Abstract
Purpose/Aim :
Luteinizing hormone (LH) is known to function as a key regulator of VEGF expression in reproductive organs. In recent years, LH has also been detected in human vitreous and LH receptors have been identified in human retina. This study was aimed to investigate a potential correlation between LH and VEGF levels in healthy mammalian eyes to provide supporting evidence of LH’s potential involvement in intraocular VEGF regulation.
Methods:
18 bovine and 30 porcine eyes were procured from an abattoire and VEGF and LH levels were measured in the vitreous extracted from these eyes by commercially-available bovine & porcine ELISA assay kits. Total protein of the vitreous was measured by using Micro BSA protein assay kit.
Results:
After total protein normalization, the Pearson Correlation Coefficients (PCC) showed a strong and significant correlation between LH and VEGF levels. (Bovine LH/VEGF PCC:0.89, p<0.001; Porcine LH/VEGF PCC: 0.80, p<0.001). Linear regression analyses, adjusted for gender, showed significant linear relationships between LH and VEGF levels in both bovine and porcine vitreous. (Bovine: t-value = 7.69, p < 0.0001, adjusted r2= .79; Porcine: t-value= 6.71, p<0.001, adjusted r2= .62)
Conclusions;
We show that VEGF and LH are strongly correlated in healthy, adult mammalian eyes. The robustness of the correlation is shown both by its strength of association and reproducibility in two species. Given that LH is well known to regulate VEGF levels in several tissue types, the LH/VEGF linear relationship in vitreous potentially implicates LH in homeostatic VEGF regulation of the eye. Because we also found that the correlation between LH and VEGF only became manifest when our targeted analytes were normalized by total amount of protein, preclinical and clinical investigators should consider normalizing analytes in vitreous by total protein when assessing potential correlations amongst them.
Keywords: vitreous VEGF, intraocular VEGF, luteinizing hormone, vascular endothelial growth factor, VEGF eye
Vascular endothelial growth factor (VEGF) regulates angiogenesis and vascular permeability in the eye.(1) Ischemic retinopathies are strongly associated with elevated levels of intraocular VEGF.(1–6) Ischemia clearly influences VEGF expression under pathophysiologic circumstances. (7–14) However, the factors that govern intraocular VEGF production under non-pathologic conditions are not as well understood. Eye researchers widely accept that oxygen levels are the most likely driver of retinal VEGF under physiologic conditions. For example, the current consensus of the scientific community is that “physiologic hypoxia” [term coined by Chan-Ling and Stone(14)] is responsible for VEGF induction during fetal retinal vascularization.(10–12) However, all factors that control VEGF production under homeostatic conditions in the eye have not been fully investigated.
Gonadotropins play an important role in angiogenesis and VEGF regulation in reproductive organs. In particular, luteinizing hormone (LH), produced by the pituitary, functions as a key regulator of VEGF expression in the testis and the ovary.(15–20) In recent years, LH has also been detected in human vitreous fluid (21) and LH retinal receptors have been identified in rats, rabbits, bovine animals and humans. These LH retinal receptors are primarily expressed by cone photoceptors. (22, 23) The role of the LH retinal receptor is not known. 19 (22, 24) We hypothesized that LH (most likely via interaction with its retinal receptor) plays a role in intraocular VEGF regulation.
If our hypothesis holds, eyes with less cone photoreceptors (and thus less LH receptors), should exhibit less VEGF-mediated angiogenesis. There is evidence in the literature to support this. For example, diabetic mice lacking cone photoreceptors do feature a lower density of retinal vessels compared to those diabetic mice with a normal distribution of cone photoreceptors. (25) In addition, diabetic patients with degenerated photoreceptors (i.e. those with concomitant retinitis pigmentosa) have less proliferative retinopathy than diabetics with intact photoreceptors.(26) The present study was aimed to investigate a possible correlation between LH and VEGF levels in the vitreous of healthy, adult mammalian eyes to provide supporting evidence of LH’s potential involvement in intraocular VEGF regulation during homeostasis.
Materials and Methods
No human or live animals were used as part of this study. Bovine and porcine whole eyes, were procured immediately after slaughter from Scholl Slaughterhouse (Blissfield, Michigan) and were transported to the laboratory on wet ice, and rinsed in ice cold PBS. After cornea was cut open and the lens removed, vitreous was collected into microfuge tubes with 1 ml pipet with wide bore pipet tips. All samples were snap frozen upon collection in liquid nitrogen and stored at −80°C until further analysis.
