Abstract
Introduction:
In Head and Neck (HN) cancer, the High-Risk Human Papillomavirus (hr HPV) infection has been associated in about 40% of these tumors. The hr HPV infection is one of the etiological factors of several epithelial tumors; however, its association with the prognosis has not yet been established for patients with Laryngeal Squamous Cell Carcinoma (LSCC). On the other hand, Epidermal Growth Factor Receptor (EGFR) is a molecular marker widely studied in cancer and its overexpression has been associated with poor prognosis in some types of cancer, including the HN cancer. In the present study, we analyzed EGFR expression and HPV detection in a cohort of Mexican patients with LSCC and define their association with clinical-pathological and survival parameters.
Methods:
EGFR expression analysis was performed by immunohistochemistry assay. A tissue array was constructed based on 30 paraffin-embedded tissue samples. HPV detection was performed by PCR. The results were then compared with the clinical-pathological variables and outcome measures (Kaplan Meier and Cox analysis).
Results:
High expression of EGFR was observed in 43% of the samples and 20% of HPV detection. The statistical analyses provided evidence of disassociation between clinical-pathological parameters and EGFR expression, but there was an association with poor prognosis. Interestingly, HPV detection is slightly associated with good prognosis.
Conclusion:
Both, EGFR overexpression and HPV presence could be associated with an unfavorable prognosis in patients with LSCC, independently of other clinical-pathological factors.
Keywords: HPV, EGFR, prognosis, laryngeal cancer
Introduction
Laryngeal Squamous Cell Carcinoma (LSCC) is the most common cancer type in the Head and Neck (HN) region (Vokes et al., 1993). In 2013, the number of LSCC related-deaths worldwide was estimated as 88,000 representing a health public concern. It has been widely described that the primary risk factor for LSCC is tobacco use, and there was an association between increased incidence with tobacco use since the decade 1940s, and interestingly there has been a steady decrease correlating with its declining use (The Global Burden of Cancer, 2013). The etiological role of High-Risk Human Papillomavirus (hr HPV) infection in the development of LSCC has been recognized, and its frequency in LSCC varies since 0% until 80% of cases.
In a recent meta-analysis about relationship between HPV and larynx cancer, was reported that as in cervical carcinomas, HPV16 type appears to be the most common genotype, while in nearly 34% for LSCC and 11% for HN tumors (Li et al., 2013). However, the role of hr HPV infection in the development of LSCC has not been clearly defined.
The main prognosis clinical factor in these patients is indicated by the tumor’s extension or by its Tumor-Node-Metastasis (TNM) classification (Megwalu et al., 2014). Recently, some molecular markers in LSCC have been described with potential prognostic value (Peralta et al., 2010, Peralta et al., 2012). By instance, it has been widely reported that Epidermal Growth Factor Receptor (EGFR) is overexpressed in a high percentage of HN tumors, similarly to bladder, brain, breast, cervix, colon, esophagus, glioma, lung, ovary, pancreas, and kidney carcinomas (Gaffney et al., 2014). EGFR is a transmembrane receptor with tyrosine kinase activity, its biological function occurs through the transmission of molecular cellular signals, participates in essential processes such as cellular proliferation and differentiation, and generally its overexpression correlates with poor prognosis (Maurizi et al., 1992, Blume-Jensen et al., 2001, Gao et al., 2016). EGFR detection is highly important due to its prognosis value in some types of cancer. Several drugs and antibodies have the ability to inhibit the EGFR activity and confer to patients a good treatment alternative (Sundvall et al., 2010, Quon et al., 2001, Levitzki et al., 1995). But in tumors negative to EGFR expression is necessary to delimit potential prognosis factors, mainly due to the fact that a subgroup of laryngeal tumors are negative to EGFR expression. In the present study, EGFR expression and HPV detection were analyzed in a Mexican cohort affected with LSCC to define their correlation with clinical-pathological and survival parameters.
