Abstract
Cruciferous vegetables are a rich source of glucosinolates that have established anti-carcinogenic activity. Naturally-occurring glucosinolates and their derivative isothiocyanates (ITCs), generated as a result of their enzymatic degradation catalysed by myrosinase, have been linked to low cancer incidence in epidemiological studies, and in animal models isothiocyanates suppressed chemically-induced tumorigenesis. The prospective effect of isothiocyanates as anti-carcinogenic agent has been much explored as cytotoxic against wide array of cancer cell lines and being explored for the development of new anticancer drugs. However, the mechanisms of isothiocyanates in inducing apoptosis against tumor cell lines are still largely disregarded. A number of mechanisms are believed to be involved in the glucosinolate-induced suppression of carcinogenesis, including the induction of apoptosis, biotransformation of xenobiotic metabolism, oxidative stress, alteration of caspase activity, angiogenesis, histone deacytylation and cell cycle arrest. The molecular mechanisms through which isothiocyanates stimulate apoptosis in cancer cell lines have not so far been clearly defined. This review summarizes the underlying mechanisms through which isothiocyanates modify the apoptotic pathway leading to cell death.
Keywords: Cruciferous vegetables, glucosinolates, isothiocyanates, chemoprevention, apoptosis
Introduction
Glucosinolates (GLs) are anionic, hydrophilic plant secondary metabolites consisting of a thioglycosides moiety that have been isolated from vegetables of the family Brassicaceae (Cruciferae) (Wu et al., 2009). Breakdown products of glucosinolates have been identified in many cruciferous plants and comprised an important group of secondary metabolites that serve as a natural source of defence against pests (Takasugi et al., 1986). Amino acids function as precursors in the biosynthesis of glucosinolates and are modified in the following order: amino acid > N-hydroxy-amino acid > aldoxime > thio-hydoxy acid > de-sulpho glucosinolate > glucosinolate (Halkier and Du, 1997). Amino acids such as methionine, phenylalanine, tyrosine or tryptophan act as the precursors of glucosinolate synthesis that varies based on the characteristic of the R side chain in plants (Wittstock and Halkier, 2002). Glucosinolates are not detrimental to human but were hydrolysed to different type of products catalysed by plant enzyme myrosinase as shown in Figure 1. Glucosinolates are substrates of the enzyme myrosinase (β-thioglucoside glucohydrolase) which coexists in the plant, but in different compartments. When the cell structure is disrupted, they interact to form a complex leading to the production of glucose and an aglycone moiety which further hydrolyse in the presence of water to produce metabolites such as isothiocyanate (ITCs), thiocyanates and nitrile (Anderson et al., 2009). Animal studies have established that isothiocyanates are effective antagonists of chemically-induced carcinogenesis (Xiang et al., 2009).
Figure 1.
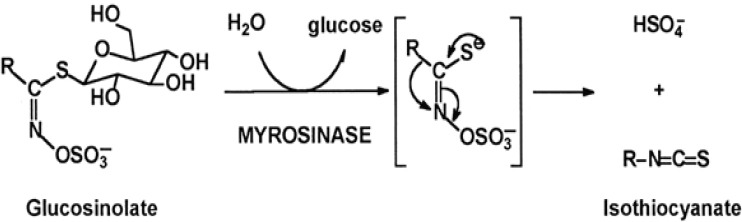
Hydrolysis of Glucosinolate by Myrosinase and Formation of Isothiocyanate (ITC). Myrosinase was expressed upon rupture of plant tissue by chewing or chopping hydrolysing glucosinolates to isothiocyantes; R represents side chains of other element in the molecules; C, carbon; S, sulphur; N-nitrogen (Xiang et al. 2009).
Numerous studies established that isothiocyanates derived from glucosinolates are biologically active compounds (Molina et al., 2013). Glucosinolates are inactive, it was structurally diverse side chains that accounts for the presence of over 130 different glucosinolates (Fabre et al., 2007). In vitro and in vivo studies have been reported focusing on health benefits of glucosinolates and their bioactive isothiocyanates such as, antimicrobial (Mithen et al., 2002), antioxidants and antifungal (Mithen et al., 1992), biopesticides (Vig et al., 2009), anticarcinogenic (Yan and Chen, 2007) and thyroidal activities (Mithen et al., 1992). Studies by Pasini et al. (2012) suggested that glucosinolates and its derivatives are relatively responsible for the spicy flavour and characteristics odor of Brassicaceae that differs from other species of the similar family.
Mechanism of action of isothiocyanates
Apoptosis or programmed cell death is a conserved cellular suicidal program to facilitate the removal of the damaged and dysfunctional cells while preserving the integrity and structure of surrounding tissue (Sangkari et al., 2012). Cell death is a widespread phenomenon that plays a vital role in cancer treatment. Cancer, a complex genetic disease triggered by mutated forms oncogenes and tumour suppressor genes that lead to alteration of death signalling pathway (Ouyang et al., 2012).
Apoptosis develop a remarkable typical morphology that occurs in three varying stages: condensation of nuclear heterochromatin, changes in cell membrane by shrinkage and changes in the orientation of intra cytoplasmic organelles (Kerr et al., 1972; Chamond et al., 1999). These morphological changes were ideal markers that allow the direct identification of cells undergoing apoptosis and other cellular mechanisms associated to apoptosis (Costa et al., 2012). Accordingly, glucosinolates were found to synthesize impressive array of metabolites which could induce apoptosis to inhibit the development of cancer (Abdull Razis and Mohd Noor, 2013b).
It should be emphasized that isothiocyanate clearly targets cancer cells of various organs through multiple apoptotic pathways leading to controlled proliferation and cancer cell death, thus this review present current knowledge on the glucosinolates hydrolysis products, namely isothiocyanates (ITCs) that exhibit promising cancer chemoprotective attributes against the most common cancer types, such as lung, prostate, breast and colon cancer by inducing apoptosis. The summary of data describing the different types of the potential glucosinolates and their common dietary sources of isothiocyanates (ITCs) are illustrated in Table 1.
Table 1.
