Abstract
Introduction:
Lung cancer (LC) has been one of the most commonly diagnosed cancers worldwide, both in terms of new cases and mortality. Exponential growth of economic and industrial activities in recent decades in the Delhi urban area may have increased the incidence of LC. The primary objective of this study was to evaluate the time trend according to gender.
Method:
LC incidence data over 25 years were obtained from the population based urban Delhi cancer registry. Joinpoint regression analysis was applied for evaluating the time trend of age-standardized incidence rates. The age-period-cohort (APC) model was employed using Poisson distribution with a log link function and the intrinsic estimator method.
Results:
During the 25 years, 13,489 male and 3,259 female LC cases were registered, accounting for 9.78% of male and 2.53% of female total cancer cases. Joinpoint regression analysis revealed that LC incidence in males continued to increase during the entire period, a sharp acceleration being observed starting from 2009. In females the LC incidence rate remained a plateau during 1988-2002 and thereafter increased. The cumulative risks for 1988-2012 were 1.79% and 0.45%. The full APC (IE) model showed best fit for an age-period-cohort effect on LC incidence, with significant increase with age peaking at 70-74 years in males and 65-69 years in females. A rising period effect was observed after adjusting for age and cohort effects in both genders and a declining cohort effect was identified after controlling for age and period effects.
Conclusion:
The incidence of LC in urban Delhi showed increasing trend from 1988-2012. Known factors such as environmental conservation, tobacco control, physical activity awareness and medical security should be implemented more vigorously over the long term in our population.
Keywords: Lung cancer, incidence, population based cancer registry, annual percentage change
Introduction
Lung cancer (LC) is a leading cancer site for incidence as well as for mortality among the men and ranked third among women across all site of cancer worldwide (Ferlay et al., 2015). The estimated incidence of lung cancer was 1.8 million new cases in 2012, both sexes combined, approximately 12.9% of total cancer cases. This cancer remains most common in men worldwide (1.2 million, 16.7% of total cancer cases). Incidence rate and pattern of LC varies according to the geographically area, ethnicity and duration and intensity of smoking as well as environmental factors of the concerned region. The less developed regions had high number of cases than developed region and contributed 63% of LC cases in males and 54% in females respectively. The age-adjusted incidence rate (AAR) of males is highest in Central and Eastern Europe (53.5 per 100,000) and East Asia (50.4 per 100, 000) on the other hand lowest incidence rate in middle and western area (2.0 and 1.7 per 100, 000). World-wide males have higher incidence of LC than females with sex ratio 2.1:1 (Ferlay et al., 2015). The AAR among the females is highest in Northern America (33.8 per 100, 000) and North Europe (23.7 per 100,000) and lowest in western and middle Africa (1.1 and 1.8 pr 100, 000, respectively) (Ferlay et al., 2015).
In India LC is also the leading diagnosed cancer among men and had seventh commonly diagnosed cancer among women (Ferlay et al., 2014). The estimated lung cancer cases in India during 2012 was 53,728 in males and 16,547 in females totaling 70,275 with AAR of incidence is 11.0 per 100,000 in males and 3.1 per 100,0000 in females, and overall 6.9 per 100,000 (Ferlay et al., 2014). The LC cases in Delhi urban during 2012 was 1064 in men and 295 in women, totaling 1,359, with AAR 17.29, and 4.91 per 100,000 in males and females respectively and had ranked first in males and ranked seventh in females. The population-based cancer registries (PBCRs) in India revealed increasing trend of LC incidence in males with estimated annual percentage change (EAPC) varying from 1.0% to 4.8% except one registry (Mumbai) which had decreasing trend (-1.7%). In females, all PCBRs showed increasing trend of LC, the EAPC change varying from 0.8% to 4.1% (NCRP, 2013).
The time trend analysis by age-period-cohort analysis helps in investigating the in-depth effects of age, period, and cohort on LC incidence and an opportunity to form the hypothesis regarding effective measures to address LC incidence in Delhi urban region. Age-Period-Cohort (APC) analysis helps to investigate the effect of three dimensions independently and may be helpful in effective action plans for controlling the LC. The objective of the present research is to investigate the time trends analysis of lung cancer from 1988-2012 according to gender in Delhi urban using APC and joinpoint regression analysis. Lastly estimated cumulative risk (0-74) and life term risk of LC were also calculated for 25 years period.
Materials and Methods
Data source
The study is based on data collected by Delhi PBCR, one of the oldest cancer registries of India. The data on LC incidence during 1988 through 2012 are obtained from this registry and does not need ethical requirement for publication of this research paper. This registry fulfilled the IARC quality standard and inclusion in their volumes of five continent series (Curado et al., 2007; Forman et al., 2014). The international classification of disease (ICD) code 9th revision with code 162 used for the time period 1988-2000 and 10th revision with code C33 and C34 was utilized for the period 2001-2012. According to 2011 census, the total population of Delhi was 1,67,53,235 with 97.5% of people living in urban area. The registry covers only the urban population of Delhi which is 1, 63, 33, 946 with 87, 49, 440 males and 75, 84, 506 females as on 2012. The population at risk during of respective year was estimated from the 1981, 1991, 2001, 2011 census reports of India using difference distribution method (Takiar and Shobana, 2009).
