Abstract
Background and aims:
The pediatric Critical Illness Stress-induced Immune Suppression (CRISIS) trial compared the effectiveness of 2 nutraceutical supplementation strategies and found no difference in the development of nosocomial infection and sepsis in the overall population. We performed an exploratory post hoc analysis of interaction between nutraceutical treatments and host immune status related to the development of nosocomial infection/sepsis.
Methods:
Children from the CRISIS trial were analyzed according to 3 admission immune status categories marked by decreasing immune competence: immune competent without lymphopenia, immune competent with lymphopenia, and previously immunocompromised. The comparative effectiveness of the 2 treatments was analyzed for interaction with immune status category.
Results:
There were 134 immune-competent children without lymphopenia, 79 previously immune-competent children with lymphopenia, and 27 immunocompromised children who received 1 of the 2 treatments. A significant interaction was found between treatment arms and immune status on the time to development of nosocomial infection and sepsis (P < .05) and on the rate of nosocomial infection and sepsis per 100 patient days (P < .05). Whey protein treatment protected immune-competent patients without lymphopenia from infection and sepsis, both nutraceutical strategies were equivalent in immune-competent patients with lymphopenia, and zinc, selenium, glutamine, and metoclopramide treatment protected immunocompromised patients from infection and sepsis.
Conclusions:
The science of immune nutrition is more complex than previously thought. Future trial design should consider immune status at the time oftrial entry because differential effects of nutraceuticals may be related to this patient characteristic. (JPENJParenterEnteralNutr. 2017;41:1325–1335)
Keywords: nutraceutical, immune status, nosocomial infection
Introduction
Despite widespread implementation of Centers for Disease Control and Prevention (CDC) recommendations, including hand washing and infection prevention bundles, hospital-acquired nosocomial infection and sepsis remain important problems in critically ill children.1 Hospital-acquired infections and sepsis are more common in long-stay patients in part related to exposures to invasive procedures.2,3 Among these children, factors that predispose to the development of nosocomial infection in immune-competent children include breakdown of the epithelial barrier in conditions such as trauma.4–7 The presence of lymphopenia is an additional risk factor in otherwise immune-competent children. Immune-competent children with lymphopenia have more nosocomial infections than immune-competent children with normal lymphocyte counts.8–13 This is believed to be related in part to an increased stress response with a relative reduced ability to clear infections. Immunocompromised children with diagnoses such as cancer, AIDS, solid organ transplantation, stem cell transplantation, autoimmune disease, primary immunodeficiency syndromes, or chronic use of immune-suppressant therapies are considered at greatest risk of developing nosocomial infection and sepsis due to the inability of their immune systems to fight infections.
The role of nutraceuticals in the protection against the development of nosocomial infection and sepsis is a highly studied field with positive trials found using whey protein, zinc, selenium, and glutamine in premature neonates and in malnourished infants.14–41 Despite this body of work, the role of immune nutrition in critically ill children as a whole and across varied immune status categories of critically ill children remains an important knowledge gap. In this regard, we previously performed the comparative effectiveness pediatric Critical Illness Stress-induced Immune Suppression (CRISIS) prevention trial and evaluated 2 different nutraceutical strategies comparing daily supplementation with whey protein to daily supplementation with zinc, selenium, glutamine, and metoclopramide for the prevention of nosocomial infection and sepsis.3 The trial was stopped early because there was no difference between treatment arms in the overall population in the development of nosocomial infection and sepsis. Nevertheless, a reduction in the rate of nosocomial infection and sepsis in the pre hoc stratified immunocompromised group of patients with the use of zinc, selenium, glutamine, and metoclopramide was observed. This finding might signal an interaction between host immune status and the effects of nutraceutical treatments.
To assess this possibility, we performed a post hoc exploratory analysis of the CRISIS trial database and reassessed the study population according to 3 post hoc admission categories of decreasing immune competence: (1) immune competent without lymphopenia, (2) immune competent with lymphopenia, and (3) immunocompromised. We reason that if the 2 nutraceutical regimens interacted with immune status, then their effects on the development of nosocomial infection and sepsis should differ across these ordered categories.
Materials and Methods
All children from our previously published prospective randomized comparative effectiveness trial3 who received nutraceutical treatments for the prevention of critical illness stress-induced immune suppression-related nosocomial infection and sepsis, with available absolute lymphocyte count (ALC) data to allow categorization for this report, were included. After obtaining institutional review board approval from each institution, informed consents were obtained from the parents of each of the children who were subsequently randomized to 1 of the 2 treatment arms. Patients were randomized to receive enteral whey protein powder and intravenous (IV) saline (WHEY group) or enteral zinc, selenium, and glutamine and IV metoclopramide (a prolactin secretalogue) (ZSGM group). Patients assigned to the WHEY group received 0.3 g/kg Beneprotein (Nestle Health Science, Vevey, Switzerland) each morning and IV saline every 12 hours. Patients assigned to the ZSGM group received enteral zinc (20 mg), selenium (40 μg for ages 1–3 years, 100 μg for ages 3–5 years, 200 pg for ages 5–12 years, and 400 pg for adolescents) and glutamine (0.3 g/kg) each morning and IV metoclopramide (0.2 mg/kg, maximum 10 mg) every 12 hours. Entry criteria in the randomized trial were an expected pediatric intensive care unit (PICU) stay >3 days with indwelling invasive devices, including endotracheal tubes, central venous catheters, and indwelling urinary catheters. The patient age had to be >1 year but <18 years. Exclusion criteria included the following: (1) a known allergy to metoclopramide; (2) planned removal of endotracheal tube, central venous, and urinary catheters within 72 hours after study enrollment; (3) suspected intestinal obstruction; (4) intestinal surgery or bowel disruption; (5) other contraindications to the enteral administration of drugs or nutrients; (6) chronic metoclopramide therapy prior to enrollment; (7) a known allergy to whey (cow’s milk) or soy-based products; (8) discharged from the PICU in the previous 28 days; (9) previously enrolled in this study; or (10) a positive pregnancy test. Patients were also excluded if their parents indicated a lack of commitment to aggressive intensive care therapies.