Different Types of Potential Glucosinolates and Their Common Dietary Sources (Adapted from Verkerk et al., 2009)
| Glucosinolate (precursor) | Isothiocyanate | Structure | Dietary source |
|---|---|---|---|
| Sinigrin | Allyl Isothiocyanate (AITC) | 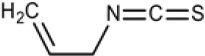 |
Broccoli, Brussels sprouts, cabbage, horseradish, mustard, radish |
| Glucotropaeolin | Benzyl Isothiocyanate (BITC) | 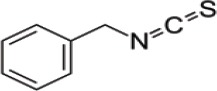 |
Cabbage, garden cress, Indian cress |
| Gluconasturtiin | Phenethyl-Isothiocyanate (PEITC) | 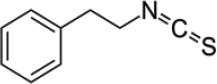 |
Watercress/ turnip |
| Glucoraphenin | Sulforaphane (SFN) | 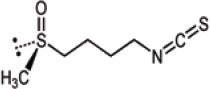 |
Broccoli, Brussels sprouts, cabbage |
| - | Phenylpropyl-ITC (PITC) | 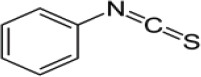 |
Horseradish |
| Glucoraphasatin | 4-methylsulfanyl-3-butenyl isothiocyanate (GRH-ITC) | 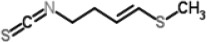 |
White radish |
| Glucoiberin | 3-Methylsulfinylpropyl-ITC | 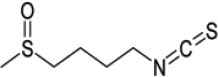 |
Brassica sp. |
Meanwhile, considerable amount of studies suggested that ITCs target on cancer cells in multiple orders that include inhibition of carcinogen-activating enzymes, induction of carcinogen-detoxifying enzymes, induction of apoptosis and arrest of cell cycle progression, as well as other mechanisms yet to be discovered in tumor cell lines (Abdull Razis and Mohd Noor, (2013b); Abdull Razis and Mohd Noor, (2015). As a result of the intense research interest in studying the protective effects of glucosinolates (GLs), this review aims to provide an overview on the series of mechanisms involved in apoptosis induction ability by GLs hydrolyzed metabolites and the signalling pathways attributed to chemoprotective properties. The review is focusing on phenethyl isothiocyanate (PEITC), sulforaphane (SFN), allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC) and their parent glucosinolates gluconasturtiin, glucoraphanin, sinigrin and glucotropaeolin.
Phenethyl isothiocyanate (PEITC)
Phenethyl isothiocyanate (PEITC), a breakdown product of naturally occurring gluconasturtiin liberated upon disruption tissue via maceration or chewing of cruciferous vegetables (Conaway et al., 2002). It was predominantly found in watercress. Over the past decades, extensive amount of epidemiological studies have been devoted to demonstrate the positive correlation between PEITC and its anti-carcinogenic effects. Large number of in vitro and in vivo investigations proposed mechanisms associated with PEITC effects on many stages of cancer development including biotransformation of phase II detoxification enzyme, mitochondrial dysfunction and oxidative stress, alteration of caspase activity, antiangiogenesis and inhibition of histone deacetylation on a panel of cancer cell lines and animal model including blood, liver (Chung et al., 1985), prostate, lung (Hecht et al., 2002), colon (Chung et al., 2000) and bladder (Nishikawa et al., 2004; Tang and Zhang, 2004).
a.Regulation of cell cycle arrest by PEITC
The cell cycle is characterized by as an orderly and tightly regulated set of stages including growth and cell division where two daughter cells are presented upon completion. Cellular division involves multiple checkpoints that evaluate the growth signals, availability of nutrients, and the integrity of DNA. Exposure of cells to PEITC has been demonstrated to modulate the cell cycle progression and regulate cell proliferation that accounts for potential anti-cancer target. Inhibition of cell cycle progression and proliferation was achieved mainly via targeting the key proteins responsible for the cell cycle progression and expression of the cyclin-dependent kinase (CDK) inhibitors (Traka and Mithen, 2009). Researches have termed the two gap as G1, phase before DNA replication, where the cell recovers from previous division, and G2, phase where completion of DNA replication to the onset of mitosis. It has been identified a family of internal signalling protein called cyclin and its associate cyclin-dependent kinases (CDK) modulates the sequential events of the cell cycle. These protein kinases serves as the signal that regulate G1 and G2 checkpoints for the transition from G1 phase to S phase and from G2 phase to M phase (Singhal et al., 2015). There was evidence indicating the mechanistic basis behind glucosinolates derived isothiocyanates associated with cell cycle arrest at various phases of cell cycle depending on the cell type used in the study. Consistent with these, Hasegawa et al., (1993) was the first to report the potency of isothiocyanates in modulating the cell cycle progression. In this case, treatment with PEITC along with other ITCs resulted in delayed cell cycle progression, therefore inhibiting the cell growth in HeLa cells at G2/M phase. Study done by Visanji et al., (2004) revealed that induced expression of the cyclin-dependent kinase (CDK) inhibitor p21waf1/cip1 caused by treatment with PEITC may play the role in cell cycle arrest at the G2 phase in Caco-2 colon cells. Report suggested that PEITC was electrophile that bind covalently to certain cysteine residues in tubulin, protein constituent of microtubules, inducing disruption in the formation and collapse of the same, which triggering cell cycle arrest in G2/M and apoptosis (Mi et al., 2009). It was interesting that PEITC down regulated the protein levels of cyclin-dependent kinase 1 (Cdk1) and Cdc25C in a dose dependent manner causing G2/M phase arrest and apoptosis in PC-3 prostate cells (Xiao et al., 2004). However, PITC, which has similar structure to PEITC, did not exhibit the same effects as this indicating that even a subtle change in the structure of the ITC may affect the efficacy of the biological activity (Xiao et al., 2004). The potential protective mechanisms of PEITC that lead to cell death is summarized in Table 2.
Table 2.
Summary of Potential Action Mechanism of PEITC in Relation to Its Apoptosis-inducing Ability Against Various Cell Lines
| Action mechanism | Cell lines/ animal model | References |
|---|---|---|
| a. Cell cycle arrest | ||
| G0/G1arrest and increase of p21 protein | DU-14 cells, Human prostate cancer | Chiao et al., 2000 |
| G0/G1arrest and increase of p21 protein | LNCaP cells, Human prostate cancer | Chiao et al., 2000 |
| G2/M phase arrest and decrease in Bcl-2 and, Bcl-X(L) | PC-3 cells, Human prostate cancer | |
| G2/M phase arrest | Hela cells | Hasegawa et al., 1993 |
| G2phase arrest, induction of CDK inhibitor p21waf1/cip1 | Caco-2 cells, human colon cancer | Visanji et al., 2004 |
| G2/M phase arrest | PC-3, Human prostate cancer | Xiao et al., 2004 |
| b. Oxidative stress | ||
| Depletion of glutathione (GSH) | Ovarian epithelial cells | Trachootham et al., 2006 |
| c. Induction of apoptosis | ||
| Induced p53 independent apoptotic pathway | PC-3 cells, Human prostate cancer | Xiao and Singh, 2002 |
| Activation of JNK, ERK and p38 | HT-29, human colon cells | Hu et al., 2003 |
| Activation of JNK | Various of cell lines | Chen et al., 1998; 2002 |
| ROS mediated apoptosis | PC-3 cells, Human prostate cancer | Xiao et al., 2006 |
| Inhibition of angiogenesis | HUVEC, human umbilical vein endothelial cells | Xu et al., 2005 |
b.Defence against mitochondrial dysfunction and oxidative stress
Mitochondria also played a prominent regulatory role in aerobic cells primarily on the integration and propagation of death signals initiated intracellular such as DNA damage, oxidative stress, starvation, as well as those induced by chemotherapeutic drugs (Kaufmann, 2000; Wang, 2001). A dissipation of the mitochondrial inner transmembrane potential (Dy) and the permeability transition (PT), by sudden increase of the inner mitochondrial membrane permeability to solutes may cause imbalance in the redox potential, inducing halt in ATP synthesis, oxidation of redox molecules namely NADH, NADPH, and glutathione and generation of harmful free radicals mediating and enhancing apoptotic pathways (Kroemer, 2000).