Materials and Methods
Biological samples
The retrospective and pilot study comprised 30 cases of patients diagnosed with LSCC who were seen at the Head and Neck Service of the Oncology Hospital, National Medical Center (CMN-SXXI) of the Mexican Institute of Social Security (IMSS) during the 2004–2008 period. The histopathological diagnosis was confirmed at the Department of Pathology of the same hospital. The clinical and follow-up data collected focused on age, tumor stage, histological differentiation degree, and treatment type, as well as the Disease-Free Survival (DFS) and Overall Survival (OS) of the patient (48 months of median follow-up time). Previous to conduct the study, the Oncology Hospital’s Research and Ethical Committee approved the protocol. According to the procedure, the results do not modify the patients’ treatments in its being a safe and non-risk protocol.
DNA extraction and HPV detection
For DNA extraction, the Wizard Genomic kit (Promega, Madison, WI, USA) was used according to the manufacturer’s instructions. DNA was purified, then quantified in a NanoDrop Spectrophotometer ND-1000 and resolved in 1% ethidium bromide-stained agarose gel. HPV detection was performed by Polymerase Chain Reaction (PCR) using two sets of primers. First, to identify HPV16 DNA sequences the E6 primers of HPV16 were utilized. These primers amplify a 126 bp fragment of the E6 gene of HPV16 (De Roda et al., 1995). The PCR solution contained 200 ng of tumor DNA, 1X buffer (50 mM KCl, 10 mM Tris- HCl, 0.1% Triton X-100), 2 mM MgCl2, 0.2 mM of each dNTP, 50 pmol of each primer, and 2 units of Taq DNA polymerase (Promega) in 50 μl of the final volume. The reaction tubes were placed in a thermal cycler (MJ Research Minicycler) with the following program: one cycle of denaturing at 94°C for 30 s; 40 cycles of denaturation at 94°C for 4 min; annealing at 55°C for 1.5 min, an extension at 72°C for 1.5 min, with a final extension at 72°C for 1.5 min. CaSKi DNA (HPV16+) was employed as positive control, while C33 (HPV-) and lymphocyte DNA were included as negative controls. Prior to HPV detection, primers for human D-loop mitochondrial region genes were used as internal controls for monitoring DNA quality. The negative samples for the initial PCR were then subjected to second PCR with consensus GP5+/GP6+ primers for the L1 gene of HPV with an initial denaturation of 94ºC for 4 min, followed by 40 amplification cycles, 94ºC for 1 min, 40ºC for 2 min, 72ºC for 2 min, and a final extension of 72ºC for 10 min. The amplification products were visualized in an ethidium bromide-stained agarose gel.
EGFR expression analysis
EGFR expression analysis was performed by means of immunohistochemistry assay. For this, a tissue array was constructed based on 30 paraffin-embedded tissue samples. Briefly, 4-μl sections were constructed from the blocks and the histological technique was performed to determine tumoral area. Using a semi-automatic tissue microarrayer (Chemicon, Co.), we proceeded to take the tumoral area and place it in a paraffin receptor successively for all samples. Once the tissue array was finished, histological sections were made, which were in turn mounted on 3-AminoPropylTriEthoxySilane (APTES) slides specially prepared for immunohistochemistry staining. Histological samples were then incubated for 1 h at a temperature of 60°C, after being deparaffinized in xylene and rehydrated with ethanol at different degrees successively. Antigenic exposure was performed by incubating the samples in a pot at maximum pressure with DAKO Target Retrieval solution. Endogenous peroxidase was removed with H2O2 at 3%. With the purpose of avoiding unspecified bindings, samples were incubated in a biotin-avidin solution for 30 min (Protein Block Serum-Free, DAKO, Carpinteria, CA, USA), although staining was reduced by incubation with goat serum (1:20) for 60 min. The primary antibody was set in place and incubated for 1 h at room temperature. Later, the secondary antibody was utilized and conjugated with Streptadivin/HRP (LSAB2, DAKO). Counter-staining was performed with hematoxylin. Immunohistochemistry studies were conducted employing rabbit IgG monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) against EGFR. The antibody was diluted 1:100 in Phosphate-Buffered Saline (PBS) solution. Breast carcinoma EGFR + samples were used as positive controls. Negative controls were performed with the same tissue but without antibodies or ovary skin. Uninjured laryngeal tissue was also utilized. Cases were considered positive if staining was homogeneous throughout the selected tissue (with at least 80% of tumor cells). In addition to negative controls resulting in negative staining, all positive cases exhibited cytoplasmic staining with membranous accentuation in most of the stained cases. Samples were evaluated as positive or negative for the same pathologist.
