlated) parameters. However, due to the correlation between parameters, the true complexity is usually much lower, for examples when parameters are estimated to be zero, i.e., are not needed in the model. The number of effective parameters pD is an estimate of the number of parameters effectively used in the model, that is, it estimates the complexity of the model. More details can be found in (Spiegelhalter et al., 2002).
Results
Table 1 provides a brief summary of the data on incidence of the seven considered cancers in each year. Among the seven cancers considered in this study, the most and least common cancers were breast and lung cancers.
Table 1.
Registered Number of the Cancers in Years 2004-2008
| Cancer | 2004 | 2005 | 2006 | 2007 | 2008 | Total |
|---|---|---|---|---|---|---|
| Esophagus | 2,584 | 3,046 | 3,176 | 3,164 | 3,859 | 15,829 |
| Stomach | 5,209 | 5,836 | 5,903 | 6,235 | 7,710 | 30,893 |
| Bladder | 3,301 | 3,936 | 4,053 | 4,417 | 4,833 | 20,540 |
| Colorectal | 3,407 | 4,056 | 4,493 | 4,887 | 6,178 | 23,021 |
| Lung | 1,508 | 1,788 | 1,922 | 2,066 | 3,048 | 10,332 |
| Prostate | 2,072 | 2,722 | 2,815 | 3,164 | 3,732 | 14,505 |
| Breast | 4,683 | 5,981 | 6,675 | 7,192 | 8,589 | 33,120 |
In Table 2 model comparison criteria for the four models and also for the multivariate spatial shared component are presented. The first column of the table D¯ can be considered as a Bayesian measure of fit gives the expectation of the posterior deviance. The second column pD is the number of effective parameters and can be considered as a measure of complexity. Among the models, Model A had the poorest overall fit and the lowest complexity. Great improvements in the DIC values are seen by including heterogeneity or space-time interaction parameters. Model D, which includes both space-time interaction and heterogeneity terms has the best absolute model fit, but at the expense of many more effective parameters. Interestingly, this model has more effective parameters than the model B resulting in a slightly larger DIC. This suggests that the heterogeneity and interaction terms are competing to explain the space-time structure not captured by the main effects. For reasons of brevity, we only present maps and graphs resulting from model B. In Table 2 we also reported sum of the DIC values from the corresponding multivariate spatial shared component model in each year. This model was worse than all the models except model A. This suggested that the model can indeed be improved by considering the temporal structure of the data.
Table 2.
DIC for Comparing the Models
| Model | D¯ | pd | DIC |
|---|---|---|---|
| Model A: no interaction + no heterogeneity | 13327.40 | 119.01 | 13446.40 |
| Model B : heterogeneity | 7318.99 | 764.68 | 8083.67 |
| Model C: interaction | 7360.73 | 788.166 | 8148.90 |
| Model D: interaction + heterogeneity | 7314.78 | 774.97 | 8089.75 |
| Spatial shared component model | 7318.54 | 891.96 | 8210.49 |
The map of the smoothed RRs for the cancers corresponding to model B is presented in Figure 1. The maps for other cancers are available in the online appendix. According to the maps for esophagus and stomach cancers the northern part of Iran was the area of high risk. For bladder cancer Gilan, Semnan, Fars, Isfahan, Yazd and Eastern Azerbaijan were found as the provinces with higher risk. For bladder and lung cancer, the areas with higher risk were the northwest. For prostate and breast cancers, Isfahan, Yazd, Fars, Tehran, Semnan, Mazandaran and Razavi Khorasan were recognized as the areas with higher risk.
Figure 1.