The children were considered immune competent if they had no known immunocompromised condition at admission to the PICU such as cancer, AIDS, solid organ transplantation, stem cell transplantation, autoimmune disease, primary immunodeficiency syndromes, or chronic use of immune-suppressant therapies. In this report, review of patient study data rather than the “as-randomized” immunocompromised stratum was used for this classification, resulting in 2 additional children being classified as immune compromised as they had admission diagnoses of cancer. The children were considered to have lymphopenia if they had an ALC <1000 mm3 any time after PICU admission and before they received nutraceutical treatment. Nosocomial infection and sepsis were defined according to the CDC criteria. Nosocomial was defined by onset >48 hours after PICU admission during the hospital stay until 5 days after discharge from the PICU; for children remaining in the PICU for >28 days, events were counted for up to 33 days. Follow-up ceased if the child died, withdrew from follow-up, or was discharged from the hospital prior to the above time points. Infection was defined by a positive culture, polymerase chain reaction (PCR) assay, or antigen test identifying an organism in a patient with fever, systolic blood pressure <90 mm Hg, or urine output <20 mL/h considered to be the cause of these symptoms for which the primary clinician chose to treat the patient with antimicrobial therapy. Sepsis was defined similarly except that there was no identified organism.
Patients were recruited from and enrolled in the PICU of the 8 centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. These sites included Arkansas Children’s Hospital, Children’s Hospital of Los Angeles, Mattel Children’s Hospital UCLA, Seattle Children’s Hospital, Harborview Medical Center, Children’s Hospital of Michigan, Children’s National Medical Center, and Children’s Hospital of Pittsburgh. After obtaining informed written consent from the parent, the children were randomized to receive (in a double-blinded fashion) 1 of the 2 therapies. Zinc, selenium, and prolactin levels were measured before treatment (study day 1). Zinc deficiency was defined as a level <0.60 μg/mL in children aged <10 years and <0.66 μg/ mL in children aged at least 11 years. Selenium deficiency was identified by a level <70 ng/mL in children aged <10 years and <95 ng/mL in children ages at least 11 years. Prolactin deficiency was defined as a level <3 ng/mL across all ages. The primary end point was time to nosocomial infection/sepsis. The secondary end point was nosocomial infection/sepsis rate per 100 days. Nosocomial infections and sepsis were identified by the site principal investigators and clinical research coordinators. All identified nosocomial infections were then adjudicated by the network steering committee, which included the site principal investigators, representatives of the data coordinating center (University of Utah), and the network scientific research officer (National Institutes of Child Health and Development) who were blinded to the treatment arm. Weight-for-age, height-for-age, and body mass index (BMI)-for-age percentiles were calculated based on 2006 World Health Organization (WHO) growth charts for children younger than 2 years and 2000 CDC growth charts for children 2 years or older.
In this post hoc analysis, patients were divided into 3 cohorts according to decreasing immune function: immune competent without lymphopenia, immune competent with lymphopenia, and immunocompromised. The patients were analyzed according to the treatment arm received rather than to the intention-to-treat analysis (as was performed in the primary analysis).
Statistical Analysis
Descriptive statistics were used to illustrate PICU admission characteristics, nosocomial infection sites, and nosocomial infection organisms among the 3 immune status cohorts. The statistical significance of association between immune status and each characteristic was assessed with methods treating immune status as ordered according to decreasing immune function—specifically, the Jonckheere-Terpstra test for continuous/ordinal characteristics, Mantel-Haenszel χ2 test for binary characteristics, and Pearson χ2 or Fisher exact test for categorical characteristics. Within each immune status cohort, we compared PICU admission characteristics by treatment group with descriptive statistics, assessing the statistical significance of associations using the Pearson χ2 or Fisher exact test for categorical characteristics and the Wilcoxon rank-sum test for continuous/ordinal characteristics. Monte Carlo simulations were used to compute Fisher exact test P values when direct computation was not feasible. We evaluated the interaction effect of immune status and treatment on the development of nosocomial infection/sepsis by comparing 2 outcome measures of nosocomial infection/sepsis: (1) the time to development of nosocomial infection/sepsis and (2) the rate of nosocomial infection/sepsis per 100 days. Reported significance levels are not adjusted for multiple comparisons as this analysis is considered exploratory.
Kaplan-Meier curves comparing the time to the development of nosocomial infection/sepsis between the 2 treatment groups are presented separately for each immune status cohort. The log-rank test was used to compare the survival curves between treatment groups within each cohort. The interaction effect of immune status and treatment on the time to the development of nosocomial infection/sepsis was assessed using the Wald X test for interaction between treatment and immune status using Cox regression analysis with treatment and immune status also included in the model. The interaction effect of prolactin deficiency, selenium deficiency, zinc deficiency, height-for-age percentile, or weight-for-age percentile and treatment on time to development of nosocomial infection/sepsis was similarly assessed.
Rates of nosocomial infection/sepsis events per 100 days were calculated, counting multiple events per patient. Event rates were compared by treatment within each immune status cohort. The significance of treatment effects within immune status cohort was assessed using Poisson regression and the Wald X test; associated 95% CIs are also reported. The interaction effect of immune status and treatment on event rates was assessed using a Wald χ2 test for the interaction between treatment and immune status using Poisson regression with treatment and immune status also included in the model. The interaction effect of prolactin deficiency, selenium deficiency, zinc deficiency, height-for-age percentile, or weight-for-age percentile and treatment on time to development of nosocomial infection/sepsis was similarly assessed.
Results
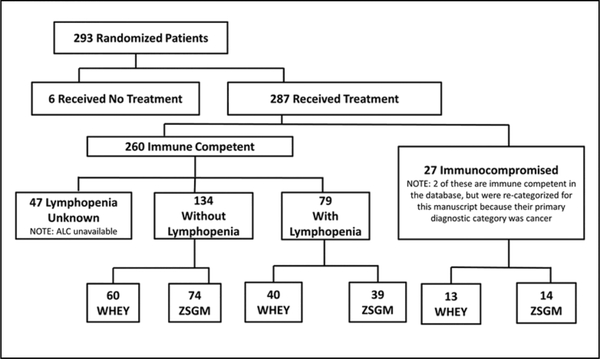
Among treated study children with available lymphocyte count data to allow categorization for this report, there were 134 immune-competent children without lymphopenia, 79 previously immune-competent children with lymphopenia, and 27 immunocompromised children enrolled who had received 1 of the 2 treatments (WHEY, n = 113; ZSGM, n = 127) (Figure 1). Tables 1–4 demonstrate the admission characteristics of the 3 cohorts as a whole and according to treatment arms. Day 1 minimum white blood cell counts (103/mm3) were available for 230 children overall (median, 9.8 [range, 0.0–31.3]) and were 10.4 (2.3–28.3) in the immune competent without existing lymphopenia group (n = 128), 9.6 (2.1–24.7) in the immune competent with existing lymphopenia group (n = 77), and 8.0 (0.0–31.3) in the immunocompromised group (n = 25). Day 1 maximum glucose levels (mg/dL) were available for 208 children overall (134 [55–449]) and were 128 (55–298) in the immune competent without existing lymphopenia group (n = 118), 148 (60–449) in the immune competent with existing lymphopenia group (n = 68), and 143 (79–284) in the immunocompromised group (n = 22). As immune function decreased, there was a trend for age (P = .02), PELOD (P = .03), PRISM (P = .004), and day 1 glucose (P = .03) to be increased and day 1 white blood cell counts to be decreased (P = .002). Immunocompromised patients were more likely to have existing infection and sepsis, whereas those in the immune-competent groups were more likely to have trauma as a primary diagnosis. Within the treatment arms, there was good balance for admission characteristics among the immunocompromised patients; however, the immune-competent patients had some discrepancies. For example, patients who were immune competent without lymphopenia and received ZSGM were more likely to have trauma and less likely to have pneumonia/bronchiolitis, tended to be male, tended to be taller for age, and tended not to have existing sepsis compared with those receiving whey protein. Table 5 displays the nosocomial infections found in the 3 cohorts.