Regulation of glutathione (GSH) levels by isothiocyanates (ICTs) was achieved via induction of phase II biotransformation enzymes or through elevation of glutathione (GSH) levels responsible for the cellular antioxidant defencing mechanism (Traka and Mitchen, 2009). In support of this mechanism, exposure of transformed ovarian epithelial cells to PEITC showed inactivation of glutathione (GSH) antioxidant system and accumulation of ROS specifically in the transformed cells that lead to malignant cell death (Trachootham et al., 2006). Additionally, this mechanism has been studied in animal models, where following administration of PEITC in adult male F344 rats for a week demonstrated elevation in hepatic glutathione (GSH) level (Staack et al., 1998). Despite clear studies done on the ability of PEITC inducing protective antioxidant capacity against carcinogens, it was also demonstrated that ITCs themselves were able to induce cellular stress treating drug resistant cancer cells. In accordance to this, PEITC was found to be highly sensitive against both fludarabine-resistant and -sensitive Chronic Lymphocytic Leukemia (CLL) cells (Trachootham et al., 2008). As a result, PEITC showed lesser sensitivity towards normal lymphocytes and extensive sensitivity to fludarabine-resistant CLL via inducing apoptotic pathways (Trachootham et al., 2008).
c.Induction of apoptosis
Apoptosis is organized machinery that facilitates the maintenance of homeostasis in multicellular organisms. Therefore, disruption in the regulation apoptosis often lead to multiple disorders namely degenerative disorders, autoimmune disorders and cancer. In accordance of this concept, studies have revealed the contribution of isothiocyanates (ITCs) namely PEITC and sulforaphane in the regulation of apoptosis through Bcl-2 family regulation, mitochondrial release of cytochrome c, and MAPK signalling lines (Traka and Mithen, 2009). The Bcl-2 protein family is an oncogene found to be one of the key apoptosis regulators that modulate the outer mitochondrial membrane (Ulukaya et al., 2011). The antiapoptotic members Bcl-2 and Bcl-xL protect the membrane integrity and avoid the release of the cytochrome c, but their activity can be disrupted by the pro-apoptotic members Bax, Bad and Bid (Ulukaya et al., 2011). To be brief, overexpression of Bax, a proapoptotic homodimer led to apoptosis and conversely overexpression of Bcl-2 homodimers contributed to the cell survival mechanism. The finding that glucosinolates derivatives are a good apoptosis inducer, fuelled up the search for possible mechanism for the anticancer study. It appeared that PEITC has equivalent apoptosis inducing potency on animal model and cancer cell lines (Traka and Mithen, 2009). Studies have showed that mouse epidermal and human prostate cancer cells have employed p53-dependent and independent pathways, respectively (Huang et al., 1998; Xiao and Singh, 2002). Hu et al., (2003) demonstrated that PEITC could induce apoptosis in colon cells HT29 on the basis of several protein kinase activation namely MAPKs, JNK (c-Jun N-terminal kinase), extracellular protein kinase (ERK) and p38 kinase. Nevertheless, Chen and co-workers have stated that down regulation of phosphatase expression may be involved in JNK activation by PEITC that leads to apoptosis initiation (Chen et al., 1998; Chen et al., 2002). ROS generation have been well studied and considered to be a key player in the control of cellular survival mechanism. Human prostate cancer PC-3 cells treated with PEITC demonstrated overexpression of ROS that mediates apoptosis in cell (Xiao et al., 2006).
d.Anti-angiogenesis effect by PEITC
Other studies have also considered the role of PEITC. In such study, this type of isothiocyanates has shown impressive angiogenic inhibitory effect by suppressing the growth factors that responsible for the formation of blood capillary was observed. As expansion of the vascular network is important for the proliferation, angiogenesis served as a platform for building new capillary beds to deliver oxygen and other nutrients to cells (Singhal et al., 2005). Therefore to clarify the anti-angiogenesis ability of glucosinolates hydrolysed product, groups of researcher examined the effects of PEITC on angiogenesis models. PEITC treatment on human umbilical vein endothelial cells (HUVEC) inhibited significantly the neovascularization and migration at a concentrations of <1 μmol/L. Importantly, this findings indicated that the anti-angiogenic action of PEITC may be partly due to inactivation of Akt, depletion in vascular endothelial growth factor (VEGF) and suppression of VEGF receptor 2 protein levels (Xu et al., 2005; Xiao and Singh, 2007).
e. Biotransformation of phase II detoxification enzyme
Naturally, all humans were exposed to the dietary and environmental carcinogenic factors. These carcinogens are subjected to be metabolized through phases of enzymatic reaction once they entered blood circulation. Inhibition of members of the cytochrome P-450 (CYP) enzymes that was responsible for the bioactivation of procarcinogens through phase I and phase II metabolism has been a putative chemopreventive mechanism (Ioannides et al., 2010). Nevertheless, the naturally occurring compound PEITC has been one of the most studied isothiocyanates and has substantial evidence in preventing cancer (Lawson et al., 2015). It involved the modification in the expression of the phase II enzymes as one of the mechanisms that play key roles in chemically-induced cancer (Xiang et al., 2009; Abdul Razis and Mohd Noor, 2013a). In vivo incubation of chemically induced rat liver with PEITC showed a dose-dependent inhibition of CYPA1 mRNA (Abdull Razis et al., 2015). Similarly, PEITC was shown to inhibit cytochrome P-450 mediated bioactivation of carcinogens with the latter promoting detoxifying enzymes. The inactivation of enzyme activity of several members of the cytochrome P-450 family caused the reduction of highly reactive intermediates that can be toxic when bound to critical macromolecules such RNA, DNA and proteins (Konsue and Ioannides, 2008; 2010a; 2010b; Abdul Razis and Mohd Noor, 2013a; 2013b; Abdul Razis et al., 2014; Yoshigae et al., 2014).