VEGF and LH levels were measured in the vitreous by commercially-available ELISA assays (Bovine VEGF ELISA: MyBiosource, San Diego, CA; Catalogue # MBS2887434; Porcine VEGF ELISA: Ray Biotech, Norcross, GA; Catalogue # ELP-VEGF-1; Bovine LH ELISA: MyBiosource, San Diego, CA; Catalogue # MBS700951; Porcine LH ELISA: MBS009739). To test the reproducibility of all ELISA kits, vitreous samples were chosen randomly from the original data set and assayed multiple times in duplicate under the same conditions. Spike recovery and dilution curves were also established to validate the kits. The lowest limit of detection for the VEGF ELISA kits were 31.25 pg/ml for bovine VEGF and 40 pg/ml for porcine VEGF. The lowest limit of detection for the LH ELISA kits were 1.25 mIU/ml for bovine LH and 3.12 mIU/ml for porcine LH.
Total protein of the VH was measured by using Micro BSA protein assay kit (Thermo Fisher, Catalogue # 23235). VEGF and LH levels were normalized to total protein levels. All statistical analyses will be performed using SAS statistical software.
Results:
Mean LH levels (pg/total protein) of porcine animals (M: 58.6 F: 84.1) were higher than that of bovine animals (Males: 7.5, F 7.6). However, mean VEGF levels of bovine animals (M:1396.8, F:1404.9) were higher that of the porcine animals (M: 172.2, F: 256.2). T-test results did not show any significant differences in mean levels of LH, VEGF or protein levels between male and female animals of either species (Table 1). Before total protein normalization, LH and VEGF levels did not demonstrate a correlation in either species (Table 2). However, after total protein normalization, the Pearson Correlation Coefficients (PCC) showed a strong and significant correlation between LH and VEGF levels. (Bovine LH/VEGF PCC:0.89, p<0.001; Porcine LH/VEGF PCC: 0.80, p<0.001 (Table 2)
Table 1:
Mean Levels of Luteinizing Hormone, VEGF and Total Proteins in Bovine/Porcine Vitreous Fluid
| Male Bovine (steer) N=6 eyes |
Female Bovine (heifer) N=12 eyes |
Male vs Female T Test results (t value, p value) |
|
|---|---|---|---|
|
Mean LH (pg/total protein) |
7.5 (SD 3.2) | 7.6 (SD 3.0) | t=0.04, p=0.96 |
|
Mean VEGF (pg/total protein) |
1396.8 (SD 573.0) | 1404.9 (SD 451.4) | t=0.03, p=0.98 |
|
Total protein (mg/ml) |
1.8 (SD 0.7) | 2.1 (SD 0.7) | t=0.07, p=0.35 |
|
Male Porcine (barrow) N=14 eyes |
Female Porcine (gilt) N=16 eyes |
Male vs Females T-test results (t value, p value) |
|
|
Mean LH (pg/total protein) |
58.6 (SD 41.1) | 84.1 (SD 72.4) | t=1.22, p=0.24 |
|
Mean VEGF (pg/total protein) |
172.2 (SD 139.5) | 256.2 (SD 239.9) | t=1.18, p= 0.25 |
| Mean Total Protein | 1.3 (SD 0.9) | 1.2 (SD 1.2) | t=−0.19, p=0.85 |
Table 2:
Correlation between Luteinizing Hormone (LH) and Vascular Endothelial Growth Factor (VEGF) in Bovine/Porcine Vitreous Fluid.
| LH/VEGF Correlation in Bovine Vitreous Fluid N=18 |
LH/VEGF Correlation in Porcine Vitreous Fluid N=30 |
|---|---|
|
Analyte concentrations expressed as: picogram of analyte/ml of vitreous Pearson Correlation Coefficient : 0.19 p=0.457 |
Analyte concentrations expressed as: picogram of analyte/ml of vitreous Pearson Correlation Coefficient : 0.15 p=0.413 |
|
Analytes normalized by total protein: picogram of analyte/mg of total protein Pearson Correlation Coefficient : 0.89 p<0.001 |
Analytes normalized by total protein: picogram of analyte/mg of total protein Pearson Correlation Coefficient : 0.80 p<0.001 |
Pearson correlation coefficients (PCC) did not show any significant correlation between LH levels of right eyes paired with the corresponding LH levels of left eyes in either bovine (PCC 0.56, p= 0.11) or porcine animals. (PCC −0.13, p=0.64). Because of this, the LH value of each eye of each animal were considered to be independent entries in the linear regression analyses described below. However, additional linear regression analyses were also performed with the use of only one eye from each animal and yielded equivalent results. (Data not shown).