Figure 1.
Patient flow diagram. ALC, available absolute lymphocyte count; WHEY, whey protein powder and intravenous saline group; ZSGM, zinc selenium, glutamine, and metoclopramide group.
Table 1.
Admission Characteristics According to Immune Status Category.
| Characteristic | Immune Competent Without Characteristic Lymphopenia (n = 134) | Immune Competent With Lymphopenia (n = 79) | Immunocompromised (n = 27) | P Value |
|---|---|---|---|---|
| Age, median (range), y | 5.1 (1–18) | 9.9 (1,18) | 7.9 (1–18) | .02a |
| Female, % | 48 | 51 | 44 | .96b |
| Weight-for-age percentile, median (range) | 48 (0–100) n = 125 | 53 (0–100) n = 76 | 19 (0–100) n = 26 | .81a |
| Height-for-age percentile, median (range) | 22 (0–100) | 51 (0–100) | 5 (0–100) | .51a |
| BMI-for-age percentile, median (range) | 66 (0–100) | 75 (0–100) | 50 (0–100) | .78a |
| PELOD, median (range) | 11 (0–50) | 11 (1–31) | 12 (0–31) | .03a |
| PRISM, median (range) | 7 (0–31) | 9(0–28) | 12 (0–26) | .004a |
| OFI, median (range) | 2 (0–6) | 2(0–5) | 2 (0–4) | .97a |
| Postoperative PICU admit, % | 25 | 25 | 19 | .64b |
| Primary diagnosis, % | .0001c | |||
| Asthma | 1 | 3 | 0 | |
| Cancer | 0 | 0 | 22 | |
| Cardiac arrest | 3 | 4 | 4 | |
| Cardiovascular disease—acquired | 1 | 1 | 4 | |
| Cardiovascular disease—congenital | 4 | 1 | 7 | |
| Drug overdose | 1 | 0 | 0 | |
| HIV | 0 | 0 | 4 | |
| Hypoxic-ischemic encephalopathy | 0 | 3 | 0 | |
| Meningitis | 1 | 1 | 0 | |
| Pneumonia/bronchiolitis | 24 | 16 | 11 | |
| Seizures | 4 | 6 | 0 | |
| Sepsis | 7 | 5 | 15 | |
| Shock | 2 | 6 | 4 | |
| Transplant | 0 | 0 | 4 | |
| Trauma | 22 | 24 | 0 | |
| Other | 30 | 29 | 26 | |
| Chronic diagnoses, % | 46 | 39 | 74 | .12b |
| Infection status at entry, % | .12d | |||
| Existing infection | 41 | 27 | 48 | |
| Existing sepsis | 29 | 34 | 33 | |
| No infection or sepsis | 30 | 39 | 19 |
BMI, body mass index; HIV, human immunodeficiency virus; OFI, Organ Failure Index; PELOD, Pediatric Logistic Organ Dysfunction score; PICU, pediatric intensive care unit; PRISM, Pediatric Risk of Mortality.
P value is based on the Jonckheere-Terpstra test for continuous/ordinal characteristics.
P value is based on the Mantel-Haenszel X test for binary characteristics.
P value is based on the Fisher exact test for categorical characteristics.
P value is based on the Pearson X test for categorical characteristics.
Table 4.
Admission Characteristics by Treatment Received and Immune Status: Immunocompromised.
| Characteristic | WHEY (n = 13) | ZSGM (n = 14) | P Value |
|---|---|---|---|
| Age, median (range), y | 8.6 (1–18) | 6.7 (1–18) | .32a |
| Female, % | 46 | 43 | .86b |
| Weight-for-age percentile, median (range) | 22 (0–100) | 11 (0–86) | .23a |
| n = 13 | n = 13 | ||
| Height-for-age percentile, median (range) | 9 (0–100) | 1 (0–39) | .36a |
| BMI-for-age percentile, median (range) | 74 (0–99) | 41 (0–100) | .18a |
| PELOD, median (range) | 11 (1–31) | 12 (0–22) | .96a |
| PRISM, median (range) | 12 (3–17) | 12 (0–26) | .98a |
| OFI, median (range) | 2(1–3) | 2 (0–4) | .72a |
| Postoperative PICU admit, % | 31 | 7 | .16c |
| Primary diagnosis, % | .84c | ||
| Cancer | 31 | 14 | |
| Cardiac arrest | 8 | 0 | |
| Cardiovascular disease—acquired | 8 | 0 | |
| Cardiovascular disease—congenital | 8 | 7 | |
| HIV infection | 0 | 7 | |
| Pneumonia/bronchiolitis | 8 | 14 | |
| Sepsis | 8 | 21 | |
| Shock | 0 | 7 | |
| Transplant | 8 | 0 | |
| Other | 23 | 29 | |
| Chronic diagnoses, % | 69 | 79 | .68c |
| Infection status at entry, % | .68c | ||
| Existing infection | 38 | 57 | |
| Existing sepsis | 38 | 29 | |
| No infection or sepsis | 23 | 14 |
BMI, body mass index; HIV, human immunodeficiency virus; OFI, Organ Failure Index; PELOD, Pediatric Logistic Organ Dysfunction score; PICU, pediatric intensive care unit; PRISM, Pediatric Risk of Mortality; WHEY, whey protein powder and intravenous saline group; ZSGM, zinc, selenium, glutamine, and metoclopramide group.
P value is based on the Wilcoxon rank-sum test for continuous/ordinal characteristics.
P value is based on the Pearson χ2 test for categorical characteristics.
P value is based on the Fisher exact test for categorical characteristics.
Table 5.