Sulforaphane (SFN)
Another isothiocyanates that has recently been particular interest of researches worldwide is sulforaphane. It was known to be derived from glucoraphenin, belonging to an important group of phytochemicals known as glucosinolates and found in broccoli and other cruciferous vegetables (Fimognari et al., 2007). SFN, one of the most comprehensively studied aromatic isothiocyanates that has been addressed as an anti-cancer agent mainly through regulation of biotransformation enzymes and cell cycle arrest (Abdull Razis and Mohd Noor, 2013a). Scientific literatures referred to SFN as an antimutagenic agent and attributed to the pungent property found in radish roots. Based on recent epidemiological studies, the role of dietary consumption of vegetables containing SFN in prevention of human cancers and human cancer susceptibility is vastly discussed (Fimognari et al., 2007).
a.Regulation of cell cycle arrest by SFN
Fimognari et al., (2002) suggested that exposure to sulforaphane led to increased accumulation of apoptotic cells during G2/M phase arrest along with time and concentration, with necrosis after prolonged exposure to high doses. In this case, sulforaphane altered cell cycle progression and induced apoptosis, increasing the expression of p53 and Bax and negatively affected the expression of Bcl-2. However, cell cycle arrest was initiated in the checkpoints when the DNA is found to be damaged and also offered the opportunity for DNA to be repaired. Despite clear evidence that glucosinolates targeted cell cycle arrest at various checkpoints there was evident demonstrating the potential of inducing phase arrest in a dose dependent manner. In LM8 cells, sulforaphane was associated with dose-dependent inhibition of cell growth at a 20 μmol/L concentration, subsequently increasing the expression of CDK inhibitor protein p21 causing cell cycle arrest at G2/M-phase (Matsui et al., 2007). In addition, suppression of cell growth and G2/M-phase arrest as a result of sulforaphane treatment was reported in human lung adenocarcinoma LTEP-A2 cells (Liang et al., 2008).
b. Biotransformation of phase II detoxification enzyme
Substantial evidence indicated that SFN and AITC were found to interact directly by inhibiting of members of the cytochrome P-450 family in phase I and inducing phase II enzymes (Zhang et al., 1994). Zhang et al. (1994) studied the chemoprotective potential of several isothiocyanates in inducing quinone reductase (QR) and GST activity in several rodent tissues. These compounds were administered through diet (3-4 µmol/g of diet) for 5-28 days via intragastric administration (5-100 µmol in single or several daily doses). The results indicated that SFN and AITC were found to elevate the level of QR and GST in several colon, bowel, kidney, lung, stomach and nasal mucosa whereby the expression of GST and QR in the cytosol of these organs was elevated by 1.2 to 9.4 times in control animals (Zhang et al., 1994).
c.Oxidative stress
Imbalance between the generation and elimination of reactive oxygen species (ROS) is often regarded as an adverse event such as mitochondrial damage, cytochrome c release leading to various diseases (Traka and Mitchen, 2009). Excessive ROS mediated oxidative stress was thought to underlie the mitochondrial damage and suppression of redox molecules that leads to abnormal cell proliferation and cell death. Using HepG2 cell lines, sulforaphane treatment caused substantial increase in GSH level (Ye and Zhang, 2001).
d.Histone deacetylation activity
Apart from all other relative anti-carcinogenic mechanisms, SFN have been considered to act on acetylation and deacetylation of histones (Myzak et al., 2004; Dashwood and Ho, 2008). A histone is a highly alkaline protein that provides structural support to a chromosome for the thread-like DNA to wrap around (Mei et al., 2004). Generally, acetylation and deacetylation of nuclear histones are two essential cellular mechanisms for the regulation of gene transcription. Therefore, any disruption in balance between acetylation and deacetylation of histone may lead to irregular gene transcription (Mei et al., 2004). In compliance with this regulatory mechanism, compounds that can actively inhibit histone deacetylases can also potentially suppress the development of cancer by up-regulating the transcription of tumor suppressor proteins that promotes apoptosis in malignant cells (Marks et al., 2004). Several studies demonstrated the potential of SFN to inhibit histone deacetylase despite other apoptotic pathways. Sulforaphane have been found to inhibit histone deacetylase activity in human colon cancer cells (Fimognari et al., 2007). There have been attempts to demonstrate the enhanced expression of beta-catenin-responsive reporter protein level human embryonic kidney 293 cells and colorectal cancer HCT116 cell lines as a consequence of exposure to SFN (Myzak et al., 2004). In a comparison of different cell lines, Pledgie et al., (2007) reported that SFN caused substantial disruption in histone deacetylase (HDAC) activity against the ER-negative cell lines compared to a panel of breast cancer cell lines that includes MDA-MB-231, MDA-MB-468, MCF-7, and T47.
e.Modulation of caspase activity
Apart from apoptosis inducing ability, SFN have been demonstrated as a potent angiogenesis inhibitor that prevents the formation of the new capillary may lead the tumors become necrotic or even apoptotic. An in vitro study conducted by Asakage et al., (2006) reported that SFN has the tendency to induce a dose dependent anti-proliferative effect on an angiogenesis model. Accordingly, Table 3 briefly summarized the studies undertaken to investigate the relative potential of SFN associated to various apoptotic mechanisms.
Table 3.
Summary of Potential Action Mechanism of SFN in Relation to Apoptosis-inducing Ability Against Various Cell Lines
| Action mechanism | Cell lines/ animal model | References |
|---|---|---|
| a.Cell cycle arrest | ||
| Activation of Chk2 leading to G2/M phase arrest | PC-3 cells, Human prostate cancer | Singh et al., 2004 |
| Increased expression of cyclin B1 and p21waf1/cip1 | HT-29 cells, human colon cancer | Gamet-Payrastre et al., 2000 |
| S-phase arrest | UM-UC3, bladder cells | Tang and Zhang, 2004 |
| Decreased expression of cyclin D1 and increased p21waf1/cip1 | PC-3 cells, Human prostate cancer | Chiao et al., 2002 |
| Increased expression of p21 and G2/M phase arrest | LM8 cells | Matsui et al., 2007 |
| G2/M phase arrest | LTEP-A2, human lung adenocarcinoma cells | Liang et al., 2008 |
| b.Biotransformation of phase II detoxification enzyme | ||
| Depletion of glutathione (GSH) | Ovarian epithelial cells | Trachootham et al., 2006 |
| c.Induction of apoptosis | ||
| Activation of caspase-8 and -9 | PC-3 cells, Human prostate cancer | Singh et al., 2002 |
| Increased expression of Bax protein, decrease in Bcl-2 expression | Human T lymphocytes | Fimognari et al., 2003 |
| Down regulation of Bcl-2 | DU-145 cells, human prostate cancer | Wang et al., 2004 |
| d.Histone deacetylation | ||
| Enhanced expression of beta-catenin-responsive reporter protein | 293 cells, human embryonic kidney, HCT-116 cells human colorectal cancer | |
| Disruption in HDAC | MDA-MB-231 cells, MDA-MB-468, MCF-7, and T47, human breast cancer | Pledgie et al., 2007 |
| e.Caspase activity | ||
| Inhibited neovascularization | Angiogenesis model | Asakage et al., 2006 |
Benzyl isothiocyanate (BITC)
Apart from the natural existence of gluconasturtiin and glucoraphanin, benzyl isothiocyanate is a yield of the endogenous enzymatic hydrolysis of glucotropaeolin glucosinolates present in the Brassicaceae family (Bones et al., 1996). Glucotropaeolin, a precursor to the formation of benzyl isothiocyanate, was isolated particularly from garden cress, has been regarded as one of the well-studied ITC compound for its potential protective effects (Bones et al., 1996).