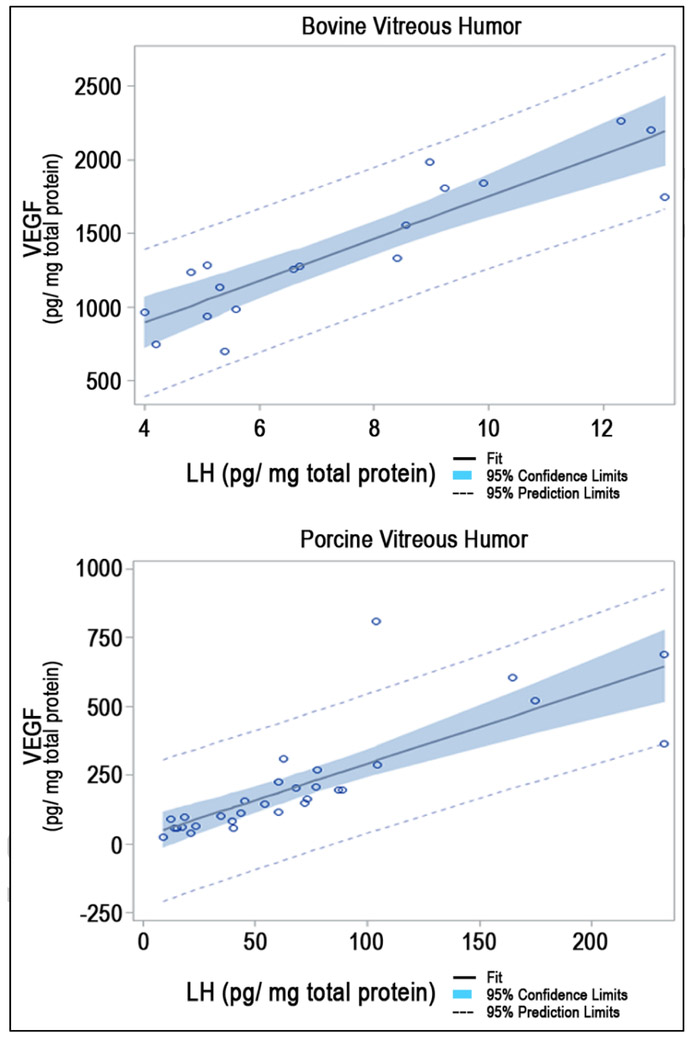
In bovine vitreous fluid samples (N=18), linear regression analysis, adjusted for gender, showed a significant linear relationship between LH and VEGF levels (t-value = 7.69, p <0.0001, adjusted r2= .79). In porcine vitreous samples (N=30), linear regression analysis, adjusted for gender, also showed a significant linear relationship between LH and VEGF levels, (t-value= 6.71, p<0.001, adjusted r2= .62) (Figure 1).
Figure 1.
The VEGF/LH Linear Relationship in Vitreous Fluid from Healthy Adult Bovine Eyes (N=18; adjusted r2= 0.79, p<0.0001) and Porcine Eyes (N=30, adjusted r2= 0.62; p<0.001)
Discussion:
To the best of our knowledge, this study is the first to show that VEGF and LH are strongly correlated in the vitreous from healthy, adult mammalian eyes. We demonstrated this linear relationship utilizing VH from bovine and porcine animals. Of note, both bovine and porcine VEGF has >90% homology with the human VEGF sequence.(13) Our findings shows that porcine eyes have relatively high LH levels compared to their VEGF levels, whereas the opposite is true for bovine eyes. One explanation for this may be that porcine eyes may have a greater density of cells that express the LH receptor (i.e. cone photoreceptors) than bovine eyes. A potential, alternative explanation may be that bovine LH is more bioactive than porcine LH, thus, allowing a lower LH level to yield a relatively higher VEGF concentration.
The protein content of the vitreous fluid is widely accepted to be a reflection of the biochemical processes that are occurring at the retinal level; in other words, the vitreous proteome is understood to represent the retinal state of affairs.(27) Given that LH is already known to regulate VEGF levels in several tissue types, the LH/VEGF linear relationship in vitreous fluid potentially implicates LH in retinal VEGF regulation. (15–20) The robustness of the LH/VEGF correlation is shown both by its strength of association and reproducibility in two different species. Thus, our study supports the potential participation by LH in homeostatic VEGF regulation in the adult eye. That said, mechanistic confirmation that LH does indeed regulate retinal VEGF levels requires further laboratory investigation. Our findings suggest that a neuroendocrine circuit may exist in the retina which involves signaling from cone photoreceptors cells (site of LH receptors) to VEGF-producing cells (RPE, astrocytes, Muller cells, vascular endothelial cells, ganglion cells and ciliary epithelium.) Further studies are needed to characterize this potential circuit.