Statistical analyses
Age-adjusted incidence rates (AAR) per 100, 000 was determined by direct method using the world standard population as reference (Esteve et al., 1994). The cumulative risk (0-74) and life term risk was computed for both gender for each year. Life term risk expressed as one out of the total number of persons who develop cancer during any time during the whole life span.
The likelihood of lung cancer in younger age groups under 20 is rare and incidence of LC cases after 74 years were given in open-ended interval and difficult to get in 5-year interval. Joinpoint regression model using joinpoint regression programme (Version 4.4.0.0 - January 4, 2017) Kim et al., (2000) was applied to study the time trends on the truncated AAR (20-74), and on truncated incidence rate ASR (20-74) according to gender. The trend coefficient was considered significant increase or decrease if P-value was less than 5%.
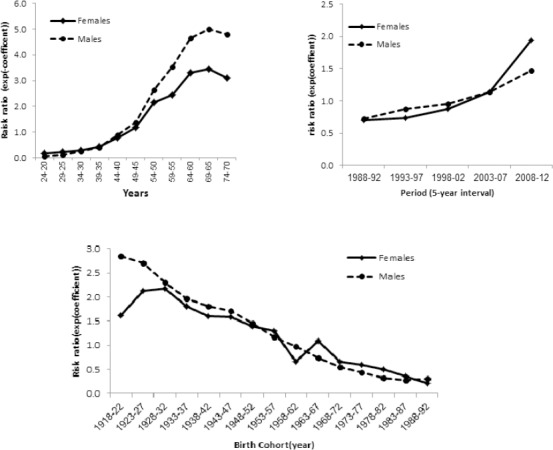
APC analysis used to evaluate the age, period and cohort effect and most frequently applied in the demography, sociology, and epidemiology. The patients were classified into eleven 5-year intervals from 20-24 to 70-74 years, study period was divided into five equal periods cohort (five-year) 1988-1992, 1993-1997, 1998-2002, 2003-2007, and 2008-2012 and 15 birth cohorts starting from 1918-1922 to 1983-1988. The basic APC model is log-linear regression and have the following form (Mason et al., 1973).
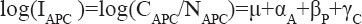
Where IAPC: Expected incidence rate in APC-group, CAPC represents number of Expected cases: NAPC is population at risk; μ is intercept, αA is regression coefficient of age A=1, …a; βP is regression coefficient of period P = 1,…,p; γC is regression coefficient of cohort C= 1,…,c. APC analysis suffers from the identification problem causing the prefect collinearity between the age, period and cohort (C=A+P) which precludes a unique solution. Several solutions have been proposed in the literature to tackle this issue (Holford et al., 1991). The one most frequently used method is adding the constraint but this requires prior knowledge about the outcome under the consideration and model solution varying to the constraint chosen. The Intrinsic estimator (IE) method proposed by Yang et al. do not require the prior knowledge of constraint and model determine by Moore-Penrose generalize inverse. many studies have been shown that IE method provides meaningful and reliable results and coefficient can be interpreted like conventional regression coefficient (Yang et al., 2008) and use minimum assumptions. The approach also not free from the limitation and validity of this approach has been recently challenged (O’Brien, 2011). This approach compared each category value with mean of respective dimension variable, thus in IE overall mean is reference category. Various series of log-linear model assuming LC incidence cases in age-period specific followed Poisson distribution and log ‘link’ function were fitted considering exposure as age-period specific population. This includes one-factor model; only age, Age+drift, two factor models; Age-period, age-cohort, and APC-IE model. A formal decision of fitting of APC models to trend is discussed elsewhere (Cyayton and Schifflers, 1987).
The overall model goodness-of-fit was tested by the chi-square test and sub-models comparison was evaluated by the difference of residual deviance and assessed as a approximate chi-square distribution using change in model degree of freedom to obtains the P-value. Akaike information criteria (AIC) with parameter penalty was also considered to assess the better fit of model. Statistical analysis were performed using STATA ver. 12.0 (Stat Corp, LP, College Station, TX, USA) and “apcie” command was applied to generate APC-IE model. In addition, the MS-office Excel was used to prepare the diagrams. Over-dispersion of Poisson model was checked by dividing the residual deviance by residual degree of freedom, closer to one indicates no over dispersion and also shows better fit of data.