a.Regulation of cell cycle arrest by BITC
The regulation of CDK activity includes modulation of diverse class of inhibiting proteins that bind to and inactivate CDK’s (Sunil et al., 2005). These proteins are generally classified into two major groups based on their structure and function that includes Cip/Kipfamily (p21, p27, p57) and the INK4 family (p16, p18, p19), collectively known as CDK inhibitors (Keum et al., 2004). Interestingly, most type of cancers regularly demonstrated the expression of key members of cell cycle regulatory gene families such as cyclin D1 at the G1/S phase. Further study in human Capan-2 pancreatic cancer cells, BITC treatment resulted in activation of checkpoint kinase 2 and at the same time reducing the Cyclin B1, Cdc2, and Cdc25C regulatory protein level that led to a G2/M phase arrest (Zhang et al., 2006). Additionally, when BITC induced HL-60 cell cycle arrest at the G2/M phase, the expression of p21, GADD45, and 14-3-3 sigma regulatory genes were increased (Miyoshi et al., 2004).
b. Biotransformation of phase II detoxification enzyme
Cytochrome P-450 (CYP) enzymes of phase I enzymes family plays a major role in the normal metabolic processing of numerous endogenous and exogenous compounds, simultaneously might as well activated certain carcinogens. Conversely, phase II metabolism consisting of conjugators and metabolizing enzymes including glutathione-S-transferase (GST), UDP-glucuronosyl transferase (UGT), thioredoxin reductase 1 (TR1), NAD(P)H:Quinone Oxidoreductase (NQO1), and heme oxygenase 1 (HO-1) that deactivated the carcinogens (Kensler et al., 2004; Traka and Mitchen, 2009). Consistent with these, induction of phase II enzymes in Wistar rats by BITC reported that the activities of GST, QR and UDP-glucuronyl transferase in liver and small intestinal were increased by 1.7- to 11-fold. BITC also demonstrated an increase in GSH levels in the esophagus and small bowel of ICR/Ha mice by 63%-75% (Talalay et al., 1996).
Expression of most of the phase II genes such as glutathione-S-transferase (GST) and quinone reductase (QR) led to protection of tissues and cells against endogenous and/or exogenous carcinogenic intermediates. ARE/EpRE (anti-oxidant response element/ electrophile element) found in the 5’ flanking region of these phase II genes played an important role in mediating their induction by xenobiotics including chemopreventive agents (Kong et al., 2001). Molecular studies done by Prawan et al. (2008) suggested that isothiocyanates was a potent inducer of phase II enzyme inducing transcription of phase II genes through common antioxidant/electrophile enhancer element (ARE/EpRE) that occured in the upstream regions of several phase II enzyme genes (Prawan et al., 2008). These findings suggested that the metabolites of isothiocyanates exhibited anti-carcinogenic activities through multiple pathways and modulation of numerous transcriptional factors that induced apoptosis.
c. Induction of apoptosis
Likewise, ITCs exhibited their action through members of antiapoptotic Bcl-2 family by disrupting the mitochondrial membrane integrity. The aromatic ITCs, BITC and PEITC treatment caused more damage to the mitochondria by stimulating phosphorylation of Bcl-2 at a dose of 7.5 mmol L-1but only in high concentrations at about 15-30 mmol L-1 compared to more soluble AITC and SFN exhibiting less damage to the membrane (Tang and Zhang, 2005). Interestingly, BITC treated cells were found to induce cytochrome c release from mitochondria (Nakamura et al., 2002), while conversely causing Bcl-2 to lose its ability to bind with Bax, by phosphorylation, increased the susceptibility of the cells to events of apoptosis. In support of this concept, BITC induced Bcl-2 phosphorylation has caused the dissociation of Bcl-2 and Bax complex, consequently leading to induction of apoptosis triggered by the signal of mitochondrial death (Miyoshi et al., 2004).
d. Modulation of caspase activity
Glucosinolate glucotropaeolin was an established class of naturally occurring chemopreventive agents that were able to modulate the caspase activity inducing apoptotic pathway (Bones et al., 1996). Caspases were the product of inactive zymogens known as procaspases that activated through cleavage of a pro-domain aided by other caspases (Zhang et al., 2006). There were two groups of caspases: initiator caspases and effector caspases. From the initiation of apoptotic pathway, the proapoptotic caspases were subdivided into the group of up-stream initiator caspases that includes procaspases -8, -9 and -10 (Byrd, 2015). Accordingly, downstream executioner caspases including procaspases -3, -6, and -7 were activated through cleavage of the pro-domain by up-stream caspase protein that can then trigger the degradation of enzymes that destroyed the cell (Byrd, 2015). Evidence showed that BITC has induced apoptosis in epithelial rat liver cells dependent on the mitochondrial death pathway-activating caspase-9/-3 activities (Nakamura et al., 2002).
e.Mitochondrial dysfunctions and oxidative stress
In addition to other potential action mechanism, BITC has also been related to complex antioxidant pathways. BITC induced expression of GSTP1 (GST isoenzyme) to mediate intracellular ROI (reactive oxygen intermediates) within short periods of time (1 h) that were sufficient to elevate GST activity where ROI production activities were highly correlated with the induction of GST, whereas the antioxidant GSH inhibited them (Miyoshi et al., 2004).
Allyl isothiocyanate (AITC)
AITC was obtained from sinigrin, the glucosinolates precursor, and act towards protecting against cancer through initiating multiple apoptotic pathways (Xiao et al., 2003; Xu et al., 2006; Zhang et al., 2006). Brussels sprouts have been a rich source of sinigrin and dietary intakes for a week resulted in significant elevation in plasma and intestinal GST (Nijhoff et al., 1995). Undoubtedly, AITC have been one of the intensely studied compounds for its chemoprotective attributes. As suggested by Zhang et al., (2003), various isothiocyanates, including AITC have the ability to initiate cell cycle arrest in cancer cell lines. As discussed earlier, sinigrin itself remains intact and inactive but displayed anti-carcinogenic properties upon exposure to endogenous enzyme myrosinase. Whereas, the hydrolysis that yielded AITC from sinigrin-myrosinase complex were suspected to possess the significant cancer chemopreventive properties. Studies undertaken to explore AITC have showed significant activities in modulating caspases, cell cycle phase arrest and induction of apoptosis as shown in Table 4.