Statistical and survival analysis
All analyses were carried out using SPSS ver. 15.0 statistical software. Chi-square test with Yates correction was used to compare qualitative variables. The estimation of the survival distribution of patients with LSCC was done according to the methods of Kaplan–Meier, and log-rank test were employed to compare the survival curves of two groups (p <0.05). Cox regression analysis was used for univariate analysis to explore the effect of clinical-pathological variables with EGFR overexpression and HPV detection on survival. An interval estimate for the hazard ratio was obtained from 20,000 bootstrap samples. A 95% BCa confidence interval for the hazard ratio for age, smoking, clinical stage, lymph node and EGFR expression was estimated.
Results
Analysis of the patients’ clinical data is summarized in Table 1. This cohort of patients, in advanced stages and with an equal number with positive (13/30) or negative lymph nodes (17/30), was observed. DNA of the same samples was analyzed for the presence of HPV16 (primers yielding a 126-bp fragment) and with consensus primers GP5+/GP6+. HPV16 was the sole type detected in LSCC samples. This primer set allowed detection of HPV DNA in 20% of LSCC samples (6/30). However, HPV-negative status had a higher number of patients (24/30) than HPV-positive (6/30). The results demonstrated no statistical difference.
Table 1.
Correlation between EGFR Overexpression and Clinical-pathological Variables in Mexican Patients Affected by LSCC
| Clinical-pathological variables | n | EGFR | P value | |
|---|---|---|---|---|
| Positive | Negative | |||
| All cases | 30 | 13 | 17 | |
| Age | 0.509 | |||
| < 60 | 5 | 1 | 4 | |
| > 60 | 25 | 12 | 13 | |
| Lymph nodes | 0.921 | |||
| Positive | 13 | 5 | 8 | |
| Negative | 17 | 8 | 9 | |
| Smoking | 0.869 | |||
| Positive | 25 | 11 | 14 | |
| Negative | 5 | 2 | 3 | |
| HPV status | 0.926 | |||
| Positive | 6 | 2 | 4 | |
| Negative | 24 | 11 | 13 | |
Test performed; Chi-square with Yates correction
Forty-three percent of the samples (n=13) were strongly immunostained with EGFR. Interestingly no significant difference between EGFR overexpression and lymph-node positivity or HPV-positive status (p >0.05) was observed. In cases of positive cytoplasm, immunostaining was evident with accentuation on the cytoplasmic membrane of tumor cells (Figure 1). No statistical correlation between EGFR expression and smoking or with the patient’s age was found. Findings from the study on possible correlation with OS or DFS support the existence of an unfavorable prognosis in patients who presented EGFR overexpression, but this prognosis is favorable in patients with positive HPV.
Figure 1.