Nosocomial Infections According to Immune Status Category.
| Variable | Immune Competent Without Lymphopenia (n = 134) | Immune Competent With Lymphopenia (n = 79) | Immunocompromised (n = 27) |
|---|---|---|---|
| Patients with 1 or more infections, No. (%) | 37 (28) | 37 (47) | 7 (26) |
| Total No. of infections | 58 | 62 | 10 |
| Site of infection, No. (%)a | |||
| Lower respiratory | 42 (72) | 34 (55) | 1 (10) |
| Upper respiratory | 0 (0) | 2 (3) | 0 (0) |
| Urinary tract | 8 (14) | 6 (10) | 3 (30) |
| Skin or soft tissue | 3 (5) | 9 (15) | 0 (0) |
| Bacteremia | 4 (7) | 7 (11) | 6 (60) |
| Other | 1 (2) | 4 (6) | 0 (0) |
| Total No. of infecting organismsb | 79 | 82 | 10 |
| Fungi, No. (%) | 15 (19) | 14 (17) | 2 (20) |
| Candida albicans | 2 (3) | 3 (4) | 1 (10) |
| Candida tropicalis | 5 (6) | 0 (0) | 1 (10) |
| Yeast | 3 (4) | 3 (4) | 0 (0) |
| Candida lusitanae | 0 (0) | 4 (5) | 0 (0) |
| Candida glabrata | 2 (3) | 2 (2) | 0 (0) |
| Other | 3 (4) | 2 (2) | 0 (0) |
| Gram-negative bacilli, No. (%) | 34 (43) | 33 (40) | 3 (30) |
| Pseudomonas aeruginosa | 12 (15) | 12 (15) | 1 (10) |
| Haemophilus influenzae | 6 (8) | 3 (4) | 0 (0) |
| Stenotrophomonas maltophilia | 3 (4) | 4 (5) | 0 (0) |
| Enterobacter cloacae | 3 (4) | 0 (0) | 0 (0) |
| Klebsiella pneumoniae | 1 (1) | 3 (4) | 0 (0) |
| Other | 9 (11) | 11 (13) | 2 (20) |
| Gram-positive bacilli, No. (%) | 0 (0) | 2 (2) | 0 (0) |
| Clostridium dificile | 0 (0) | 2 (2) | 0 (0) |
| Gram-negative cocci, No. (%) | 3 (4) | 1 (1) | 0 (0) |
| Moraxella catarrhalis | 3 (4) | 1 (1) | 0 (0) |
| Gram-positive cocci, No. (%) | 24 (30) | 27 (33) | 5 (50) |
| Staphylococcus aureus | 15 (19) | 10 (12) | 0 (0) |
| Staphylococcus coagulase negative | 1 (1) | 5 (6) | 0 (0) |
| Staphylococcus epidermidis | 4 (5) | 0 (0) | 0 (0) |
| Enterococcus faecalis | 1 (1) | 3 (4) | 0 (0) |
| Other | 3 (4) | 9 (11) | 5 (50) |
| Virus, No. (%) | 1 (1) | 3 (4) | 0 (0) |
| Human herpes virus 6 | 0 (0) | 1 (1) | 0 (0) |
| Parainfluenza virus type 3 | 0 (0) | 1 (1) | 0 (0) |
| Respiratory syncytial virus | 1 (1) | 1 (1) | 0 (0) |
| Undetermined, No. (%) | 2 (3) | 2 (2) | 0 (0) |
Percentages are out of total infections because patients have multiple infections.
Percentages are out of total infecting organisms because infections have multiple infecting organisms.
Figure 2 depicts the relationships between the 2 treatment arms and the 3 host immune status categories as it relates to the time to development of the first nosocomial infection and sepsis episode. Among the patients who received whey, the immune-competent patients without lymphopenia had the longest time to the development of nosocomial infection or sepsis (median time not reached at 33 days, with 54% event free at 16.2 days; Figure 2A), and the immunocompromised patients had the shortest time to the development of nosocomial infection or sepsis (median time 10.0 days; Figure 2C). Whey protein treatment delayed the time until the first nosocomial infection or sepsis episode in the immune-competent children without lymphopenia (median time for those receiving whey was >16 days compared with 12.1 days in those receiving ZSGM; P = .02) but not in the immunocompromised patients. In immunocompromised patients, whey protein therapy resulted in earlier onset of nosocomial infection or sepsis (median 10.0 days) vs ZSGM therapy (median 32.4 days; P = .16). Overall, this analysis suggests a significant interaction between treatment arm and immune status and the time to the development of nosocomial infection and sepsis (P = .0497). There were no significant interactions between treatment arm and prolactin deficiency (P = .59), selenium deficiency (P = .30), zinc deficiency (P = .17), height-for-age percentile (P = .85), or weight-for-age percentile (P = .45) and the time to development of nosocomial infection and sepsis.
Figure 2.