Table 4.
Summary of Various Action Mechanism Exerted of AITC Associated to Apoptosis Inducing Ability Against Various Cell Lines
| ction mechanism | Cell lines/ animal model | References |
|---|---|---|
| a. Cell cycle arrest | ||
| Increased expression of cyclin B1 and p21waf1/cip1 | UM-UC-3 cells, human bladder carcinoma | Tang et al., 2004 |
| G1-phase arrest | HL60 cells, human leukemia | Zhang et al., 2003 |
| Decreased expression of cyclin B1 and G2/M phase arrest | LNCaP cells, human prostate cancer | Xiao et al., 2003; Smith et al., 2004 |
| G2/M phase arrest | HeLa cells, human cervical cancer | Hasegawa et al., 1993 |
| M phase arrest | HT29, human colorectal cancer | Smith et al., 2004 |
| b. Induction of apoptosis | ||
| Activation of JNK; decrease in Bcl-2, Bcl-xl protein expression | PC-3 cells, Human prostate cancer; LNCaP cells | Xiao et al., 2003; Xu et al., 2006 |
| Down regulation of Bcl-2 | HL60 cells, human leukemia cancer | Zhang et al., 2003 |
| c.Caspase activity | ||
| Activation of pro-apoptotic caspase enzyme, caspase-3, -8 and -9 | HL60 cells, human leukemia cancer | Zhang et al., 2006 |
a.Cell cycle arrest
There were clear indications that AITC has strong positive correlation associated with cell cycle arrest in particular. In vitro studies have reported the potency of AITC in blocking the cancer progress at various stages. Study pursued by Smith et al., (2004) demonstrated that in the case of HT29, human colorectal cancer cells, AITC showed cell cycle arrest at M phase. Other studies have however evaluated the potency of AITC. In one such study, AITC have been shown to block cell cycle progression at G1 phase in HL60, human leukemia cancer cells (Zhang et al., 2003). In contrast, several studies have revealed that AITC showed G2/M phase arrest in human bladder carcinoma cells (Tang et al., 2004), human cancer cells of prostate (Xiao et al., 2003), colon cancer cells (Smith et al., 2004) and cervical cancer cells (Hasegawa et al., 1993). It was concluded that the findings were due to the regulation of proteins involved in G2/M phase which includes cyclin B1, cdk1, cdc25B and cdc25C (Xiao et al., 2003; Smith et al., 2004).
b. Induction of apoptosis
In parallel to these studies, among isothiocyanates, exposure of BITC and AITC against a panel of human cancer cell lines (human prostate, colon and leukaemia cancer cells) resulted in activation of JNK and /or suppression of the anti-apoptotic proteins Bcl-2 and Bcl-XL directing apoptotic pathways (Patten and DeLong, 1999; Xiao et al., 2003). These findings suggested that glucosinolates, in addition to their various extrinsic and intrinsic anticancer pathways, can also induce apoptosis in certain types of tumor cells. In some investigations, it have been shown that AITC caused increase in the pro-apoptotic caspase enzyme, caspase-3,-8 and -9 (Zhang et al., 2003). In the case of HL60 leukemia cells, treatment with 10 M of AITC for 3 hours caused the activation of caspase enzymes (Zhang et al., 2003). Suppression of anti-apoptotic protein Bcl-2, Bcl-Xl and JNK mediated apoptosis also occurred as a result of exposure of AITC to both prostate cancer cells and LNCaP cells (Xiao et al., 2003; Xu et al., 2006).
Discussion
Glucosinolates and isothiocyanates have been part of our daily dietary consumption. Remarkable amount of experimental research suggest impressive credentials showing significant activities of metabolites yield from upon glucosinolates degradation. Among hundreds of glucosinolates, PEITC, BITC, SFN and AITC were most strongly associated with potential chemopreventive effects targeting multiple biological pathways by modulating cellular events leading to apoptotic cell death. However, the mechanisms facilitated by glucosinolates in inducing apoptotic pathways are not fully understood. In summary, glucosinolates and its derivatives isothiocyanates were found to be a potent source of chemopreventive therapy. Nevertheless, more investigations are encouraged in the upcoming time to clarify and demonstrate the evidence pointing the involvement of glucosinolates in relative to their target molecule and mechanisms involved in the apoptotic events.
Acknowledgements
This project was funded by Ministry of Higher Education, Malaysia under Fundamental Research Grant Scheme (FRGS) FRGS/1/2014/SKK10/UPM/02/11 as well as TWAS-COMSTECH Joint Research Grants 14-377RG/PHA/AS_C; UNESCO FR:3240283437.
References
- 1.Abdull Razis AF Mohd, Noor N, Konsue N. Induction of epoxide hydrolase, glucuronosyl transferase and sulfotransferase by phenethyl isothiocyanate in male Wistar albino rats. Biomed Res Int. 2014 doi: 10.1155/2014/391528. dx.doi.org/10.1155/2013/391528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdull Razis AF Mohd, Noor N. Sulforaphane is superior to glucoraphanin in modulating carcinogen-metabolising enzymes in HepG2 cells. Asian Pacific J Cancer Prev. 2013a;14:4325–8. doi: 10.7314/apjcp.2013.14.7.4235. [DOI] [PubMed] [Google Scholar]
- 3.Abdull Razis AF Mohd, Noor N. Cruciferous vegetables:dietary phytochemicals for cancer prevention. Asian Pacific J Cancer Prev. 2013b;14:1565–70. doi: 10.7314/apjcp.2013.14.3.1565. [DOI] [PubMed] [Google Scholar]
- 4.Abdull Razis AF Mohd, Noor N. Naturally-occurring glucosinolates, glucoraphanin and glucoerucin, are antagonists to aryl hydrocarbon receptor as their chemopreventive potency. Asian Pac J Cancer Prev. 2015;16:5801–5. doi: 10.7314/apjcp.2015.16.14.5801. [DOI] [PubMed] [Google Scholar]
- 5.Andersson D, Chakrabarty R, Bejai S, et al. Myrosinases from root and leaves of Arabidopsis thaliana have different catalytic properties. Phytochemistry. 2009;708:1345–54. doi: 10.1016/j.phytochem.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 6.Asakage M, Tsuno NH, Takahashi K, Nagawa H. Sulforaphane induces inhibition of human umbilical vein endothelial cells proliferation by apoptosis. Angiogenesis. 2006;9:83–91. doi: 10.1007/s10456-006-9034-0. [DOI] [PubMed] [Google Scholar]
- 7.Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plant. 1996;97:194–208. [Google Scholar]
- 8.Chamond RR, Anon JC, Guerra P, et al. Apoptosis and disease. Alergol Immunol Clin. 1999;14:367–74. [Google Scholar]
- 9.Chen YR, Han J, Kori R, Kong AN, Tan TH. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J Biol Chem. 2002;277:39334–42. doi: 10.1074/jbc.M202070200. [DOI] [PubMed] [Google Scholar]
- 10.Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;273:1769–75. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 11.Chiao JW, Chung FL, Krzeminski J, et al. Modulation of growth of human prostate cancer cells by the N-acetylcysteine conjugate of phenethyl isothiocyanate. Int J Oncol. 2000;16:1215–19. doi: 10.3892/ijo.16.6.1215. [DOI] [PubMed] [Google Scholar]