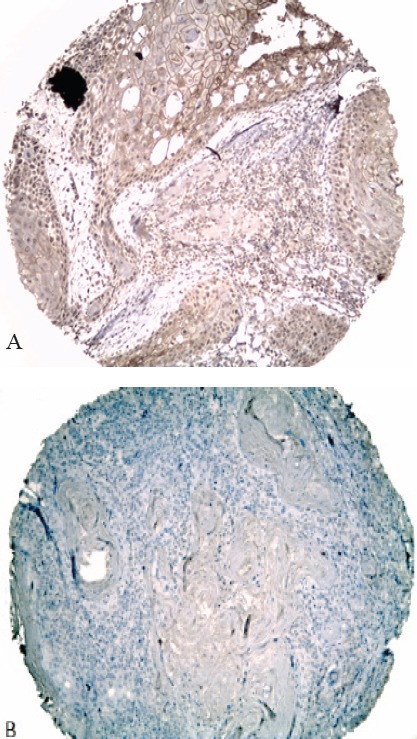
EGFR Protein Immunodetection in Human Larynx Tissues. (A) EGFR expression was observed in the cell membrane of tumoral cells as a brownish reaction. (B) In some tissues this reaction was absent. All tissue sections were counterstained with hematoxilyn. Original amplification x100
When log-rank test was used, HPV positive status was found to be related significantly to good overall survival (P=0.448) in the patients included in the study (the survival distribution shows that HPV positive cases survive longer than HPV negative cases), while cases with HPV negative cases shows survival distribution with poor clinical outcome. In the EGFR overexpression cases showed a poor survival (P=0.122) (the survival distribution shows that EGFR overexpression survive longer than EGFR negative expression cases) (Figures 2 and 3). Univariate Cox regression analysis also identified that clinical variables including age, smoking and lymph node (positive) were not significantly associated with overall survival. However, clinical stage (T1-T2) presented a hazard ratio of 0.104 (95% BCa CI: 0.021-0.350) (Tables 2 and 3).
Figure 2.
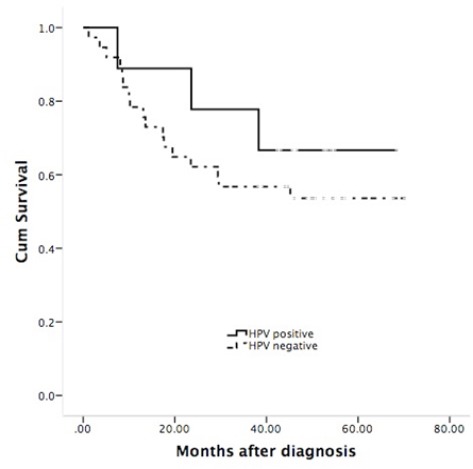
Survival Probability of LSCC Patients with HPV Positive; Favorable Pognosis in Patients’ HPV-Positive, Log-rank; P=0.448
Figure 3.
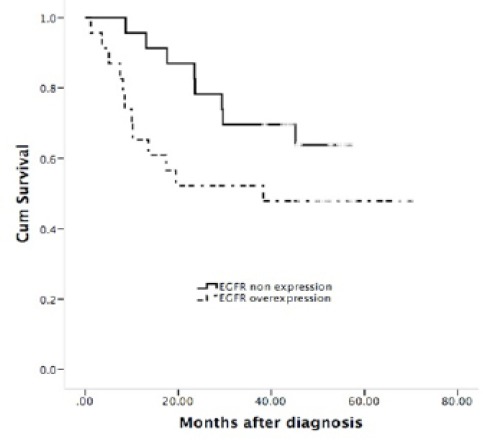
Survival Probability of LSCC Patients with EGFR Overexpression; Unfavorable Prognosis in Patients with EGFR Overexpression, Log-rank; P=0.122
Table 2.
Survival Analysis
| Factor | P value§ | HR (95% BCa CI) §§ |
|---|---|---|
| Age (<60) | 0.801 | 0.854 (0.043- 2.595) |
| Clinical stage (T1-T2) | 0.007* | 0.104 (0.021-0.350) |
| Lymph node (positive) | 0.163 | 1.856 (0.701-5.030) |
| EGFR (overexpression) | 0.122 | 0.500 (0.191- 1.189) |
| Smoking (yes) | 0.198 | 0.455 (0.034- 22.261) |
, Cox regression univariate analysis;
, Log-rank test; HR, Hazard ratio;
, Statistical significant.
Table 3.