There is a second prominent finding from this study; we discovered that the LH/VEGF correlation becomes manifest only after the targeted analytes were normalized by total protein Though other researchers have previously suggested that intravitreal concentrations of targeted analytes be corrected by total protein content,(28) our study clearly demonstrates the necessity of this. Given that vitreous humor is a non-homogenous viscoelastic gel containing > 1200 proteins, the importance of total protein correction should come as no surprise. After all, it’s well known that the content of the vitreous proteome differs substantially from one individual to the next under both physiologic and pathophysiologic circumstances.(28) Furthermore, VEGF levels in other non-homogenous matrices such as urine have also been normalized by total protein.(29) All this said, the protein correction step in vitreous is not frequently carried out; this is especially true when candidate proteins are quantified by ELISA testing. In a published review of 9 human vitreous studies, mean VEGF levels (all assessed by ELISA methodology) were expressed as picogram (or nanogram) per milliliter in every study; not a single one of the 9 studies corrected for either total protein content or by any other method of analyte concentration correction (such as by estimation of the ratio of vitreous and plasma concentration). (28) (30) Perhaps, this common omission of vitreous standardization accounts for some of the unexplained difficulty that vision researchers have had in validating VEGF as a biomarker.
Though affirmation of the LH/VEGF correlation in human eye would be ideal, it will offer challenges. Cadavers are the main source of human eyes without retinal pathology. However, there is usually a several hour delay before the recovery of a cadaver eye. Given the short halflife of LH (i.e. just few minutes), LH measurements from cadaver viteous cannot be relied upon to reflect physiological levels. Another vitreous source would be from live individuals undergoing scheduled vitrectomies. It may seem that vitreous sampling should come from eyes with epiretinal membranes or macular holes. These types of eyes are frequently used as normal controls. However, in the human eye, the retinal LH receptor is mainly expressed by cone photoreceptors centered around the macula;(22, 23) Therefore, macular holes may affect the expression of the LH receptor and extraretinal membranes may affect LH receptor access. Thus, these types of retinal pathologies may interfere with the interaction between LH with its retinal receptor.
In summary, this study provides compelling evidence of a strong and significant LH/VEGF relationship in the eye; this finding supports our original hypothesis that LH may play a role in homeostatic retinal VEGF regulation. More studies are needed to further investigate the potential role of LH in controlling retinal VEGF levels. Another important observation of this study is that the LH/VEGF correlation only became manifest when the analytes were normalized by total protein. Thus, laboratory and clinical investigators should consider normalizing vitreous analytes of interest by total protein when assessing potential correlations amongst them.
Acknowledgments
Funding: This study was funded by NIH/NEI Grant # R43EY026281-01
Footnotes
Disclosure of Potential Conflicts of Interest: Zietchick Research Institute (ZRI) is a private, (for-profit) research institute and Dr. Tammy Movsas (Founder and Director of ZRI) has a pending patent application for the use of gonadotropin antagonists for the treatment of ocular diseases. Other than being a fulltime employee (ocular biochemist) at ZRI, Dr. A Muthusamy has no other financial conflicts to report. Dr. Robert Sigler is the Director of Unit Laboratory of Animal Medicine at University of Michigan and has no financial or other potential conflicts to report.