Results
During the last 25 years period 1988-2012, a total of 16748 lung cancer cases (13,489 males and 3,259 females) were registered in Delhi registry, accounting 9.78% of male’s cancer and 2.53% female’s cancer. The average AAR was 14.02 among males and 3.7 among females per 100, 000 population, respectively Table 1. The median age of diagnosing the LC in Delhi was 62 years for males and 60.5 years for females, respectively Table 2. The AAR (0-74) for males and females LC in Delhi increases over the period of 25 years. In males, overall LC incidence AAR increased by 52.3% with AAR 11.35 per 100,000 in 1988 to 17.29 per 100,000 in 2012, while higher increase 72.9% was observed in females with 2.84 per 100,000 in 1988 to 4.91 per 100,000 in females (Table 1). The respective truncated AAR (20-74) values for males and females were 18.42 and 4.22 in 1988 and increased to 25.35 and 7.53 in 2012, respectively.
Table 1.
AAR(0-75+), Truncated AAR(20-74), Cumulative Risk(0-74) and LTR of Developing the Lung Cancer in Males and Females
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Year | AAR | Truncated AAR | Cumulative risk (0-74) | LTR | AAR | Truncated AAR | Cumulative risk (0-74) | LTA |
| 1988 | 11.35 | 18.42 | 1.56 | 65 | 2.84 | 4.22 | 0.29 | 345 |
| 1989 | 12.41 | 18.86 | 1.53 | 66 | 2.26 | 3.64 | 0.29 | 345 |
| 1990 | 13.35 | 20.23 | 1.72 | 59 | 3.43 | 5.81 | 0.44 | 227 |
| 1991 | 13.36 | 20.78 | 1.69 | 60 | 2.37 | 3.95 | 0.31 | 323 |
| 1992 | 12.58 | 19.94 | 1.66 | 61 | 3.19 | 5.21 | 0.44 | 227 |
| 1993 | 15.07 | 23.81 | 2.01 | 50 | 2.99 | 4.57 | 0.34 | 294 |
| 1994 | 13.6 | 20.19 | 1.7 | 59 | 2.9 | 4.72 | 0.41 | 244 |
| 1995 | 11.03 | 17.07 | 1.37 | 73 | 2.72 | 4.32 | 0.36 | 278 |
| 1996 | 13.83 | 21.69 | 1.83 | 55 | 2.91 | 4.34 | 0.34 | 294 |
| 1997 | 14.05 | 21.9 | 1.8 | 56 | 2.94 | 4.68 | 0.34 | 294 |
| 1998 | 12.61 | 19.58 | 1.62 | 62 | 3.1 | 4.77 | 0.42 | 238 |
| 1999 | 12.31 | 18.53 | 1.59 | 63 | 3.57 | 5.8 | 0.46 | 217 |
| 2000 | 12.32 | 18.45 | 1.56 | 65 | 2.89 | 4.34 | 0.35 | 286 |
| 2001 | 13.88 | 21.33 | 1.77 | 57 | 2.99 | 4.5 | 0.36 | 278 |
| 2002 | 14.62 | 22.82 | 1.93 | 52 | 3.24 | 4.98 | 0.37 | 270 |
| 2003 | 13.2 | 20.55 | 1.65 | 61 | 3.42 | 5.26 | 0.41 | 244 |
| 2004 | 13.03 | 19.57 | 1.65 | 61 | 3.53 | 5.43 | 0.42 | 238 |
| 2005 | 14.32 | 21.59 | 1.8 | 56 | 3.58 | 5.68 | 0.43 | 233 |
| 2006 | 14.23 | 21.57 | 1.81 | 56 | 3.63 | 5.65 | 0.46 | 217 |
| 2007 | 13.68 | 20.87 | 1.72 | 59 | 3.6 | 5.63 | 0.46 | 217 |
| 2008 | 14.06 | 21.8 | 1.83 | 55 | 4.23 | 6.57 | 0.5 | 200 |
| 2009 | 13.94 | 20.77 | 1.76 | 57 | 4.16 | 6.31 | 0.48 | 208 |
| 2010 | 15.65 | 23.45 | 2.02 | 50 | 4.4 | 6.72 | 0.56 | 179 |
| 2011 | 16.81 | 25.06 | 2.15 | 47 | 5.26 | 7.96 | 0.63 | 159 |
| 2012 | 17.29 | 25.35 | 2.19 | 46 | 4.91 | 7.53 | 0.61 | 164 |
| 1988-2012 | 14.02 | 21.3 | 1.79 | 56 | 3.7 | 5.64 | 0.54 | 222 |
AAR, Age-Adjusted Rate; LTR, Life time risk
Table 2.