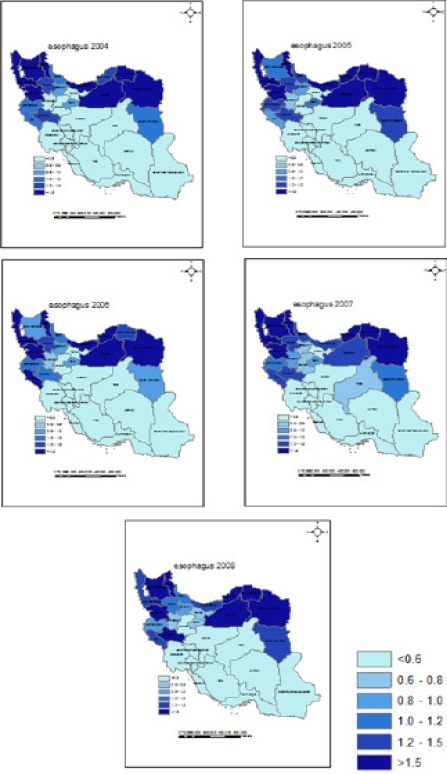
Posterior Median Relative Risk for Esophagus Cancer in Iran Provinces between 2004 and 2008
The estimated effects of the four shared spatial components λl are mapped in Figure 2. The component A, shared by esophagus, stomach, bladder and lung, can be considered to represent the effect of smoking and had more effect in Gilan, Mazandaran, Chaharmahal and Bakhtiari, Kohgilouyeh and Boyerahmad, Ardebil and Tehran provinces respectively. Component B, shared by esophagus, colorectal, prostate and breast cancers can be considered to represent the effect of overweight and obesity. For this component the largest effect was found for Tehran, Razavi Khorasan, Semnan, Yazd, Isfahan, Fars, Mazandaran and Gilan, respectively. The component C is shared by esophagus and stomach cancers and is considered to represent the effect of inadequate fruit and vegetable consumption. It did not show any significant differences among the provinces. For the component D, shared by colorectal and breast cancers and considered to represent the effect of low physical activity, North Khorasan, Ardebil, Golestan, Ilam, Razavi Khorasan and Southern Khorasan were found to have the largest effects respectively.
Figure 2.
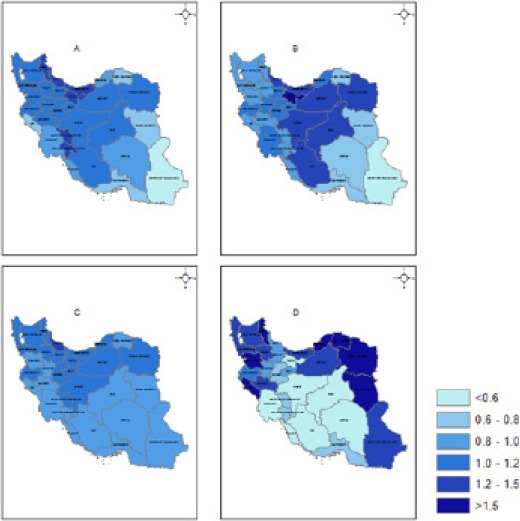
Estimated Relative Risks for the Spatial Effects of the Four Components. A: Sharing for esophagus, stomach, bladder and lung (representing smoking), B: Sharing for esophagus, colorectal, prostate and breast (representing overweight and obesity), C: Sharing for esophagus and stomach (representing inadequate fruit and vegetables consumption), D: Sharing for colorectal and breast (representing low physical activity).
Table 3 represents the temporal effect of the shared components. As expected, all relative risks in each time period were near 1 and none of them were significant.
Table 3.
Estimated Relative Risks for the Temporal Effects of the Four Components in each Time Period
| Posterior median (95% CI) in year: | |||||
|---|---|---|---|---|---|
| Component | 2004 | 2005 | 2006 | 2007 | 2008 |
| 1 | 0.99 (0.94-1.05) | 1.00 (0.97-1.04) | 1.01 (0.98-1.05) | 1.00 (0.97-1.04) | 1.00 (0.94-1.04) |
| 2 | 1.01 (0.96-1.06) | 1.00 (0.97-1.04) | 1.00 (0.97-1.03 | 1.00 (0.97-1.04) | 0.99 (0.94-1.03) |
| 3 | 1.00 (0.95-1.06) | 1.00 (0.95-1.03) | 1.00 (0.96-1.03) | 1.00 (0.97-1.05) | 1.00 (0.95-1.05) |
| 4 | 0.99 (0.93-1.04) | 1.00 (0.96-1.04) | 1.00 (0.96-1.03) | 1.01 (0.97-1.05) | 1.01 (0.96-1.06) |
Table 4 shows the level of importance or relative weight δ, that each shared component has for the spatial and temporal variation of the relevant cancers. The first component (considered to represent smoking) is significantly more important for stomach than for esophagus, bladder and lung cancers. The effect of second component (representing overweight and obesity) was significantly more for colorectal than of esophagus cancer. The two last components (representing low physical activity and inadequate consumption of fruits and vegetables) did not show any significant differences according to the weights on the relevant cancers. The figures in the main body of the table represent the weight of the cancer listed along the columns to the cancers listed along the rows (with 95% confidence intervals). If the RR is greater than 1.00 the cancer along the columns has more weight, if the RR is less than 1.00 the cancer along the rows has more weight.