Prognosis between HPV and EGFR Status in Mexican Patients with LSCC
| HPV Status | EGFR Status | n | Favorable Prognosis |
|---|---|---|---|
| + | + | 2 | Yes |
| + | - | 4 | Yes |
| - | + | 11 | No |
| - | - | 13 | No |
Discussion
EGFR gene encodes for a transmembrane receptor with tyrosine kinase activity that can be stimulated by EGF (Epidermal Growth Factor). Once stimulated, the receptor induces the phosphorylation of several effectors involved in the MAP Kinase pathways, which are implicated in the cellular proliferation (Okano et al., 2000). EGFR overexpression has been observed in 40–80% of HN tumors. This overexpression appears to be linked with mutations EGFR and amplification (Grandis et al., 1993a, Grandis et al., 1993b); EGFR gene is mapped in the short arm of human chromosome 7p12.10, this region is frequently amplified in epithelial tumors. Several molecular cytogenetic studies (e.g. comparative genomic hybridization) have showed an amplification of EGFR in various tumors, particularly in LSCC (Cancer Genome Atlas Network, 2015).
Molecular markers of clinical importance in cancer research such as EGFR are widely used to determine the prognosis and to have therapeutic strategies depending on their expression in a tumor. Several works in cancer attribute a poor prognosis value to EGFR overexpression, but fortunately in the clinic, different therapeutic options are already available in patients with this molecular alteration (Grandis et al., 1998, Wen et al., 1996, Lee et al., 1997). At present, treatment options include monoclonal antibodies and inhibitors of tyrosine kinases. In the first case, an antibody available is Cetuximab®, with good results in clinical trials performed on patients with advanced stages of HN cancer, and in the second case, an inhibitor is Iressa®, which specifically inhibits phosphorylation of the receptor’s catalytic domain (Ugurluer et al., 2014, Campbell et al., 2016). Therapy to achieve tumor growth inhibition, based on EGFR inhibition with molecular agents, represents a therapeutic option in the treatment of laryngeal cancer. These treatments could be substantially superior (as personalized medicine) to the conventional alternatives currently employed based on the traditional chemotherapy regimen. While studying the association between patients’ survival and EGFR overexpression, we found an unfavorable prognosis in EGFR overexpression. Based in our data, we could suggest that the implementation of a fast-molecular technique to achieve EGFR detection for patients diagnosed with LSCC is necessary. The hope is that these kinds of patients will be treated with therapeutic options as previously described. In the present study, EGFR overexpression was detected (43%), supporting previous results (Grandis et al., 1993a, Grandis et al., 1993b). We also observed that EGFR overexpression was associated with unfavorable prognosis, but neither lymph-node positivity nor smoking was correlated with reduced survival. Most of the patients corresponded to advanced stages (T3-T4 stages). This independent molecular factor related to poor prognosis has been reported (Ugurluer et al., 2014). Thus, the observation could robust that the EGFR expression as molecular target. The impact of different prognostic factors such as EGFR, clinical factors such as tumor stage (TNM) and age are not fully known for HN cancer. The reported survival rates vary and the optimal treatment has not yet been established.
In the last decade, the HPV infection has been identified as an important prognostic factor in oropharyngeal cancer (Bersani et al., 2017, Shimura et al., 2017), and there is now a growing interest in the importance of HPV for HN cancer (Schroeder et al., 2017, Linge et al., 2016). Axelsson et al., report that HPV infection is common in HN tumors with good prognosis. Independent of others prognostic factors for survival such as the age of more than 70 years, and N3 stage (Axelsson et al., 2017). Zhang et al., (2016) finds that patients with HPV-positive HN carcinomas have a better prognosis than the HPV-negative cases and hypothesizes that the replication of HPV genome into the genomes of cancer cells may enhance DNA repair mechanisms, which in turn limit the accumulation of lethal somatic mutations. Another author hypothesizes that in HPV-positive cases, it would be interesting to study the expression of E5/HPV protein, which may modulate EGFR turnover (Keck et al., 2015, Rampias et al., 2013). A large-scale study is still necessary to reach satisfactory and concrete conclusions as well as to elucidate the molecular mechanism that favors prognosis in HPV-positive cases in HN.