Comparison of Lung Cancer Statistics -2012 between Delhi and All India
| Variable | Delhi* | India* | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Crude Incidence rate | 11.8 | 3.7 | 8.3 | 2.7 |
| Age-standardized Rate(World adjusted) | 17.29 | 4.9 | 11 | 3.1 |
| Cumulative risk (0-74) | 2.19 | 0.61 | 1.36 | 0.37 |
| Life time risk | 46 | 164 | 74 | 270 |
| Male to Female ratio | 3.61 | 1 | 3.25 | 1 |
| % of LC <40 years | 3.38% | 4.75% | 2.46% | 6.72% |
| % of LC 40 to 60 years | 36.00% | 42.03% | 35.05% | 42.35% |
| % of LC >60 years | 60.62% | 53.22% | 62.49% | 50.94% |
| Maximum LC incidence (%) achieved | 60-64 (20.5%) | 60-64 (20%) | 60-64 (16.5%) | 60-64 (15.6%) |
| Median age at diagnose | 62 | 60.5 | 63.3 | 59.8 |
Source, GLOBOCAN--2012 and Delhi Cancer Registry
The estimated average cumulative incidence risk (0-74 years) was found to be 1.79% of the male population and 0.45% of female population. To express this average into life time risk to develop the LC is 1 in 56 during the life time in men and 1 out of 222 in women providing they continue to experience similar age-specific rate in future. (The cumulative incidence risk and life time risk to develop the LC in absence of any competing risk from 1988 to 2012 are given in Table 1).
Results of Joinpoint regression
The joinpoint analysis was done on both truncated AAR and ASR incidence rates. The joinpoint analysis was done on ASR so that trend of ASR could be correlated with APC model results later. In males trend of AAR has no joinpoint and demonstrated steady growth with EAPC 0.89 (95% CI: 0.42%-1.37%, P=0.001). Significant positive temporal trend showed the trend of AAR among males was increased during the 25 years period. However, the trend for truncated ASR incidence rate had one joinpoint in 2009, the EAPC for 1988-2009 was 0.21(95%CI: -0.33 to 0.76, P=0.48) reflecting a plateau during this period and trend accelerated between 2009-2012 with an EAPC 16.60(95% CI:9.10 to 24.61, P<0.001) respectively (Figure 1).
Figure 1.
Age-Specific Lung Cancer Incidence Rate by Gender in Delhi Urban, 1988-2012.
The one joinpoint was observed in females AAR in 2002, the EAPC from 1988-2002 was 0.45 (95% CI: -1.16 to 2.09, P=0.570) and in 2002-2012 it was 4.58 (95% CI: 3.01-6.18, P<0.001) showed AAR remain consistent during 1988-2002 and increased significantly in recent decade. The joinpoint analysis of ASR also suggested one jointpoint in 2001 and the corresponding EPAC of ASR was 0.47(95% CI: -1.70 to 2.68, p=0.66) and 5.45(95% CI: 3.46 to 7.29, P<0.001) (Figure 1). For both AAR and ASR the trend represented a significant during recent 10 years period.
Descriptive period effect
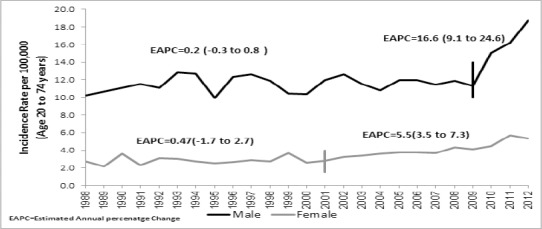
The age-specific of LC incidence rates for five periods of five-year interval for males and females is displayed in Figure 2a and b respectively. There is a non-linear increase in LC incidence rate according to age in all the five periods and incidence increased with steady speed after 40 years of age for both the genders and achieved the peak at 70-74 years in males and at 65-69 in females. Among the males, the pattern of incidence is approximately similar in all five periods from 20 to 60 years of age that showed LC incidence rate increases with age regardless of period. The LC incidence for age 60 year old and above showed an increasing trend with period. The increase of LC incidence was more in recent period 2008-2012.
Figure 2.
a) Age-specific males LC incidence rate in different periods,1988-2012, (b) Age-specific females LC incidence rate in different periods, 1988-2012.
The age pattern of LC incidence rate is different in females and found lower incidence rate compared to males. The period 2008-2012 showed higher ASR rate as compared to other four periods and the difference start increasing from age 45 years onwards. In this period the ASR increased from 2 in 40 years to 35 in 70 years of age.
Results of Age-Period-Cohort Analysis
The APC full model explained the best variation in the LC incidence rate in both the gender. The period and cohort effects represents significant non-linear trend in males as well as females. In males cohort had higher non-linear effect than period and in females period had higher non-linear effect than cohort (Table 3). For both gender APC-IE model had residual deviance roughly equal to residual degree of freedom indicate good fitness of model and also there is no over-dispersion and Poisson with log link function seems correct for this data.
Table 3.