Table 4.
Posterior Median (95% CI) Relative Weights of the Shared Components on the Spatial and Temporal Variation of the Relevant Cancers
| Esophagus | Stomach | Bladder | Colorectal | Lung | Prostate | Breast | |||
|---|---|---|---|---|---|---|---|---|---|
| Spatial | Esophagus | 1 | 1 | ||||||
| 2 | 1 | ||||||||
| 3 | 1 | ||||||||
| Stomach | 1 | 0.36 (0.18-0.62) | 1 | ||||||
| 3 | 0.88 (0.40-1.91) | 1 | |||||||
| Bladder | 1 | 0.58 (0.26-1.17) | 1.61 (1.26-2.14) | 1 | |||||
| Colorectal | 2 | 0.31 (0.15-0.62) | - | - | 1 | ||||
| Lung | 1 | 0.63 (0.28-1.39) | 1.75 (1.19-2.91) | 1.08 (0.74-1.77) | - | 1 | |||
| Prostate | 2 | 0.50 (0.22-1.07) | - | - | 1.00 (0.50-1.95) | - | 1 | ||
| Breast | 2 | 0.54 (0.24-1.23) | - | - | 1.09 (0.53-2.26) | - | 1.68 (0.85-3.44) | 1 | |
| 4 | - | - | - | 0.93 (0.35-2.28) | - | - | - | ||
| Temporal | Esophagus | 1 | 1 | ||||||
| 2 | 1 | ||||||||
| 3 | 1 | ||||||||
| Stomach | 1 | 1.03 (0.35-3.04) | 1 | ||||||
| 3 | 0.88 (0.40-1.91) | 1 | |||||||
| Bladder | 1 | 0.97 (0.33-2.93) | 0.97 (0.34-2.79) | 1 | |||||
| Colorectal | 2 | 0.95 (0.32-2.82) | - | - | 1 | ||||
| Lung | 1 | 0.94 (0.32-2.77) | 0.94 (0.32-2.74) | 0.97 (0.32-2.90) | - | 1 | |||
| Prostate | 2 | 0.92 (0.31-2.78) | - | - | 0.98 (0.34-2.90) | - | 1 | ||
| Breast | 2 | 0.89 (0.30-2.66) | - | - | 0.96 (0.32-2.81) | - | 1.01 (0.33-3.13) | 1 | |
| 4 | - | - | - | 0.96 (0.33-2.79) | - | - | - |
None of the components shows significant differences according to the weights on the temporal changes of the relevant cancers.
Discussion
In this paper, we have combined the ideas of multivariate spatial shared components and bivariate spatio-temporal shared component models. This way, we have presented a novel and valuable model that is capable to include any desired number of diseases, geographical areas, time periods and shared components representing the risk factors.
The proposed models allows for better estimation of the spatial pattern and of the temporal trend of the diseases by incorporating joint information from multiple diseases. Additionally, fitting this latent variables model enables us to estimate the effect of shared components representing the risk factors in all the spatial areas and time periods without the need of having real data for these factors. We also have the possibility to compare the relative weight of each component for the spatial and temporal variations of its relevant diseases using the scaling parameters.
Our proposed model has benefits over the pure spatial shared component models in addition to make improvement according to Deviance Information Criterion (DIC) and model fit. The results illustrate the changes over time by including temporal effects and hereby increasing the epidemiological interpretability the results. The model allows to investigate the persistence of pat- terns over time and highlight unusual patterns. In addition suitable space-time interaction terms can be included, allowing for the detection of localized clusters and strengthening inference (Manda et al., 2009; Richardson et al., 2006).