Some authors also use EGFR and HPV expression to perform molecular sub-classification in oropharyngeal tumors (Rivera-Peña et al., 2016). HPV infection is an indicator of good response to chemoradiotherapy, and EGFR is a molecular target in HN carcinoma, so, the molecular sub-classification based on HPV infection and EGFR expression could serve as important for appropriate therapeutic strategy (Nakano et al., 2016). In the present study, the LSCC exhibited that HPV status is independent to EGFR status. Recently was reported that HPV 16 may be associated with a favorable prognosis in patients with LSCC (Zhang et al., 2016). According to our results, the cases HPV-negative cases and EGFR positive expression, an appropriate therapeutic option would be available.
In conclusion, EGFR overexpression in patients with LSCC could be associated with an unfavorable prognosis, independently of other clinical-pathological factors. It is also possible to find a group associated with HPV infection, with a favorable prognosis. More research is needed to establish and define the molecular mechanism, and to strengthen the present findings.
Acknowledgements
The present work was partially supported by 3602-0024 FIS-IMSS and 202222 CONACyT México grants (MS). RP wishes to thank the PRODEP-SEP for their support. During this work, MS was a Fundación IMSS, A.C. fellowship.
References
- 1.Axelsson L, Nyman J, Haugen-Cange H, et al. Prognostic factors for head and neck cancer of unknown primary including the impact of human papilloma virus infection. J Otolaryngol Head Neck Surg. 2017;46:45. doi: 10.1186/s40463-017-0223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bersani C, Mints M, Tertipis N, et al. A model using concomitant markers for predicting outcome in human papillomavirus positive oropharyngeal cancer. Oral Oncol. 2017;68:53–9. doi: 10.1016/j.oraloncology.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;28:67–79. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 4.Campbell NP, Hensing TA, Bhayani MK, Shaikh AY, Brockstein BE. Targeting pathways mediating resistance to anti-EGFR therapy in squamous cell carcinoma of the head and neck. Expert Rev Anticancer Ther. 2016;16:847–8. doi: 10.1080/14737140.2016.1202116. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Roda AM, Wallboomers JM, Van Den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3'ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–62. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 7.Gaffney DC, Soyer HP, Simpson F. The epidermal growth factor receptor in squamous cell carcinoma:An emerging drug target. Australas J Dermatol. 2014;55:24–34. doi: 10.1111/ajd.12025. [DOI] [PubMed] [Google Scholar]
- 8.Global Burden of Disease Cancer Collaboration. The Global burden of cancer 2013. JAMA Oncol. 2013;1:505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J, Ulekleiv CH, Halstensen TS. Epidermal growth factor (EGF) receptor-ligand based molecular staging predicts prognosis in head and neck squamous cell carcinoma partly due to deregulated EGF-induced amphiregulin expression. J Exp Clin Cancer Res. 2016;35:151. doi: 10.1186/s13046-016-0422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandis JR, Tewardy DJ. Elevated levels of TGF-a and EGFR messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–84. [PubMed] [Google Scholar]
- 11.Grandis JR, Tewardy DJ. TGF-alpha and EGFR in head and neck cancer. J Cell Biochem Suppl. 1993;17:188–91. doi: 10.1002/jcb.240531027. [DOI] [PubMed] [Google Scholar]
- 12.Grandis JR, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 13.Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21:870–81. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 14.Lee CS, Redshaw A, Boag G. Epidermal growth factor receptor immunoreactivity in human laryngeal squamous cell carcinoma. Pathology. 1997;29:251–4. doi: 10.1080/00313029700169005. [DOI] [PubMed] [Google Scholar]
- 15.Levitzki A, Gazit A. Tyrosine kinase inhibition:an approach to drug development. Science. 1995;267:1782–8. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Gao L, Li H, et al. Human papillomavirus infection and laryngeal cancer risk:a systematic review and meta-analysis. J Infect Dis. 2013;207:479–88. doi: 10.1093/infdis/jis698. [DOI] [PubMed] [Google Scholar]