Comparison of Nested APC Models, Ages 20-74 for Lung Cancer in Delhi-1988-2012
| Age-Period-Cohort Models | Males aged 20-74 years | |||||||
|---|---|---|---|---|---|---|---|---|
| DOF | Residual deviance | AIC | Model comparison | Comparison effect | Change in deviance (~Chi-square) | DOF | P-value$ | |
| Age(A) | 44 | 132.24 | 9.35 | |||||
| Age + drift (AD) | 43 | 114.92 | 9.07 | A vs AD | Drift | 17.32 | 1 | <0.001 |
| Age-Period (AP) | 40 | 99.56 | 8.9 | AD vs AP* | Non-linear period | 15.36 | 3 | 0.0015 |
| Age-Cohort (AC) | 30 | 54.96 | 8.45 | AD vs AC* | Non-linear cohort | 59.96 | 13 | <0.001 |
| Age-Period-Cohort (IE) | 27 | 34.73 | 8.19 | AP vs. APC AC vs. APC |
Non-linear cohort Non-linear period |
64.83 20.23 | 13 3 | <0.001 <0.001 |
| Female aged 20-74 years | ||||||||
| Age(A) | 44 | 241.56 | 10.18 | |||||
| Age + drift (AD) | 43 | 97.97 | 7.6 | A vs AD | Drift | 143.59 | 1 | <0.001 |
| Age-Period (AP) | 40 | 55.86 | 6.95 | AD vs AP* | Non-linear period | 42.11 | 3 | <0.001 |
| Age-Cohort (AC) | 30 | 73.92 | 7.64 | AD vs AC* | Non-linear cohort | 25.05 | 13 | 0.023 |
| Age-Period-Cohort (IE) | 27 | 27.4 | 6.9 | AP vs. APC AC vs. APC |
Non-linear cohort Non-linear period |
28.46 46.52 | 13 3 | 0.007 <0.001 |
DOF, Degree of freedom; AIC, Akaike Information Criteria (-2LL+2k)/N [small the value of AIC, the better the model fit]; LL, Log-likelihood value; k, number parameters and N is number of total; IE, Intrinsic Estimator
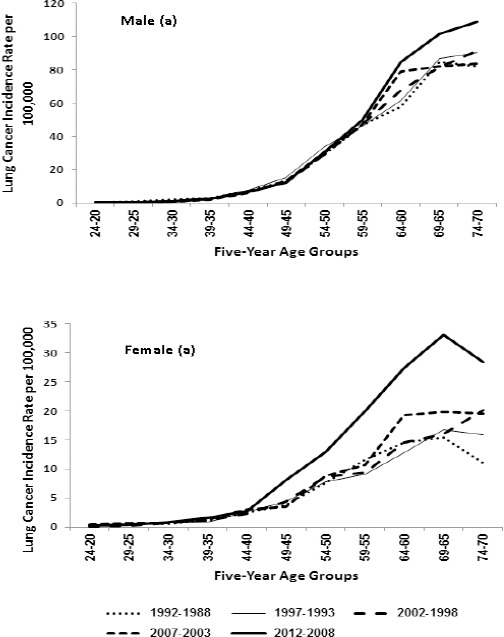
The results of coefficient estimation of full APC (IE) model were presented in Table 3 among the males and females. Figure 3 displays the risk ratio (exponeitated of coefficient) for individual effect of age, period, and cohort. The results confirms descriptive analysis presented earlier in the results section. The Figure 3a depicts the age effect after adjusting the period and cohort effect. LC incidence monotonically increasing after 40 and had a peaked at 65-69 years in both males and females as compared to average value. The male showed consistently higher risk of LC incidence then females, albeit the pattern of age effect was similar till 50 years between males and females. The gap between gender increases as age progresses (Figure 3a). Relative risk of one-age group compared to another age group was computed to get age-effect more interpretable. The RR of 65-69 year old group and 70-74 year old group was 81.89 times [exp(coefficient) of 65-69 years/exp(coefficient of 20-24)] and 74.41 times as compared to 20-24 years old group. While in the females these values were 18.29 times and 16.52 times respectively.
Figure 3.
APC-IE Estimate Effect of Lung Cancer Incidence for Age 20-74 Years in Delhi 1988-2012. a) Age effects on Lung cancer incidence, by gender. b) Period effect of lung cancer incidence, by gender c) Cohort effect on lung cancer incidence, by gender
The Period effect on LC incidence rate is presented in the Figure 3b and agree with period graph as shown in Figure 2 that displayed the LC truncated ASR and joinpoint regression analysis to examine the trend over 25 years. The period effect revealed approximate similar linear pattern till 2007 for both gender and found a sharp increase in females than males during 2008-2012 period. The risk of lung cancer incidence among males in 2008-2012 was 2.02 times the risk of LC incidence in 1988-1992, in other words risk LC incidence increased by 102% indicating the annual average growth 4.12%. However, in females the risk of LC incidence increased by 174% with annual average growth 6.96%.
Finally, Figure 3c depicts the net cohort effect on LC incidence between males and females born in different birth cohort where they exhibited slightly different at early birth cohorts. Among the males the incidence rate is consistently decreasing from birth 1918 to 1987 demonstrating improvement across the Cohort and in females there is increase initially in birth cohort 1918-1922 to 1928-1932 and then decreasing upto birth cohort 1958-1962 and observed sharp increase in birth cohort 1963-67 and then decrease till birth cohort 1988-1992. Except, initially three cohort the pattern are similar in both sexes. The RR of birth cohorts 1988-1992 was 0.11 (0.326/2.843) in males and indicating the incidence decreased by 88.5% from 1918-1922 to 1988-1992 in females as compared with oldest birth cohort 1918-1922.