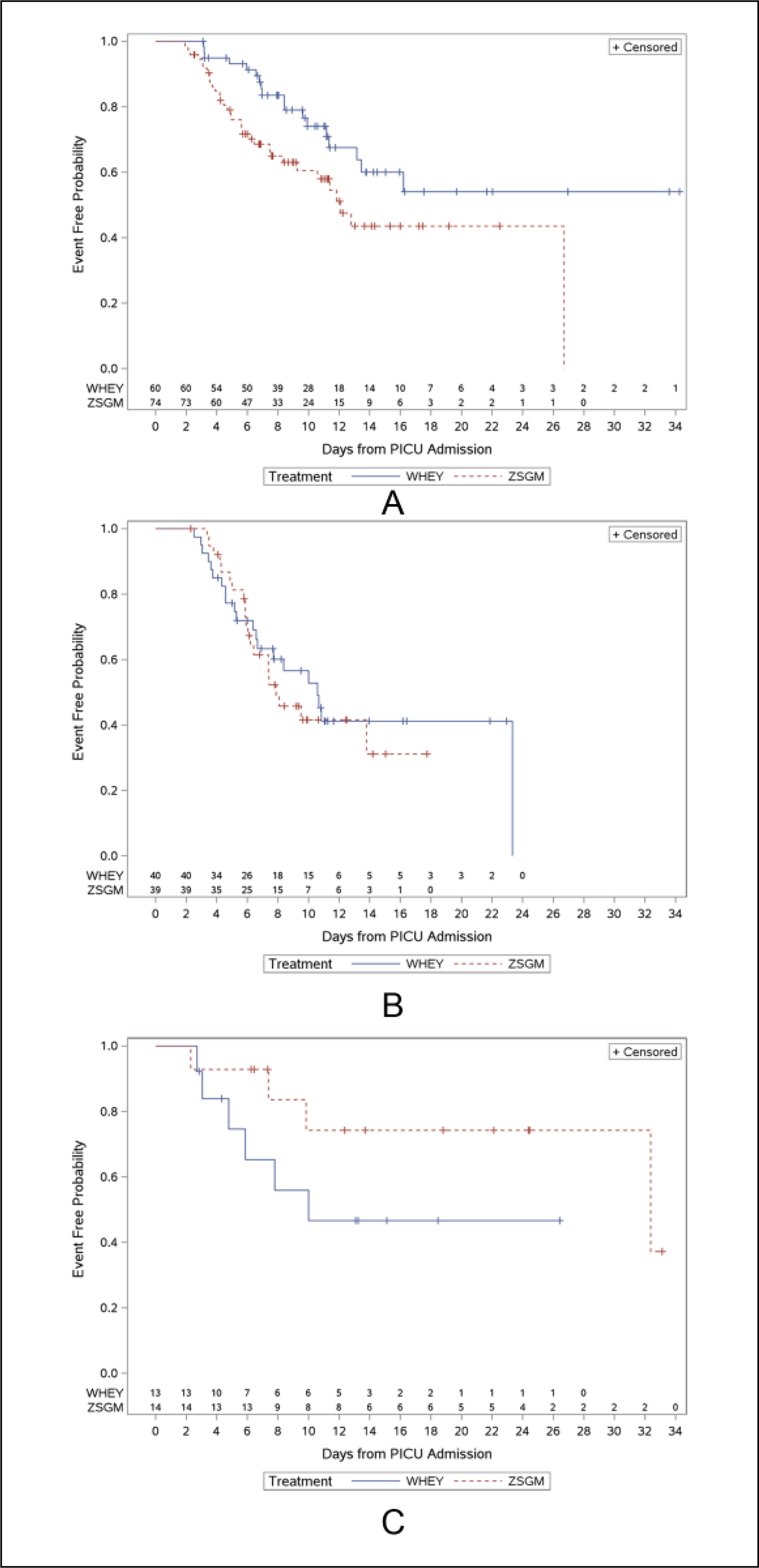
(A) Time to first nosocomial infection and sepsis episode in immune-competent hosts without lymphopenia. Median time to nosocomial infection and sepsis was not reached at 33 days for whey protein compared with 12.1 days for ZSGM (P = .02). (B) Time to first nosocomial infection and sepsis episode in immune-competent hosts with lymphopenia. Median time to nosocomial infection and sepsis episode was 10.6 days with whey protein compared with 7.9 days with ZSGM (P = .62). (C) Time to first nosocomial infection and sepsis episode in immunocompromised hosts. Median time to nosocomial infection and sepsis was 10.0 days with whey protein compared with 32.4 days with ZSGM (P = .16). Hash lines represent ZSGM and solid lines represent WHEY. PICU, pediatric intensive care unit; WHEY, whey protein powder and intravenous saline group; ZSGM, zinc, selenium, glutamine, and metoclopramide group.
Table 6 reveals the relationships between the treatment arms and the host immune status categories and rate of nosocomial infection and sepsis per 100 days. Among children receiving whey, the rate of nosocomial infection and sepsis was least in immune-competent children without lymphopenia (3.27 per 100 days) and greatest in immunocompromised children (6.33 per 100 days). ZSGM appeared to protect the immunocompromised children from nosocomial infection and sepsis (1.57 per 100 days with ZSGM vs 6.33 per 100 days with whey protein; P = .01). The rate of nosocomial infection and sepsis in the immune-competent children without lymphopenia was 3.27 per 100 days with whey protein compared with 4.74 per 100 days with ZSGM (P = .09). There was a significant interaction between treatment arms and immune status and the rate of nosocomial infection and sepsis per 100 days (P = .01). There were no significant interactions between treatment arm and prolactin deficiency (P = .58), selenium deficiency (P = .76), zinc deficiency (P = .99), height-for-age percentile (P = .07), or weight-for-age percentile (0.78) and the rate of nosocomial infection and sepsis per 100 days.
Table 6.
Rates of Nosocomial Infection/Sepsis by Treatment and Immune Status.
| Immune Status | WHEY (n = 113) | ZSGM (n = 127) | Within Subgroup, P Value | Interaction, P Value |
|---|---|---|---|---|
| Immune competent without lymphopenia | n = 60 | n = 74 | .09 | .01 |
| Total No. of events (infection or sepsis) | 33 | 54 | ||
| Total No. of days | 1008 | 1139 | ||
| Mean events/patient/100 days (95% CI) | 3.27 (2.33–4.60) | 4.74 (3.63–6.19) | ||
| Immune competent with lymphopenia | n = 40 | n = 39 | .49 | |
| Total No. of events (infection or sepsis) | 40 | 40 | ||
| Total No. of days | 672 | 576 | ||
| Mean events/patient/100 days (95% CI) | 5.95 (4.37–8.11) | 6.94 (5.09–9.47) | ||
| Immunocompromised | n = 13 | n = 14 | .01 | |
| Total No. of events (infection or sepsis) | 15 | 4 | ||
| Total No. of days | 237 | 255 | ||
| Mean events/patient/100 days (95% CI) | 6.33 (3.82–10.50) | 1.57 (0.59–4.18) |
WHEY, whey protein powder and intravenous saline group; ZSGM, zinc, selenium, glutamine, and metoclopramide group.
Discussion
In this analysis, the effects of the 2 different nutraceuticals on the development of nosocomial infection and sepsis were not the same across the 3 immune status categories assessed. The protective treatment effects of each nutraceutical varied in opposite directions across each end of the immune status spectrum. One was most protective in the most immune-competent population, while the other was most protective in the most immunocompromised population, with no difference observed between the 2 treatments in the in-between immune status population. These post hoc exploratory findings suggest that the contention that nutraceuticals are equally effective regardless of the host immune status category requires further study.
Whey protein is derived from the “fast protein” or easily digestible portion of cow’s milk. It contains all of the known essential amino acids. These essential amino acids are very important for repair of epithelial barriers as well as maintenance of immune cell function.16,17 In addition, whey protein has high concentrations of lactoferrin. This protein is produced in cow and human breast milk to prevent infection in newborns.42 Lactoferrin sequesters iron from bacteria without inducing anemia.43 This effect prevents the overgrowth of iron-loving microbial pathogens and prevents late-onset infection and sepsis in very low-birth-weight infants.44 If whey protein is protective in the immune-competent host, then it is biologically plausible that it is related to these qualities because epithelial breakdown and exposure to pathogens are thought to be important predisposing causes of nosocomial infection and sepsis in this population. Unfortunately, in our study, the protective effects of whey protein appeared to be limited to the immune-competent host. Within the whey-treated groups, we observed that the time to nosocomial infection and sepsis decreased and the rate of nosocomial infection and sepsis increased as patients became more immunocompromised going across the categories from the immune-competent host without lymphopenia, to the immune-competent host with lymphopenia, to the immunocompromised host.