Our model also has some advantages over other multivariate spatio-temporal models, mainly the ability to estimate the spatial and temporal effects of shared components as surrogates of the risk factors. We can also estimate the relative importance of each component on the relevant diseases through including the spatial and temporal scaling parameters. It is possible include different structures of space-time, space-disease and disease-time interactions and to include data on environmental, social, economic, etc. covariates, (Manda et al., 2009; Richardson et al., 2006).
The final results will depend on the number of shared components representing the risk factors and their relevant diseases. So, using this model one needs to define the relationship between the diseases and risk factors in advance. To do so, we need to apply the epidemiological background of the diseases (Held et al., 2005; Downing et al., 2008).
One constraint of our model is the independency assumption between the shared components, and impossibility of assessing the interactions among the covariates (Knorr-Held and Best, 2001; Held et al., 2005, Best and Hansell, 2009).
When observed numbers of diseases in each geographical area small, the model has more strength in compare with mapping crude or standardized rates or simple Besag/York/Mollie models. The smaller the areas the less the observed counts and the better the estimates of our model. Also, when information is available for more time periods, our model should work better. With long latency diseases like cancers, investigating the observed rates for lots of time period or for few long time periods such as 5-year periods are ideal because we do not expect remarkable changes over a short time period for cancer (Manda et al., 2009; Richardson et al., 2006).
From an epidemiological point of view we found a clearer spatial pattern with an obvious distinction between the high risk and low risk areas for the stomach and esophagus cancers in comparison with other cancer types. In comparison with less frequent cancers smaller changes over the time periods were observed. We have found notable similarities between the geographical patterns of the relative risks of esophagus and stomach cancers and also for bladder and colorectal cancers and again for breast and prostate cancers. The patterns for bladder and colorectal cancers are different from that of esophagus and stomach cancers. The spatial pattern for the relative risk of lung cancer is quite different from all others cancers. The results were in correspondence to other previous studies about geographical pattern of these cancers in Iran (Asmarian et al., 2013; Haddad-Khoshkar et al., 2015; Haddad-Khoshkar et al., 2015; Mahaki et al., 2014).
Sistan and Baluchestan had the lowest relative risk for all the seven cancer in all the time periods for. After this province, Hormozgan, North Khorasan, South Khorasan, Kohgilouyeh and Boyerahmad, Kerman and Bushehr were found as the lowest risk areas respectively. Also, Razavi Khorasan, Semnan, Gilan, Mazandaran, Yazd, Isfahan, East Azerbaijan, Fars, West Azerbaijan, Kurdistan, Tehran, Ardebil and Golestan were recognized as the areas with highest risks. These results are in accordance with the results of previous studies about incidence and prevalence of the cancers in provinces, and similarity between the spatial patterns of the cancers (Azadeh et al., 2008; Islami et al., 2009; Mousavi et al., 2009; Sadjadi et al., 2003; Sadjadi et al., 2010).
Temporal effects of the shared components representing the four latent risk factors were almost constant. According to the type of disease and the short and few time periods in this study, this was predictable. The result revealed the need for further studies including more time periods. Due to the lack of accurate data for cancer registration system in the county level, we considered Iran provinces as geographical areas. Also, since the cancer registration has been implemented in recent years, we restricted the data only for years 2004 to 2008. Due to some unexplained dispersion in the model and also due to other possible risk factors associated with the cancers, it would be helpful to include some other components to the model or to add real data of some important variables as ecological covariates. In this regard, adding socio-economic background as an important factor for all cancers can be considered (Dabney and Wakefield, 2005).
In summary our presented model is a valuable model to model geographical and temporal variation among diseases and has some interesting potential features and benefits over other joint models.
Acknowledgements
The authors are grateful to express their sincere thanks to the office of non-communicable disease, Ministry of Health and Medical Education, Iran.