- 17.Linge A, Lohaus F, Löck S, et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy:A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG) Radiother Oncol. 2016;121:364–73. doi: 10.1016/j.radonc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Maurizi M, Scambia G, Benedetti Panici P, et al. EGFR expression in primary laryngeal cancer:correlation with clinico-pathological features and prognostic significance. Int J Cancer. 1992;52:862–6. doi: 10.1002/ijc.2910520605. [DOI] [PubMed] [Google Scholar]
- 19.Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:855–60. doi: 10.1001/jamaoto.2014.1671. [DOI] [PubMed] [Google Scholar]
- 20.Nakano T, Yamamoto H, Nakashima T, et al. Molecular subclasssfication determined by human papillomavirus and epidermal growth factor receptor status is associated with the prognosis of oropharyngeal squamous cell carcinoma. Hum Pathol. 2016;50:51–61. doi: 10.1016/j.humpath.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. Akt/PKB isoforms are differentially regulated by EGF stimulation. J Biol Chem. 2000;275:30934–2. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- 22.Peralta R, Baudis M, Vazquez G, Juárez S, et al. Increased expression of cellular retinol-binding protein 1 in laryngeal squamous cell carcinoma. J Cancer Res Clin Oncol. 2010;136:931–8. doi: 10.1007/s00432-009-0735-9. [DOI] [PubMed] [Google Scholar]
- 23.Peralta R, Valdivia A, Alvarado-Cabrero I, et al. Correlation between expression of cellular retinol-binding protein 1 and its methylation status in larynx cancer. J Clin Pathol. 2012;65:46–50. doi: 10.1136/jclinpath-2011-200304. [DOI] [PubMed] [Google Scholar]
- 24.Quon H, Liu FF, Cummings BJ. Potential molecular prognostic markers in head and neck squamous cell carcinomas. Head Neck. 2001;23:147–59. doi: 10.1002/1097-0347(200102)23:2<147::aid-hed1010>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Rampias T, Pectasides E, Prasad M, et al. Molecular profile of head and neck squamous cell carcinomas bearing p16 high phenotype. Ann Oncol. 2013;24:2124–31. doi: 10.1093/annonc/mdt013. [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Peña B, Ruíz-Fullana FJ, Vélez-Reyes GL, et al. HPV-16 infection modifies overall survival of Puerto Rican HNSCC patients. Infect Agent Cancer. 2016;11:47. doi: 10.1186/s13027-016-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder L, Boscolo-Rizzo P, Dal Cin E, et al. Human papillomavirus as prognostic marker with rising prevalence in neck squamous cell carcinoma of unknown primary:A retrospective multicenter study. Eur J Cancer. 2017;74:73–81. doi: 10.1016/j.ejca.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Shimura E, Hama T, Suda T, et al. The presence of HPV DNA in neck lymph node metastasis correlates with improved overall survival of patients with oropharyngeal cancer undergoing surgical tratment. Oncology. 2017;92:87–93. doi: 10.1159/000452420. [DOI] [PubMed] [Google Scholar]
- 29.Sundvall M, Karrila A, Nordberg J, Grénman R, Elenius K. EGFR targeting drugs in the treatment of head and neck squamous cell carcinoma. Expert Opin Emerg Drugs. 2010;15:185–201. doi: 10.1517/14728211003716442. [DOI] [PubMed] [Google Scholar]
- 30.Ugurluer G, Ozsahin M. Early investigational drugs that target epidermal growth factor receptors for the treatment of head and neck cancer. Expert Opin Investig Drugs. 2014;23:1637–54. doi: 10.1517/13543784.2014.951435. [DOI] [PubMed] [Google Scholar]
- 31.Vokes EE, Weichselbaum RR, Lippman SM, Honk WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 32.Wen QH, Miwa T, Yoshizaki T, et al. Prognostic value of EGFR and TGF-alpha in early laryngeal cancer treated with radiotherapy. Laryngoscope. 1996;106:884–8. doi: 10.1097/00005537-199607000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Edwards A, Fang Z, Flemington EK, Zhang K. Integrative genomics and transcriptomics analysis reveals potential mechanisms for favorable prognosis of patients with HPV-Positive head and neck carcinomas. Sci R. 2016 doi: 10.1038/srep24927. [DOI] [PMC free article] [PubMed] [Google Scholar]