The immunocompromised population appeared to be most protected from nosocomial infection and sepsis by supplementation with ZSGM. The inability to fight infection in these children may be related in part to the use of immune suppressants and chemotherapy, which can result in lymphopenia and neutropenia. However, it may also be related to a profound Th2 state.12,45 A properly functioning immune response requires a balanced Th1/Th2 axis. The Th1 response is responsible for killing bacteria, fungus, and virus, whereas the Th2 response is responsible for making antibodies and dampening the Th1 response. Zinc, selenium, glutamine, and prolactin (secreted in response to metoclopramide) can all prevent stress-induced lymphopenia. Glutamine and prolactin can also restore the Th1/Th2 balance.27,46,47 It is biologically plausible that this Th1-like effect might make this nutraceutical approach more effective in the Th2-dominant immunocompromised host than the whey protein approach.
Our study suggests that many factors could be related to the differential responses found among the arms related to the immune status groups. For example, stress-induced hypermetabolism and the catabolic state (insulin resistance) has been associated with infectious complications, particularly in burn patients. In this regard, we found more hyperglycemia (median glucose >140 mg/dL) in the patients with lymphopenia or known conditions associated with immunocompromise than in the immune-competent patients without lymphopenia (median glucose <130 mg/dL). Patients with known baseline immunocompromise had reduced weight-for-age percentile, height-for-age percentile, and BMI-for-age percentile, suggesting that there was more malnutrition with chronic illness in these children compared with the immune-competent patients with or without lymphopenia. The immunocompromised group also had lower white blood cell counts, and a greater percentage had existing sepsis or infection, suggesting further that iatrogenic immune suppression as well as malnutrition could be important in this process. Severity of illness (PELOD and PRISM) and presumably stress also increased in a graded fashion from the immune competent without lymphopenia, to the immune competent with lymphopenia, to the immunocompromised groups. With regard to primary diagnoses, besides the obvious finding that cancer, transplantation, and HIV accounted for many immunocompromised patients and none of the immune-competent patients, trauma accounted for many immune-competent patients and no immunocompromised patients. It is biologically plausible that nutraceutical strategies aimed at improving immune competence in the previously immunocompromised patient are very likely different from those aimed at improving epithelial function in trauma patients.
There are several important limitations to consider in this post hoc analysis. First, we used an ALC <1000/mm3 as a biomarker of immune deficiency but did not measure immune function. However, we and others previously demonstrated that this cutoff identifies children at increased risk for nosocomial infection and sepsis.7–13 Second, we did not perform a nutrition assessment with measurements of metabolic rate, protein, prealbumin, triglycerides, or free amino acids. However, we found no interaction between treatment arm and selenium or zinc deficiency status and the development of nosocomial infection or sepsis. Third, we did not measure the proinflammatory or anti-inflammatory response or lymphocyte subsets. However, we found no interaction between treatment arm and prolactin (counterregulatory stress hormone) deficiency and the development of nosocomial infection and sepsis. It is desirable to perform more in-depth immune function, nutrition, and cytokine analyses and assessments in future studies. Fourth, the immune-competent patients without lymphopenia had some imbalance in admission characteristics according to treatment arm. Those who were treated with ZSGM were more likely to have trauma compared with pneumonia/bronchiolitis and to be male. However, this article is an exploratory post hoc analysis that is hypothesis generating rather than definitive. Future randomized controlled studies will be needed to determine whether whey protein is protective in the immune-competent population without lymphopenia and/or ZSGM is protective in the immunocompromised host. Fifth, when comparing results in this post hoc analysis with those published in the primary analysis, it is important to note that the present article used a “treatment-received” analysis among children with available lymphopenia status, whereas the primary manuscript used an intention-to-treat analysis on all randomized children. Therefore, slight data discrepancies exist between the 2 analyses. Treatment-received analyses are most appropriately used for hypothesis generation, rather than treatment decision making, because unmeasured factors related to discrepancies between intention to treat and treatment received could unexpectedly skew results.
In summary, the findings of this study suggest a previously unappreciated interaction between 2 nutraceutical approaches and immune status categories in the development of nosocomial infection and sepsis. These findings suggest that the science of immune nutrition in critically ill children may be more complex than previously thought. This has several implications. Future trial designs may be improved if immune status at the time of trial entry is incorporated in stratification schemas because differential effects of nutraceuticals might be related to this patient characteristic. Three easily identified categories for stratification randomization strategies include the immune competent without lymphopenia, immune competent with lymphopenia, and immunocompromised hosts. Because this is a post hoc treatment-received analysis, further randomized studies of whey protein supplementation to protect against nosocomial infection and sepsis in the immune-competent host without lymphopenia and/or studies of ZSGM supplementation to protect against nosocomial infection and sepsis in the immunocompromised host will be needed to determine whether the hypotheses generated herein are valid.
Table 2.
Admission Characteristics by Treatment Received and Immune Status: Immune Competent Without Lymphopenia.
| Characteristic | WHEY (n = 60) | ZSGM (n = 74) | P Value |
|---|---|---|---|
| Age, median (range), y | 5.1 (1–18) | 5.2 (1–18) | .53a |
| Female (%) | 58 | 39 | .03b |
| Weight-for-age percentile, median (range) | 34 (0–100) n = 54 | 52 (0–100) n = 71 | .15a |
| Height-for-age percentile, median (range) | 13 (0–100) | 46 (0–100) | .07a |
| BMI-for-age percentile, median (range) | 72 (0–100) | 64 (0–100) | .47a |
| PELOD, median (range) | 11 (0–31) | 11 (1–50) | .55a |
| PRISM, median (range) | 8 (0–27) | 7 (0–31) | .87a |
| OFI, median (range) | 2 (0–5) | 2 (0–6) | .78a |
| Postoperative PICU admit, % | 22 | 27 | .47b |
| Primary diagnosis, % | .04c | ||
| Asthma | 2 | 0 | |
| Cardiac arrest | 2 | 4 | |
| Cardiovascular disease—acquired | 0 | 1 | |
| Cardiovascular disease—congenital | 5 | 4 | |
| Drug overdose | 2 | 0 | |
| Meningitis | 2 | 1 | |
| Pneumonia/bronchiolitis | 33 | 16 | |
| Seizures | 2 | 5 | |
| Sepsis | 8 | 7 | |
| Shock | 5 | 0 | |
| Trauma | 12 | 30 | |
| Other | 28 | 31 | |
| Chronic diagnoses, % | 48 | 45 | .67b |
| Infection status at entry, % | .055b | ||
| Existing infection | 43 | 39 | |
| Existing sepsis | 37 | 23 | |
| No infection or sepsis | 20 | 38 |
BMI, body mass index; HIV, human immunodeficiency virus; OFI, Organ Failure Index; PELOD, Pediatric Logistic Organ Dysfunction score; PICU, pediatric intensive care unit; PRISM, Pediatric Risk of Mortality; WHEY, whey protein powder and intravenous saline group; ZSGM, zinc, selenium, glutamine, and metoclopramide group.