Discussion
This study revealed the increasing trend of LC incidence in Delhi urban population over the last 25 years. The increasing trend of LC are largely attributable to increase in life expectancy, smoking (active or passive), indoor and outdoor air pollution, and may be other known risk factors such as physical inactivity, family history of LC, history of tuberculosis and inflammatory respiratory disease. The smoking, physical inactivity and air pollution are preventable factors and can be controlled with honestly implementing suitable strategies and by increasing awareness about drastic effect of these factors on LC.
The crude incidence rate and AAR of Delhi region is comparatively higher than all India level in both sexes. The cumulative risk (0-74) was also higher in Delhi region than all India level for both males (2.19% vs. 1.36%) and in females (0.61% vs. 0.37%). Comparison of LC statistics between Delhi and India was presented in Table 2.
The median age at diagnosis in males found in the present study was similar to some Arab countries such as Jordan (62-64 years), Omen (64 years), Tunisia (64 years) (Missaoui et al., 2011) and found lower median age of diagnose from Australia(70.5 years) (Australian Institute of Health and Welfare and Cancer Australia, 2011), Iran (69 years) (Al-Hashimi MMY and Wang, 2014). The median age at diagnosis in females was approximate equal to Tunisia (61 years) and lower than Australia (70 years) (Australian Institute of Health and Welfare & cancer Australia, 2011).
The incidence of LC is relatively less among the younger population (<40 years) than older age group (Tas et al., 2012). The average percentage of LC incidence in present study was 3.4% and 4.7% in males and females respectively that agreed with the literature percentage (Tas and Keskin, 2012).
Worldwide estimated cumulative risk (0-74) of LC in males is 3.9% and 1.6% in females which leads to probability of estimates that one in 26 males and one in 63 females will have LC during their life time in 2012 (Ferlay et al., 2014). In Delhi, the present study showed one in 46 for males and one in 164 for females will be diagnosed with LC during their life time in the year 2012. However, these values were lower than Mumbai one in 74 and one in 286 in males and in females respectively (Agarwal et al., 2008).
The incidence of LC varies according to the geographical variation and ethnicity globally as well as between the region within the same country like India that had highest AAR incidence in males at Mizoram 32.6 per 100, 000 to lowest AAR rate at Dibrugrah district 5.0 per 100,000) while the similar AAR in females was 29.3 and 2.3 respectively (NCRP, 2016). Recent study of tobacco related survey revealed that north-east population is most vulnerable with respect to tobacco use in India, Mizoram state had ranked second in smoking among the males and ranked first among the females while Meghalaya state had highest prevalence of smoking among males. Illiteracy and unawareness about the effect of smoking on health is most common cause of smoking in these state. The prevalence rate of smoking was maximum in 50-54 years of age among the males and among the females the prevalence was found maximum in 65+years (Singh et al, 2014). The trend of Age adjusted rate among females was increasing over the time and mainly due to the approximately half of the state in India are experiencing an increase in smoking among the females (Singh and ladusingh, 2014).
The incidence of LC is closely associated with age for both males and females in APC model. The gap between males and females starts increasing from age 50 years and increase along with age. The latency period of LC in smoking is around 30 years for both males and females and median age of smoking initiation in India is 20 years and prevalence of smoking ratio (males:females) is 7:1(Mishra et al., 2016). The increase in gap between males and females may be due to this reason. There may be other factors for increasing the difference between the gender are outdoor air population, occupational exposure, indoor pollution, second hand smoking. Males are more vulnerable to first two factors while females are for last two factors.
The period effect showed significant effect and attributable to several reasons. Recent two five years period showed higher risk ratio in both the gender. Females showed higher risk than males independent of age. This may due to change in coding scheme in Delhi PBCR from 2001 onwards, increase in screening facilities, spread of awareness about LC, in smoking prevalence, and also increase in outdoor pollution level. Study showed that smoking is predominant cause of LC and likelihood of LC increase with duration of smoking and intensity of smoking per-day and male smoker had 9-fold to 10-fold likelihood of LC compared to non-smoker(Dela Cruz et al., 2011). The Delhi had 14th rank in males with 48.2% of all cancers are tobacco related cancer in males while females had 24th rank with 11.6% (NCRP, 2016). A recent survey showed the age-standardized prevalence (ASP) of smoking among males in all India of age-15-69 years fell from 27% in 1998 to 24% in 2010 however ASP in Delhi at similar age-group rose significantly by 220% (13.9% to 31.4%) during the same period (Mishra et. al., 2016). The highest ASP of any smoking in men aged 15-69 was in illiterate men in both 1998 and 2010. The ASP of any smoking in women aged 15-69 years rose from 1.4% to 2.7% in 2010 (Mishra et al., 2016). Control of smoking and use of tobacco product is big challenge in India because most of the stakeholder of these products are illiterate population (Kaur and Jain, 2011). The increase in tobacco products among the women are growing not only in India but also in other developing countries and have a big challenge for the policymakers to control.