- 12.Chiao JW, Chung FL, Kancherla R, et al. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 2002;20:631–6. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 13.Chung FL, Wang MY, Hecht SS. Effects of dietary indoles and isothiocyanates on N-nitrosodimethylamine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone alpha-hydroxylation and DNA methylation in rat liver. Carcinogenesis. 1985;6:539–43. doi: 10.1093/carcin/6.4.539. [DOI] [PubMed] [Google Scholar]
- 14.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–91. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 15.Costa M, Costa-Rodrigues J, Fernandes MH, et al. Marine cyanobacteria compounds with anticancer properties:A review on the implication of apoptosis. Marine Drugs. 2012;10:2181–2207. doi: 10.3390/md10102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dashwood RH, Ho E. Dietary agents as histone deacetylase inhibitors:sulforaphane and structurally related isothiocyanates. Nutr Rev. 2008;66:36–8. doi: 10.1111/j.1753-4887.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabre N, Poinsot V, Debrauwer L, et al. Characterisation of glucosinolates using electrospray ion trap and electrospray quadrupole time-of-flight mass spectrometry. Phytochem Anal. 2007;18:306–19. doi: 10.1002/pca.983. [DOI] [PubMed] [Google Scholar]
- 18.Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Fimognari C, Nusse M, Berti F, et al. Sulforaphane modulates cell cycle and apoptosis in transformed and non-transformed human T lymphocytes. Ann N Y Acad Sci. 2003;1010:393–8. doi: 10.1196/annals.1299.072. [DOI] [PubMed] [Google Scholar]
- 20.Fimognari C, Nusse M, Cesari R, et al. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23:581–6. doi: 10.1093/carcin/23.4.581. [DOI] [PubMed] [Google Scholar]
- 21.Gamet-Payrastre L, Li P, Lumeau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–33. [PubMed] [Google Scholar]
- 22.Halkier BA, Du L. The biosynthesis of glucosinolates. Trends Plant Sci. 1997;2:425–31. doi: 10.1016/j.tplants.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa T, Nishino H, Iwashima A. Isothiocyanates inhibit cell cycle progression of HeLa cells at G2/M phase. Anticancer Drugs. 1993;4:273–9. doi: 10.1097/00001813-199304000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Hecht SS, Upadhyaya P, Wang M, et al. Inhibition of lung tumorigenesis in A/J mice by N -acetyl- S -(N -2-phenethylthiocarbamoyl)-L-cysteine and myo -inositol, individually and in combination. Carcinogenesis. 2002;23:1455–61. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- 25.Hu R, Kim BR, Chen C, et al. The roles of JNK and apoptosis signalling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis. 2003;24:1361–7. doi: 10.1093/carcin/bgg092. [DOI] [PubMed] [Google Scholar]
- 26.Huang C, Ma WY, Li J, Hecht SS, Dong Z. Essential role of p53 in phenethyl isothiocyanate-induced apoptosis. Cancer Res. 1998;58:4102–6. [PubMed] [Google Scholar]
- 27.Ioannides C, Hanlon N, Konsue N. Isothiocyanates:a chemical class of potential nutraceuticals. Open Nutraceuticals J. 2010;3:55–62. [Google Scholar]
- 28.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–9. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 29.Kensler TW, Talalay P. In: Inducers of enzymes that protect against carcinogens and oxidants:Drug- and food-based approaches with dithiolethiones and sulforaphane promising cancer chemopreventive agents, Volume 1:Cancer chemopreventive agents. Kelloff GJ, Hawk ET, Sigman CC, editors. Totowa, NJ: Humana Press; 2004. pp. 3–20. [Google Scholar]
- 30.Kerr JF, Wyllie AH, Currie AR. Apoptosis:a basic, biological phenomenon with wide ranging implications, in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keum YS, Jeong WS, Kong ANT. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutation Res. 2004;555:191–02. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Kong AN, Yu R, Hebbar V, et al. Signal transduction events elicited by cancer prevention compounds. Mutation Res. 2001;480:231–41. doi: 10.1016/s0027-5107(01)00182-8. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 34.Lawson AP, Long MJ, Coffey RT, et al. Naturally occurring isothiocyanates exert anticancer effects by inhibiting deubiquitinating enzymes. Cancer Res. 2015;1:5130–42. doi: 10.1158/0008-5472.CAN-15-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang H, Lai B, Yuan Q. Sulforaphane induces cell-cycle arrest and apoptosis in cultured human lung adenocarcinoma LTEP-A2 cells and retards growth of LTEP-A2 xenografts in vivo. J Nat Prod. 2008;71:1911–4. doi: 10.1021/np800233q. [DOI] [PubMed] [Google Scholar]
- 36.Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–68. doi: 10.1016/S0065-230X(04)91004-4. [DOI] [PubMed] [Google Scholar]
- 37.Matsui TA, Murata H, Sakabe T, et al. Sulforaphane induces cell cycle arrest and apoptosis in murine osteosarcoma cells in vitro and inhibits tumor growth in vivo. Oncol Rep. 2007;18:1263–8. [PubMed] [Google Scholar]
- 38.Mi L, Gan N, Chung F. Aggresome-like structure induced by isothiocyanates is novel proteasome-dependent degradation machinery. Biochem Biophys Res Commun. 2009;388:456–62. doi: 10.1016/j.bbrc.2009.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei S, Ho AD, Mahlknecht U. Role of histone deacetylase inhibitors in the treatment of cancer (Review) Int J Oncol. 2004;25:1509–19. [PubMed] [Google Scholar]
- 40.Molina-Vargas Luis F. Mechanism of action of isothiocyanates. A review. Agronomía Colombiana. 2013;31:68–75. [Google Scholar]
- 41.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane:inhibition of histone deacetylase. Cancer Res. 2004;64:5767–74. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 42.Mithen R. Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica. 1992;63:71–83. [Google Scholar]
- 43.Miyoshi N, Uchida K, Osawa T, Nakamura Y. Benzyl isothiocyanate modifies expression of the G2/M arrest-related genes. Bio Factors. 2004;21:23–6. doi: 10.1002/biof.552210106. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura Y, Kawakami M, Yoshihiro A, et al. Involvement of the mitochondrial death pathway in chemopreventive benzyl isothiocyanate-induced apoptosis. J Biol Chem. 2002;277:8492–9. doi: 10.1074/jbc.M109760200. [DOI] [PubMed] [Google Scholar]
- 45.Nijhoff WA, Grubben MJ, Nagengast FM, et al. Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans. Carcinogenesis. 1995;16:2125–28. doi: 10.1093/carcin/16.9.2125. [DOI] [PubMed] [Google Scholar]