P value is based on the Wilcoxon rank-sum test for continuous/ordinal characteristics.
P value is based on the Pearson X test for categorical characteristics.
P value is based on the Fisher exact test for categorical characteristics.
Table 3.
Admission Characteristics by Treatment Received and Immune Status: Immune Competent With Lymphopenia.
| Characteristic | WHEY (n = 40) | ZSGM (n = 39) | P Value |
|---|---|---|---|
| Age, median (range), y | 11.4 (1–18) | 9.4 (1–18) | .56a |
| Female, % | 53 | 49 | .74b |
| Weight-for-age percentile, median (range) | 53 (0–100) n = 39 | 64 (0–100) n = 37 | .67a |
| Height-for-age percentile, median (range) | 50 (0–100) | 54 (0–100) | .80a |
| BMI-for-age percentile, median (range) | 72 (0–100) | 75 (0–100) | .88a |
| PELOD, median (range) | 11 (1–31) | 11 (1–31) | .34a |
| PRISM, median (range) | 9(0–28) | 9 (0–24) | .79a |
| OFI, median (range) | 2(0–5) | 2 (0–4) | .04a |
| Postoperative PICU admit, % | 28 | 23 | .65b |
| Primary diagnosis, % | .70c | ||
| Asthma | 5 | 0 | |
| Cardiac arrest | 3 | 5 | |
| Cardiovascular disease—acquired | 0 | 3 | |
| Cardiovascular disease—congenital | 3 | 0 | |
| Hypoxic-ischemic encephalopathy | 3 | 3 | |
| Meningitis | 0 | 3 | |
| Pneumonia/bronchiolitis | 15 | 18 | |
| Seizures | 8 | 5 | |
| Sepsis | 3 | 8 | |
| Shock | 3 | 10 | |
| Trauma | 28 | 21 | |
| Other | 33 | 26 | |
| Chronic diagnoses, % | 40 | 38 | .89b |
| Infection status at entry, % | .31b | ||
| Existing infection | 23 | 31 | |
| Existing sepsis | 30 | 38 | |
| No infection or sepsis | 48 | 31 |
BMI, body mass index; HIV, human immunodeficiency virus; OFI, Organ Failure Index; PELOD, Pediatric Logistic Organ Dysfunction score; PICU, pediatric intensive care unit; PRISM, Pediatric Risk of Mortality; WHEY, whey protein powder and intravenous saline group; ZSGM, zinc, selenium, glutamine, and metoclopramide group.
P value is based on the Wilcoxon rank-sum test for continuous/ordinal characteristics.
P value is based on the Pearson χ2 test for categorical characteristics.
P value is based on the Fisher exact test for categorical characteristics
Acknowledgments
Financial disclosure: This work was supported, in part, by cooperative agreements (U10HD049983, U10HD049981, U10HD050096, U10HD500009, U10HD049945, U10HD050012, U10HD063108, U10HD063106, U10HD063114, and U01HD049934) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Department of Health and Human Services.
Footnotes
Conflicts of interest: None declared.
Clinical Relevancy Statement
It may be important to consider host immune status when planning nutraceutical clinical trials aimed at reducing nosocomial infection risk.
References
- 1.Gravel D, Matlow A, Ofner-Agostini M, et al. A point prevalence survey of health care associated infections in pediatric population in major Canadian acute care hospitals. Am J Infect Control 2007;35(3):157–162. [DOI] [PubMed] [Google Scholar]
- 2.LeClerc F, Leteutre S, Duhamel A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Resp Crit Care Med 2005;171(4):348–353. [DOI] [PubMed] [Google Scholar]
- 3.Carcillo JA, Dean JM, Holubkov R, et al. The randomized comparative pediatric Critical Illness Stress-induced Immune Suppression (CRISIS) prevention trial. Pediatr Crit Care Med 2012;13(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taira BR, Fenton KE, Lee TK, et al. Ventilator associated pneumonia in pediatric trauma patients. Pediatr Crit Care Med 2009;10(4):491–494. [DOI] [PubMed] [Google Scholar]
- 5.Upperman JS, Sheridan R. Pediatric susceptibility to sepsis. Pediatr Crit Care Med 2005;6(3):S108–S111. [DOI] [PubMed] [Google Scholar]
- 6.Urrea M, Torner F, Pons M, Latorre C, Huguet R. Incidence study of nosocomial infection in pediatric trauma patients. J Pediatr Orthop B 2005;17(5):37–44. [DOI] [PubMed] [Google Scholar]
- 7.Brook I Infectious complications following trauma in children. Adv Pediatr. 1997;44:429–451. [PubMed] [Google Scholar]
- 8.Meert KL, Offenstein JP, Sarnaik AP. Altered T cell cytokine production following mechanical trauma. Ann Clin Lab Sci 1998;28(5):283–288. [PubMed] [Google Scholar]
- 9.Meert KL, Long M, Kaplan J, Sarnaik AP. Alterations in immune function following head injury in children. Crit Care Med. 1995;23(5):822–828. [DOI] [PubMed] [Google Scholar]
- 10.Heffernan DS, Monaghan SF, Thakka RK, Machan ST, Cioffi WG, Ayala R. Failure to normalize lymphopenia following trauma is associated with increased mortality independent of leukocytosis pattern. Crit Care 2012;16(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucin KM, Sanders VM. Stress hormones collaborate to induce lymphocyte apoptosis after high spinal cord injury. J Neurochem 2009;110(5):1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intens Care Med 2011;37(3):525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felmet KA, Hall MW, Clark RS, et al. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol 2005;174(6):3765–3772. [DOI] [PubMed] [Google Scholar]
- 14.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. [DOI] [PubMed] [Google Scholar]
- 15.Stone CA, Kawai K, Kuphra R, Fawzi WW. Role of selenium in HIV infection. Nutr Rev. 2010;68(1):671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth E Immune and cell modulation by amino acids. Clin Nutr. 2007;26(5):535–544. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Yin YL, Li D, et al. Amino acids and immune function. Br J Nutr. 2007;98(2):237–252. [DOI] [PubMed] [Google Scholar]
- 18.Yavagal DR, Karnad DR, Oak JL. Metoclopramide for preventing pneumonia in critically ill patients receiving enteral tube feeding: a randomized controlled trial. Crit Care Med. 2000;28(5):1408–1411. [DOI] [PubMed] [Google Scholar]