Nonetheless, LC in never smoker is also cause of concern, albeit the data on never smoker is limited and study showed women are more likely than men to have non-smoking associated LC. In India, the incidence of LC among the non-smokers are increasing (Krishnamurthy et al., 2014), however not much studies were found on non-smokers but now risk of other factors need to be investigated (Noronha et al., 2012). Economic growth led better lifestyle but this also led to change in unhealthy behaviours like reduction in physical activity and increase in high calorific food during the last two decades. The recent survey on physical inactivity in Indian major states revealed that 58.7% males and 71.2% of females were inactive (MET<600 met-minutes). The physical activity reduces likelihood of LC in both the gender(Emaus and Thune, 2011). There is urgent need to increase the awareness on physical activity because physical inactivity are also associated with other cancers and chronic diseases. Announcing the 21st June as the international “Yoga” day worldwide in 2015 is first step to increase the awareness by Indian Government. The yoga not only improves the physical fitness but also increase mental strength.
Air pollution level caused by vehicular emission, and expansion of industries are increasing day by day and degrading the environment very fastly in Delhi and nearby areas. Various studies has been be conducted to find the association of air pollution and LC. The meta analysis showed a significant association between the risk of LC incidence and per 10 µg/m3 increase in PM10 would increase the LC incidence by 44% (Raaschon-Niclsen at al., 2013). Another meta analysis of traffic air-pollution and LC showed exposure to air pollution significantly increase the likelihood of LC (nitrogen dioxide OR=1.06 (0.99-1.13), nitrogen oxide OR=1.04(1.01-1.07), Sulfur dioxide OR=1.03 (1.02-1.05), and fine particular matter OR 1.11 (1.00-1.22) (Chen G et al., 2015). As per the recent World Health Organization Delhi has exceeded the PM10 level by almost 11 times and reached at 229 µg/m3 (WHO, 2016). The capacity of lung function was reduced in 40.3% individuals in Delhi urban area as compared to 20.1% in control group (Rizwan et al., 2013). Several steps have been taken to reduce the air pollution in Delhi urban during the last decade by Central Pollution Control Board (CPCB) and the National Green Tribunal (NGT) government agencies to monitor and control of the pollution level in India. However, steps taken by CPCB and NGT seems to be inadequate to reduce the air pollution levels. Strict implementation, stringent regulation, awareness about health hazards due to air pollution, and suitable alternatives of transport to reduce the vehicular congestion are necessary to improve the air quality.
The present study had LC incidence data available for Delhi urban area only. Since in-migration rate from various states in Delhi is very high, the exact cohort effect is difficult to discern because within a country there is large variation in LC incidence (NCRB, 2016). The net migrants steadily increased from 6.34 laks during 1961-71 to 17.64 lakhs during 1991-2001 (NCRPB, 2016). The histological type trend of LC cancer incidence cannot be determined due to non availability of adequate data.
In conclusion, the trend of LC is increasing in both males and females during the 25 years period in Delhi urban area and continuing peak in age 60 and 70 years in both the gender. The respective government should increase the smoking prevention and cessation programs and strictly adhere the rules made for controlling the smoking, and increase awareness in illiterate population about the consequence of smoking. Although, Indian government restricted the sale of smoking tobacco to less than 18 years individual in India and also educating by pasting the picture and warning on cigarette pack but still stringent implementation is lacking. Tobacco control and air pollution control should be given high priority, this will reduce not only LC but also other various chronic diseases. Air pollution is an important issue in the recent years and need urgent attention to reduce the future LC incidence burden.
References
- 1.Agarwal N, Yeole BB, Ram U. Lifetime risk and trends in lung cancer incidence in greater Mumbai. Asian Pac J Cancer Prev. 2008;10:75–82. [PubMed] [Google Scholar]
- 2.Al-Hashimi MMY, Wang XJ. Trend analysis of lung cancer incidence rates in Ninawa province, Iraq, from 2000 to 2010 - Decrease and recent stability. Asian pac J Cancer Prev. 2014;15:385–90. doi: 10.7314/apjcp.2014.15.1.385. [DOI] [PubMed] [Google Scholar]
- 3.Australian Institute of Health and welfare and cancer Australia. Cancer series no. 64. Cat. no. CAN 58. Canberra: AIHW; 2011. Lung cancer in Australia:a overview. [Google Scholar]
- 4.Census of India. D-series Migration tables. 2001. [assessed on 14 October 2017]. http://censusindia.gov.in/Tables_Published/D-Series/Tables_on_Migration_Census_of_India_2001.aspx .