References:
- 1.Ferrara N Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. [DOI] [PubMed] [Google Scholar]
- 2.Amadio M, Govoni S, Pascale A. Targeting VEGF in eye neovascularization: What’s new?: A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol Res. 2016;103:253–69. [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–7. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell RB, Bartoli M, Behzadian MA, ElRemessy AE, Alâ Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19(6):442–55. [DOI] [PubMed] [Google Scholar]
- 5.Semeraro F, Cancarini A, Morescalchi F, Romano M, dellOmo R, Ruggeri G, et al. Serum and intraocular concentrations of erythropoietin and vascular endothelial growth factor in patients with type 2 diabetes and proliferative retinopathy. Diabetes & metabolism. 2014;40(6):445–51. [DOI] [PubMed] [Google Scholar]
- 6.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN ophthalmology. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol. 2001;280(6):C1367–74. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. [DOI] [PubMed] [Google Scholar]
- 9.Aly H, Hassanein S, Nada A, Mohamed MH, Atef SH, Atiea W. Vascular endothelial growth factor in neonates with perinatal asphyxia. Brain Dev. 2009;31(8):600–4. [DOI] [PubMed] [Google Scholar]
- 10.Baba T, McLeod DS, Edwards MM, Merges C, Sen T, Sinha D, et al. VEGF 165 b in the developing vasculatures of the fetal human eye. Dev Dyn. 2012;241(3):595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318(2):666–75. [DOI] [PubMed] [Google Scholar]
- 12.Hartnett ME. Pathophysiology and Mechanisms of Severe Retinopathy of Prematurity. Ophthalmology. 2015;122(1):200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung CY. Vascular endothelial growth factor: possible role in fetal development and placental function. J Soc Gynecol Investig. 1997;4(4):169–77. [PubMed] [Google Scholar]
- 14.Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7 Pt 1): 4738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsampalas M, Gridelet V, Berndt S, Foidart J-M, Geenen V, d’Hauterive SP Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. Journal of reproductive immunology. 2010;85(1):93–8. [DOI] [PubMed] [Google Scholar]
- 16.Licht P, Losch A, Dittrich R, Neuwinger J, Siebzehnrübl E, Wildt L Novel insights into human endometrial paracrinology and embryo-maternal communication by intrauterine microdialysis. Human reproduction update. 1998;4(5):532–8. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen K, Laugesen C, Ringholm L, Vestgaard M, Damm P, Mathiesen E. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia. 2010;53(6):1076–83. [DOI] [PubMed] [Google Scholar]
- 18.Babitha V, Yadav V, Chouhan V, Hyder I, Dangi S, Gupta M, et al. Luteinizing hormone, insulin like growth factor-1, and epidermal growth factor stimulate vascular endothelial growth factor production in cultured bubaline granulosa cells. General and comparative endocrinology. 2014;198:1–12. [DOI] [PubMed] [Google Scholar]
- 19.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trau HA, Davis JS, Duffy DM. Angiogenesis in the Primate Ovulatory Follicle Is Stimulated by Luteinizing Hormone via Prostaglandin E2. Biology of reproduction. 2015;92(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong A, Aw S. Postmortem endocrine levels in the vitreous humor. Annals of the Academy of Medicine, Singapore. 1986;15(4):606–9. [PubMed] [Google Scholar]
- 22.Dukic-Stefanovic S, Walther J, Wosch S, Zimmermann G, Wiedemann P, Alexander H, et al. Chorionic gonadotropin and its receptor are both expressed in human retina, possible implications in normal and pathological conditions. PloS one. 2012;7(12):e52567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson D, Othman M, Lei Z, Li X, Huang Z-H, Eadie D, et al. Localization of receptors for luteinizing hormone/chorionic gonadotropin in neural retina. Life Sci. 1998;63(12):1057–64. [DOI] [PubMed] [Google Scholar]
- 24.Thompson DA, Othman MI, Lei Z, Li X, Huang ZH, Eadie DM, et al. Localization of receptors for luteinizing hormone/chorionic gonadotropin in neural retina. Life Sci. 1998;63(12):1057–64. [DOI] [PubMed] [Google Scholar]
- 25.De Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. IOVS. 2006;47(12):5561–8. [DOI] [PubMed] [Google Scholar]
- 26.Arden G The absence of diabetic retinopathy in patients with retinitis pigmentosa: implications for pathophysiology and possible treatment. Br J Ophthalmol. 2001;85(3):366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteiro JP, Santos FM, Rocha AS, Castro-de-Sousa JP, Queiroz JA, Passarinha LA, et al. Vitreous humor in the pathologic scope: insights from proteomic approaches. Proteomics Clin Appl. 2015;9(1–2):187–202. [DOI] [PubMed] [Google Scholar]
- 28.Simo-Servat O, Hernandez C, Simo R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators Inflamm. 2012;2012:872978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levesque BM, Kalish LA, Winston AB, Parad RB, Hernandez-Diaz S, Phillips M, et al. Low urine vascular endothelial growth factor levels are associated with mechanical ventilation, bronchopulmonary dysplasia and retinopathy of prematurity. Neonatology. 2013;104(1):56–64. [DOI] [PubMed] [Google Scholar]
- 30.Sharma RK, Rowe-Rendleman CL. Validation of molecular and genomic biomarkers of retinal drug efficacy: use of ocular fluid sampling to evaluate VEGF. Neurochem Res. 2011;36(4):655–67. [DOI] [PubMed] [Google Scholar]