- 19.Brooks WA, Yunus M, Santosham M, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet. 2004;363(9422):1683–1688. [DOI] [PubMed] [Google Scholar]
- 20.Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–275. [DOI] [PubMed] [Google Scholar]
- 21.Baqui AH, Black RE, El Arifeen S, et al. Zinc therapy for diarrhoea increased the use of oral rehydration therapy and reduced the use of antibiotics in Bangladeshi children. J Health Popul Nutr. 2004;22(4):440–442. [PubMed] [Google Scholar]
- 22.Bhatnagar S, Bahl R, Sharma PK, et al. Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: a randomized controlled trial. J Pediatr Gastroenterol Nutr 2004;38(1): 34–40. [DOI] [PubMed] [Google Scholar]
- 23.Raqib R, Roy SK, Rahman MJ, et al. Effect of zinc supplementation on immune and inflammatory responses in pediatric patients with shigellosis. Am J Clin Nutr. 2004;79(3):444–450. [DOI] [PubMed] [Google Scholar]
- 24.Sazawal S, Black RE, Menon VP, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics. 2001;108(6):1280–1286. [DOI] [PubMed] [Google Scholar]
- 25.Darlow BA, Austin NC. Selenium supplementation to prevent short term morbidity in preterm neonates. Cochrane Database Syst Rev 2003;4:CD003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Berg A, van Elburg RM, Westerbeek EA, et al. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: a randomized controlled trial. Am J Clin Nutr. 2005;81(6):1397–1404. [DOI] [PubMed] [Google Scholar]
- 27.Boelens PG, Houdijk AP, Fonk JC, et al. Glutamine-enriched enteral nutrition increases in vitro interferon-gamma production but does not influence the in vivo specific antibody response to KLH after severe trauma: a prospective, double blind, randomized clinical study. Clin Nutr. 2004;23(3):391–400. [DOI] [PubMed] [Google Scholar]
- 28.Yalcin SS, Yurdakok K, Tezcan I, et al. Effect of glutamine supplementation on diarrhea, interleukin-8 and secretory immunoglobulin A in children with acute diarrhea. J Pediatr Gastroenterol Nutr. 2004;38(5):494–501. [DOI] [PubMed] [Google Scholar]
- 29.Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review). J Nutr Biochem 2003;14(5):251–258. [DOI] [PubMed] [Google Scholar]
- 30.Low PP, Rutherford KJ, Gill HS, et al. Effect of dietary whey protein concentrate on primary and secondary antibody responses in immunized BALB/c mice. Int Immunopharmacol 2003;3(3):393–401. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Cano FJ, Marin-Gallen S, Castell M, et al. Supplementing suckling rats with whey protein concentrate modulates the immune response and ameliorates rat rotavirus-induced diarrhea. J Nutr. 2008;138(12):2392–2398. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Cano FJ, Marin-Gallen S, Castell M, et al. Bovine whey protein concentrate supplementation modulates maturation of immune system in suckling rats. Br J Nutr. 2007;98(1):S80–S84. [DOI] [PubMed] [Google Scholar]
- 33.Wong CW, Watson DL. Immunomodulatory effects of dietary whey proteins in mice. J Dairy Res. 1995;62(2):359–368. [DOI] [PubMed] [Google Scholar]
- 34.Micke P, Beeh KM, Buhl R. Effects of long-term supplementation with whey proteins on plasma glutathione levels of HIV-infected patients. Eur J Nutr. 2002;41(1):12–18. [DOI] [PubMed] [Google Scholar]
- 35.Micke P, Beeh KM, Schlaak JF, et al. Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. Eur J Clin Invest 2001;31(2):171–178. [DOI] [PubMed] [Google Scholar]
- 36.Moreno YF, Sgarbieri VC, da Silva MN, et al. Features of whey protein concentrate supplementation in children with rapidly progressive HIV infection. J Trop Pediatr 2006;52(1):34–38. [DOI] [PubMed] [Google Scholar]
- 37.Alexander JW, MacMillan BG, Stinnett JD, et al. Beneficial effects of aggressive protein feeding in severely burned children. Ann Surg 1980;192(4):505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeman SL, Fisher L, German JB, et al. Dairy proteins and the response to pneumovax in senior citizens: a randomized, double-blind, placebocontrolled pilot study. Ann N Y Acad Sci 2010;1190(1):97–103. [DOI] [PubMed] [Google Scholar]
- 39.Skelton JA, Havens PL, Werlin JL. Nutrient deficiencies in tube fed children. Clin Pediatr. 2006;45(1):37–41. [DOI] [PubMed] [Google Scholar]
- 40.Jeejeebhoy K Zinc essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5):S7–S12. [DOI] [PubMed] [Google Scholar]
- 41.Shenkin A Selenium in intravenous nutrition. Gastroenterology 2009;137(5):S61–S69. [DOI] [PubMed] [Google Scholar]
- 42.Farnaud S, Evans RW. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol Immunol. 2003;40(7):395–405. [DOI] [PubMed] [Google Scholar]
- 43.Hernell O, Lönnerdal B. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am J Clin Nutr. 2002;76(4):858–864. [DOI] [PubMed] [Google Scholar]
- 44.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H; Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302(13):1421–1428. [DOI] [PubMed] [Google Scholar]
- 45.Voiculescu C, Avrâmescu C, Radu E, Balasoiu M, Turculeanu A. Current laboratory assays and in vitro intracellular Th1 and Th2 cytokine synthesis in monitoring antiretroviral therapy of pediatric HIV infection. FEMS Immunol Med Microbiol. 2000;27(1):67–71. [DOI] [PubMed] [Google Scholar]
- 46.Bernton E, Bryant H, Holaday J, Dave J. Prolactin and prolactin secreta-gogues reverse immunosuppression in mice treated with cysteamine, glucocorticoids, or cyclosporin-A. Brain Behav Immun 1992;6(4):394–408. [DOI] [PubMed] [Google Scholar]
- 47.Bayoumi NK, Elhassan EM, Elbashir MI, Adam I. Cortisol, prolactin, cytokines and the susceptibility of pregnant Sudanese women to Plasmodium falciparum malaria. Ann Trop Med Parasitol 2009;103(2):111–117. [DOI] [PubMed] [Google Scholar]