- 5.Chen G, Wan X, Yang G, Zou X. Traffic-related air pollution and lung cancer:A meta-analysis. Thoracic Cancer. 2015;6:307–18. doi: 10.1111/1759-7714.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curado MP, Edwards B, Shin HR, et al. IX IARC scientific publication no. 164. lyon, France: IARC; 2007. Cancer Incidence in five continets Vol; p. 229. [Google Scholar]
- 7.Cyayton D, Schifflers E. Models for temporal variation in cancer rates I:Age-period and age-cohort models. Stat Med. 1987;6:449–67. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 8.Cyayton D, Schifflers E. Models for temporal variation in cancer rates II:Age-period and age-cohort models. Stat Med. 1987;6:469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 9.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer:Epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–44. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emaus A, Thune I. physical activity and lung cancer prevention. Recent Results Cancer Res. 2011;186:101–33. doi: 10.1007/978-3-642-04231-7_5. [DOI] [PubMed] [Google Scholar]
- 11.Esteve J, Benhamou E, Raymond L. Editors IV. France:IARC scientific publications No 128; Statistical methods in cancer research. Descriptive Epidemiology. 1994:313. [PubMed] [Google Scholar]
- 12.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 13.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.1, Cancer Incidence and Mortality Worldwide:IARC Cancer Base No. 11 [Internet] Vol. 2014. Lyon, France: International Agency for Research on Cancer; 2012. [accessed on 16/01/2017]. Available from: http://globocan.iarc.fr . [Google Scholar]
- 14.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in five continents Vol. X IARC scientific publication no. 164. lyon, France: IARC; 2014. p. 550. [Google Scholar]
- 15.Holford TR. Understanding the effect of age, period, and cohort on incidence and mortality rates. Annu Rev Publ Health. 1991;12:425–57. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 16.Kaur J, Jain DC. Tobacco control polices in India:Implementation and challenges. Indian J Public Health. 2011;55:220–7. doi: 10.4103/0019-557X.89941. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurthy A, Vijayalakshmi R, Gadigi V, Ranganathan R, Sagar TG. The relevance of non-smoking associated of lung cancer in India:A single centre experience. India J Cancer. 2012;49:82–8. doi: 10.4103/0019-509X.98928. [DOI] [PubMed] [Google Scholar]
- 19.Mason KO, Mason WM, Winsborough HH, Poole WK. Some methodological issue in cohort analysis of archival data. Am Social Rev. 1973;38:242–58. [Google Scholar]
- 20.Mishra S, Joseph RA, Gupta PC, et al. Trends in bidi and cigarette smoking in India from 1998 to 2015, by age, gender and education. BMJ Glob Health. 2016;1:e000005. doi: 10.1136/bmjgh-2015-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missaoui N, Hmissa S, Landolsi H, et al. Lung cancer in central Tunisia:Epidemiology and clinicopathological features. Asian Pac J of Cancer Prev. 2011;12:2305–09. [PubMed] [Google Scholar]
- 22.National Cancer Registry Programme. Bangalore, India: Time trends in cancer incidence rates 1982-2010; 2013. p. 22. [Google Scholar]
- 23.National Cancer Registry Programme. Bangalore, India: Three year report of population based cancer registries 2012-2014 Report of 27 PBCRs in India; 2016. pp. 9–16. [Google Scholar]
- 24.Noronha V, Dikshit R, Raut N, et al. Epidemiology of lung cancer in India:Focus on the difference between non-smokers and smokers:A single centre experience. India J Cancer. 2012;49:74–81. doi: 10.4103/0019-509X.98925. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien RM. Constrained estimators and age-period-cohort models. Social Methods Res. 2011;40:419–52. [Google Scholar]
- 26.Rizwan SA, Nogkynrih B, Gupta SK. Air pollution in Delhi:Its magnitude and effects on health. Indian J Community Med. 2013;38:4–8. doi: 10.4103/0970-0218.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raaschou-Nielsen O, Anderson ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European Cohorts:prospective analysis from Europe study of cohorts for air pollution effects (ESCAPE) Lung Oncol. 2013;14:813–22. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 28.Singh A, ladusingh L. Prevalence and determinants of tobacco use in India:Evidence from recent global adult tobacco survey data. PLoS One. 2014;12:e114073. doi: 10.1371/journal.pone.0114073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takiar R, Shobana B. Cancer Incidence rates and the problem of denominators - a new approach in Indian cancer registries. Asian Pacific J cancer Prev. 2009;10:123–6. [PubMed] [Google Scholar]
- 30.Tas F, Keskin S. Age specific incidence ratio of lung cancer in Turkey:Lung cancer in older people is increasing. Arch Gerontal Geriatrics. 2012;55:276–8. doi: 10.1016/j.archger.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Weiss W. Cigarette smoking and lung cancer trends. A light at the end of the tunnel? Chest. 1997;111:1414–6. doi: 10.1378/chest.111.5.1414. [DOI] [PubMed] [Google Scholar]
- 32.WHO. World health organization global urban ambient air pollution database. 2016. Retrieved June 10, 2017 from http://www.who.int/phe/health_topics/outdoorair/databases/cities/en/
- 33.Yang Y, Schulhofer-Wohl S, Fu WJ, Land KC. The intrinsic estimator for age-period-cohort analysis:what it is and how to use it. Am J Sociol. 2008;113:1697–736. [Google Scholar]