- 46.Nishikawa A, Furukawa F, Lee IS, et al. Potent chemopreventive agents against pancreatic cancer. Curr Cancer Drug Targets. 2004;4:373–84. doi: 10.2174/1568009043332970. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer:a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487–98. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasini F, Verardo V, Caboni MF, et al. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD-MS:evaluation of Eruca sativa Mill and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012;133:1025–33. [Google Scholar]
- 49.Patten EJ, DeLong MJ. Temporal effects of the detoxification enzyme inducer, benzyl isothiocyanate:activation of c-Jun N-terminal kinase prior to the transcription factors AP-1 and NFkappaB. Biochem Biophys Res Commun. 1999;257:149–55. doi: 10.1006/bbrc.1999.0422. [DOI] [PubMed] [Google Scholar]
- 50.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–21. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 51.Prawan A, Keum YS, Khor TO, et al. Structural influence of isothiocyanates on the antioxidant response element (ARE)-mediated heme oxygenase-1 (HO-1) expression. Pharm Res. 2008;25:836–44. doi: 10.1007/s11095-007-9370-9. [DOI] [PubMed] [Google Scholar]
- 52.Sangkari LS, Masthan KMK, Babu NA, et al. Apoptosis in cancer- An update. Asian Pac J Cancer Prev. 2012;13:4873–8. doi: 10.7314/apjcp.2012.13.10.4873. [DOI] [PubMed] [Google Scholar]
- 53.Singh AV, Xiao D, Lew KL, et al. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 54.Singhal S, Vachani A, Antin-Ozerkis D, et al. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer:A Review. Clin Cancer Res. 2005;11:3974–87. doi: 10.1158/1078-0432.CCR-04-2661. [DOI] [PubMed] [Google Scholar]
- 55.Smith TK, Lund EK, Parker ML, et al. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis. 2004;25:1409–15. doi: 10.1093/carcin/bgh149. [DOI] [PubMed] [Google Scholar]
- 56.Staack R, Kingston S, Wallig MA, et al. A comparison of the individual and collective effects of four glucosinolate breakdown products from brussels sprouts on induction of detoxification enzymes. Toxicol Appl Pharmacol. 1998;149:17–23. doi: 10.1006/taap.1997.8340. [DOI] [PubMed] [Google Scholar]
- 57.Takasugi M, Katsui N, Shirata A. Isolation of three novel sulphur-containing phytoalexins from the Chinese cabbage Brassica campestris L. spp.pekinensis. J Chem Soc Chem Commun. 1986;20:1077–8. [Google Scholar]
- 58.Talalay P, Zhang Y. Chemoprevention against cancer by isothiocyanates and glucosinates. Biochem Soc Trans. 1996;24:806–10. doi: 10.1042/bst0240806. [DOI] [PubMed] [Google Scholar]
- 59.Tang L, Li G, Song L, et al. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anticancer Drugs. 2006;17:297–305. doi: 10.1097/00001813-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 2004;134:2004–10. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 61.Tang L, Zhang Y. Mitochondria are the primary target in isothiocyanate induced apoptosis in human bladder cancer cells. Mol Cancer Ther. 2005;5:1250–59. doi: 10.1158/1535-7163.MCT-05-0041. [DOI] [PubMed] [Google Scholar]
- 62.Trachootham D, Zhang H, Zhang W, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–22. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–52. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem Rev. 2009;8:269–82. [Google Scholar]
- 65.Ulukaya E, Acilan C, Yilmaz Y. Apoptosis:Why and how does it occur in biology? Cell Biochem Funct. 2011;29:468–80. doi: 10.1002/cbf.1774. [DOI] [PubMed] [Google Scholar]
- 66.Vig AP, Rampal G, Thind TS, et al. Bio-protective effects of glucosinolates–A review. LWT - Food Sci Technol. 2009;42:1561–72. [Google Scholar]
- 67.Villarreal-García D, Jacobo-Velázquez DA. Glucosinolates from broccoli:Nutraceutical properties and their purification. Curr Trends Nutraceuticals. 2016;1:1–6. [Google Scholar]
- 68.Visanji JM, Duthie SJ, Pirie L, et al. Dietary isothiocyanates inhibit Caco-2 cell proliferation and induce G2/M phase cell cycle arrest, DNA damage, and G2/M checkpoint activation. J Nutr. 2004;134:3121–6. doi: 10.1093/jn/134.11.3121. [DOI] [PubMed] [Google Scholar]
- 69.Wang L, Liu D, Ahmed T, et al. Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int J Oncol. 2004;24:187–92. [PubMed] [Google Scholar]
- 70.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 71.Wittstock U, Halkier BA. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 2002;7:263–70. doi: 10.1016/s1360-1385(02)02273-2. [DOI] [PubMed] [Google Scholar]
- 72.Wu X, Zhou Q-H, Xu K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol Sin. 2009;30:501–12. doi: 10.1038/aps.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiang Wu, Qing-hua Zhou, Ke Xu. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol Sin. 2009;30:501–12. doi: 10.1038/aps.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao D, Johnson CS, Trump DL, et al. Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in phenethyl isothiocyanate induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol Cancer Ther. 2004;3:567–75. [PubMed] [Google Scholar]
- 75.Xiao D, Lew KL, Zeng Y, et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–34. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 76.Xiao D, Singh SV. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002;62:3615–19. [PubMed] [Google Scholar]
- 77.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67:2239–46. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 78.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 79.Xu C, Li YT, Kong AN. Induction of phase i, ii and iii drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–68. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 80.Xu C, Shen G, Yuan X, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–45. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 81.Yan X, Chen S. Regulation of plant glucosinolate metabolism. Planta. 2007;226:1343–52. doi: 10.1007/s00425-007-0627-7. [DOI] [PubMed] [Google Scholar]
- 82.Ye L, Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxyfication enzymes. Carcinogenesis. 2001;22:1987–92. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 83.Yoshigae Y, Sridar C, Kent UM, Hollenberg PF. The inactivation of human CYP2E1 by phenethyl isothiocyanate, a naturally occurring chemopreventive agent, and its oxidative bioactivation. Drug Metab Dispos. 2014;41:858–69. doi: 10.1124/dmd.112.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang RF, Loganathan S, Humphreys I. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J Nutr. 2006;136:2728–34. doi: 10.1093/jn/136.11.2728. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y. Cancer-preventive isothiocyanates:measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–90. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Kensler TW, Cho CG, et al. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–50. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang YS, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–52. [PubMed] [Google Scholar]