References
- 1.Ahmadipanahmehrabadi V, Hassanzadeh A, Mahaki B. Bivariate spatiotemporal shared component modeling:Mapping of relative death risk due to colorectal and stomach cancers in Iran provinces. Int J Prev Med In press. 2018 doi: 10.4103/ijpvm.IJPVM_31_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asmarian NS, Ruzitalab A, Amir K, Masoud S, Mahaki B. Area-to-area poisson kriging analysis of mapping of county-level esophageal cancer incidence rates in Iran. Asian Pac J Cancer Prev. 2013;14:11–3. doi: 10.7314/apjcp.2013.14.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Assunção RM, Castro MSM. Multiple cancer sites incidence rates estimation using a multivariate bayesian model. Int J Epidemiol. 2004;33:508–16. doi: 10.1093/ije/dyh040. [DOI] [PubMed] [Google Scholar]
- 4.Azadeh S, Moghimi-Dehkordi B, Fatem S, et al. Colorectal cancer in Iran:An epidemiological study. Asian Pac J Cancer Prev. 2008;9:123. [PubMed] [Google Scholar]
- 5.Bernardinelli L, Clayton D, Pascutto C, et al. Bayesian analysis of space-time variation in disease risk. Stat In Med. 1995;14:2433–43. doi: 10.1002/sim.4780142112. [DOI] [PubMed] [Google Scholar]
- 6.Best N, Hansell AL. Geographic variations in risk:Adjusting for unmeasured confounders through joint modeling of multiple diseases. Epidemiology (Cambridge, Mass.) 2009;20:400. doi: 10.1097/EDE.0b013e31819d90f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabney AR, Wakefield JC. Issues in the mapping of two diseases. Stat Methods Med Res. 2005;14:83–112. doi: 10.1191/0962280205sm340oa. [DOI] [PubMed] [Google Scholar]
- 8.Downing A, Forman D, Gilthorpe MS, Edwards KL, Manda SOM. Joint disease mapping using six cancers in the Yorkshire region of England. Int J Health Geogr. 2008;7:41. doi: 10.1186/1476-072X-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreassi E. Polytomous disease mapping to detect uncommon risk factors for related diseases. Biom J. 2007;49:520–9. doi: 10.1002/bimj.200610295. [DOI] [PubMed] [Google Scholar]
- 10.Haddad-Khoshkar A, Jafari-Koshki T, Mahaki B. Investigating the incidence of prostate cancer in Iran 2005-2008 using bayesian spatial ecological regression models. Asian Pac J Cancer Prev. 2015;16:5917–21. doi: 10.7314/apjcp.2015.16.14.5917. [DOI] [PubMed] [Google Scholar]
- 11.Held L, Natário I, Fenton SE, Rue H, Becker N. Towards joint disease mapping. Stat Meth In Med Res. 2005;14:61–82. doi: 10.1191/0962280205sm389oa. [DOI] [PubMed] [Google Scholar]
- 12.Iranian Annual National Cancer Registration Report 2005-2006, Provincial report. Iran (Tehran): Noncommunicable diseases Office, Ministry of Health and Medical Education; 2007. [Google Scholar]
- 13.Iranian Annual National Cancer Registration Report 2006-2007, Provincial report. Iran (Tehran): Noncommunicable Diseases Office, Ministry of Health and Medical Education; 2008. [Google Scholar]
- 14.Iranian Annual National Cancer Registration Report 2007-2008, Provincial report. Iran (Tehran): Noncommunicable Diseases Office, Ministry of Health and Medical Education; 2009. [Google Scholar]
- 15.Iranian Annual National Cancer Registration Report 2008-2009, Provincial repor. Iran (Tehran): Noncommunicable Diseases Office, Ministry of Health and Medical Education; 2010. [Google Scholar]
- 16.Iranian Annual National Cancer Registration Report 2009-2010, Provincial report. Iran (Tehran): Noncommunicable Diseases Office, Ministry of Health and Medical Education; 2011. [Google Scholar]
- 17.Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and oesophageal cancer:Results from a population-based case–control study in a high-risk area. Int J Epidemiol. 2009;38:978. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jafari Koshki T, Schmid VJ, Mahaki B. Trends of breast cancer incidence in Iran during 2004 2008:A bayesian space time model. Asian Pac J Cancer Prev. 2014;15:1557–61. doi: 10.7314/apjcp.2014.15.4.1557. [DOI] [PubMed] [Google Scholar]
- 19.Jafari-Koshki T, Arsang-Jang S, Mahaki B. Bladder cancer in Iran:Geographical distribution and risk factors. Int J Cancer Manag. 2017;10:e5610. [Google Scholar]
- 20.Khoshkar AH, Koshki TJ, Mahaki B. Comparison of Bayesian spatial ecological regression models for investigating the incidence of breast cancer in Iran, 2005. Asian Pac J Cancer Prev. 2015;16:5669–73. doi: 10.7314/apjcp.2015.16.14.5669. [DOI] [PubMed] [Google Scholar]
- 21.Knorr-Held L, Besag J. Modelling risk from a disease in time and space. Stat Med. 1998;17:2045–60. doi: 10.1002/(sici)1097-0258(19980930)17:18<2045::aid-sim943>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Knorr-Held L, Best NG. A shared component model for detecting joint and selective clustering of two diseases. J R Stat Soc Ser A Stat. 2001;164:73–85. [Google Scholar]
- 23.Lawson A, Biggeri A, Boehning D, et al. Disease mapping models:An empirical evaluation. Disease mapping collaborative group. Stat Med. 2000;19:2217–41. doi: 10.1002/1097-0258(20000915/30)19:17/18<2217::aid-sim565>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Lawson AB, Browne WJ, Rodeiro CLV, Wiley J. Disease mapping with winbugs and Mlwin. London: Wiley Online Library; 2003. [Google Scholar]
- 25.Lunn DJ, Thomas A, Best N, Spiegelhalter D. Winbugs-A bayesian modelling framework:concepts, structure, and extensibility. Stat Comput. 2000;10:325–7. [Google Scholar]
- 26.Mahaki B, Mehrabi Y, Kavousi A, et al. Multivariate disease mapping of seven prevalent cancers in Iran using a shared component model. Asian Pac J Cancer Prev. 2011;12:2353–8. [PubMed] [Google Scholar]
- 27.Manda SOM, Feltbower RG, Gilthorpe MS. Investigating spatio-temporal similarities in the epidemiology of childhood leukaemia and diabetes. Eur J Epidemiol. 2009;24:743–52. doi: 10.1007/s10654-009-9391-2. [DOI] [PubMed] [Google Scholar]
- 28.Mousavi S, Gouya M, Ramazani R, et al. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20:556. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 29.Nasrazadani M, Maracy MR, Dreassi E, Mahaki B. Mapping of stomach, colorectal, and bladder cancers in Iran, 2004–2009:Applying Bayesian polytomous logit model. Int J Prev Med In press. 2018 doi: 10.4103/ijpvm.IJPVM_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oleson JJ, Smith BJ, Kim H. Joint spatio-temporal modeling of low incidence cancers sharing common risk factors. J Data Sci. 2008;6:105–23. [Google Scholar]
- 31.Rastaghi S, Jafari-Koshki T, Mahaki B. Application of bayesian multilevel space-time models to study relative risk of esophageal cancer in Iran 2005-2007 at a county level. Asian Pac J Cancer Prev. 2015;16:5787–92. doi: 10.7314/apjcp.2015.16.14.5787. [DOI] [PubMed] [Google Scholar]
- 32.Rastaghi R, Jafari-Koshki T, Mahaki B, et al. Trends and risk factors of gastric cancer in Iran (2005-2010) Int J Prev Med In press. 2018 doi: 10.4103/ijpvm.IJPVM_188_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson S, Abellan JJ, Best N. Bayesian spatio-temporal analysis of joint patterns of male and female lung cancer risks in Yorkshire (Uk) Stat Methods Med Res. 2006;15:385–407. doi: 10.1191/0962280206sm458oa. [DOI] [PubMed] [Google Scholar]
- 34.Sadjadi A, Malekzadeh R, Derakhshan MH. Cancer occurrence in Ardabil:Results of a population-based cancer registry from Iran. Int J Cancer. 2003;107:113–18. doi: 10.1002/ijc.11359. [DOI] [PubMed] [Google Scholar]
- 35.Sadjadi A, Marjani H, Semnani S, Nasseri-Moghaddam S. Esophageal cancer in Iran:A review. Middle East J Cancer. 2010;1:5–14. [Google Scholar]
- 36.Wakefield J, Best N, Waller L. Bayesian approaches to disease mapping. Spat Epidemiol. 2007;1:104–28. [Google Scholar]


